Abstract
Previous studies have shown that when the cytosolic domains of the type I membrane proteins TGN38 and lysosomal glycoprotein 120 (lgp120) are added to a variety of reporter molecules, the resultant chimeric molecules are localized to the trans-Golgi network (TGN) and to lysosomes, respectively. In the present study we expressed chimeric constructs of rat TGN38 and rat lgp120 in HeLa cells. We found that targeting information in the cytosolic domain of TGN38 could be overridden by the presence of the lumenal and transmembrane domains of lgp120. In contrast, the presence of the transmembrane and cytosolic domains of TGN38 was sufficient to deliver the lumenal domain of lgp120 to the trans-Golgi network. On the basis of steady-state localization of the various chimeras and antibody uptake experiments, we propose that there is a hierarchy of targeting information in each molecule contributing to sorting within the endocytic pathway. The lumenal and cytosolic domains of lgp120 contribute to sorting and delivery to lysosomes, whereas the transmembrane and cytosolic domains of TGN38 contribute to sorting and delivery to the trans-Golgi network.
INTRODUCTION
Receptor-mediated endocytosis via clathrin-coated pits is the major and best understood entry route to the endosomal system (Robinson, 1994; Lamaze and Schmid, 1995; Mellman, 1996). Several sequence motifs have been defined in the cytosolic tails of cell surface membrane proteins that act as targeting signals for sorting into clathrin-coated pits and thereby delivery to the early endosomal compartment (Trowbridge et al., 1993; Robinson, 1994). Sequence motifs of this sort include YXXØ (where X is any amino acid, and Ø is a hydrophobic amino acid), NPXY and LL (Trowbridge et al., 1993). It has been shown, using fusion protein-binding assays and the yeast two-hybrid system, that YXXØ motifs can bind to clathrin adaptor subunits (Beltzer and Spiess, 1991; Ohno et al., 1995, 1996; Stephens et al., 1997), suggesting a mechanism for sorting into coated pits. Once membrane proteins and attached ligands have entered the early endosome, several fates are possible (Mellman, 1996). Many ligands dissociate from their receptors in the acid lumen of the endosome and are delivered to late endosomes and lysosomes for degradation (Pastan and Willingham, 1985). Others remain bound to their receptors and recycle to the plasma membrane (Mayor et al., 1993) or, in polarized cells, may be transcytosed (Sztul et al., 1991; Aroeti and Mostov, 1994). In addition to recycling and transcytosis, two other fates have been described for endocytosed membrane proteins. Some are delivered to the trans-Golgi network (TGN), and others are delivered to late endosomes and lysosomes. The type I membrane proteins TGN38 (Luzio et al., 1990) and lysosomal glycoprotein 120 (lgp120) (Howe et al., 1988) have been regarded, respectively, as resident membrane proteins of the TGN and late endosomes and lysosomes. Most newly synthesized lgp120 is directly targeted from the TGN to endosomes and lysosomes (Harter and Mellman, 1992). However, in at least some cell types, it is clear that a proportion of both TGN38 and lgp120 undergoes a membrane traffic itinerary via the plasma membrane (Ladinsky and Howell, 1992; Reaves et al., 1993; Chapman and Munro, 1994; Rajasekaran et al., 1994; Honing et al., 1996). Both molecules possess classical YXXØ internalization motifs in their cytosolic tails for endocytosis from the cell surface via clathrin-coated pits. When “added” to a variety of “neutral” plasma membrane reporter proteins, these cytosolic tail motifs have also been shown to act as TGN and lysosomal targeting signals. The initial evidence that the cytosolic tail of TGN38 plays an important role in its TGN localization and retrieval from the cell surface came from transfection experiments in which tail-deleted rat TGN38 overexpressed in monkey cells was observed at the cell surface (Luzio et al., 1990; Reaves and Banting, 1994a). Subsequent experiments in three laboratories showed that chimeras of the ectodomains and transmembrane domains (TMDs) of three different type I plasma membrane proteins (Tac, low-density lipoprotein receptor, and glycophorin) together with the 33-amino acid cytosolic tail of TGN38 were localized to the TGN (Bos et al., 1993; Humphrey et al., 1993; Wong and Hong, 1993). Deletion analysis and mutagenesis of these chimeras confirmed that YQRL, or possibly SDYQRL, is necessary and sufficient for retrieval and localization in the TGN even when present in a cytosolic tail constructed otherwise entirely of G or S (Bos et al., 1993; Wong and Hong, 1993). It was later discovered that the localization of TGN38 to the TGN was dependent not only on a cytosolic tail retrieval signal but also on a TMD retention signal (Ponnambalam et al., 1994; Reaves and Banting, 1994a). This is not strong enough to prevent TGN38 molecules leaving the TGN, and indeed after chloroquine treatment, effectively all of the cellular content of TGN38 may be trapped in an endosomal compartment (Chapman and Munro, 1994; Reaves and Banting, 1994b).
In contrast to TGN38, the only targeting signal identified so far in lgp120 is in the cytosolic tail, which consists of 11 amino acids ending with the sequence YQTI. Mutagenesis of the tyrosine residue in this motif results in inhibition of internalization and accumulation at the cell surface (Guarnieri et al., 1993). The conclusion that YQTI also functions as a lysosomal targeting motif was drawn from experiments in which addition of this sequence to the truncated cytosolic domain of CD44, a cell surface hyaluronate receptor, resulted in its delivery to lysosomes (Guarnieri et al., 1993). Mutagenesis studies on the TMD of lgp120 have suggested that it plays only a subtle role in lysosomal targeting (Wimer-Mackin and Granger, 1996). Several factors have been suggested to be involved in sorting lgps within endosomes for targeting and localization to lysosomes. These include the spacing of the YXXØ motif relative to the membrane (Rohrer et al., 1996), the identity of the carboxyl-terminal amino acid (Gough and Fambrough, 1997), and the proteolytic modification of the cytosolic tail (Guarnieri et al., 1993; Akasaki et al., 1995). It has also been observed that the delivery mechanism to the lysosome mediated by the YQTI motif is saturable (Marks et al., 1996).
Despite the above suggestions and the data on which they are based, it remains unclear where and how the YXXØ motifs in TGN38 and lgp120 are distinguished in the endosomal system after being recognized equally at the cell surface for endocytosis via clathrin-coated pits. To study these problems, we have followed the endocytosis of endogenous cell surface lgp120, endogenous TGN38, and chimeric proteins by examining the uptake of extracellularly added antibodies. We have prepared a series of chimeric molecules containing the lumenal domain of lgp120, either the TMD of lgp120 or TGN38, and various modifications of the cytosolic tail of either protein. After transfection and selection of stable cell lines, we examined both the steady-state localization of the chimeric molecules and their membrane traffic routes from the cell surface. The data we obtained are compatible with the hypotheses that the lumenal domain of lgp120 and the TMD of TGN38 play a role in addition to that of the cytosolic tail YXXØ motif in the endosomal sorting of these molecules. They are also consistent with the hypothesis that the sorting event takes place in the early endosome.
MATERIALS AND METHODS
Recombinant DNA Procedures
Standard molecular biology procedures (Sambrook et al., 1989) were performed unless otherwise stated. Wizard prep kits (Promega, Southampton, United Kingdom) were used for high-purity plasmid isolation, and Qiaex II kits (Qiagen, Chatsworth, CA) were used for purification of DNA from agarose gels. Rat TGN38 cDNA in the mammalian expression vector pUEX1 (Luzio et al., 1990) and rat lgp120 cDNA (a gift from Dr. I. Mellman, Yale University, New Haven, CT) were used as templates for PCR amplification of the wild-type molecules. PCR was performed for 30 cycles using Vent DNA polymerase (New England Biolabs Inc., Beverly, MA) according to the manufacturer’s instructions with the oligonucleotides shown in Table 1. The component parts of each chimeric cDNA were joined at a SalI site. Control TGN38 and lgp120 constructs containing SalI sites on either side of the TMD were expressed in transfected HeLa cells and were faithfully localized in the TGN and in late endosomes and lysosomes, respectively (our unpublished observations). All constructs were ligated into the PvuII site in the mammalian expression vector ΔpMEP (Girotti and Banting, 1996), and DNA sequence was confirmed by dideoxy chain termination sequencing, using the service provided by the Department of Biochemistry, University of Cambridge. The ΔpMEP vector contains the human metallothionein II promoter, which can be induced by heavy metals, and the hygromycin B resistance gene for selection in eukaryotic cells.
