Abstract
Background
Although approximately 80% of individuals with alcohol use disorders are chronic smokers and despite reported associations between chronic cigarette smoking and lower cerebral perfusion in nonalcoholics, previous brain perfusion studies with alcoholics did not account for the potential effects of concurrent chronic cigarette smoking.
Methods
One-week-abstinent alcohol-dependent individuals in treatment (ALC) [19 smokers (sALC) and 10 nonsmokers (nsALC)] and 19 healthy light drinking, nonsmoking control participants (nsLD) were scanned with a pulsed arterial spin labeling method to measure cerebral perfusion without an exogenous contrast agent. Studies were performed with 2 different postlabeling delay times (time from labeling pulse to the excitation pulse; PLD = 1,500 ms and PLD = 1,200 ms) to assess the potential effect of arterial blood transit time on the perfusion. Average gray matter (GM) and white matter (WM) perfusion for the frontal and parietal lobes were calculated for each hemisphere from voxels containing at least 90% GM and 100% WM.
Results
At PLD = 1,500 ms, multivariate analyses compared ALC (combined sALC and nsALC) with nsLD (p = 0.04) and contrasted sALC, nsALC, and nsLD (p = 0.006). ALC, as a group, showed 13% lower frontal GM perfusion (p = 0.005) and 8% lower parietal GM perfusion than nsLD (p = 0.03). With ALC separated into smokers and nonsmokers, sALC showed 19% lower frontal GM perfusion (p = 0.001) and 12% lower parietal GM perfusion than nsLD (p = 0.004). In sALC, a higher number of cigarettes smoked per day was associated with lower perfusion. Overall, regional perfusion did not differ significantly between nsALC and nsLD. Results obtained with PLD = 1,200 ms generally confirmed the 1,500 ms findings.
Conclusions
This study provides preliminary evidence that chronic cigarette smoking adversely affects cerebral perfusion in frontal and parietal GM of 1-week-abstinent alcohol-dependent individuals. These results are in line with our spectroscopic and structural magnetic resonance studies that suggest chronic cigarette smoking compounds the detrimental effects of alcohol dependence on brain neurobiology.
Keywords: Alcohol Dependence, Cigarette Smoking, Regional Brain Perfusion, Cerebral Blood Flow, Magnetic Resonance (MR), Neurocognition
Brain perfusion quantitates blood flow to tissue per unit time and is typically tightly coupled with brain metabolic activity (e.g., Raichle et al., 1976; Silverman et al., 2001). Previous studies of the effects of chronic alcohol dependence on cerebral perfusion employing 133Xe inhalation tomography and 99mTc-HMPAO single photon emission tomography (SPECT) demonstrated perfusion abnormalities in the frontal, parietal, and temporal lobes. Specifically, these studies indicated larger perfusion deficits in the right hemisphere (Berglund et al., 1987; Tutus et al., 1998), left hemisphere (Erbas et al., 1992), or bilateral abnormalities (Demir et al., 2002; Mampunza et al., 1995; Nicolas et al., 1993). Similarly, abnormal glucose metabolism has been shown with 18FDG-positron emission tomography (PET), especially in frontal lobes of alcohol-dependent individuals (Gilman et al., 1990; Volkow et al., 1994). Lower frontal cerebral perfusion among individuals in alcohol treatment was associated with deficits in working memory and executive functions (Demir et al., 2002; Nicolas et al., 1993; Noel et al., 2001, 2002).
