Abstract
Background
The impact of L-arginine on atherogenesis and its ability to prevent endothelial dysfunction have been studied extensively during the past years. L-arginine is a substance for nitric oxide synthesis which involves in apoptosis. Hypercholesterolemia promotes endothelial dysfunction, and it is hypothesized that L-arginine prevents endothelial dysfunction through endothelial cells apoptosis inhibition. To test this hypothesis, thirty rabbits were assigned into two groups. The control group received 1% cholesterol diet for 4 weeks, and the L-arginine group received same diets plus 3% L-arginine in drinking water.
Results
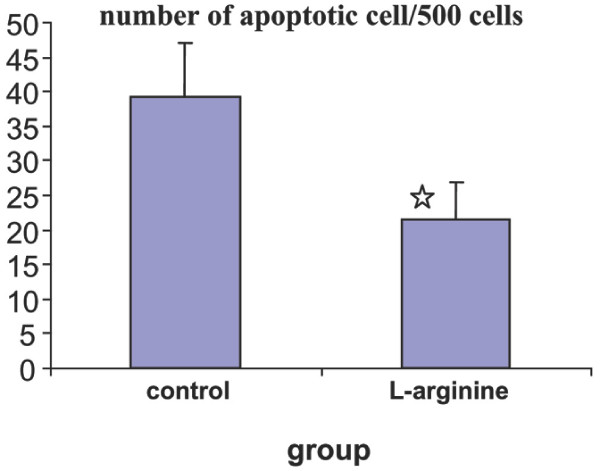
No significant differences were observed in cholesterol level between two groups, but the nitrite concentration in L-arginine group was significantly higher than other group (control group: 11.8 ± 1; L-arginine group: 14.7 ± 0.5 μmol/l); (p < 0.05). The aorta score of fatty streak in control group was 0.875 ± 0.35, but no fatty streak lesion was detected in L-arginine group (p < 0.05). The number of intimal apoptotic cells/500 cells of aorta in two groups of experiment were statistically different (control group: 39.3 ± 7.6; L-arginine group: 21.5 ± 5.3) (p < 0.05).
Conclusion
The inhibition of endothelial cells apoptosis by L-arginine restores endothelial function in a model of hypercholesterolemia.
Background
The concept of programmed cell death was introduced to describe cell death during normal development [1], and apoptosis is the most common form of cell death. Apoptosis is characterized by cell shrinkage, nuclear fragmentation and membrane blabbing [2,3].
Atherosclerotic lesions develop in the tunica intima of the arteries, in which accumulation of cellular components, lipids, and extracellular matrix yields a fibro fatty plaque that focally thickens the artery wall [4]. Apoptosis is a feature of human atherosclerosis which is associated with development of the lesion necrotic core as well as instability of complex plaques [4-9].
The first evidence that endothelial cell (EC) apoptosis might contribute to the initiation of atherogenesis came from the observation that all classical risk factors known to promote endothelial dysfunction (ED) and atherogenesis can induce vascular cell apoptosis [10]. However, there is some in vivo evidence for a pro-atherogenic effect of apoptosis. A study in monkeys revealed that vascular ED was present without evidence of atherosclerosis, which may be due to endothelial apoptosis [11]. Apoptotic vascular cells are also found in hypercholesterolemic pigs and mice [12]. On the other hand, shear stress leads to physiologic low concentrations of nitric oxide (NO) within ECs [13]. The continuous generation of NO can prevent ECs apoptosis, thereby protecting the endothelial monolayer from injury [14]. Intervention with NO donor; L-arginine, has induced beneficial effects on atherosclerosis [15]. These findings strongly support the current clinical concept that ED precedes plaque formation and disease progression in patients [16].
