Abstract
Background
The need for pulsatility in the circulation during long-term mechanical support has been a subject of debate. We compared histological changes in calf renal arteries subjected to various degrees of pulsatile circulation in vivo. We addressed the hypothesis that the local reninangiotensin system (RAS) may be implicated in these histological changes.
Methods and Results
Sixteen calves were implanted with devices giving differing degrees of pulsatile circulation: six had a continuous flow left ventricular assist device (LVAD); six had a continuous flow right ventricular assist device (RVAD); and four had a pulsatile total artificial heart (TAH). Six other calves were histological and immunohistochemical controls. In the LVAD group, the pulsatility index was significantly lower (0.28 ± 0.07 LVAD vs 0.56 ± 0.08 RVAD, vs 0.53 ± 0.10 TAH; p < 0.01), and we observed severe periarteritis in all cases in the LVAD group. The number of angiotensin II type 1 receptor (AT1R)-positive cells and angiotensin converting enzyme (ACE)-positive cells in periarterial areas was significantly higher in the LVAD group (AT1R: 350 ± 139 LVAD vs 8 ± 6 RVAD, vs 3 ± 2 TAH, vs 3 ± 2 in control; p < 0.001 and ACE: 325 ± 59 LVAD vs 6 ± 4 RVAD, vs 6 ± 5 TAH, vs 3 ± 1 control; p < 0.001).
Conclusions
The reduced pulsatility produced by a continuous flow LVAD implantation induced severe periarteritis in the kidney. The local RAS was upregulated in the inflammatory cells only in the continuous flow LVAD group.
ULTAMINI-ABSTRACT
We compared histological changes in calf renal arteries subjected to various degrees of pulsatile circulation; continuous flow left ventricular assist device (LVAD), continuous flow right ventricular assist device, pulsatile total artificial heart and control. We observed severe periarteritis, and upregulation of local renin angiotensin system only in the LVAD group. The necessity of maintaining pulsatility in the systemic circulation during long-term mechanical support has been a subject of debate. Recently, simpler and smaller continuous flow blood pumps have become more prevalent. The diminished pulsatility created by support from a continuous flow left ventricular assist device (LVAD) is physiologically abnormal, and some changes to the morphology of the aortic wall and the renal artery have been reported.1,2 Continuous flow LVAD support has been reported to cause renal cortical artery hypertrophy and inflammatory cell infiltration in the renal cortex.2 However, the mechanisms leading to those morphological changes are still unclear.
In this study, we examined the renal arteries of calves implanted with several different types of circulatory support devices delivering various degrees of systemic arterial pulsatility. We observed severe inflammatory and morphological changes in the kidney only in those calves with continuous flow LVADs. In addition to its pressor effect, angiotensin II (Ang II), the physiologically active component of the renin-angiotensin system (RAS), has a variety of nonhemodynamic actions, including cell growth as well as pro-inflammatory and pro-fibrogenic actions through the Ang II type 1 receptors (AT1R).3 Many of these actions are associated with cardiovascular and renal pathology.4 Many tissues are thought to be capable of local Ang II production via tissue-specific local RAS.5–9 This locally produced Ang II acts on tissue through resident Ang II receptors. We directed our attention to the local RAS as a possible mechanism for those pathological changes, and we demonstrated by immunohistochemical analysis that AT1R and angiotensin converting enzyme (ACE) were found in inflammatory cells that had infiltrated the kidneys of calves with continuous flow LVADs.
Methods
Animals and Device Description
Twenty two male Holstein calves (99.8 ± 16.7 kg) were used in this study. A continuous flow LVAD was implanted in six calves creating the condition of reduced systemic arterial pulsatile perfusion; a continuous flow right ventricular assist device (RVAD) was implanted in six calves representing the condition of pulsatile perfusion and as a biomaterial control. The RVAD did produce reduced pulsatility in the pulmonary circulation; however, pulsatility was maintained in the systemic circulation. A pulsatile total artificial heart (TAH) was implanted in four calves to study pulsatile perfusion. Six normal calves were used as normal histological and immunohistochemical controls. As shown in Table 1, the devices were implanted for various durations ranging from 22 to 95 days.
TABLE 1.
Characteristics of Calves Implanted with Mechanical Support Devices and Normal Control Calves
| No. | Implant Duration (days) | Body Weight (kg) | |
|---|---|---|---|
| LVAD (n = 6) | 1 | 39 | 84.0 |
| 2 | 29 | 93.0 | |
| 3 | 31 | 83.0 | |
| 4 | 31 | 86.0 | |
| 5 | 30 | 92.0 | |
| 6 | 95 | 83.0 | |
| AVE. | 42.5 ± 26.0 | 86.8 ± 4.5 | |
| RVAD (n = 6) | 1 | 30 | 93.0 |
| 2 | 29 | 96.0 | |
| 3 | 25 | 160.0 | |
| 4 | 22 | 95.6 | |
| 5 | 30 | 96.9 | |
| 6 | 30 | 113.2 | |
| AVE. | 27.7 ± 3.4 | 109.1 ± 26.0 | |
| TAH (n = 4) | 1 | 83 | 105.0 |
| 2 | 28 | 113.0 | |
| 3 | 92 | 110.5 | |
| 4 | 67 | 110.0 | |
| AVE. | 67.5 ± 28.3 | 109.6 ± 3.4 | |
| Control (n = 6) | 1 | - | 100.0 |
| 2 | - | 90.0 | |
| 3 | - | 90.0 | |
| 4 | - | 110.5 | |
| 5 | - | 90.0 | |
| 6 | - | 101.0 | |
| AVE. | - | 96.9 ± 8.4 | |
LVAD, left ventricular assist device; RVAD, right ventricular assist device; TAH, total artificial heart.