Table 1.
Oligonucleotides used in constructing lgp120 mutants and chimaeras
| Lumenal domain lgp120-5′: |
| GCAACCGCCGTCCTTCGGCCTCGGC |
| Lumenal domain lgp120-3′: |
| GGGGTCGACGCCGATGAGGTAGGCGATGAGGACGAT |
| Transmembrane domain lgp120-5′: |
| TTTGTCGACCTGATCCCCATTGCTGTGGGCGGG |
| Transmembrane domain lgp120-3′: |
| GGGGTCGACGCCGATGAGGTAGGCGATGAGGACGAT |
| Cytoplasmic domain lgp120-5′: |
| TTTGTCGACAGGAAGAGGAGTCACGCGGGCTATCAG |
| Cytoplasmic domain lgp120-3′: |
| GCCCTCGAGGCGCACCTGCCCACCAGGCTAGATGGT |
| lgp120-SDYQRL-3′: |
| CAGGCTAGAGGCGCTGATAGTCCGAGTGACTCCTC |
| Lumenal domain TGN38-5′: |
| AGGGACCAAGCTAGCATGCAGTTCCTGGTTCGGTTGCTC |
| Lumenal domain TGN38-3′: |
| GGGGTCGACGTAAGCAATATAGAGGACAGCAACAAG |
| Transmembrane domain TGN38-5′: |
| TTTGTCGACTTCTTTGCTTATCTGGTGACCGCT |
| Transmembrane domain TGN38-3′: |
| GGGGTCGACGTAAGCAATATAGAGGACAGCAACAAG |
| Cytosolic domain TGN38-5′: |
| TTTGTCGACCACAACAAACGAAAGATTATTGCT |
| Cytosolic domain TGN38-3′: |
| GCCCTCGAGTCAAAGCTTTAGGTTCAAACGTTGGTA |
| TGN38-YQTI-3′: |
| TCAAAGCTTTAGGTTGATAGTTTGGTGTCACT |
Cell Culture and Transfection
Normal rat kidney (NRK) cells and HeLa cells were grown in tissue culture flasks or on glass coverslips in DMEM and 10% FCS containing 100 μg/ml streptomycin and 100 U/ml penicillin.
ΔpMEP constructs were transfected into HeLa cells by electroporation or lipofection. For electroporation (Chu et al., 1987), ∼5 × 106 HeLa cells grown to 70% confluence were washed three times in ice-cold PBS, resuspended in 0.8 ml of ice-cold buffer containing 20 mM HEPES (pH 7.0), 137 mM sodium chloride, 5 mM potassium chloride, 0.7 mM disodium orthophosphate, 272 mM sucrose, and 20 μg of plasmid DNA added. The cell suspension was then electroporated at room temperature in a 0.4-cm cuvette at 220 V, 960 μF, using a Bio-Rad (Hercules, CA) Gene Pulser. After a recovery period of 10 min at room temperature, the electroporated cells were transferred to a 150-mm-diameter tissue culture dish containing DMEM and 10% FCS. For lipofection, ∼106 HeLa cells cells were washed once with PBS, and then 0.5 ml serum-free DMEM was added. Purified plasmid DNA (5 μg) was resuspended in 1 ml of serum-free DMEM containing 10 μl of Transfectam reagent (Promega) and applied to the washed cells. After 16–18 h at 37°C, the transfection medium was removed and replaced with DMEM and 10% FCS. Forty-eight hours after electroporation or lipofection, selection was begun by the addition of hygromycin B (Boehringer Mannheim, East Sussex, United Kingdom) at a final concentration of 400 μg/ml. Stable clones were selected 2–3 wk later and maintained in DMEM and 10%FCS containing 200 mg/ml hygromycin B.
Expression of protein in the transfected cells was stimulated by addition of 5 μM cadmium chloride to the cultured cells in DMEM and 10% FCS for 16–18 h at 37°C before the cells were used in an experiment. No significant differences in the steady-state localization of any expressed chimeras after induction with cadmium chloride in the range of 1–5 μM, a range causing a 3.5-fold variation in protein expression (Reaves and Banting, 1994a).
Antibodies
The mouse monoclonal antibody (mAb) to rat lgp120, designated GM10 (Grimaldi et al.., 1987), was a gift from Prof. K. Siddle (Department of Clinical Biochemistry, University of Cambridge) and Dr. J.C. Hutton (Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO) and was used as described previously (Reaves et al., 1996). The mouse mAb to rat TGN38, designated 2F7.1, was as described (Horn and Banting, 1994). Neither GM10 nor 2F7.1 reacted with endogenous proteins in HeLa cells. The rabbit polyclonal antibodies (pAbs) to rat lgp110 (Reaves et al., 1996), rat cation-independent mannose 6-phosphate receptor (Reaves et al., 1996), and rat TGN38 (Luzio et al., 1990) were as described previously. The rabbit anti-human TGN46, designated GB2, was as described previously (Ponnambalam et al., 1996). Rabbit anti-human cathepsin D was from Dako (High Wycombe, Bucks, United Kingdom). FITC-labeled goat anti-mouse IgG and Texas Red-labeled donkey anti-rabbit IgG were obtained from Amersham (Little Chalfont, Bucks, United Kingdom).
Antibody Uptake Experiments
Antibody uptake experiments were carried out by incubating cells cultured on glass coverslips with antibodies (1:100 dilution of ascites fluid for mouse monoclonal antibodies or rabbit polyclonal antiserum) for 30 min at 4°C followed by 2 h at 37°C before washing, fixation, and processing for microscopy (Reaves et al., 1996). When required, cultured cells were incubated with chloroquine, nocodazole, or brefeldin A (all from Sigma) prepared as described previously (Reaves and Banting, 1994b). Cells were incubated with these agents for 1 h at 37°C before addition of antibodies to the extracellular medium. The cells were then incubated for a further 2 h in the continued presence of the agents and antibodies before washing, fixation, and processing for microscopy.
Fluorescence Microscopy
Indirect immunofluorescence microscopy was performed as described previously (Reaves and Banting, 1992; Reaves et al., 1993) using cells grown on glass coverslips. Cells were rinsed in PBS and fixed for 5 min in methanol at −20°C. After incubation with antibodies and mounting, the cells were examined using a Planapochromat 63×, 1.4 lens on a Zeiss (Thornwood, NY) Axiophot microscope (Figures 1 and 2) or for confocal microscopy, a Nikon (Garden City, NY) Optiphot-2 epifluorescence microscope equipped with a Bio-Rad (Hemel Hempstead, United Kingdom) MRC 1000 confocal laser scanning attachment. Extended focus projections of various z-series of images obtained by confocal microscopy are shown in Figures 4–8. In double-labeling experiments using the mouse monoclonal anti-lgp120 antibody and the rabbit antibodies to other antigens, coverslips were first fixed with 4% paraformaldehyde in PBS for 20 min before permeabilizing with methanol.