The role of L-arginine and NO in apoptosis have been studied in different conditions (17–30). NO has also been demonstrated to be involved in the regulation of apoptosis, and recent evidence indicates that NO is a potent modulator of homeostasis operationally preventing or inducing apoptosis [31,32]. It is also reported that in some cell types, NO can promote apoptosis, whereas in others it inhibits apoptosis [33]. L-arginine as a NO donor is a potent substance to reverse ED [34-37]. Otsuji et al. studied the relationship between L-arginine and the progression of atherosclerosis. They found that exogenous L-arginine reverses acetylcholine-induced vasoconstriction in human coronary arteries in the early stages of atherosclerosis [38]. In hypercholesterolaemic rabbits treated with L-arginine, platelet aggregation, myointimal cell proliferation and vascular monocyte accumulation were attenuated while endothelium dependent vasoreactivity was improved [39]. Therefore, it is a hypothesized that L-arginine prevents ED through EC apoptosis inhibition in a model of hypercholesterolemia, and this hypothesis was tested in this study.
Results
Cholesterol and Nitrite Concentrations
The data for the cholesterol and nitrite concentrations are tabulated in table 1. The statistical analyses indicate that no significant difference was observed between the cholesterol levels of two groups, but the nitrite concentration in L-arginine group was significantly higher than control group (p < 0.05).
Table 1.
The mean of serum cholesterol, LDL and nitrite levels in two groups of experiments.
| group | cholesterol (mg/dl) | nitrite (μmol/l) | ||
| before | after | before | after | |
| Control (n = 16) | 111.7 ± 14.1 | 2129.1 ± 176.2 | 10 ± 0.7 | 11.8 ± 1 |
| L-arginine (n = 14) | 125.4 ± 14.5 | 2109.1 ± 166.9 | 11.6 ± 0.5 | 14.7 ± 0.5 |
| p | >0.05 | >0.05 | >0.05 | <0.05 |
Fatty streak formation
The score of aorta fatty streak in first group was 0.875 ± 0.35, but no fatty streak lesion was detected in L-arginine group. The statistical analysis indicates that fatty streak formation is significantly lower in L-arginine group (p < 0.05).
The number of intimal apoptotic cells
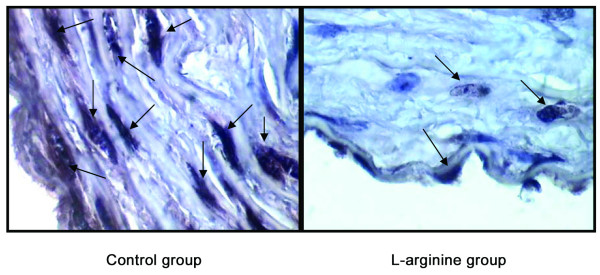
The number of intimal apoptotic cells/500 cells in rabbit's aortas is demonstrated in figure 1 (control group: 39.3 ± 7.6, L-arginine group: 21.5 ± 5.3) (p < 0.05). In situ detection of apoptotic cells indicate that in aorta section from control group, the apoptosis of intimal cells is a prominent feature of atherosclerotic lesions, but less apoptosis cells were observed in L-arginine group (figure 2).
Figure 1.
The intimal apoptotic cells in rabbit's aortas in two groups of animals. Figure shows that less apoptosis cells were observed in the aorta of L-arginine group. ☆ Indicate significant difference from control group (p < 0.05).
Figure 2.
TUNEL performed in rabbit's aorta. Aorta section from rabbit that apoptosis of arterial cells is a prominent feature of atherosclerotic lesions (control group), but less apoptotic cells were observed in the aorta of L-arginine group.
Discussion
The role of L-arginine in ED prevention through EC apoptosis inhibition was the main objective of this study. There was no fatty streak in the aorta of L-arginine treated group while significant fatty streak lesions were found in control group. Subsequently, L-arginine supplementation successfully prevented atherosclerosis process, and this is in agreement with the results of several other studies in which L-arginine supplementation restores endothelial function [40-47].
Among the NO metabolites, nitrite is a major oxidative metabolite, which was implicated to be both an indicator for NO synthase (NOS) activity [48,49] and a circulating NO donor [50]. It has been shown that up to 70–90% of plasma nitrite derived from eNOS activity in fasted humans and other mammals [49,50].