However, the aforementioned studies did not account for the potential effects of concurrent chronic cigarette smoking on cerebral perfusion and/or metabolism. Tobacco products are the most frequently used substances among alcoholics, with an estimated 80% of alcohol-dependent individuals smoking regularly (Hurt et al., 1994; Pomerleau et al., 1997; Romberger and Grant, 2004) and 50 to 90% demonstrating nicotine dependence (Daeppen et al., 2000; Marks et al., 1997). Active cigarette smoking in alcoholics has been associated with significantly higher quantity and frequency of alcohol consumption (John et al., 2003), particularly compared with nonsmoking or former-smoking alcohol-dependent individuals (York and Hirsch, 1995). Chronic cigarette smoking has been associated with increased risk for arteriosclerosis (e.g., Bolego et al., 2002; Haustein et al., 2004), compromised vasomotor reactivity (Bolego et al., 2002; Terborg et al., 2002), and impaired pulmonary function (Yamashita et al., 1988). After at least 2 hours of abstinence from smoking, chronic nonalcoholic smokers demonstrated 4 to 12% lower global brain perfusion, compared with nonsmokers, as measured by 133Xe inhalation (Rogers et al., 1983; Yamashita et al., 1988). However, another study without any constrains on smoking before an IMP–SPECT scan demonstrated 22% lower global perfusion in smokers (Rourke et al., 1997), with perfusion inversely related to cigarette pack-years. The lower perfusion reported in smokers in the latter study may be partially accounted for by acute effects of smoking, consistent with 7 to 10% global decrease in glucose utilization following acute nicotine administration (Domino et al., 2000; Stapleton et al., 2003). Smoking also elicits different patterns of relative perfusion responses, with increases of the order of 6 to 8% in a number of brain regions including prefrontal and cingulate cortices as well as decreases in cerebellum and occipital lobes that were associated with plasma nicotine levels (Brody, 2005; Domino et al., 2004; Rose et al., 2003). Thus, it is not clear if acute cigarette smoking affects absolute perfusion in prefrontal and cingulate cortices (Brody, 2005).
Chronic cigarette smoking, independent of chronic and heavy alcohol consumption, was also linked to compromised memory, cognitive flexibility, executive functions, psychomotor speed, general intellectual abilities, and working memory (see Durazzo et al., 2004, and references therein). Moreover, smoking appears to modulate relationships between brain morphology, metabolites, and neurocognition in recently detoxified and short-term abstinent alcoholics (Durazzo et al., 2004, 2006; Gazdzinski et al., 2005). This possibly reflects a reorganization of neural networks or compensatory increase in the activity of other functional networks (see Desmond et al., 2003; Pfefferbaum et al., 2001) to counter the additional adverse neurobiologic effects of chronic smoking.
Perfusion deficits may be related to metabolic and/or structural abnormalities observed in recently detoxified alcoholics (see Sullivan, 2000, for review). Brain perfusion is closely coupled with brain glucose metabolism (Silverman et al., 2001), and we showed that glucose metabolism was positively associated with the concentration of N-acetylaspartate (NAA; putative marker of neuronal viability) measured with magnetic resonance spectroscopy (O'Neill et al., 2000). Some studies also reported associations between cerebral hypoperfusion and atrophy (Nicolas et al., 1993; Oishi et al., 1999; Yamaguchi et al., 1983). Thus, perfusion deficits may be associated with decreased levels of NAA and/or brain shrinkage reported in alcoholics. Via proton magnetic resonance spectroscopic imaging, we demonstrated that cigarette smoking exacerbated alcohol-induced neuronal dysfunction in the frontal lobe of alcohol-dependent individuals and that chronic smoking had independent, detrimental effects on tissue in select subcortical nuclei and the cerebellum (Durazzo et al., 2004). We also showed comorbid chronic cigarette smoking and alcoholism were associated with parietal, temporal, and occipital gray matter (GM) volume reductions that were greater than GM shrinkage induced by alcohol dependence alone (Gazdzinski et al., 2005).
Given the high comorbidity between alcohol dependence and chronic cigarette smoking and their adverse effects on cerebral perfusion, it is not clear if the perfusion deficits reported in previous studies of alcohol-dependent individuals are solely attributable to alcohol consumption or if the combination of both chronic alcohol dependence and cigarette smoking produce greater adverse effects on brain perfusion than alcohol dependence alone. Furthermore, it is unclear if smoking affects the relationships between cerebral perfusion and cognitive function in alcohol dependence.
In this cross-sectional study, we used magnetic resonance imaging (MRI) to noninvasively measure cerebral perfusion in a group of 1-week-abstinent alcohol-dependent individuals (ALC), who were retrospectively classified into smokers (sALC) and nonsmokers (nsALC), and in an age-matched group of nonsmoking light drinking individuals (nsLD). We compared groups on mean brain perfusion in GM and white matter (WM) of the frontal and parietal lobes, bilaterally, and related regional perfusion to performance on a brief neuropsychological test battery assessing visuospatial learning and memory, working memory, and visuomotor scanning speed and incidental learning. We tested the following hypotheses:
Alcohol-dependent participants, as a group, demonstrate lower frontal and parietal GM and WM perfusion than nonsmoking light drinkers.