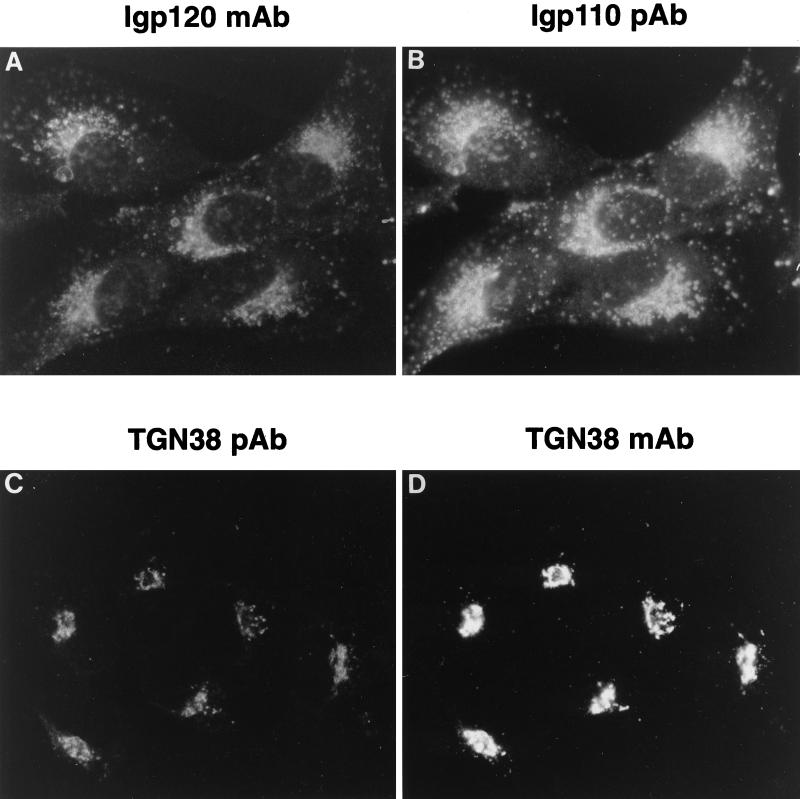
Figure 1.
The uptake and endocytic delivery of monoclonal antibodies in NRK cells. NRK cells cultured on glass coverslips were incubated with exogenously added monoclonal antibodies to lgp120 (GM10; A and B) or TGN38 (2F7.1; C and D) for 2 h at 37°C. Localization of the internalized antibodies (A and C) was visualized by indirect immunofluorescence and compared with the steady-state localization of lgp110 (B) and TGN38 (D) observed with pAbs to these proteins. Labeling with the pAbs defined the localization of late endosomes and lysosomes and TGN, respectively.
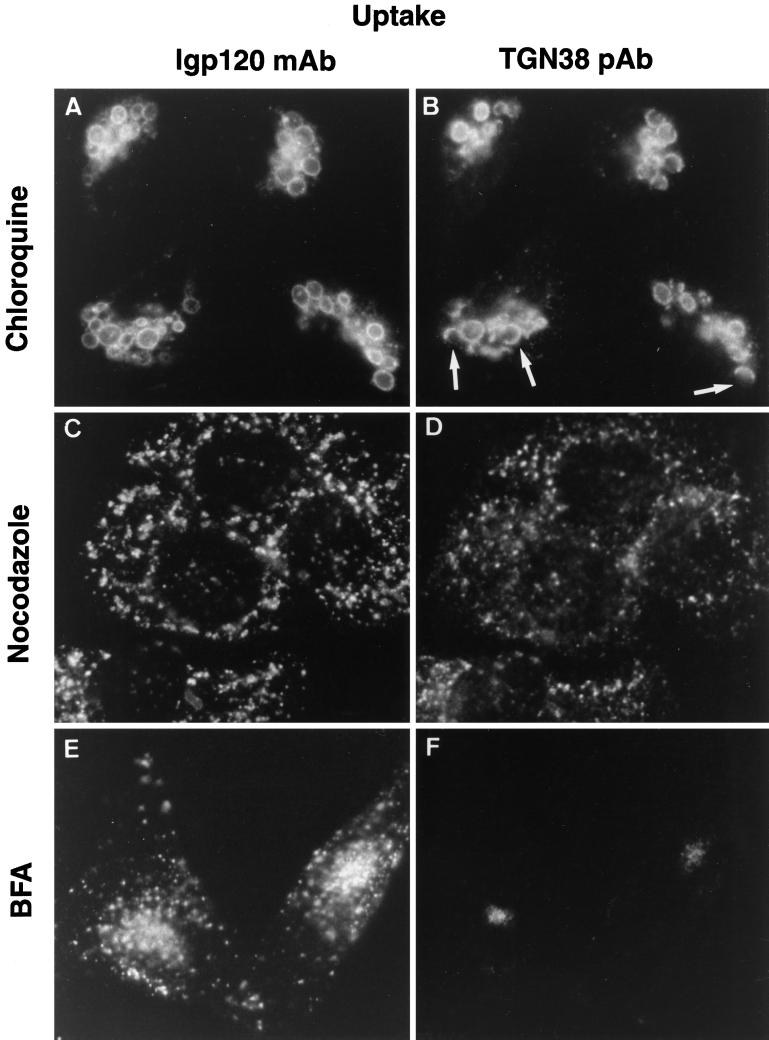
Figure 2.
Uptake and delivery of antibodies in cells treated with agents blocking endocytic traffic. NRK cells were treated with chloroquine (A and B), nocodazole (C and D) or brefeldin A (E and F) for 1 h before addition of both an mAb to lgp120 (A, C, and E) and a pAb to TGN38 (B, D, and F) to the extracellular medium. After a further incubation for 2 h in the continued presence of the drugs, the cells were fixed, permeabilized, and processed for indirect immunofluorescence with fluorescent-labeled second antibodies. The localization of the internalized antibodies to lgp120 and TGN38 is shown. Arrows (A and B) indicate the presence of lgp120-positive structures that are only partially labeled with TGN38.
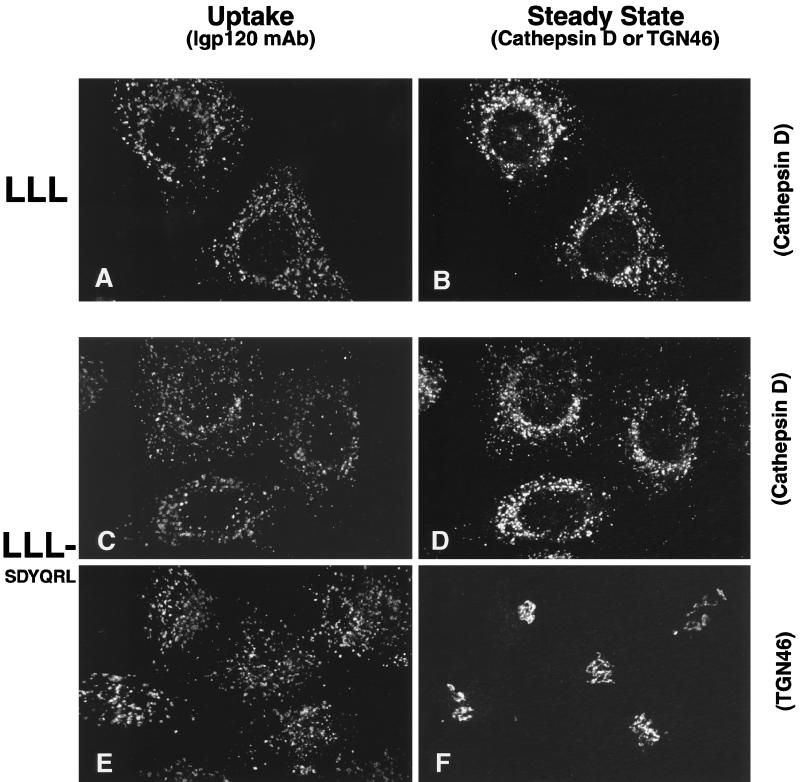
Figure 4.
Replacement of AGYQTI in the cytosolic domain of lgp120 with the TGN38 steady-state localization signal, SDYQRL, has no effect on the delivery of lgp120 to lysosomes. Hela cells were stably transfected with either a wild-type lgp120 construct (A and B) or a construct containing a mutated lgp120 cytosolic targeting sequence (C–F). Uptake experiments were performed using the mAb to lgp120. Both the wild-type lgp120 protein and the protein containing the TGN38 localization signal were internalized and delivered to a lysosomal/endosomal compartment as demonstrated by colocalization with endogenous human cathepsin D (B and D). The mutated protein showed no colocalization with the endogenous TGN46 (F).