The assumption of L-arginine efficacy theoretically has been based on eNOS activation and nitrite production enhancement. Our results were in line of this assumption, in which L-arginine supplementation led to significantly higher plasma nitrite concentration. Other studies have reported NO metabolites increasing in hypercholesterolemic animals [51-53]. Although decreased NO bioactivity (stems from flow mediated dilation studies) has been attributed to ED [54], increased plasma level of nitrite has been reported in hypercholesterolemic patients too [55]. It has been suggested that enhanced NO synthesis might be a defense mechanism to compensate continuous inactivation of NO and to protect from damaging factors such as hypercholesterolemia [56,57]. Another proposed mechanism for the elevation of nitrite may be NO production by other isoforms of NOS enzymes [57,58]. Although decreased activity of eNOS has been indicated in atherosclerosis but NO may be produced by iNOS in macrophages and other cell types in the atherosclerosis [57,58].
NO is also an essential signaling molecule for endothelial integrity and growth [59]. A moderate basal NO production can protect ECs from damaging effects of risk factor [49]. We hypothesized that if intrinsic protective mechanisms could be activated by moderate NO production in ECs, these cells could be better prepared to the ensuing risk factor (hypercholesterolemia) assault. Our results corroborate the protective role as the L-arginine group had significantly lower apoptotic cells in aorta intimal layer after 4 weeks of diet consumption. The precise mechanism responsible for inhibition of apoptosis by NO is not clear. Several possibilities exist that may explain the anti-apoptotic effects of NO. NO has been shown to increase Bcl-2, thioredoxin, and heat-shock protein-70 and -32 expression, and therefore it inhibits the release of mitochondrial cytochrome c and apoptosis inducing factors [33,60,61]. The activation of cGMP and cGMP-dependent protein kinase by NO increases a major intracellular anti-apoptotic protein, both directly and indirectly [62].
Also, it has been shown that NO inhibits the caspases-3 and 8 activations in L-arginine treated ECs and consequently inhibits apoptosis, which is consistent with our findings [63-65]. It should be further acknowledged, however, that the protective effect of L-arginine could also be mediated through non-eNOS-dependent pathways, since L-arginine has anti oxidant effects too [66,67]. Of course more studies are warranted in this field, and for future researches, using different doses of L-arginine and cholesterol diet in acute and chronic models of hypercholesterolemia are suggested.
Conclusion
L-arginine attenuates the number of apoptosis cells in the aorta of a model of hypercholesterolemia. The inhibition of EC apoptosis may be the underlying mechanism of restore endothelial function by L-arginine.
Methods
Animals and Experimental design
This study was reviewed and approved by the Ethics Committee of Isfahan University of Medical Sciences. Thirty white male rabbits weighing 1.95 ± 0.25 kg were obtained from the Pasteur Institute of Iran. All animals were housed three per cage with free access to food and water. After 1-week acclimation period and an overnight fasting, blood samples were taken as pre-experimental sampling to obtain baseline data. Collected blood samples were centrifuged (10,000 _ g), and the resulting serum was stored at -70°C until measurements. The animals were then randomly assigned to 2 groups. The rabbits were fed rabbit chow supplemented with 1% cholesterol (hypercholesterolemic diet; control group, n = 16) or high-cholesterol diet with oral L-arginine (3% in drinking water) (L-arginine group, n = 14) for 4 weeks. Pure cholesterol and L-arginine were obtained from Scharlau Chemie (Barcelona, Spain) and Ajinomoto Co (Japan) respectively. At the end of experiment, fasting blood samplings were obtained, and half of the animals of each group randomly were selected and euthanized by an overdose of sodium pentobarbital (50 mg/kg) and ex-sanguinated. The animal's aortas were harvested for pathological investigation. The serum levels of cholesterol, and nitrite were measured. The fatty streak formation and the number of apoptotic cells also were determined as previously described [53,68,2,3].