Smoking alcoholics demonstrate lower frontal and parietal perfusion than nonsmoking alcoholics and nonsmoking light drinkers in GM and WM.
Nonsmoking alcoholics demonstrate lower frontal and parietal perfusion than nonsmoking light drinkers in the frontal and parietal lobes, bilaterally.
Regional perfusion is negatively correlated with measures of drinking and smoking severity.
Lower frontal and parietal perfusion are associated with worse performance on measures of visuospatial learning and memory, working memory, and visuomotor scanning speed and incidental learning.
Materials and Methods
Participants
Twenty-nine alcohol-dependent individuals in treatment were recruited from the San Francisco VA Medical Center Substance Abuse Day Hospital and the San Francisco Kaiser Permanente Chemical Dependence Recovery Program. All participants were between the ages of 26 and 66 years at the time of enrollment. ALC were retrospectively divided into smokers (sALC, n = 19, 1 female; 1 left-handed participant) and nonsmokers (nsALC, n = 10, all right-handed males). The sALC had their last alcoholic drink 6.0 ± 3.2 and the nsALC 5.5 ± 2.6 days before the MRI study (p = NS). Nineteen healthy nonsmoking light drinkers (nsLD, 3 females; 1 left-handed participant) were recruited from the San Francisco Bay Area community. Twenty-two ALC and 6 nsLD of this study were also participants in corresponding MR spectroscopic imaging (Durazzo et al., 2004) and structural MRI studies (Gazdzinski et al., 2005). There were no restrictions on tobacco and caffeine use before the scanning session; however, time since last use of each substance was recorded.
The inclusion and exclusion criteria are fully described in Durazzo et al., (2004). In summary, all ALC met DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, fourth edition) criteria for alcohol dependence with physiological dependence and consumed more than 150 standard alcoholic drinks per month for at least 8 years before enrollment into the study. A standard drink contains 13.6 g of pure ethanol, equivalent of 12 oz. beer, 5 oz. wine, or 1.5 oz. liquor. All participants were free of general medical, neurologic, and psychiatric conditions (except unipolar mood disorders, controlled hypertension, and hepatitis C in ALC) known or suspected to influence brain morphology, brain perfusion, or neurocognition. Unipolar mood disorders were not exclusionary for ALC given their high incidence reported in alcohol-dependent individuals (e.g., Gilman and Abraham, 2001; Hasin and Grant, 2002) and chronic cigarette smokers (e.g., Dursun and Kutcher, 1999; Fergusson et al., 2003).
To assess hepatocellular injury and red blood cell (RBC) status, standard liver and blood panels were performed within 1 day of the perfusion study. Two participants in the sALC group and 3 in the nsALC group tested positive for hepatitis C. Serum albumin and prealbumin were used as indicators of nutritional status (Weinrebe et al., 2002).
Participants completed the clinical interview for DSM-IV Axis I Disorders Patient Edition, Version 2.0 (American Psychiatric Association, 1994), and standardized questionnaires assessing alcohol withdrawal (CIWA-Ar; Addiction Research Foundation Clinical Institute of Withdrawal Assessment for Alcohol; Sullivan et al., 1989), depressive (Beck Depression Inventory; Beck, 1978), and anxiety symptomatology (State-Trait Anxiety Inventory, Y-2, STAI Y-2; Spielberger et al., 1977) within 1 day of the scanning session. Participants were not studied if their CIWA-Ar indicated clinically significant withdrawal symptoms (i.e., total score greater than 8). In the sALC group, 4 participants met DSM-IV criteria for substance-induced (alcohol) mood disorder with depressive features and were not taking antidepressant medications at the time of study. One sALC was diagnosed with recurrent major depression with mood congruent psychotic symptoms and was taking buproprion at the time of the study. In the nsALC group, 2 participants met DSM-IV criteria for substance-induced (alcohol) mood disorder with depressive features (1 took mirtazapine at the time of study), whereas 1 participant met criteria for recurrent major depression. The same proportion of sALC (6/19, 32%) and nsALC (3/10, 30%; χ2 = 0.004, p = 0.95) was diagnosed with recurrent major depression or substance induced mood disorders.
