Since its discovery in the 1950's, olefin metathesis has emerged as a valuable synthetic tool for the construction of carbon-carbon bonds.1 In particular, well-defined ruthenium catalysts 1 and 2 have received significant attention due to their high reactivity, commercial availability, and functional group tolerance.2,3
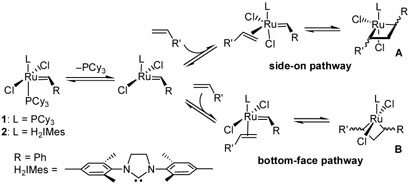
Extensive investigation into the mechanism of olefin metathesis has determined that the principal steps occur according to the Chauvin mechanism, where the reaction proceeds via the intermediacy of a metallacyclobutane complex, which subsequently collapses via a [2+2] cycloreversion to afford the olefinic product and the propagating metal carbene catalyst.4 However, for catalysts 1 and 2, there is still an ongoing discussion as to the nature of the metallacyclobutane intermediate, particularly with regard to the stereochemical orientation of the ligands (Scheme 1).5 Unfortunately, acquiring detailed data on this has proven elusive, and until recently, experimental evidence supporting metallacycle orientation in ruthenium-based systems has been indirect, relying primarily upon the translation of stereochemistry from olefinic complexes.6 While compelling, the study of such complexes primarily indicates the thermodynamic preference for olefin binding (either side-on or bottomface), not the kinetic preference for subsequent metallacycle formation.
Scheme 1.
Initial steps of the olefin metathesis mechanism.
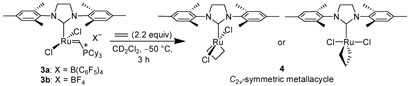
In 2005, Piers and coworkers reported the first direct observation of a ruthenium metallacycle. Upon the reaction of catalyst 3a with 2.2 equivalents of ethylene at −50 °C, a C2v-symmetric ruthenacyclobutane (4) was observed via 1H NMR spectroscopy (Scheme 2).7,8 Piers' result is suggestive of bottom-face olefin coordination and metallacycle generation, as additional shifts that could be attributed to protons lying above and below the plane of a side-bound structure were not observed (Scheme 1; A vs. B).7,9 Such an orientation is consistent with previous computational5 and crystallographic6a evidence. However, the ligand dynamics of rotation about the ruthenium-NHC (N-heterocyclic carbene) bond as well as the stereochemical orientation of substituted metallacycles remained unknown. For these reasons, we initiated a comprehensive investigation to elucidate this information.
Scheme 2.
Reaction of 3a with ethylene.
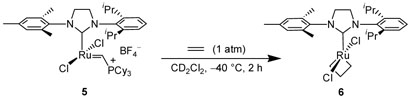
To study the dynamics of the NHC ligand, we envisioned the preparation of an unsymmetrical catalyst, thereby breaking the C2v-symmetry previously observed with metallacycle 4. To this effect, complex 5 was prepared in seven steps in 16% overall yield.9 Upon the reaction of 5 with ethylene (1 atm) at −40 °C, complete conversion to metallacycle 6 was observed after 1.5 h (Scheme 3). Metallacycle 6 possesses a distinctive two-proton signal at −2.66 ppm that corresponds to the enantiotopic βhydrogens.10,11 Analysis of the reaction mixture via 2D-COSY and ROESY NMR identified the metallacycle α-hydrogen shifts at 6.75 and 6.60 ppm, indicating that Ru-NHC rotation is sufficiently slow on an NMR timescale at −40 °C that the α-methylene groups are diastereotopic. HMQC 1H-13C analysis correlated the 6.75 and 6.60 ppm shifts with 13C shifts at 94.9 and 93.7 ppm, respectively, allowing the assignment of each α-hydrogen signal to a unique carbon resonance. These results support the trigonal bipyramidal structure proposed by Piers and coworkers7 and computationally predicted by Chen.5
Scheme 3.
Reaction of 5 with ethylene.