Serum cholesterol and nitrite measurements
Total cholesterol level was measured using standard enzymatic kit (Pars Azmoon Co, Iran). The serum level of nitrite (stable NO metabolite) was measured using a colorimetric assay kit (R&D Systems, Minneapolis, USA) that involves the Griess reaction. Briefly, serums were added into wells (96-well enzymatic assay plate). A sulphanilamide solution was added to all experimental samples, and after incubation, N-1-naphtylethylenediamine dihydrochloride solution was added. Then, absorbance was measured by a microreader in 540 nm wavelength. The samples nitrite concentration was determined by comparison to nitrite standard reference curve. The detection limit was 0.25 μM nitrite.
Fatty streak determination
The abdominal aortas were subjected to pathological investigation to verify fatty dot or fatty streak lesions formation. The entire aorta, from the aortic arch to the external iliac arteries, was dissected out and cleaned of excess adventitial tissue. The aortas were fixed in buffered 10% formalin for 24 h, and then embedded in paraffin. The paraffin-embedded specimens were sectioned at 5 μm (20 sections in succession) and stained with haematoxylin and eosin, and examined by light microscopy to measure fatty streak by two pathologists in a double-blinded manner.
Fatty streak lesions were graded as zero for no fatty streak, 1 for existence of fatty streak in 1–4 sections, 2 for existence of fatty streak in 5–9 sections, 3 for existence of fatty streak in 10–14 sections and 4 for existence of fatty streak in 15 to all 20 sections of vessels.
In situ detection of apoptotic cells by TUNEL method
The Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) method was used for in situ detection of apoptotic cells by in situ cell death detection kit (Roche Applied Science, Indianopolis, IN, USA) as the manufacturer's instructions. Breifly, after dewaxation of formalin-fixed tissue sections; the slides were placed in a plastic jar containing 200 ml 0.1 M citrate buffer, pH 6.0, and were heated applying 350 W microwave irradiation for 5 min. After rinsing the slide with PBS (20°–25°C), they were immersed in a blocking solution containing 0.1 M Tris-HCl, 3% BSA, and 20% normal bovine serum, pH 7.5 for 30 min at room temperature, and washed again with PBS. Then TUNEL reaction mixture was added and incubated for 60 min at 37°C in a humidified chamber. The slides were washed, and anti fluorescein conjugated with alkaline phosphatase were added, and incubated again for 30 min. After rinsing in PBS, BCIP-NBT substrate solution was added and incubated for 15 min. The slides were subjected to wash extensively in tap water and were counterstained with hematoxylin. For apoptotic cells enumeration, at least 500 intimal cells were counted and the number of apoptotic cells was determined per 500 cells using light microscope.
Statistical Analysis
The data are reported as the mean ± SEM. A statistical software package, SPSS (version 13), was used to perform statistical analysis. The data were tested for normality and homogeneity of variance. Otherwise, unpaired Student's t-test (equal or unequal variance assumed accordingly) was used to assess the significance of any change between groups. Statistical significance was accepted at p < 0.05.
Abbreviations
EC: Endothelial Cell; ED: Endothelial Dysfunction; NO: Nitrix Oxide; NOS: Nitric Oxide Synthase; eNOS: Endothelial Nitric Oxide Synthase; TUNEL: Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MN carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript. SH provide assistance in the design of the study, coordinated and carried out all the experiments and participated in manuscript preparation. FM and ALM provides assistance for all experiments. All authors have read and approved the content of the manuscript.
Acknowledgments
Acknowledgements
This study was supported by Isfahan University of Medical sciences, Isfahan, Iran (Grant #384151). We acknowledge Mehrzad Ghadesi for his technical assistance. We also thank Mr Hasan Sadeghi, Mr Asgar Sayadi and Mansoor Karimi for their help in animal laboratory.
Contributor Information
Mehdi Nematbakhsh, Email: nematbakhsh@gmail.com.
Shaghayegh Haghjooyjavanmard, Email: shaghayeghhaghjoo@yahoo.com.