Animal Care
This study was approved by the Cleveland Clinic’s Institutional Animal Care and Use Committee, and all animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press, revised 1996.
Implant Procedure
Upon arrival at the facility, all calves were quarantined for at least 14 days in the Biological Resources Unit. The calves were fasted 12 hours prior to surgery. Anesthesia was induced with ketamine, 10 mg/kg i.m., and isoflurane via mask inhalation. The animal was then intubated, and anesthesia was maintained with isoflurane (1.0–2.0%) and oxygen.
LVAD Implantation
The CorAide™ continuous flow left ventricular assist system (LVAS; Arrow International, Reading, PA), originally developed at the Cleveland Clinic, is a centrifugal continuous flow pump.10 Through a left thoracotomy, the outflow graft of the pump was anastomosed to the descending aorta, and the inflow cannula of the pump was inserted into the left ventricle. An ultrasonic perivascular flow probe (Transonic Systems Inc, Ithaca, NY) was placed around the outflow graft for continuous monitoring of the pump output. Arterial pressure was measured in the carotid artery by a fluid-filled pressure monitoring line. After surgery, intravenous nitroprusside was administered to maintain mean arterial pressure at less than 125 mm Hg and pump flows at greater than 3.5 L/min.
RVAD Implantation
The Cleveland Clinic’s DexAide blood pump is a centrifugal, continuous flow blood pump, constructed of the same materials as CorAide LVAS,11 but with a modified design. Through a left thoracotomy, the outflow graft of the pump was anastomosed to the pulmonary artery, and the inflow cannula of the pump was inserted into the right ventricle under cardiopulmonary bypass (CPB) support. An ultrasonic perivascular flow probe was placed around the outflow graft. Systemic arterial pressure was measured in the carotid artery by a fluid-filled pressure monitoring line. After CPB was discontinued, the RVAD was initiated.
TAH Implantation
The Cleveland Clinic’s MagScrew TAH is an implantable pulsatile TAH system.12 A right fourth intercostal thoracotomy was performed. Under CPB support, the native ventricles were removed. The right and left outflow grafts were anastomosed to the main pulmonary artery and the ascending aorta, respectively, and the inflow cuffs were anastomosed to the left and right atria. Bioprosthetic valves (Edwards Lifesciences 6900 P series, Edwards Lifesciences, Irvine, CA) were placed into the inflow and outflow portions of the TAH. Systemic arterial pressure was measured in the carotid artery by a fluid-filled pressure monitoring line. As CPB was discontinued, the TAH was initiated.
Postoperative Hemodynamic Data and Renal Function
After surgery, the calves were transferred to a chronic care facility and maintained in cages with continuous hemodynamic monitoring. An Astro-Med MT95K2, 16-channel data acquisition and recording system (Astro-Med, Inc, West Warwick, RI) continuously recorded arterial pressure, electrocardiogram, and pump flow. Mean values for arterial pressure, pump flow, and pump speed were recorded hourly. Systemic arterial pressure pulsatility was quantified by pulse pressure (systolic pressure - diastolic pressure) and pulsatility index (pulse pressure/mean arterial pressure). Renal function was evaluated weekly by blood urea nitrogen and serum creatinine analysis.
Autopsy
At the completion of each study, after full heparinization (500 U/kg bolus injection), the animal was sacrificed with an overdose of sodium pentobarbital (5,000 mg) and potassium chloride (240 mEq), and a thorough autopsy was performed. The renal issue specimens included medulla and cortex samples from both the right and left kidneys.