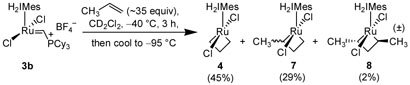
With the Ru-NHC ligand dynamics better understood, we looked to investigate the influence of metallacycle substitution on ruthenium complex stability and stereochemistry. The exposure of propene to a metathesis catalyst results in the formation of butene and ethylene via cross-metathesis. Hence, when catalyst 3b was reacted with an excess of propene at −40 °C, the presence of the ethylene-derived metallacycle 4 in 45% conversion was readily explained (Scheme 4 and Figure 1a).9,12 However, much of the mass balance of the reaction remained to be accounted for, as >95% of the starting catalyst had been consumed.
Scheme 4.
Reaction of 3b with propene.16
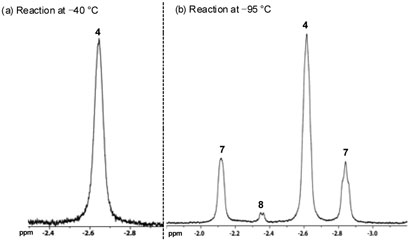
Figure 1.
Reaction of catalyst 3b with propene.
During our 1H NMR studies on metallacycle 4, it had been observed that, upon warming the reaction mixture to −20 °C, sufficient line broadening of the metallacycle peaks had occurred such that they disappeared into the baseline. Thinking that a similar scenario might be in effect, the reaction of 3b with propene was observed at lower temperatures. Gratifyingly, three new peaks at −2.11, −2.35, and −2.84 ppm were observed in the upfield region of the 1H NMR upon cooling the reaction to −95 °C (Figure 1b). The formation of these new species was found to be reversible, as warming the reaction mixture back to −40 °C afforded solely metallacycle 4 without any visible decomposition. In addition, the presence of these new peaks was found not to be solely due to ethylene, as cooling a solution of 4 to −95 °C afforded no new species. Instead, these new complexes were ultimately attributed to metallacycles derived from propene (Scheme 4).
2D-COSY, ROESY, and HMQC analysis of the reaction mixture identified the protons at −2.11 and −2.84 ppm to occupy the same carbon on propene-derived metallacycle 7, which was found to be present in 29% conversion. The doublet observed at −2.35 ppm was attributed the β-hydrogens of the C2-symmetric metallacycle 8. As with 4, metallacycles 7 and 8 lie in a bottomface orientation. Repeating the reaction in the presence of 3-13C-labeled propene confirmed that no metallacycles derived from either cis-or trans-2-butene are observable at these reaction temperatures—a result consistent with what Piers had observed in the reaction of 3a with butene at −50 °C.7 It is important to note that the trans orientation of the methyl groups in metallacycle 8 is in marked contrast to the preferred cis orientation that had been previously postulated based upon analogy to cyclobutane puckering.1b As the structure of 8 indicates, a cis/trans argument based solely upon the configurational preferences of cyclobutane is not entirely valid, particularly in view of experimental evidence that suggests the metallacycle ring is a distorted kite-shape due to a M…C² interaction.7
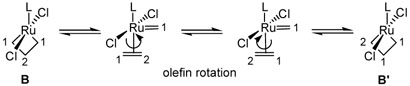
At this point, we had acquired significant insight into the Ru- NHC ligand dynamics and the configurational preferences of ruthenium metallacycles. During the course of our 2D-NMR investigations, however, we had additionally found the ±-and ²-positions of metallacycles 4 and 6 to possess exchange crosspeaks, 13,9 raising the issue of metallacycle dynamics. Taking previous mechanistic studies into account,1,4 the presence of exchange cross-peaks was strongly indicative that metallacycle cycloreversion and reformation was occurring on the NMR timescale, leading to an equilibrium between B and B' (Scheme 5). In addition, as no exchange was observed between the metallacycle and free olefin within this time period, exchange between the α- and β-positions must be attributed to rotation of the π-bound olefin between successive reaction cycles. Two-dimensional EXSY experiments on 4 were utilized to determine the rate constant (kB↔B')14 for this process to be 26 ± 2 s−1, corresponding to a ΔG≠233K = 12.18 ± 0.04 kcal/mol. Experimental evidence indicates that metallacycles derived from propene are also dynamic structures; however, further investigation is required to fully ascertain their exchange behavior. The extension of these studies to include the dynamics of substituted metallacycles derived from 5 also remains to be investigated.15
Scheme 5.