Farzaneh Mahmoodi, Email: darkhaste_1@yahoo.com.
Ali Reza Monajemi, Email: alirezamonajemi@yahoo.com.
References
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Morris RG, Smith A, Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984;142:67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Kockx MM. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol. 1998;18:1519–22. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- Isner JM, Kearney M, Bortman S, Passeri J. Apoptosis in human atherosclerosis and resteNOSis. Circulation. 1995;91:2703–2711. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- Hegyi L, Skepper JN, Cary NR, Mitchinson MJ. Foam cell apoptosis and the development of the lipid core of human atherosclerosi. J Pathol. 1996;180:423–9. doi: 10.1002/(SICI)1096-9896(199612)180:4<423::AID-PATH677>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kockx MM, De Meyer GR, Muhring J, Bult H, Bultinck J, Herman A. Distribution of cell replication and apoptosis in atherosclerotic plaques of cholesterol-fed rabbits. Atherosclerosis. 1996;120:115–124. doi: 10.1016/0021-9150(95)05691-2. [DOI] [PubMed] [Google Scholar]
- Geng YJ, Libby P. Evidence for apoptosis in advanced human atheroma: colocalization with interleukin 1β converting enzyme. Am J Pathol. 1995;147:251–66. [PMC free article] [PubMed] [Google Scholar]
- Han DK, Haudenschild CC, Hong MK, Tinkle BT, Leon MB, Liau G. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am J Pathol. 1995;147:267–77. [PMC free article] [PubMed] [Google Scholar]
- Björkerud S, Björkerud B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol. 1996;149:367–80. [PMC free article] [PubMed] [Google Scholar]
- Stefanec T. Endothelial apoptosis: could it have a role in the pathogenesis and treatment of disease? Chest. 2000;117:841–54. doi: 10.1378/chest.117.3.841. [DOI] [PubMed] [Google Scholar]
- Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, Natividad F, Bishop SP, Vatner SF. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–99. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104:2649–52. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83:334–41. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- Tricot O, Mallat Z, Heymes C, Belmin J, Lesèche G, Tedgui A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–3. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- Napoli C, Ignarro LJ. Nitric oxide-releasing drugs. Annu Rev Pharmacol Toxicol. 2003;43:97–123. doi: 10.1146/annurev.pharmtox.43.100901.140226. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse longterm outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Wu CT, Ren YF, Liu JF, Zhang JH, Lei ST. L-arginine reduces intestinal epithelial cell apoptosis in rats with severe abdominal infection. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1728–30. [PubMed] [Google Scholar]
- Sumou IK, Du JB, Wei B, Zhang CY, Qi JG, Tang CS. Effect of L-arginine on pulmonary artery smooth muscle cell apoptosis in rats with hypoxic pulmonary vascular structural remodeling. Acta Biochim Biophys Sin (Shanghai) 2006;38:15–21. doi: 10.1111/j.1745-7270.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- Tan X, Pan JQ, Li JC, Liu YJ, Sun WD, Wang XL. L-arginine inhibiting pulmonary vascular remodelling is associated with promotion of apoptosis in pulmonary arterioles smooth muscle cells in broilers. Res Vet Sci. 2005;79:203–9. doi: 10.1016/j.rvsc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Holm AM, Andersen CB, Haunsø S, Hansen PR. Effects of L-arginine on vascular smooth muscle cell proliferation and apoptosis after balloon injury. Scand Cardiovasc J. 2000;34:28–32. doi: 10.1080/14017430050142369. [DOI] [PubMed] [Google Scholar]