Pathological Study
All renal tissues specimens were fixed in 10% formaldehyde. Four-micrometer-thick transverse sections were stained with hematoxylin-eosin (HE) and periodic acid/Schiff for light microscopy evaluation. To quantify morphological changes, HE-stained sections were scanned at 100x magnification with a light microscope equipped with Retiga Exi Fast 1394 camera (Qimaging, Burnaby, British Columbia, Canada). The arterial wall diameter, wall thickness, ratio of wall thickness/wall diameter, wall cross-sectional area, and ratio of smooth muscle layer crosssectional area/number of smooth muscle cells were measured on all arteries in the renal corticomedullary junction using Image-Pro Plus (Media Cybernetics, Silver Spring, ML). The average values for each animal were reported as an average of the 20–40 arterial cross-sections examined. The wall area was defined as the region between the endothelium and external elastic lamina. The smooth muscle layer was defined as the region between the internal and external elastic lamina. The wall cross-sectional area of the renal arteries was determined as the mean of the measurements of the two opposite walls in the direction of the minimal diameter, since that was the direction in which measurements are least affected by the sectioning angle.13
Immunohistochemical Study
Four-micrometer-thick sections, cut from paraffin blocks of the renal tissues, were used. The tissue sections were immunostained by rabbit anti-AT1R antibody (ab18801, Abcam, Cambridge, UK), mouse anti-ACE antibody (MAB3502, Chemicon, Temecula, CA) and by rabbit polyclonal to endothelial nitric oxide synthase (eNOS) (ab5589, Abcam, Cambridge, UK). The tissue sections were deparaffinized in xylene and descending grades of alcohol. The retrieval of the antigen was performed by treating the slides in 10 mM citrate buffer, pH 6.0, in a 120 °C autoclave for 5 min (for ACE antibody and eNOS) or in 0.025% trypsin and 0.1% CaCl2 2H20 in 50 mM Tris buffer, pH 7/6, in a 37° water bath for 30 minutes (for AT1R antibody). After blocking the endogenous peroxidase activity, the primary and then secondary antibody were added. Antibody binding was visualized by adding diaminobenzidine as a chromogen and hematoxylin for counterstaining. To quantify the number of inflammatory cells present, AT1R-positive cells and ACE-positive cells were measured on 20 different sections that included the wall of an arcuate artery or interlobular artery at a final magnification of 200:1 by using Image-Pro Plus Image plus (Media Cybernetics, Silver Spring, ML). The average number of positive cells per section was reported for each animal.
Statistical Analysis
All data were expressed as mean value ± standard deviation. Statistical analyses were performed with a commercially available software program (StatView 5.0, SAS Institute Inc, Cary, NC). Differences among groups were assessed by one-way analysis of variance followed by the Bonferroni’s multiple comparison tests. A probability value of <0.05 was considered significant.
Results
Hemodynamic Data and Renal Function
A summary of hemodynamic characteristics obtained during the course of each experiment is given in Table 2. The pulse pressures of the LVAD group were significantly smaller than in the other groups (28.3 ± 8.0 mm Hg LVAD, 52.7 ± 7.7 mm Hg RVAD, 49.5 ± 6.9 TAH; p < 0.01). In the LVAD group, the pulsatility index was also significantly lower than in other groups (0.28 ± 0.07 LVAD, 0.56 ± 0.08 RVAD, 0.53 ± 0.10 TAH; p < 0.01). The continuous flow RVAD pump produced reduced pulsatility in the pulmonary circulation; however, pulsatility was maintained in the systemic circulation. There was no significant difference in the mean arterial pressures. In the TAH group, the beat rate was significantly higher (217.8 ± 5.9 beats/min TAH, 101.3 ± 10.0 beats/min LVAD, 88.6 ± 22.7 beats/min RVAD; p < 0.001). The pump flow was 6.1 ± 1.0 L/min in the LVAD group, 6.1 ± 1.4 L/min in the RVAD group, and 10.0 ± 0.6 L/min in the TAH group.
TABLE 2.
Hemodynamics and Renal Function Data
| Variables | LVAD | RVAD | TAH |
|---|---|---|---|
| Arterial pressure (mm Hg) | |||
| Systole | 116.0 ± 9.6 | 125.8 ± 7.1 | 126.4 ± 5.7 |
| Mean | 98.5 ± 8.0 | 95.1 ± 6.3 | 97.8 ± 5.4 |
| Diastole | 89.1 ± 7.0 | 73.3 ± 4.4 | 76.9 ± 7.3 |
| Heart rate (beats/min) | 101.3 ± 10.0 | 88.6 ± 22.7 | 217.8 ± 5.9* |
| Pulse pressure (mm Hg) | 28.3 ± 8.0† | 52.7 ± 7.7 | 49.5 ± 6.9 |
| Pulsatility index | 0.28 ± 0.07† | 0.56 ± 0.08 | 0.53 ± 0.10 |
LVAD, left ventricular assist device; RVAD, right ventricular assist device; TAH, total artificial heart.
p < 0.001 vs other groups
p < 0.01 vs other groups.
Pathological Study
All six calves that received a continuous flow LVAD implantation showed periarteritis in the kidneys (Fig. 1 A). These results were not found in the other groups (Fig. 1 B–D). The major pathological findings were these: The medium-sized arteries, such as the arcuate and interlobular arteries, showed wall thickening (Fig. 2 A), with abundant mononuclear cells infiltrating the periarterial areas (Fig. 2 B). The arterial structure was relatively conserved and showed fewer inflammatory cells in the vascular walls.
Fig. 1.

Hematoxylin-eosin (HE) staining of renal arteries in the corticomedullary junction area (magnification x40). (A) Continuous flow left ventricular assist device (LVAD) implanted calf. Extensive hyperplasia of the intima, media, and adventitia of the arteries is evident, along with mononuclear inflammatory cell infiltrates. (B) Continuous flow right ventricular assist device (RVAD) implanted calf. (C) Pulsatile total artificial heart (TAH) implanted calf. (D) Control normal calf. There are no morphological changes, suggesting that periarteritis exists in the other groups (B, C, D).
Fig. 2.

HE staining of kidney in continuous flow LVAD implanted calf. (A) The corticomedullary junction area (magnification x40). The medium-size arteries, such as the arcuate artery and interlobular arteries, showed wall thickening and the presence of inflammatory cells. (B) The arcuate artery: expanded view of area encircled with yellow line in A (magnification x100), showing abundant mononuclear cells accumulating in the periarterial area. The artery structure is relatively conserved and has few inflammatory cells in the vascular wall. There is extensive hyperplasia of the smooth muscle layer of the media of the vessels.