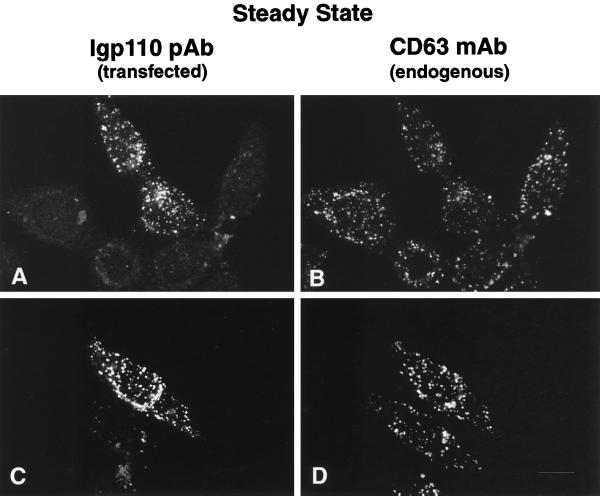
Figure 8.
Another integral membrane lysosomal protein, lgp110, is also delivered faithfully to lysosomes despite the replacement of the cytosolic domain with that of TGN38. Transiently transfected HeLa cells were analyzed by immunofluorescence using a pAb to lgp110 to identify both the wild-type lgp110 (A) and the chimeric protein (C). Both proteins colocalized with endogenous cathepsin D (B and D).
RESULTS
In a first set of experiments we showed, by double-labeling immunofluorescence microscopy, that when NRK cells were incubated with exogenously added mouse monoclonal antibodies to lgp120 and TGN38, these antibodies were respectively delivered to late endosomes and lysosomes and the TGN (Figure 1). In these experiments the late endosomes and lysosomes were identified by postfixation labeling with rabbit antibodies to lgp110 (Figure 1B), and the TGN was identified by postfixation labeling with rabbit antibodies to TGN38 (Figure 1D) Thus, uptake of the monoclonal antibodies may be used to follow the respective trafficking routes of these membrane proteins from the plasma membrane through the endocytic system. Previous experiments have shown that exogenously added pAbs to TGN38 are also delivered to the TGN (Ladinsky and Howell, 1992), and pAbs to lgps are delivered to late endosomes and lysosomes (Reaves et al., 1996). Because chloroquine and nocodazole block traffic through the endocytic system at an early stage (Rogalski et al., 1984; Chapman and Munro, 1994; Reaves and Banting, 1994b), we investigated the uptake and delivery of antibodies in cells treated with these agents. After chloroquine treatment, monoclonal anti-lgp120 antibodies were taken up into swollen vesicular structures, decorating their entire membrane circumference (Figure 2A). Polyclonal anti-TGN38 antibodies were taken up into the same structures but in many cases decorated only part of the rims (Figure 2B). After nocodazole treatment, antibodies to lgp120 and TGN38 were taken up into small vesicular structures (Figure 2, C and D). Some of these vesicles were labeled with both antibodies; others were labeled with only one. The uptake experiments on chloroquine- and nocodazole-treated cells were repeated with the mAb to TGN38 and pAbs to lgp110, which gave the same fluorescence patterns. The data from these experiments are consistent with endocytic uptake into common endosomal compartments where sorting occurs before delivery to late endosomes and lysosomes or to the TGN. As reported previously (Ladinsky and Howell, 1992; Reaves and Banting, 1992), brefeldin A treatment caused the collapse of the TGN into a structure concentrated around the microtubule-organizing center and visualized by immunofluorescence as a juxtanuclear “dot.” However, treatment of cells with brefeldin A had no effect on the delivery of extracellularly added monoclonal anti-lgp120 antibodies to late endosomes and lysosomes (Figure 2E), which were lgp110-positive structures (our unpublished observations), or of monoclonal anti-TGN38 antibodies to a juxtanuclear dot structure (Figure 2F), which could be postfixation labeled with rabbit pAbs to TGN38 (our unpublished observations).
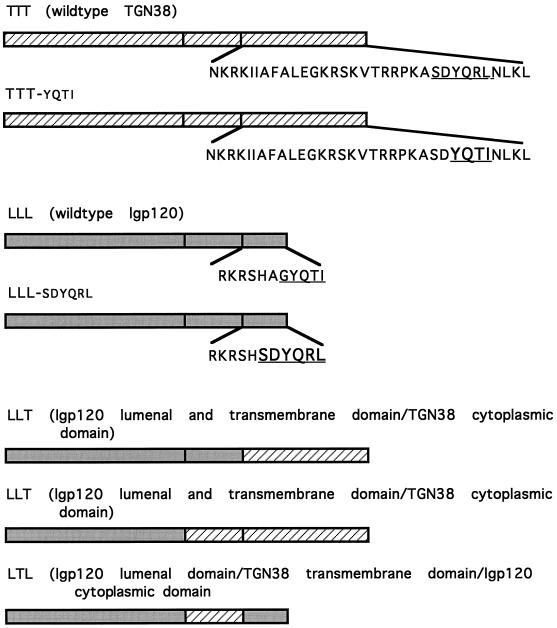
To understand the basis of this sorting, we decided to investigate steady-state localization and antibody uptake in HeLa cells expressing chimeric constructs of either rat lgp120-containing sequences from rat TGN38 or chimeric constructs of rat TGN38-containing sequences from rat lgp120 (Figure 3). In every experiment we report below, delivery of endocytosed antibodies was always to the intracellular site of steady-state localization of the transfected expressed protein (even when referring to our unpublished observations). As expected, when rat lgp120 was expressed in HeLa cells, it mostly colocalized with endogenous cathepsin D, and the mAb to lgp120 taken up by the cells was delivered to these late endosomes and lysosomes (Figure 4, A and B). When the carboxyl-terminal six amino acids of lgp120, AGYQTI, were replaced by the TGN38 sequence SDYQRL, the expressed chimeric protein was still localized in late endosomes and lysosomes, and internalized anti-lgp120 antibodies were delivered to these structures (Figure 4, C and D) and not to the TGN (Figure 4, E and F). Similar observations were made in experiments in which YQTI was replaced by YQRL (our unpublished observations). When rat TGN38 was expressed in HeLa cells, it colocalized with endogenous TGN46, the human orthologue of TGN38 (Ponnambalam et al., 1996), and the mAb to TGN38 taken up by the cells was delivered to the TGN (Figure 5, A and B). Replacement of the YQRL motif within the cytosolic tail of TGN38 by the lgp120 carboxyl-terminal sequence YQTI resulted in an expressed chimeric protein that was still localized in the TGN. Anti-TGN38 antibodies added extracellularly to cells expressing this construct were delivered to the TGN (Figure 5, C and D), not to late endosomes and lysosomes (Figure 5, E and F).
Figure 3.
Schematic representation of the construction of mutated and chimeric proteins. The full sequences of the cytosolic domains of both TGN38 and lgp120 are shown for the wild-type proteins as well as for the proteins with altered targeting signals. Tyrosine-containing steady-state localization signals are underlined, and the exchange of TGN38 and lgp120 targeting signals are depicted in large type. The nomenclature indicates either an L, for lgp120, or T, for TGN38, for the lumenal domains, TMDs, and cytoplasmic domains of each of the chimeric proteins.
Figure 5.
Replacement of YQRL in the cytosolic domain of TGN38 with the lysosomal targeting signal, YQTI, does not affect delivery of TGN38 from the cell surface to the TGN. Stably transfected Hela cells expressing TGN38 wild-type protein (A and B) or TGN38 with the lysosomal targeting signal, YQTI, replacing YQRL (C–F) were incubated in the presence of an mAb to TGN38, 2F7.1, and internalization was allowed to occur at 37°C for 2 h. The localization of the wild-type and mutant proteins is shown to coincide with the endogenous TGN46 marker (B and C). There is no overlap in distribution of the mutated protein with the endogenous lysosomal marker cathepsin D (F).