- Dodd F, Limoges M, Boudreau RT, Rowden G, Murphy PR, Too CK. L-arginine inhibits apoptosis via a NO-dependent mechanism in Nb2 lymphoma cells. J Cell Biochem. 2000;77:624–34. doi: 10.1002/(SICI)1097-4644(20000615)77:4<624::AID-JCB10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bagci EZ, Vodovotz Y, Billiar TR, Ermentrout B, Bahar I. Computational insights on the competing effects of nitric oxide in regulating apoptosis. PLoS ONE. 2008;3:e2249. doi: 10.1371/journal.pone.0002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer DA, Das A, Semela D, Kang-Decker N, Hendrickson H, Bronk SF, Katusic ZS, Gores GJ, Shah VH. Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species. Hepatology. 2008;47:1983–93. doi: 10.1002/hep.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SD, Bakker AD, Semeins CM, Kuijpers-Jagtman AM, Klein-Nulend J. Inhibition of osteocyte apoptosis by fluid flow is mediated by nitric oxide. Biochem Biophys Res Commun. 2008;369:1150–4. doi: 10.1016/j.bbrc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Tang JR, Seedorf G, Balasubramaniam V, Maxey A, Markham N, Abman SH. Early inhaled nitric oxide treatment decreases apoptosis of endothelial cells in neonatal rat lungs after vascular endothelial growth factor inhibition. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1271–80. doi: 10.1152/ajplung.00224.2007. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kitao T, Nakagawa K, Fujisaki H, Takegawa Y, Koda K, Ago Y, Baba A, Matsuda T. Nitric oxide-induced apoptosis in cultured rat astrocytes: protection by edaravone, a radical scavenger. Glia. 2007;55:1325–33. doi: 10.1002/glia.20541. [DOI] [PubMed] [Google Scholar]
- Mori M. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J Nutr. 2007;137:1616S–1620S. doi: 10.1093/jn/137.6.1616S. [DOI] [PubMed] [Google Scholar]
- Bobba A, Atlante A, Moro L, Calissano P, Marra E. Nitric oxide has dual opposite roles during early and late phases of apoptosis in cerebellar granule neurons. Apoptosis. 2007;12:1597–610. doi: 10.1007/s10495-007-0086-4. [DOI] [PubMed] [Google Scholar]
- Mannick JB, Miao XQ, Stamler JS. Nitric oxide inhibits Fas-induced apoptosis. J Biol Chem. 1997;272:24125–8. doi: 10.1074/jbc.272.39.24125. [DOI] [PubMed] [Google Scholar]
- Kim YM, Kim TH, Seol DW, Talanian RV, Billiar TR. Nitric oxide suppression of apoptosis occurs in association with an inhibition of Bcl-2 cleavage and cytochrome c release. J Biol Chem. 1998;273:31437–41. doi: 10.1074/jbc.273.47.31437. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Bonfoco E, Brune B, Tarr JM, Eggleton P, Winyard PG. Mechanisms for nitric oxide-induced cell death: involvement of apoptosis. Adv Neuroimmunol. 1995;5:411–20. doi: 10.1016/0960-5428(95)00025-9. [DOI] [PubMed] [Google Scholar]
- Li CQ, Wogan GN. Nitric oxide as a modulator of apoptosis. Cancer Lett. 2005;226:1–15. doi: 10.1016/j.canlet.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Brune B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003;10:864–9. doi: 10.1038/sj.cdd.4401261. [DOI] [PubMed] [Google Scholar]
- Clarkson P, Adams MR, Powe AJ, Donald AE, McCredie R, Robinson J, McCarthy SN, Keech A, Celermajer DS, Deanfield JE. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest. 1996;97:1989–94. doi: 10.1172/JCI118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka Y, Egashira K, Imaizumi T, Tagawa T, Kai H, Sugimachi M, Takeshita A. Effect of L-arginine on acetylcholine-induced endothelium-dependent vasodilation differs between coronary and forearm vasculatures in humans. J Am Coll Cardiol. 1994;24:948–55. doi: 10.1016/0735-1097(94)90854-0. [DOI] [PubMed] [Google Scholar]
- Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-arginine improves endothelium dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–53. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H, Zeiher AM, Meinzer K, Just H. Correlation of endothelial dysfunction in coronary microcirculation of hypercholesterolemic patients by L-arginine. Lancet. 1991;338:1546–50. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- Otsuji S, Nakajima O, Waku S, Kojima S, Hosokawa H, Kinoshita I, Okubo T, Tamoto S, Takada K, Ishihara T. Attenuation of acetylcholine-induced vasoconstriction by L-arginine is related to the progression of atherosclerosis. Am Heart J. 1995;129:1094–100. doi: 10.1016/0002-8703(95)90388-7. [DOI] [PubMed] [Google Scholar]
- Böger RH, Bode-Böger SM, Kienke S, Stan AC, Nafe R, Frölich JC. Dietary L-arginine decreases myointimal cell proliferation and vascular monocyte accumulation in cholesterol-fed rabbits. Atherosclerosis. 1998;136:67–77. doi: 10.1016/S0021-9150(97)00183-4. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Boger RH, Antoniades C, Siasos G, Stefanadi E, Stefanadis C. Mechanisms of disease: L-arginine in coronary atherosclerosis – a clinical perspective. Nat Clin Pract Cardiovasc Med. 2007;4:274–83. doi: 10.1038/ncpcardio0878. [DOI] [PubMed] [Google Scholar]
- Siasos G, Tousoulis D, Antoniades C, Stefanadi E, Stefanadis C. L-arginine, the substrate for NO synthesis: an alternative treatment for premature atherosclerosis? Int J Cardiol. 2007;116:300–8. doi: 10.1016/j.ijcard.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Fisman EZ, Tenenbaum A, Shapira I, Pines A, Motro M. The nitric oxide pathway: is L-arginine a gate to the new millennium medicine? A meta-analysis of L-arginine effects. J Med. 1999;30:131–48. [PubMed] [Google Scholar]
- Tousoulis D, Antoniades C, Tentolouris C, Goumas G, Stefanadis C, Toutouzas P. L-arginine in cardiovascular disease: dream or reality? Vasc Med. 2002;7:203–11. doi: 10.1191/1358863x02vm434ra. [DOI] [PubMed] [Google Scholar]
- Preli RB, Klein KP, Herrington DM. Vascular effects of dietary L-arginine supplementation. Atherosclerosis. 2002;162:1–15. doi: 10.1016/S0021-9150(01)00717-1. [DOI] [PubMed] [Google Scholar]
- Le Tourneau T, Van Belle E, Corseaux D, Vallet B, Lebuffe G, Dupuis B, Lablanche JM, McFadden E, Bauters C, Bertrand ME. Role of NO in resteNOSis after experimental balloon angioplasty in the hypercholesterolemic rabbit: effects on neointimal hyperplasia and vascular remodeling. J Am Coll Cardiol. 1999;33:876–82. doi: 10.1016/S0735-1097(98)00621-4. [DOI] [PubMed] [Google Scholar]
- Corseaux D, Le Tourneau T, Six I, Ezekowitz MD, Mc Fadden EP, Meurice T, Asseman P, Bauters C, Jude B. Enhanced monocyte tissue factor response after experimental balloon angioplasty in hypercholesterolemic rabbits: inhibition with L-arginine. Circulation. 1998;98:1776–82. doi: 10.1161/01.cir.98.17.1776. [DOI] [PubMed] [Google Scholar]
- Adams MR, McCredie R, Jessup W, Robinson J, Sullivan D, Celermajer DS. Oral L-arginine improves endothelium dependent dilatation and reduces monocyte adhesion to endothelial cells in young men with coronary disease. Atherosclerosis. 1997;129:261–9. doi: 10.1016/S0021-9150(96)06044-3. [DOI] [PubMed] [Google Scholar]
- Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Gödecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–6. doi: 10.1016/S0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Bryan NS. Nitrite in nitric oxide biology: cause or consequence? A systems-based review. Free Radic Biol Med. 2006;41:691–701. doi: 10.1016/j.freeradbiomed.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Minor RL, Jr, Myers PR, Guerra R, Jr, Bates JN, Harrison DG. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest. 1990;86:2109–16. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroe H, Honda H. Comparison of endothelial function in the carotid artery between normal and short-term hypercholesterolemic rabbits. Comp Biochem Physiol C Toxicol Pharmacol. 2006;144:197–203. doi: 10.1016/j.cbpc.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Nematbakhsh M, Hayat-Davoodi P, Rajabi P, Samarian SH. The effect of estrogen on endothelial permeability of aorta and the level of serum nitrite concentration in cholesterol-fed ovariectomized rabbit. Iran Biomed J. 2002;6:77–82. [Google Scholar]