Morphometry of Renal Arteries
Compared with the other groups, calves in the continuous flow LVAD group exhibited significantly greater wall diameter, wall thickness, and wall cross-sectional area (Table 3). There was no increase in the smooth muscle layer cross-sectional area/number of smooth muscle cell count ratio in the LVAD group, suggesting that the increased wall thickness was due to hyperplasia, but not hypertrophy.
TABLE 3.
Morphometric Data
| LVAD | RVAD | TAH | Control | |
|---|---|---|---|---|
| Wall diameter (µm) | 310.8 ± 56.8‡ | 242.5 ± 36.5 | 225.7 ± 18.1 | 194.2 ± 15.6 |
| Wall thickness (µm) | 121.9 ± 27.1† | 88.7 ± 15.0 | 75.1 ± 14.0 | 63.3 ± 7.0 |
| Wall thickness/diameter | 0.38 ± 0.01 | 0.35 ± 0.01 | 0.32 ± 00.3 | 0.34 ± 0.03 |
| Wall CSA (mm2) | 13.6 ± 5.1‡ | 9.1 ± 3.1 | 6.5 ± 1.7 | 4.8 ± 0.5 |
| SML CSA/nSMC (µm/cell) | 477 ± 220 | 435 ± 107 | 615 ± 497 | 330 ± 75 |
LVAD, left ventricular assist device; RVAD, right ventricular assist device; TAH, total artificial heart; CSA, cross-sectional area; SML, smooth muscle layer; nSMC, number of smooth muscle cells.
p < 0.01 vs other groups
p < 0.05 vs other groups.
Immunohistochemical Findings
AT1R was observed in the endothelial and inflammatory cells that infiltrated the periarterial and cortical interstitial areas of all animals in the LVAD group (Fig. 3 C, D). ACE was observed in renal tubuli, endothelial cells of the renal arteries, and inflammatory cells that infiltrated the periarterial and cortical interstitial areas of all animals in the LVAD group (Fig. 3 E, F). In other groups, ACE expression was seen only in the renal tubuli and in endothelial cells of renal arteries, and there were few ACE-positive reactions in inflammatory cells.
Fig. 3.
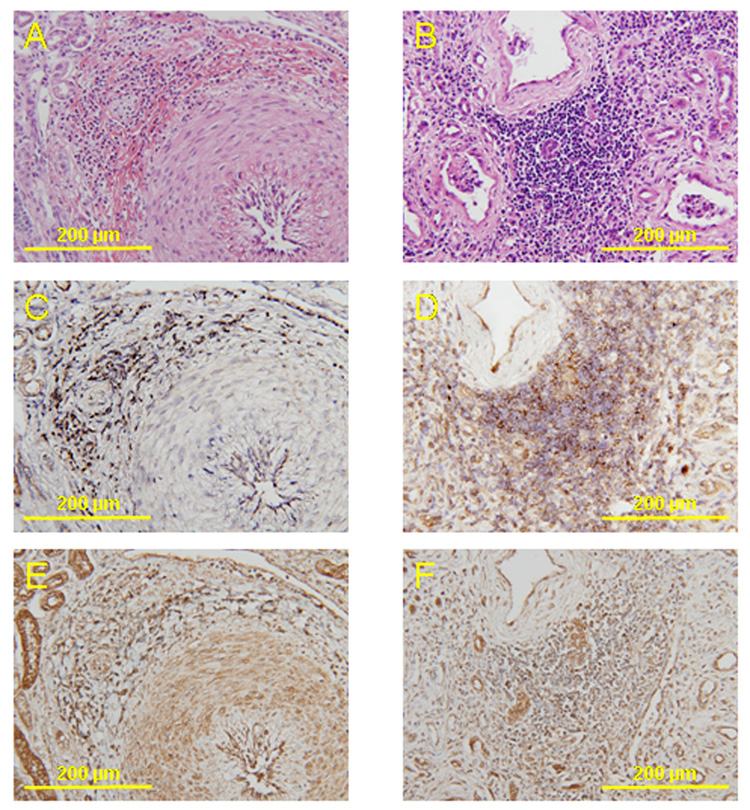
Histopathology and immunohistochemistry of serial sections of renal arteries from a calf implanted with a continuous flow LVAD (magnification x200). HE staining of the interlobular artery (A) and cortical interstitial area (B). Immunohistochemical staining for angiotensin II type 1 receptor (AT1R) (C, D) and angiotensin-converting enzyme (ACE) (E, F). AT1R is observed in the endothelial cells and inflammatory cells that infiltrated the periarterial area (C) and cortical interstitial area (D). ACE is observed in renal tubuli, endothelial cells, the smooth muscle layer and in inflammatory cells that had infiltrated the periarterial area (E) and cortical interstitial area (F).