One participant in each ALC group met criteria for past methamphetamine dependence, and 1 in the sALC group met criteria for past opioid dependence with physiologic dependence. Both were in sustained full remission, with last use 5 or more years before enrollment. Two sALC (2/19; 10%) and 3 nsALC (3/9; 30%; χ2 = 1.74, p = 0.20) were prescribed chlordiazepoxide (Librium®) for alcohol withdrawal symptoms at the time of study. Six sALC (6/19, 32%) and 2 nsALC (2/10, 20%; χ2 = 0.26, p = 0.61) had (medication controlled) hypertension.
Alcohol consumption over lifetime was assessed via the lifetime drinking history (LDH; Skinner and Sheu, 1982; Sobell and Sobell, 1992; Sobell et al., 1988). The LDH obtains quantity and frequency information about alcohol consumption from the first age of regular drinking (defined as consuming at least 1 standard drink per month) to the present. Six measures of drinking severity were calculated from the LDH: average number of drinks per month over 1 and 3 years before enrollment, average number of drinks per month over lifetime, total amount of pure ethanol consumed over lifetime, number of lifetime years of regular drinking, and onset of heavy drinking, defined as age when alcohol consumption exceeded 100 drinks per month. For sALC, smoking behavior was assessed with the Fagerstrom Tolerance Test for Nicotine Dependence (Fagerstrom et al., 1991). Pack-years were calculated as: [(number of cigarettes per day/20) × (duration of smoking at current level in years)]. All sALC participants were actively smoking at the time of study. The nsALC reported no cigarette smoking for at least 1 year before enrollment.
The Institutional Review Boards of the University of California San Francisco and the San Francisco VA Medical Center approved all procedures. Informed consent was obtained from all participants before study. ALC participants were compensated with gift certificates to a local retail store and nsLD were paid by check.
Image Acquisition
Images were acquired on a standard 1.5-T MR system (Siemens Vision, Erlangen, Germany) using a circularly polarized head coil for radiofrequency transmission and reception. Studies were usually performed early evening. Smoking participants were given ample time to smoke before the scanning session. Acquisition of perfusion data started about 90 minutes into the MR study. To reduce potential effects of sleep on perfusion (Braun et al., 1997), before the acquisition of perfusion images, the MR operator spoke to participants to ensure they were awake during data acquisition.
A pulsed arterial spin labeling method, termed DIPLOMA (double inversions with proximal labeling of both tagged and control images; see Jahng et al., 2003, for details) was used for labeling arterial blood water by changing its magnetic properties. This method is noninvasive and uses no exogenous radiotracers or contrast agents. Instead, arterial blood is labeled proximally to the region of interest (ROI, Fig. 1, region B) by changing its magnetic properties and allowed to travel to the ROI (Fig. 1, region A) over a postlabeling delay time (PLD; between tagging pulses and imaging pulses) of 1,500 ms. Then the ROI is imaged with a gradient-echo single-shot echo-planar imaging (EPI) sequence (resolution of 2.3 × 2.3 mm2 over a field of view of 225 × 300 mm2 in five 8-mm-thick slices, each, 2 mm apart, oriented 5° shallower than the orbital–meatal angle, with the most inferior slice located approximately 20 mm above the circle of Willis), yielding tagged images (see Figs. 1 and 2).
Fig. 1.
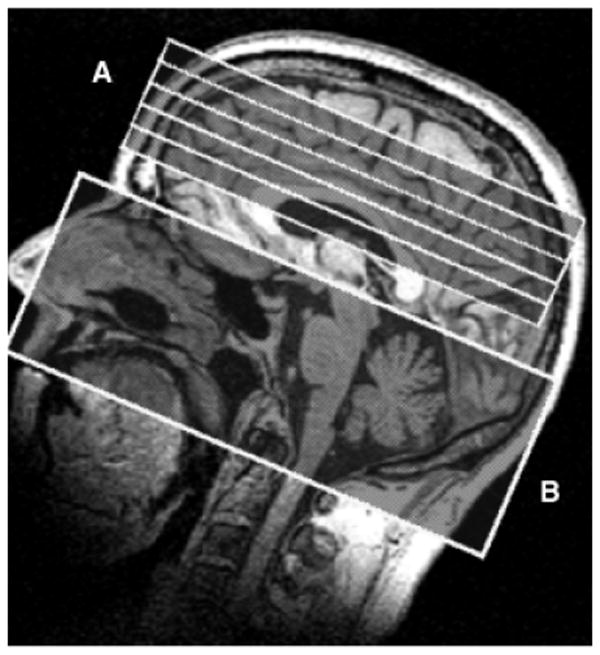
Horizontal and vertical extents of the 5 measured perfusion slices (region A) and tagging region (region B).