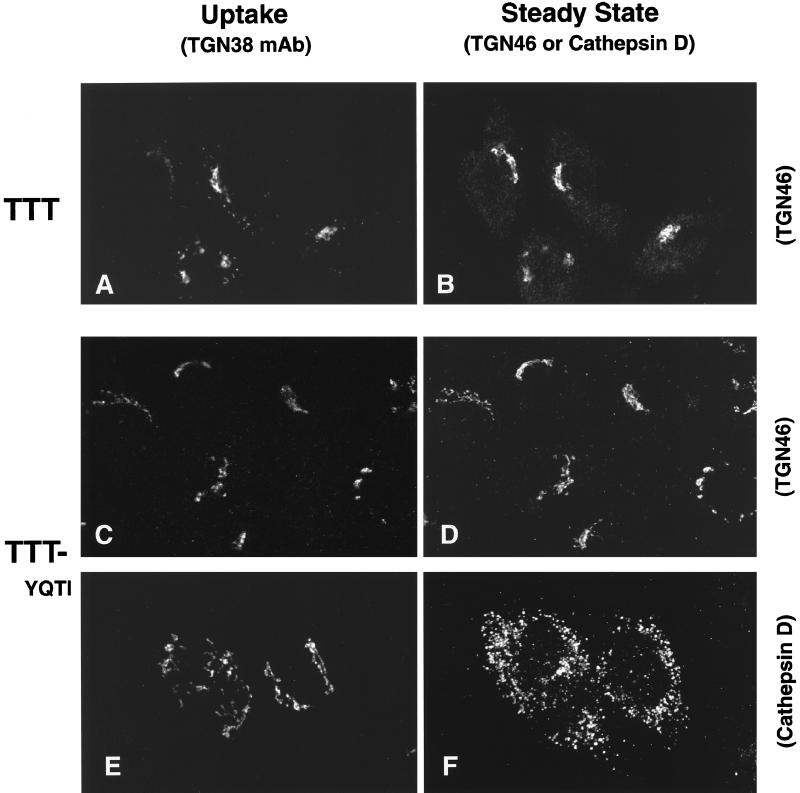
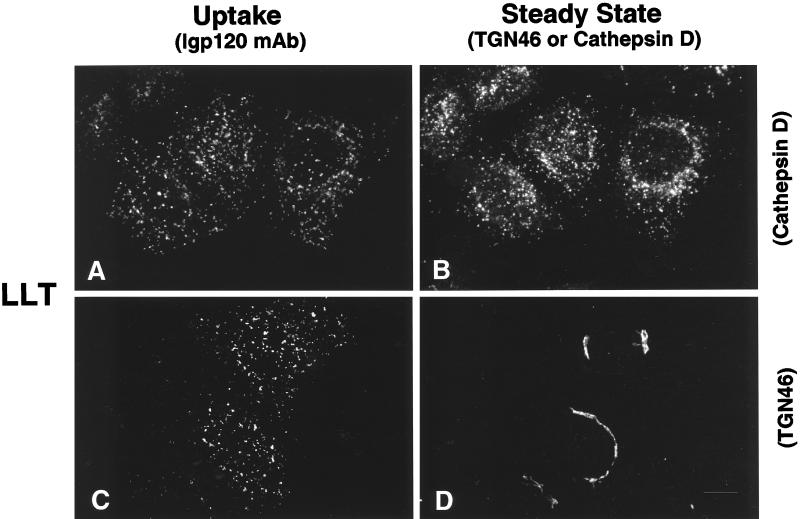
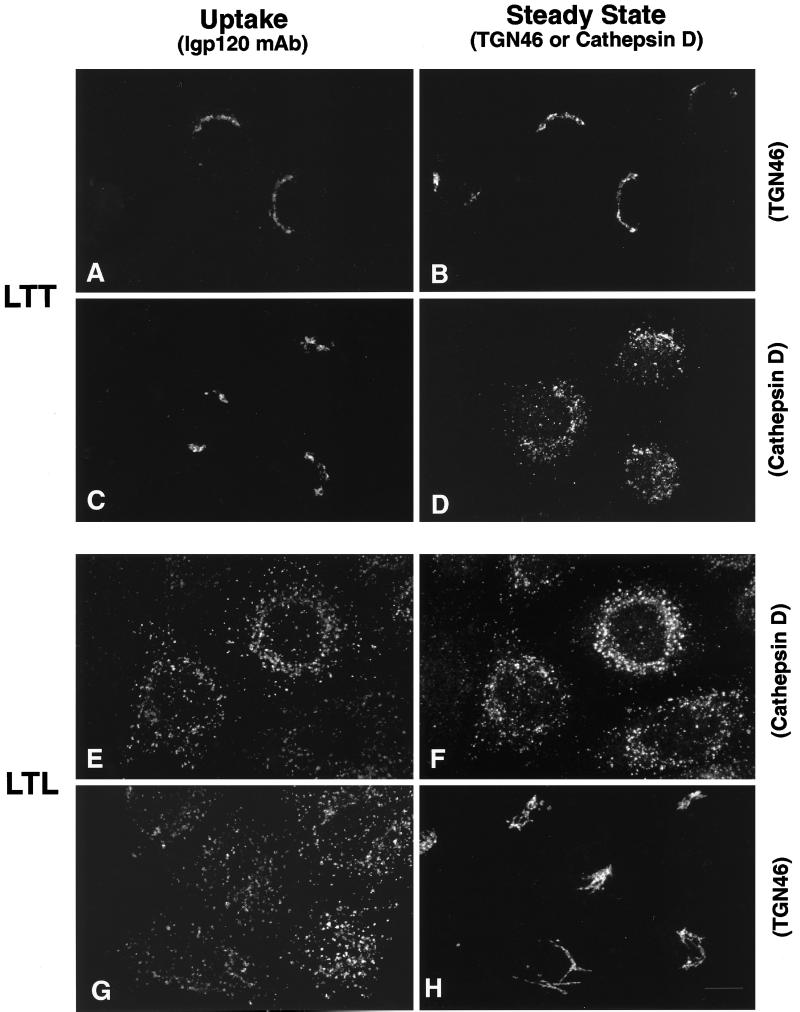
The observations that the cytosolic tail sequences YQRL and SDYQRL were not able to divert lgp120 from late endosomes and lysosomes to the TGN were a surprise in view of the literature reports indicating that these sequences are sufficient to target a number of plasma membrane protein constructs to this intracellular site. Because the data might be explained by the environment of the YQRL motif within the lgp tail sequence, we made a further chimera in which the TGN38 tail was added to the lumenal domain and TMD of lgp120 (Figure 3, LLT). When this was expressed in HeLa cells it was localized in late endosomes and lysosomes. Extracellularly added antibodies to lgp120 taken up by cells expressing this LLT construct were delivered to late endosomes and lysosomes (Figure 6, A and B) and not to the TGN (Figure 6, C and D). Two other chimeras were then expressed in HeLa cells. A chimera consisting of the lumenal domain of lgp120 together with the TMD and cytosolic domains of TGN38 (Figure 3, LLT) localized to the TGN, and extracellularly added anti-lgp120 antibodies were taken up and delivered to the TGN (Figure 7, A and B) and not to late endosomes and lysosomes (Figure 7, C and D). In contrast, a chimera consisting of the lumenal and cytosolic tail domains of lgp120 together with the TMD of TGN38 (Figure 3, LTL) was mainly localized to late endosomes and lysosomes. Extracellularly added anti-lgp120 antibodies were delivered to the late endosomes and lysosomes (Figure 7, E and F) and not to the TGN (Figure 7, G and H). The altered intracellular destination of monoclonal anti-lgp120 antibody when added to cells expressing LTT and LTL confirmed that addition of extracellular antibody did not affect the membrane traffic routes taken by these molecules.
Figure 6.
Addition of the full-length cytosolic domain of TGN38 to the lumenal domain and TMD of lgp120 is not sufficient to divert the chimeric protein to the TGN. HeLa cells stably transfected with the LLT construct were incubated in the presence of the mAb to lgp120 (A and C), and the intracellular localization was determined by indirect immunofluorescence. Colocalization with an endogenous lysosomal marker, cathepsin D (B) is shown, as well as the lack of overlap in distribution with endogenous TGN46 (D).
Figure 7.