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- Ferlito S, Gallina M, Catassi S, Bisicchia A, Di Salvo MM. Nitrite plasma levels in normolipemic and hypercholesterolemic patients with peripheral occlusive arteriopathy. Panminerva Med. 1999;41:307–9. [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Matsui-Hirai H, Fukatsu A, Sumi D, Kano-Hayashi H, Rani PJA, Iguchi A. Selective iNOS inhibitor, ONO1714 successfully retards the development of high-cholesterol diet induced atherosclerosis by novel mechanism. Atherosclerosis. 2006;187:316–24. doi: 10.1016/j.atherosclerosis.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Nachtigal P, Kopecky M, Solichova D, Zdansky P, Semecky V. The changes in the endothelial expression of cell adhesion molecules and iNOS in the vessel wall after the short-term administration of simvastatin in rabbit model of atherosclerosis. J Pharm Pharmacol. 2005;57:197–203. doi: 10.1211/0022357055353. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Napoli C. Novel features of nitric oxide, endothelial nitric oxide synthase, and atherosclerosis. Curr Diab Rep. 2005;5:17–23. doi: 10.1007/s11892-005-0062-8. [DOI] [PubMed] [Google Scholar]
- 28. Boyd CS, Cadenas E. Nitric oxide and cell signaling pathways in mitochondrial-dependent apoptosis. Biol Chem. 2002;383:411–23. doi: 10.1515/BC.2002.045. [DOI] [PubMed] [Google Scholar]
- Li CQ, Wogan GN. Nitric oxide as a modulator of apoptosis. Cancer Lett. 2005;226:1–15. doi: 10.1016/j.canlet.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Razavi HM, Hamilton JA, Feng Q. Modulation of apoptosis by nitric oxide: implications in myocardial ischemia and heart failure. Pharmacol Ther. 2005;106:147–62. doi: 10.1016/j.pharmthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem. Biophy. Res Commun. 1997;240:419–24. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- Kim YM, Chung HT, Simmons RL, Billiar TR. Cellular non-heme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition. J Biol Chem. 2000;275:10954–61. doi: 10.1074/jbc.275.15.10954. [DOI] [PubMed] [Google Scholar]
- Raveendran M, Wang J, Senthil D, Wang J, Utama B, Shen Y, Dudley D, Zhang Y, Wang XL. Endogenous nitric oxide activation protects against cigarette smoking induced apoptosis in endothelial cells. FEBS Lett. 2005;579:733–40. doi: 10.1016/j.febslet.2004.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabłecka A, Checiñski P, Krauss H, Micker M, Ast J. The influence of two different doses of L-arginine oral supplementation on nitric oxide (NO) concentration and total antioxidant status (TAS) in atherosclerotic patients. Med Sci Monit. 2004;10:CR29–32. [PubMed] [Google Scholar]
- Suessenbacher A, Lass A, Mayer B, Brunner F. Antioxidative and myocardial protective effects of L-arginine in oxygen radical-induced injury of isolated perfused rat hearts. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:269–76. doi: 10.1007/s00210-001-0523-9. [DOI] [PubMed] [Google Scholar]
- Nematbakhsh M, Ali-Hemmatti A, Dashti G, Rajabi P. Estrogen attenuates the accumulation of fatty streaks in coronary arteries of ovariectomized high cholesterol-fed rabbits. Ateroskleroza. 2002;6:13–6. [Google Scholar]