Dynamics of Ruthenium Metallacycles.
In summary, we have shown evidence supporting the bottomface orientation of ruthenium(IV) metallacycles derived from both ethylene and propene. The metallacycle structures derived from propene represent the first observed examples of substituted ruthenacyclobutanes, and offer new insight into the preferred stereochemical orientation of metathesis intermediates. In addition, we have presented new evidence that suggests that ruthenacyclobutanes derived from ethylene and propene are dynamic structures that proceed through a series of nonproductive metallacycle formations/cycloreversions prior to olefin exchange. Current efforts are underway to apply these data to the rational design of additional olefin metathesis catalysts, particularly those directed at the still unsolved problem of olefin diastereocontrol.
Supplementary Material
Acknowledgement
The authors thank Dr. Phillip Dennison and the U.C. Irvine NMR Facility for the generous donation of instrument time for the propene VT NMR experiments. Theodor Agapie is acknowledged for his instruction on Schlenk techniques for quantitated gas additions. Special thanks also goes to Professor Daniel O'Leary of Pomona College for invaluable research discussion. Funding was provided by NIH. Postdoctoral funding for AGW was provided by NIH (NRSA GM070147-02) and UNCF-Pfizer.
Footnotes
Supporting Information Available
Experimental procedures and characterization data are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Grubbs RH, editor. Handbook of Metathesis. 1-3. Wiley-VCH; Weinheim, Germany: 2003. [Google Scholar]; (b) Ivin KJ, Mol JC. Olefin Metathesis and Metathesis Polymerization. Academic Press; San Diego, CA: 1997. [Google Scholar]
- 2.(a) Schwab P, Grubbs RH, Ziller JW. J. Am. Chem. Soc. 1996;118:100–110. [Google Scholar]; (b) Nguyen ST, Grubbs RH, Ziller JW. J. Am. Chem. Soc. 1993;115:9858–9859. [Google Scholar]
- 3.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 4.Hérisson JL, Chauvin Y. Makromol. Chem. 1971;141:161–176. [Google Scholar]
- 5.Adlhart C, Chen P. J. Am. Chem. Soc. 2004;126:3496–3510. doi: 10.1021/ja0305757. [DOI] [PubMed] [Google Scholar]
- 6.(a) Tallarico JA, Bonitatebus PJ, Jr., Snapper ML. J. Am. Chem. Soc. 1997;119:7157–7158. [Google Scholar]; (b) Anderson DR, Hickstein DD, O'Leary DJ, Grubbs RH. J. Am. Chem. Soc. 2006;128:8386–8387. doi: 10.1021/ja0618090. [DOI] [PubMed] [Google Scholar]
- 7.Romero P, Piers WE. J. Am. Chem. Soc. 2005;127:5032–5033. doi: 10.1021/ja042259d. [DOI] [PubMed] [Google Scholar]
- 8. We have subsequently confirmed the presence of 4 under these conditions via mass spectrometry. Refer to the Supporting Information.
- 9. Refer to the Supporting Information.
- 10.Mashima K, Kaidzu M, Nakayama Y, Nakamura A. Organometallics. 1997;16:1345–1348. [Google Scholar]
- 11.Howard TR, Lee JB, Grubbs RH. J. Am. Chem. Soc. 1980;102:6878–6880. [Google Scholar]
- 12. Relative to anthracene as an internal standard.
- 13.Neuhaus D, Williamson MP. The Nuclear Overhauser Effect in Structural and Conformational Analysis. 2nd. Wiley-VCH; New York: 2000. Chapter 8. [Google Scholar]
- 14. The kB↔B′ rate constant includes the rates of metallacycle cycloreversion, olefin rotation, and metallacycle formation to interconvert positions 1 and 2 in Scheme 5.
- 15. Examples of additional olefins that we have thus far investigated: 1,1-difluoroethylene, vinyl fluoride, ethyl vinyl ether, neohexene, acenaphthylene, 2-butyne, and methyl acrylate.
- 16. Propene was added in excess (35 equiv) to both minimize the conversion of 3b into a bis-ruthenium μ-Cl decomposition product and prevent the reaction mixture from freezing.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.