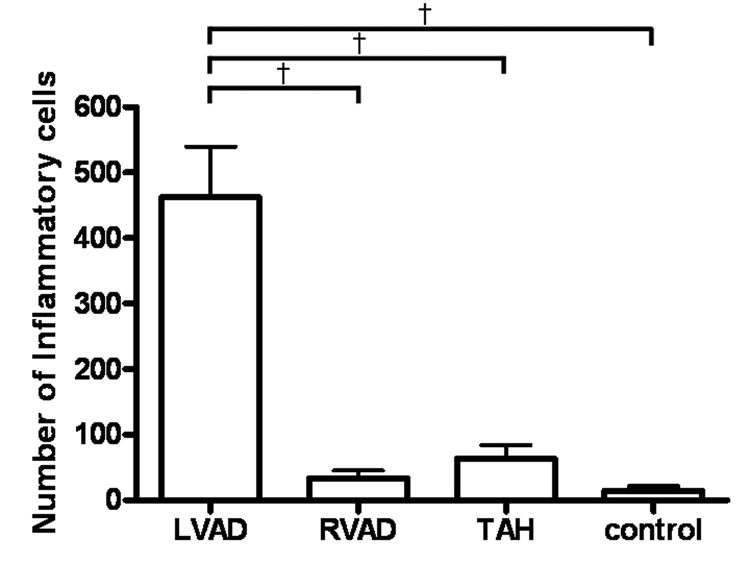
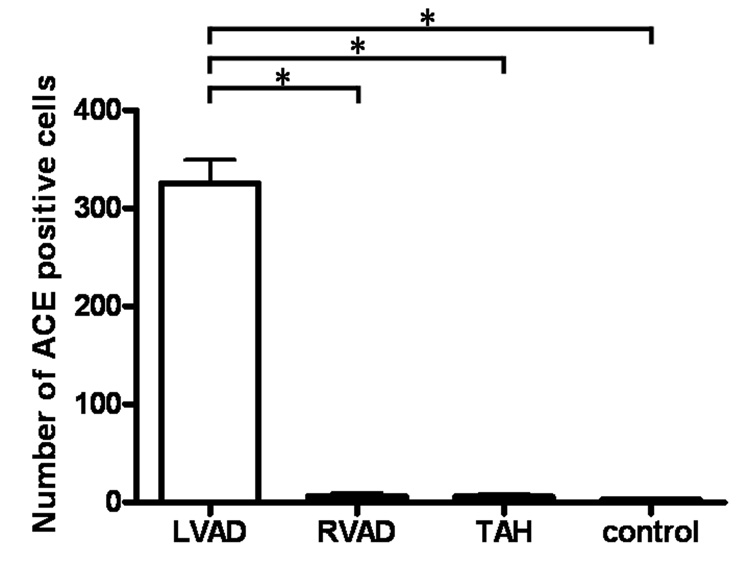
The average number of inflammatory cells in periarterial areas (pa) was significantly higher in the LVAD group (462 ± 190 cells/pa LVAD, 31 ± 21 cells/pa RVAD, 63 ± 36 cells/pa TAH, 13 ± 12 cells/pa control; p < 0.01; Fig. 4). The average number of AT1R-positive cells in periarterial areas was significantly higher in the LVAD group (350 ± 139 cells/pa LVAD, 8 ± 6 cells/pa RVAD, 3 ± 2 cells/pa TAH, 3 ± 2 cells/pa in control; p < 0.001; Fig. 5). The average number of ACE-positive cells in periarterial areas was significantly higher in the LVAD group (325 ± 59 cells/pa LVAD, 6 ± 4 cells/pa RVAD, 6 ± 5 cells/pa-TAH, 3 ± 1 cells/pa control; p < 0.001; Fig. 6). We observed a few infiltrated inflammatory cells in the TAH group; however we did not observe visible AT1R or ACE activation in those inflammatory cells.
Fig. 4.
The number of inflammatory cells in periarterial areas from the continuous flow LVAD group was significantly higher than in the other groups. † p < 0.01.
Fig. 5.
The number of AT1R-positive cells in periarterial areas from the continuous flow LVAD group was significantly higher than in the other groups. * p < 0.001.
Fig. 6.
The number of ACE-positive cells in periarterial areas from the continuous flow LVAD group was significantly higher than in the other groups. * p < 0.001.
The immunohistochemical study showed a prominent expression of eNOS in the endothelium of renal arteries from the LVAD group (Fig. 7 A) that appeared more prominent compared to the other groups (Fig. 7 B, C, D).
Fig. 7.
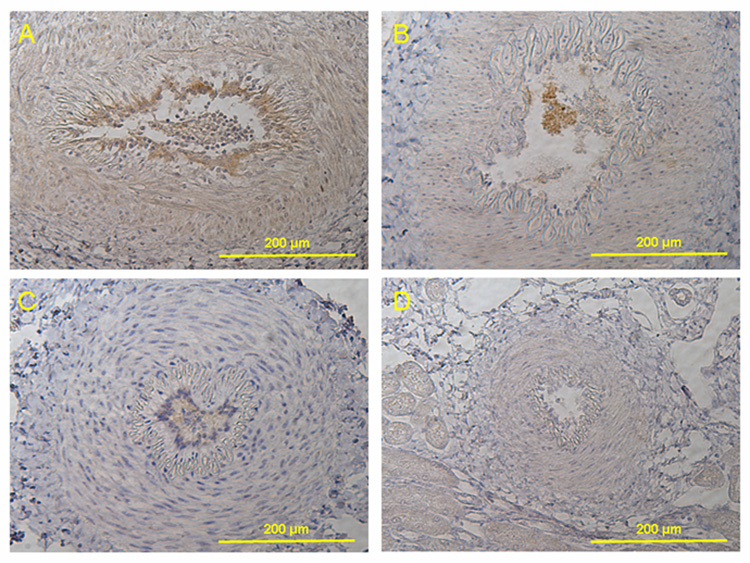
Immunohistochemical staining for endothelial nitric oxide synthase (eNOS) (magnification x200). (A) Continuous flow LVAD implanted calf. The immunohistochemical study showed a prominent expression of eNOS in the endothelium of renal arteries that appeared more prominent in LVAD group than other group. (B) Continuous flow RVAD implanted calf. (C) Pulsatile TAH implanted calf. (D) Control normal calf.