Fig. 2.

Perfusion images of nonsmoking light drinker (nsLD) and smoking alcoholic (sALC) obtained with PLT = 1,500 ms, together with coregistered T1-weighted images. The brain was divided in bilateral frontal and parietal gray matter (GM) and white matter using methods described in the Methods section. Note lower intensity in GM region of sALC suggesting lower GM perfusion than in nsLD. An offset in intensity was applied for display purposes.
The other acquisition parameters were: TR = 2.5 seconds, TE = 15 milliseconds. To control for magnetization transfer effects, a control scan was acquired with identical imaging parameters, but without labeling blood. The acquisition lasted 5 minutes with a total of 60 signal averages. Tagged and control scans were then subtracted to obtain perfusion-weighted images.
The sequence was then repeated under identical experimental conditions but with a shorter PLD (1,200 milliseconds), to evaluate potential effects of arterial transit time, which is the time the labeled blood needs to arrive in the imaging slices. Additionally, a reference image covering the entire brain with the same resolution and orientation as the perfusion scan was obtained with EPI to improve coregistration between structural and perfusion images.
Structural 3D T1-weighted images were acquired using a standard magnetization prepared rapid gradient echo (MPRAGE) sequence with TR/TE/TI = 10/7/300 ms, 15° flip angle, 1.0 × 1.0-mm2 in-plane resolution, and 1.5-mm-thick coronal partitions oriented orthogonal to the long axes of hippocampi as seen on a sagittal scout MR image. The MPRAGE images were used for tissue segmentation and coregistration of segmented images to perfusion images. Multislice T2-weighted MR images were obtained with a double spin-echo sequence (TR/TE1/TE2 = 2,500/20/80 ms, 1.00 × 1.00 mm2 in-plane resolution, 3-mm slice thickness, no slice gap, oriented as perfusion data) and were used to create brain masks necessary for segmentation. Following acquisition and image reconstruction, the data were transferred to an off-line workstation for image processing and analysis by one of the authors (S.G.).
Data Processing
Postprocessing steps are depicted in Fig. 3. Probabilities of GM and WM within particular lobes (B–E) were obtained in 2 steps. First, 3-tissue intensity-based segmentation was applied to MPRAGE images to assign a set of probabilities of GM, WM, or cerebrospinal fluid to each voxel using a home-written software described and validated by Cardenas et al. (2005). Then, major lobes were outlined by a deformable registration method using a single MRI atlas manually divided into lobes, subortical nuclei, brainstem, and cerebellum based on anatomical divisions (as described in Cardenas et al., 2005) and overlaid on segmented images.
Fig. 3.
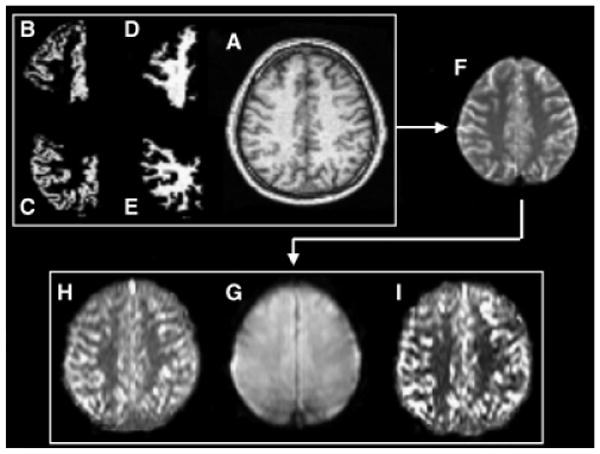
Data processing: The T1-weighted image (A), together with probabilistic maps of gray matter (GM) (B and C) and white matter (WM) (D and E) in frontal and parietal lobes, is coregistered to the control perfusion scan (G) via a reference image covering the entire brain (F). The control scan (G) has the same position and orientation as perfusion images measured with postlabeling delay time of 1,500 ms (H) and 1,200 ms (I). Only voxels containing more than 90% GM or 100% WM were included in analyses.