Both the TMD and cytosolic domain of TGN38, but not the TMD alone, are necessary to target the lumenal domain of lgp120 to the TGN. Chimeric proteins containing the lumenal domain of lgp120 together with the TMD and cytosolic domain of TGN38 were internalized from the cell surface and delivered to the TGN, as determined by following uptake of the mAb to lgp120 (A and C). This chimeric protein colocalized with endogenous TGN46 (B) but not cathepsin D (D). In contrast, replacement of the TMD of lgp120 with the TMD of TGN38 had no effect on its delivery to lysosomes from the cell surface followed by uptake of monoclonal lgp120 antibody (E and G). Delivery to lysosomes is demonstrated by colocalization with endogenous cathepsin D (F) as well as lack of colocalization with endogenous TGN46 (H).
The data showed that the targeting and localization of the chimeric membrane proteins could not simply be explained on the basis of their cytosolic tail domains, but that the TMD of TGN38 and, more surprisingly, the lumenal domain of lgp120 also played a role. To confirm that the effect of the lumenal domain of lgp120 was not unique, we expressed a chimera consisting of the lumenal domain and TMD of lgp110 with the cytosolic tail of TGN38. Transiently transfected HeLa cells were examined by immunofluorescence, and the chimera was shown to colocalize with CD63 (Figure 8, A and B) another marker for late endosomes and lysosomes (Metzelaar et al., 1991).
DISCUSSION
Our experiments on antibody uptake by NRK cells in the presence of inhibitors of membrane traffic pathways strongly suggest that the site of sorting of endocytosed TGN38 and lgp120 is the early endosome. Endosomal acidification has been suggested to be necessary for the formation of endocytic carrier vesicles from the early endosome (Clague et al., 1994) and the addition of chloroquine neutralizes endosome pH, causes endosome swelling, and blocks the traffic of membrane proteins (Mellman et al., 1986; Chapman and Munro, 1994). After chloroquine treatment we observed swollen endosomal compartments in which endocytosed antibodies to TGN38 and lgp120 were segregated. Nocodazole, by depolymerizing microtubules, prevents endocytic carrier vesicles from moving toward the microtubule-organizing center and fusing with target organelles (Pierre et al., 1992; Aniento et al., 1993). Again we observed segregation of endocytosed antibodies to TGN38 and lgp120 in the presence of nocodazole, consistent with the formation of separate carrier vesicles from the early endosomal compartment. The fungal metabolite brefeldin A has a dramatic effect on the morphology of the TGN, causing it to collapse into a collection of vesicles close to the microtubule-organizing center (Ladinsky and Howell, 1992; Reaves and Banting, 1992), and it also affects the morphology of the endosomal system (Wood et al., 1991; Hunziker et al., 1992; Tooze and Hollinshead, 1992; Wood and Brown, 1992). Addition of brefeldin A to NRK cells did not prevent segregation of endocytosed anti-lgp120 and anti-TGN38 antibodies and their respective delivery to late endosomes and lysosomes and the collapsed TGN. This is in agreement with reports that brefeldin A affects the morphology more dramatically than the function of endosomes (Hunziker et al., 1992; Wood and Brown, 1992). The present data suggesting sorting of endocytosed TGN38 in the early endosome before delivery to the TGN are consistent with our previous experiments showing that treatment of NRK cells with the phosphatidyl inositide kinase inhibitor wortmannin does not prevent delivery of endocytosed anti-TGN38 antibodies to the TGN but alters morphology and membrane traffic in the late endosome/lysosome system (Reaves et al., 1996; Bright et al., 1997).
Using chimeric constructs transfected into HeLa cells, we have been able to generate data providing insights into the mechanism of sorting of endocytosed TGN38 and lgp120 in early endosomes. Although the cytosolic tail sequence (SD)YQRL has been shown to be necessary and sufficient for targeting to the TGN when added to several type I plasma membrane proteins (see INTRODUCTION), it was unable to alter the targeting of lgp120 to late endosomes and lysosomes. This could not be explained simply by the distance of the YQRL motif from the lipid bilayer, which was identical to that of the endogenous YQTI sequence, or the context of the rest of the lgp120 cytosolic tail, because replacement of the entire tail by that of TGN38 still resulted in a chimera (LLT) that was targeted to, and localized in, late endosomes and lysosomes. This implied that there was targeting information in the lumenal domain or TMD of lgp120, and that this was capable of overriding the information in the TGN-derived cytosolic tail that was sufficient to target plasma membrane protein chimeras to the TGN. Because mutagenesis of the TMD of lgp120 has been shown to affect its intracellular localization only subtly (Wimer-Mackin and Granger, 1996), and in the present study we found that the chimera TLT had a Golgi localization in transiently transfected HeLa cells (our unpublished observations), it seems most likely that the lumenal domain of lgp120 contains targeting information. At first glance this may appear illogical, because the cytosolic coat proteins responsible for sorting and vesicle formation must interact with cytosolic tails. However, there are already precedents for targeting information in lumenal domains. A striking example is the role of protein glycosylation in targeting from the TGN to the apical domain of polarized epithelial cells (Fiedler and Simons, 1995; Scheiffele et al., 1995). This has been suggested to be mediated by a putative carbohydrate receptor, which may itself be a transmembrane protein. The aggregation of specific subsets of lumenal proteins together with membrane proteins in the TGN is also regarded as an important sorting mechanism in the formation of regulated secretory granules (Bauerfeind and Huttner, 1993; Cool et al., 1997). In contrast, aggregation of the lumenal domains of furin molecules in the TGN has been correlated with lysosomal targeting, and it has been suggested that this pathway provides a final level of quality control for proteins that become aggregated or otherwise damaged in post-Golgi compartments, in a manner analagous to endoplasmic reticulum quality control mechanisms (Wolins et al., 1997). The lumenal domain of the cation-independent mannose 6-phosphate receptor has also been proposed to play a role in determining the steady-state distribution of this molecule between the TGN and prelysosomes (Conibear and Pearse, 1994). In the endocytic pathway there is a wealth of indirect evidence linking protein aggregation with lysosomal targeting, exemplified by the common observation that cross-linking cell surface proteins with antibodies or multivalent ligands results in increased internalization and degradation as a result of lysosomal delivery (Taylor et al., 1971; Ukkonen et al., 1986; Weissman et al., 1986).
Our experiments in which the TGN38 tail was attached to the TMD and lumenal domains of lgp110 show that lumenal domain information for targeting lysosomal glycoproteins to late endosomes and lysosomes is not restricted to lgp120. An attractive hypothesis to explain the function of lumenal domains in late endosome and lysosome targeting is that it derives from the ability of these domains to aggregate under acid conditions. Solubilized lumenal domains of lgp110 (probably obtained by proteolytic cleavage close to the membrane) form aggregates as the pH is reduced below pH 7 (Jadot et al., 1996). If such aggregates were formed in the early endosome, the cytosolic tails of these molecules might be presented to novel cytosolic coat proteins in a manner different from their presentation to clathrin adaptors at the plasma membrane. A diagrammatic representation of this hypothesis is shown in Figure 9. The suggestion that endosomal acidification may be required for sorting of lgps in the early endosome is consistent with the data from our chloroquine experiments, in which anti-lgp120 antibodies internalized into the neutralized, swollen endosome decorated the whole membrane rim, whereas the endocytosed anti-TGN38 antibodies were clustered. The data from our experiments in which the (SD)YQRL signal was transplanted onto lgp120 also show that it is not always the case that the rest of a chimeric construct has no effect in targeting on membrane traffic pathways when a specific sequence motif is being examined. In this respect it is interesting to note that when the SDYQRL motif was used to replace the YTRF motif in the transferrin receptor, a type II membrane protein, the resulting chimera was not targeted to the TGN but altered the morphology and pH of the endocytic recycling compartments to which it was targeted (Johnson et al., 1996).
Figure 9.