Discussion
This study yielded two major findings: (1) Periarteritis in the kidney occurred only in the group implanted with a continuous flow LVAD and (2) AT1R and ACE were upregulated in mononuclear inflammatory cells in the kidney of the continuous flow LVAD group. The significant hemodynamic differences between the LVAD group and the other groups were lower pulsatility and pulse pressure, but not mean arterial pressure.
Whether or not one needs to maintain pulsatility in the systemic circulation has been a subject of lively discussion. It is known that flow pulsatility can influence cell signaling. For example, cyclic shear stress markedly elevates eNOS activity and is associated with more sustained cytosolic calcium transients.14,15 Sustained exposure to phasic elevated hydrostatic pressure stimulates endothelial secretion of an antiproliferative factor.16 Although numerous research efforts focus on the influence of mechanical forces on gene expression and signaling through use of in vitro models of cyclic mechanical stretching on cultured vascular cells, no reliable nonpulsatile in vivo models have yet been established. Continuous flow LVAD support has been reported to cause renal cortical artery hypertrophy and inflammatory cell infiltration in the renal cortex.2 However, the mechanisms leading to this altered morphology are still unclear. In this study, we investigated the effects of pulsatility in vivo on the kidney by comparing renal histological findings for differing degrees of pulsatility produced following the implantation of different types of circulatory support devices. Our histological analysis at multiple levels of the renal artery revealed periarteritis with smooth muscle layer hyperplasia in the animals implanted with an LVAD that produced the greatest degree of chronic reduced systemic arterial pulsatility.
We detected AT1R and ACE in mononuclear cells in the kidney after exposure to reduced pulsatile circulation. In recent years, the role of the local RAS has gained considerable attention, especially in the heart, brain, eye, testis, and kidney.5–9 Local foci of the RAS generate Ang II, which acts upon resident Ang II receptors. In addition to its pressor effect, Ang II is a factor in a variety of nonhemodynamic actions, including pro-inflammatory, pro-fibrogenic actions and cell growth through an AT1R, many of which are associated with cardiovascular and renal pathology.4 For example, several reports have indicated the possible role of Ang II in inducing chemoattractants for monocyte/macrophage infiltration in a rabbit model of early accelerated arteriosclerosis and in a rat model of immune complex nephritis.17,18 Ang II modulates the proliferation of vascular smooth muscle cells and causes marked thickening of the vascular wall.19–21 We observed many pathological changes that can be explained by Ang II actions: 1) mononuclear cell infiltration into periarterial and interstitial areas, 2) wall thickening and vascular smooth muscle cell hyperplasia in the arcuate and interlobular arteries. One report stated that shear stress reduced ACE activity in endothelial cells.22 This result suggests that hemodynamic forces regulate the local RAS. One possible explanation for the pathological changes in this study is that the state of reduced pulsatile circulation may activate the local RAS and elevate the Ang II level, which in turn may have induced an inflammatory reaction and vascular proliferation in the kidney.
In this study, the outflow graft of the LVAD was anastomosed to the descending thoracic aorta; whereas the majority of LVADs are likely to be anastomosed to the ascending aorta during a clinical implantation. Our healthy calf heart model with a descending aorta anastomosis is a study limitation. One group reported that at moderate levels of continuous flow LVAD support (25% to 40% of cardiac output), the amount of blood flow distal to the outflow graft anastomosis decreased approximately 25% due to increased regurgitant blood flow in the aorta.23 We considered the possibility of systemic RAS activation after LVAD implantation as a result of decreased renal perfusion and ischemic changes in the kidneys. However, the pathological diagnosis and the histological changes in the kidney included mainly periarteritis and not ischemic changes. The systemic RAS is an endocrine system responsible for correcting acute hypotension through changes in peripheral vascular resistance and electrolyte homeostasis.24,25 Renin release is mainly regulated by reduced renal blood pressure; renal blood flow plays only a minor role in renin release.26 We believe that our findings of AT1R and ACE upregulation in the periarterial and cortical interstitial inflammatory cells and the fact that there was no difference in the mean arterial pressure between the groups further supports the hypothesis that the local RAS might play a major role in these histological changes. However, more focused experiments will be needed to evaluate the relative contribution of systemic and local RAS factors, including measurements of serum RAS levels and renal arterial flow and pressure after continuous flow LVAD implantations.