Direct coregistration of T1-weighted MRI to perfusion images was not reliable because of limited brain coverage and lack of structural features in the perfusion images. Thus, a stepwise coregistration was done by first registering the MPRAGE image (Fig. 3A) to the reference image (F) for each participant. Then the reference image was registered to the control image of the perfusion data (G) obtained with the PLD = 1,500 ms sequence. These transformations were combined and applied to lobar GM and WM probability maps (B–E). Finally, GM and WM probability maps were resampled to the resolution of perfusion data using trilinear interpolation. All processing steps were performed with Statistical Parameter Mapping (SPM2, Wellcome Department of Cognitive Neurology, London, UK). All coregistrations used normalized mutual information as cost function. As the participants' movement between both perfusion sequences was verifiably negligible, the same definitions of lobar GM and WM were used in analysis of the perfusion data obtained at PLD = 1,500 ms (H) and PLD = 1,200 ms (I).
The perfusion image intensities were then corrected for the inter-participant variations of coil loading and signal amplification by first calculating the image brightness according to a previously described formula (Johnson et al., 2005): Brightness = α × 10(receiver gain in dB/20), where α stands for coil loading which is determined according to voltage required for a RF spin inversion pulse. The ratio of image brightness to the mean brightness of all the images in the study was then calculated, and finally individual voxel intensities in each perfusion image were divided by this ratio to obtain a common intensity scale across the study, referred to as institutional units (IU). Perfusion was averaged separately within right and left frontal and parietal WM and GM. Only voxels at the resolution of perfusion images containing at least 90% GM (but no WM) or fully volumed WM voxels were used for averaging. No corrections for partial volumes were performed. Because GM and WM masks did not completely remove artifactual hyperintensities created by arterial blood signal because of different sensitivity to flow artifacts of perfusion images and MPRAGE images and less than perfect coregistration, we excluded from analyses all voxel intensities beyond 2.5 SD from the regional perfusion means. This step removed on average between 1 and 3% of voxels for particular regions. Furthermore, only positive perfusion values were used in calculations. All studies having less than 10 perfusion voxels per bilateral lobar GM or WM were excluded. On average, we obtained approximately 100 voxels in frontal GM, 80 voxels in parietal GM, 300 voxels in frontal WM, and 200 voxels in parietal WM. These voxels were equally distributed between left and right hemispheres and across groups. All perfusion data and coregistration steps were carefully reviewed one by one to assure satisfactory data quality.
Neurocognitive Assessment
A brief neurocognitive battery, administered to ALC within 1 day of the MR study, evaluated working memory (WAIS-III Digit Span; Wechsler, 1997), visuospatial learning and memory (Brief Visual Memory Test-Revised; BVMT-R; Benedict, 1997), and visuomotor scanning speed and incidental learning (WAIS-III Digit Symbol; Wechsler, 1997). The American National Adult Reading Test (Grober and Sliwinski, 1991) estimated premorbid verbal intelligence in ALC. Participants were allowed to smoke ad libitum before cognitive evaluation. A doctoral level neuropsychologist (T.C.D.) administered all neurocognitive and behavioral tests according to standardized procedures.
Statistical Analyses
Demographic and laboratory variables among groups were compared using univariate analyses of variance (ANOVA) with no correction for multiple comparisons. Multivariate analyses of variance (MANOVA; Wilks' λ) were conducted separately for perfusion data obtained with PLD = 1,500 and 1,200 ms. As the acquisition method was optimized to measure GM perfusion, we performed separate analyses for GM and WM regions. Therefore, groups were compared with individual MANOVAs on GM and WM perfusion in the right and left frontal and parietal lobes. The use of MANOVA accounted for the intercorrelations between perfusion in assessed regions, controlled for familywise error rate across the analyzed regions, and evaluated the hypothesis that cerebral perfusion differ as a function of group assignment, hemisphere, and/or region of interest. Type III sum of squares were used in all multivariate and univariate analyses.