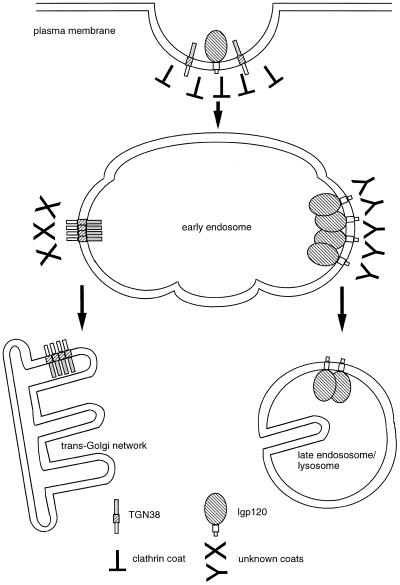
Model for the sorting of lgp120 and TGN38 in the early endosome. Specific features of the proposed model are that aggregation of the lumenal domains of lgp120 and interaction of the TMDs of TGN38 occur in the early endosome, resulting in targeting respectively to late endosomes and lysosomes and to the TGN.
Although the experiments with the LLT constructs demonstrated a role for the lumenal domain in late endosome and lysosome targeting, experiments with LTT and LTL constructs showed that the targeting information in this domain could be overridden by that provided jointly by the TMD and cytosolic tail domains of TGN38 but not by either domain alone. Thus, these membrane proteins contain a hierarchy of targeting signals in different domains. This is in contrast to proteins that have been described as having more than one signal within a single domain, i.e., the cytosolic tail (Matter et al., 1992; Aroeti and Mostov, 1994; Hamer et al., 1997). The ability to distinguish the targeting signals within the endosomal system may also give clues as to the temporal and/or spatial order of sorting events. Thus, the delivery of LTT chimeras to the TGN suggests that a required sorting event occurs in a temporal or spatial subdomain of the early endosome where acidification is insufficient to allow the dominance of aggregation of the lumenal domain for targeting to late endosomes and lysosomes. The suggestion of a hierarchy of targeting signals in different domains is entirely consistent with previous work showing that the cytosolic tails of TGN38 and lgp120 or the TMD of TGN38 contain sufficient and necessary targeting information when added to targeting-neutral plasma membrane proteins. The TMD of TGN38 has been defined as a retention signal because of its behavior in chimeric molecules when combined with the extracellular and cytosolic domains of type I plasma membrane proteins (Ponnambalam et al., 1994). The relatively short TMDs of Golgi membrane proteins are thought to cause retention either as a result of oligomerization (Nilsson et al., 1993) or, more likely, because they are trapped in a relatively thin membrane bilayer provided by a lipid composition different from that observed at the cell surface (Bretscher and Munro, 1993; Munro, 1995). There is now a wealth of evidence that lipid sorting to form localized patches of membrane of specific lipid composition plays a role in membrane protein sorting within the TGN for delivery to the apical membrane domain of polarized epithelial cells (Simons and Ikonen, 1997), and it may also play a role in sorting on transcytotic pathways (van IJzendoorn et al., 1997). The data in the present paper suggest that a similar sorting mechanism may occur in the early endosome such that the TMD of TGN38 can sort this molecule into patches. We propose that such sorting and resultant molecular aggregation results in presentation of the cytosolic tails to cytosolic coat proteins in a way that improves the ability to distinguish them and favor targeting to the TGN. The observation that LTT constructs were targeted to the TGN together with the observation of aggregation of endocytosed anti-TGN38 antibodies in the swollen endosomes of chloroquine-treated cells is consistent with this hypothesis. It should be noted that we are proposing a sorting mechanism for TGN38 in the early endosome that is consistent with what is known about the behavior of this protein in the TGN. Others have commented on the similarity in signals recognized in endosomes and in the TGN when targeting membrane proteins to the basolateral surface of polarized epithelial cells (Matter et al., 1993; Aroeti and Mostov, 1994; Mellman, 1996).
Although it is expected that endosomal sorting events are dependent on cytosolic coat proteins, we do not yet know their identity. Coats of varying composition have been observed on endosomal structures (Seaman et al., 1993; Whitney et al., 1995; Stoorvogel et al., 1996; Traub et al., 1996) but have not yet been linked to specific trafficking events. Identifying the coats involved in endosomal sorting is clearly an important task. We shall also be interested in further analyzing the mechanism by which lumenal domains of lgps become aggregated at acid pH.
ACKNOWLEDGMENTS
We thank the Medical Research Council for financial support and Sally Gray for technical assistance. We also thank Nick Bright and Margaret Robinson for much valuable advice and discussion, Paul Guest for the design and preparation of several oligonucleotides, and Helen Story for preparing the lgp110 chimeras.
Footnotes
Abbreviations used: lgp, lysosomal glycoprotein; mAb, monoclonal antibody; NRK, normal rat kidney; TGN, trans-Golgi network; TMD, transmembrane domain; pAb, polyclonal antibody.
REFERENCES
- Akasaki K, Michihara A, Mibuka K, Fujiwara Y, Tsuji H. Biosynthetic transport of a major lysosomal membrane glycoprotein, lamp- 1: convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp Cell Res. 1995;220:464–473. doi: 10.1006/excr.1995.1338. [DOI] [PubMed] [Google Scholar]
- Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B, Mostov KE. Polarized sorting of the polymeric immunoglobulin receptor in the exocytotic and endocytotic pathways is controlled by the same amino acids. EMBO J. 1994;13:2297–2304. doi: 10.1002/j.1460-2075.1994.tb06513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993;5:628–635. doi: 10.1016/0955-0674(93)90132-a. (erratum 5, 1106) [DOI] [PubMed] [Google Scholar]
- Beltzer JP, Spiess M. In vitro binding of the asialoglycoprotein receptor to the beta adaptin of plasma membrane coated vesicles. EMBO J. 1991;10:3735–3742. doi: 10.1002/j.1460-2075.1991.tb04942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos K, Wraight C, Stanley KK. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- Bright NA, Reaves BJ, Mullock BM, Luzio BM. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J Cell Sci. 1997;110:2027–2040. doi: 10.1242/jcs.110.17.2027. [DOI] [PubMed] [Google Scholar]
- Chapman RE, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G, Hayakawa H, Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987;15:1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague MJ, Urbe S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- Conibear E, Pearse BMF. A chimera of the cytoplasmic tail of the mannose 6-phosphate/IGF-II receptor and lysozyme localizes to the TGN rather than prelysosomes where the bulk of the endogenous receptor is found. J Cell Sci. 1994;107:923–932. doi: 10.1242/jcs.107.4.923. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Girotti M, Banting G. TGN38-green fluorescent protein hybrid proteins expressed in stably transfected eukaryotic cells provide a tool for the real-time, in vivo study of membrane traffic pathways and suggest a possible role for ratTGN38. J Cell Sci. 1996;109:2915–2926. doi: 10.1242/jcs.109.12.2915. [DOI] [PubMed] [Google Scholar]
- Gough NR, Fambrough DM. Different steady state subcellular distributions of the three splice variants of lysosome-associated membrane protein LAMP-2 are determined largely by the COOH-terminal amino acid residue. J Cell Biol. 1997;137:1161–1169. doi: 10.1083/jcb.137.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi KA, Hutton JC, Siddle K. Production and characterization of monoclonal antibodies to insulin secretory granule membranes. Biochem J. 1987;245:557–566. doi: 10.1042/bj2450557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri FG, Arterburn LM, Penno MB, Cha Y, August JT. The motif Tyr-X-X-hydrophobic residue mediates lysosomal membrane targeting of lysosome-associated membrane protein 1. J Biol Chem. 1993;268:1941–1946. [PubMed] [Google Scholar]
- Hamer I, Renfrew Haft C, Paccaud J-P, Maeder C, Taylor S, Carpentier J-L. Dual role of a dileucine motif in insulin receptor endocytosis. J Biol Chem. 1997;272:21685–21691. doi: 10.1074/jbc.272.35.21685. [DOI] [PubMed] [Google Scholar]