There are some reports on the positive effects of pulsatility on the eNOS gene expression in cultured endothelial cells and isolated blood vessels.14, 27–28 Nakano et al.29 showed that increased release of nitric oxide (NO) and the activation of eNOS resulted after pulsatile pump implantation in their acute studies. However, our immunohistochemical study showed a prominent expression of eNOS in the endothelium of renal arteries after chronic continuous flow LVAD implantation. Recent in vivo studies have shown that Ang II stimulates an increase in eNOS mRNA and NO production. The prominent expression of eNOS in the renal artery endothelium of this study could be explained by intervention of the local RAS.30
Implanted mechanical circulatory support systems have an inherent potential to cause systemic inflammatory responses. Ankersmit and associates31 reported that transient immunologic activation was observed in patients receiving a continuous flow LVAD. The authors suspected that the presence of inflammatory cells may indicate immunologic reactions. Since the LVAD and RVAD pumps used in this study were both centrifugal continuous flow pumps of the same volume and weight and were manufactured from the same material, both pumps can be regarded as the same antigen for the animals in this study. However, only those animals implanted with an LVAD showed inflammatory changes. Based on this result, we believe that an immunologic reaction to the LVAD blood pump was not primarily responsible for the histological changes observed in the kidney.
The positive effects of treatment with ACE inhibitors or angiotensin receptor blockers for heart failure patients have been reported.32–34 Most patients who are implanted with a continuous flow LVAD take these medications as part of a standard therapeutic regimen to suppress the progression of heart failure, and there is a possibility that these therapies incidentally prevent inflammatory changes such as periarteritis in the kidney. Further research will be needed to determine the effects of ACE inhibitors or angiotensin receptor blockers on the mechanisms of these pathological changes.
As mentioned earlier, a limitation to this study is the fact that we did not measure the systemic RAS level and that the systemic arterial pressure was measured by a fluid-filled pressure-monitoring line in the carotid artery. Assessment of the histological changes in the kidney would best be evaluated by a direct comparison renal arterial pressure and flow measurements. Also the immunohistochemical analyses have the possibility of nonspecific binding. A second method to confirm activation of the local RAS would strengthen this analysis. Six normal calves were used as normal histological and immunohistochemical controls; however we did not record hemodynamic control data from them. Incorporation of these changes into in vivo protocols is intended for further prospective studies.
In conclusion, only the continuous flow LVAD group demonstrated renal periarteritis, upregulation of the local RAS in inflammatory cells and reduced systemic arterial pressure pulsatility. The study design ruled out a systemic inflammatory response due to the blood pump biomaterials and supports the hypothesis that activity of the local RAS secondary to decreased renal arterial pressure pulsatility is the primary factor leading to these pathologic findings.
Abbreviations and Acronyms
- ACE
angiotensin converting enzyme
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- CPB
cardiopulmonary bypass
- eNOS
endothelial nitric oxide synthase
- HE
hematoxylin-eosin
- LVAD
left ventricular assist device
- LVAS
left ventricular assist system
- NO
nitric oxide
- PA
periarterial areas
- RAS
renin angiotensin system
- RVAD
right ventricular assist device
- TAH
total artificial heart
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nishimura T, Tatsumi E, Takaichi S, Taenaka Y, Wakisaka Y, Nakatani T, Masuzawa T, Takewa Y, Nakamura M, Endo S, Nakata M, Takano H. Prolonged nonpulsatile left heart bypass with reduced systemic pulse pressure causes morphological changes in the aortic wall. Artif Org. 1998;22:405–410. doi: 10.1046/j.1525-1594.1998.06137.x. [DOI] [PubMed] [Google Scholar]
- 2.Kihara S, Litwak KN, Nichols L, Litwak P, Kameneva MV, Wu Z, Kormos RL, Griffith BP. Smooth muscle cell hypertrophy of renal cortex arteries with chronic continuous flow left ventricular assist. Ann Thorac Surg. 2003;75:178–183. doi: 10.1016/s0003-4975(02)04087-0. [DOI] [PubMed] [Google Scholar]
- 3.Leung PS. The peptide hormone angiotensin II: its new functions in tissues and organs. Curr Protein Pept Sci. 2004;5:267–273. doi: 10.2174/1389203043379693. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 5.Dostal DE, Baker KM. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res. 1999;85:643–650. doi: 10.1161/01.res.85.7.643. [DOI] [PubMed] [Google Scholar]
- 6.Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, et al. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol. 1996;80:159–163. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung PS, Wong TP, Lam SY, Chan HC, Wong PY. Testicular hormonal regulation of the renin-angiotensin system in the rat epididymis. Life Sci. 2000;66:1317–1324. doi: 10.1016/s0024-3205(00)00439-2. [DOI] [PubMed] [Google Scholar]