- Harter C, Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992;117:311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S, Griffith J, Geuze HJ, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Horn M, Banting G. Okadaic acid treatment leads to a fragmentation of the trans-Golgi network and an increase in expression of TGN38 at the cell surface. Biochem J. 1994;301:69–73. doi: 10.1042/bj3010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Granger BL, Hull M, Green SA, Gabel CA, Helenius A, Mellman I. Derived protein sequence, oligosaccharides, and membrane insertion of the 120-kDa lysosomal membrane glycoprotein (lgp120): identification of a highly conserved family of lysosomal membrane glycoproteins. Proc Natl Acad Sci USA. 1988;85:7577–7581. doi: 10.1073/pnas.85.20.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JS, Peters PJ, Yuan LC, Bonifacino JS. Localization of TGN38 to the trans-Golgi network: Involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993;120:1123–1136. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Whitney JA, Mellman I. Brefeldin A and the endocytic pathway. Possible implications for membrane traffic and sorting. FEBS Lett. 1992;307:93–96. doi: 10.1016/0014-5793(92)80908-y. [DOI] [PubMed] [Google Scholar]
- Jadot M, Wattiaux R, Mainferme F, Dubois F, Claessens A, Wattiaux De Coninck S. Soluble form of Lamp II in purified rat liver lysosomes. Biochem Biophys Res Commun. 1996;223:353–359. doi: 10.1006/bbrc.1996.0898. [DOI] [PubMed] [Google Scholar]
- Johnson AO, Ghosh RN, Dunn KW, Garippa R, Park J, Mayor S, Maxfield FR, McGraw TE. Transferrin receptor containing the SDYQRL motif of TGN38 causes a reorganization of the recycling compartment but is not targeted to the TGN. J Cell Biol. 1996;135:1749–1762. doi: 10.1083/jcb.135.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky MS, Howell KE. The trans-Golgi network can be dissected structurally and functionally from the cisternae of the Golgi complex by brefeldin A. Eur J Cell Biol. 1992;59:92–105. [PubMed] [Google Scholar]
- Lamaze C, Schmid SL. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Brake B, Banting G, Howell KE, Braghetta P, Stanley KK. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38) Biochem J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: The cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Matter K, Whitney JA, Yamamoto EM, Mellman I. Common signals control low density lipoprotein receptor sorting in endosomes and the Golgi complex of MDCK cells. Cell. 1993;74:1053–1064. doi: 10.1016/0092-8674(93)90727-8. [DOI] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Metzelaar MJ, Wijngaard PLJ, Peters PJ, Sixma JJ, Nieuwenhuis HK, Clevers HC. CD63 antigen: a novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem. 1991;266:3239–3245. [PubMed] [Google Scholar]
- Munro S. An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J. 1995;14:4695–4704. doi: 10.1002/j.1460-2075.1995.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Slusarewicz P, Hoe MH, Warren G. Kin recognition. A model for the retention of Golgi enzymes. FEBS Lett. 1993;330:1–4. doi: 10.1016/0014-5793(93)80906-b. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Pastan I, Willingham MC. The pathway of endocytosis. In: Pastan I, Willingham MC, editors. Endocytosis. New York: Plenum Press; 1985. pp. 1–44. [Google Scholar]
- Pierre P, Scheel J, Rickard JE, Kreis TE. CLIP-170 links endocytic vesicles to microtubules. Cell. 1992;70:887–900. doi: 10.1016/0092-8674(92)90240-d. [DOI] [PubMed] [Google Scholar]
- Ponnambalam S, Girotti M, Yaspo ML, Owen CE, Perry ACF, Suganuma T, Nilsson T, Fried M, Banting G, Warren G. Primate homologues of rat TGN38: primary structure, expression and functional implications. J Cell Sci. 1996;109:675–685. doi: 10.1242/jcs.109.3.675. [DOI] [PubMed] [Google Scholar]
- Ponnambalam S, Rabouille C, Luzio JP, Nilsson T, Warren G. The TGN38 glycoprotein contains two non-overlapping signals that mediate localization to the trans-Golgi network. J Cell Biol. 1994;125:253–268. doi: 10.1083/jcb.125.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran AK, Humphrey JS, Wagner M, Miesenbock G, Le Bivic A, Bonifacino JS, Rodriguez Boulan E. TGN38 recycles basolaterally in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1994;5:1093–1103. doi: 10.1091/mbc.5.10.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B, Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B, Banting G. Overexpression of TGN38/41 leads to mislocalisation of gamma-adaptin. FEBS Lett. 1994a;351:448–456. doi: 10.1016/0014-5793(94)00813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B, Banting G. Vacuolar ATPase inactivation blocks recycling to the trans-Golgi network from the plasma membrane. FEBS Lett. 1994b;345:61–66. doi: 10.1016/0014-5793(94)00437-4. [DOI] [PubMed] [Google Scholar]
- Reaves B, Horn M, Banting G. TGN38/41 recycles between the cell surface and the TGN: Brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993;4:93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves BJ, Bright NA, Mullock BM, Luzio JP. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Rogalski AA, Bergmann JE, Singer SJ. Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J Cell Biol. 1984;99:1101–1109. doi: 10.1083/jcb.99.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Schweizer A, Russell D, Kornfeld S. The targeting of lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J Cell Biol. 1996;132:565–576. doi: 10.1083/jcb.132.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Scheiffele P, Peranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Seaman MN, Ball CL, Robinson MS. Targeting and mistargeting of plasma membrane adaptors in vitro. J Cell Biol. 1993;123:1093–1105. doi: 10.1083/jcb.123.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Stephens DJ, Crump CM, Clarke AR, Banting G. Serine 331 and tyrosine 333 are both involved in the interaction between the cytosolic domain of TGN38 and the μ2 subunit of the AP2 clathrin adaptor complex. J Biol Chem. 1997;272:14104–14109. doi: 10.1074/jbc.272.22.14104. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E, Kaplin A, Saucan L, Palade G. Protein traffic between distinct plasma membrane domains: isolation and characterization of vesicular carriers involved in transcytosis. Cell, 1991;64:81–89. doi: 10.1016/0092-8674(91)90210-p. [DOI] [PubMed] [Google Scholar]
- Taylor RB, Duffus PH, Raff MC, de Petris S. Redistribution and pinocytosis of lymphocyte surface immunoglobulin molecules induced by anti-immunoglobulin antibody. Nature New Biol. 1971;233:225–229. doi: 10.1038/newbio233225a0. [DOI] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M. In AtT20 and HeLa cells brefeldin A induces the fusion of tubular endosomes and changes their distribution and some of their endocytic properties. J Cell Biol. 1992;118:813–830. doi: 10.1083/jcb.118.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Bannykh SI, Rodel JE, Aridor M, Balch WE, Kornfeld S. AP-2-containing clathrin coats assemble on mature lysosomes. J Cell Biol, 1996;135:1801–1814. doi: 10.1083/jcb.135.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Ukkonen P, Lewis V, Marsh M, Helenius A, Mellman I. Transport of macrophage Fc receptors and Fc receptor-bound ligands to lysosomes. J Exp Med. 1986;163:952–971. doi: 10.1084/jem.163.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn SCD, Zegers MMP, Kok JW, Hoekstra D. Segregation of glucosylceramide and sphingomyelin occurs in the apical to basolateral transcytotic route in HepG2 cells. J Cell Biol. 1997;137:347–357. doi: 10.1083/jcb.137.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AM, Klausner RD, Rao K, Harford JB. Exposure of K562 cells to anti-receptor monoclonal antibody OKT9 results in rapid redistribution and enhanced degradation of the transferrin receptor. J Cell Biol. 1986;102:951–958. doi: 10.1083/jcb.102.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Wimer-Mackin S, Granger BL. Transmembrane domain mutations influence the cellular distribution of lysosomal membrane glycoprotein A. Biochem Biophys Res Commun. 1996;229:472–478. doi: 10.1006/bbrc.1996.1828. [DOI] [PubMed] [Google Scholar]
- Wolins N, Bosshart H, Kuster H, Bonifacino JS. Aggregation as a determinant of protein fate in post-Golgi compartments: role of the luminal domain of furin in lysosomal targeting. J Cell Biol. 1997;139:1735–1745. doi: 10.1083/jcb.139.7.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localization. J Biol Chem. 1993;268:22853–22862. [PubMed] [Google Scholar]
- Wood SA, Brown WJ. The morphology but not the function of endosomes and lysosomes is altered by brefeldin A. J Cell Biol. 1992;119:273–286. doi: 10.1083/jcb.119.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Park JE, Brown WJ. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]