- 9.Gomez RA, Lynch KR, Chevalier RL, Wilfong N, Everett A, Carey RM, et al. Renin and angiotensinogen gene expression in the maturing rat kidney. Am J Physiol. 1988;254:582–587. doi: 10.1152/ajprenal.1988.254.4.F582. [DOI] [PubMed] [Google Scholar]
- 10.Ochiai Y, Golding LA, Massiello AL, Medvedev AL, Horvath DJ, Gerhart RL, et al. Cleveland Clinic CorAide blood pump circulatory support without anticoagulation. ASAIO J. 2002;48:249–252. doi: 10.1097/00002480-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Fukamachi K, Horvath DJ, Massiello AL, Ootaki Y, Kamohara K, Akiyama M, et al. Development of a small implantable right ventricular assist device. ASAIO J. 2005;51:730–735. doi: 10.1097/01.mat.0000181031.66900.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber S, Kamohara K, Klatte RS, Luangphakdy V, Flick C, Chen JF, et al. MagScrew TAH: an update. ASAIO J. 2005;51:xxxvi–xlvi. doi: 10.1097/01.mat.0000187395.29817.36. [DOI] [PubMed] [Google Scholar]
- 13.Amann K, Gharehbaghi H, Stephen S, Mall G. Hypertrophy and hyperplasia of smooth muscle cells of small intramyocardial arteries in spontaneously hypertensive rats. Hypertension. 1995;25:124–131. doi: 10.1161/01.hyp.25.1.124. [DOI] [PubMed] [Google Scholar]
- 14.Nori M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M, et al. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res. 1995;76:536–543. doi: 10.1161/01.res.76.4.536. [DOI] [PubMed] [Google Scholar]
- 15.Helmlinger G, Berk BC, Nerem RM. Calcium responses of endothelial cell monolayers subjected to pulsatile and steady laminar flow differ. Am J Physiol. 1995;269:367–375. doi: 10.1152/ajpcell.1995.269.2.C367. [DOI] [PubMed] [Google Scholar]
- 16.Vouyouka AG, Powell RJ, Ricotta J, Chen H, Dudrick DJ, Sawmiller CJ, et al. Ambient pulsatile pressure modulates endothelial cell proliferation. J Mol Cell Cardiol. 1998;30:609–615. doi: 10.1006/jmcc.1997.0625. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Presa M, Bustos C, Ortego M, Tunon J, Renedo G, Ruiz-Ortega M, et al. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–1541. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Ortega M, Bustos C, Hernandez-Presa MA, Lorenzo O, Plaza JJ, Egido J. Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 synthesis. J Immunol. 1998;161:430–439. [PubMed] [Google Scholar]
- 19.Geisterfer AAT, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 20.Powell JS, Clozel JP, Muller RK, Kuhn H, Hefti F, Hosang M, et al. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989;245:186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- 21.Campbell-Boswell M, Robertson AL., Jr Effects of angiotensin II and vasopressin on human smooth muscle cells in vitro. Exp Mol Pathol. 1981;35:265–276. doi: 10.1016/0014-4800(81)90066-6. [DOI] [PubMed] [Google Scholar]
- 22.Rieder MJ, Carmona R, Krieger JE, Pritchard KA, Jr, Greene AS. Suppression of angiotensin-converting enzyme expression and activity by shear stress. Circ Res. 1997;80:312–319. doi: 10.1161/01.res.80.3.312. [DOI] [PubMed] [Google Scholar]
- 23.Litwak KN, Kihara S, Kameneva MV, Litwak P, Uryash A, Wu Z, et al. Effects of continuous flow left ventricular assist device support on skin tissue microcirculation and aortic hemodynamics. ASAIO J. 2003;49:103–107. doi: 10.1097/00002480-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1997;57:313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 25.Reid IA, Morris BJ, Ganong WG. The renin-angiotensin system. Annu Rev Physiol. 1978;40:377–410. doi: 10.1146/annurev.ph.40.030178.002113. [DOI] [PubMed] [Google Scholar]
- 26.Nafz B, Berthold H, Ehmke H, Hackenthal E, Kirchheim HR, Persson PB. Flow versus pressure in the control of renin release in conscious dogs. Am J Physiol. 1997;273:200–205. doi: 10.1152/ajprenal.1997.273.2.F200. [DOI] [PubMed] [Google Scholar]
- 27.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250:1145–1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 28.Hutcheson IR, Griffith TM. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol. 1991;261:257–262. doi: 10.1152/ajpheart.1991.261.1.H257. [DOI] [PubMed] [Google Scholar]
- 29.Nakano T, Tominaga R, Nagano I, Okabe H, Yasui H. Pulsatile flow enhances endothelium-derived nitric oxide release in the peripheral vasculature. Am J Physiol Heart Circ Physiol. 2000;278:1098–1104. doi: 10.1152/ajpheart.2000.278.4.H1098. [DOI] [PubMed] [Google Scholar]
- 30.Olson S, Oeckler R, Li X, Du L, Traganos F, Zhao X, et al. Angiotensin II stimulates nitric oxide production in pulmonary artery endothelium via the type 2 receptor. Am J Physiol Lung Cell Mol Physiol. 2004;287:559–568. doi: 10.1152/ajplung.00312.2003. [DOI] [PubMed] [Google Scholar]
- 31.Ankersmit HJ, Wieselthaler G, Moser B. Transitory immunologic response after implantation of the DeBakey VAD continuous axial-flow pump. J Thorac Cardiovasc Surg. 2002;123:557–561. doi: 10.1067/mtc.2002.120011. [DOI] [PubMed] [Google Scholar]
- 32.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 33.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 34.Cohn JN, Tognoni G Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]