Three main analyses were performed: in Analysis 1, perfusion was compared between the entire ALC and the nsLD group, as was typically done in previous neuroimaging research. In Analysis 2, we contrasted all groups (i.e., sALC, nsALC, and nsLD) to investigate the hypothesized effects of comorbid alcohol dependence and chronic cigarette smoking. In Analysis 3, we contrasted sALC and nsALC groups using measures of drinking severity as covariates to assess the effects of concurrent chronic cigarette smoking in alcohol dependence. As major depression is associated with decreased prefrontal cortex perfusion (Drevets, 1999; Soares and Mann, 1997), we repeated all analyses excluding participants diagnosed with recurrent major depression and substance induced mood disorders.
Nonparametric (Spearman) correlations evaluated relationships between regional perfusion and measures of drinking and cigarette smoking severity, as well as neurocognitive performance. The α levels were conservatively adjusted, separately for GM and WM, based on 2 regions (bilateral frontal and bilateral parietal lobe), 6 measures of drinking severity, 4 measures of smoking severity, and 4 measures of neurocognition. For example, in correlations between measures of smoking severity with GM perfusion, α = 0.05/(2 regions × 4 measures of smoking severity) = 0.006. Only regional perfusion obtained with PLD = 1,500 ms was used in correlational analyses, as it is less prone to confounds from arterial transit time than the 1,200-ms sequence (Li et al., 2005). All statistical tests were conducted with SPSS-12.0 for Windows (SPSS, Chicago, IL).
Results
Participant Characterization
Groups were matched on age [F(2, 45) = 0.60, p = 0.55], but not on education [F(2, 45) = 7.24, p = 0.002], with nsLD having more education than sALC and nsALC (both p < 0.02). Detailed demographics and participant characteristics for all groups are given in Table 1. nsALC consisted of 8 Caucasians, 1 Latino, and 1 Native American; sALC included 14 Caucasians, 4 African Americans, and 1 Native American; and the nsLD group consisted of 15 Caucasians, 2 Asians, 1 African American, and 1 Latino.
Table 1.
Demographics and Participant Characteristics (Mean ± Standard Deviation)
| Parameter | nsLD N = 19 (3F) | nsALC N = 10 (0F) | sALC N = 19 (1F) |
|---|---|---|---|
| Age (y) | 47.0 ± 7.9 | 50.9 ± 10.0 | 48.0 ± 9.9 |
| Education (y) | 16.9 ± 2.8#,$$ | 14.2 ± 2.6# | 13.6 ± 2.8$$ |
| AMNART | 119 ± 7 | 111 ± 10 | 113 ± 10 |
| BDI | 3.3 ± 3.9 | 17.3 ± 8.9 | 15.1 ± 10.8 |
| STAI Y-2 | – | 50 ± 10 | 51 ± 10 |
| CIWA-Ar | – | 4.5 ± 3.8 | 2.1 ± 1.8 |
| 1-y average (drinks per month) | 12 ± 17 | 414 ± 182 | 429 ± 165 |
| 3-y average (drinks per month) | 12 ± 17 | 406 ± 186 | 414 ± 180 |
| Lifetime average (drinks per month) | 15 ± 14 | 206 ± 132 | 297 ± 107 |
| Lifetime years | 28.2 ± 5.7 | 34.4 ± 10.0 | 30.8 ± 9.9 |
| Total lifetime consumption (kg) | 65 ± 63 | 1150 ± 840 | 1520 ± 820 |
| Onset of heavy drinking (y) | – | 26.0 ± 8.8 | 20.0 ± 3.9 |
| Months of heavy drinking | – | 240 ± 100 | 300 ± 110 |
| Time after last caffeinated drink (h) | 8.3 ± 2.5 | 9.5 ± 2.0 | 8.1 ± 3.5 |
| Time after last cigarette (h) | – | – | 2.2 ± 1.0 |
Contrast between sALC and nsLD: p<0.05.
Contrast between nsALC and nsLD: p<0.01.
AMNART, American National Adult Reading Test; BDI, Beck Depression Inventory; STAI Y-2, State-trait Anxiety Inventory-State; CIWA-Ar, Addiction Research Foundation Clinical Institute of Withdrawal Assessment for Alcohol; 1 y average, number of drinks per month over 1y before study; 3-y average, number of drinks per month over 3 y before study; lifetime average, number of drinks per month over lifetime; lifetime years, number of years of regular alcohol consumption over lifetime; total lifetime consumption, total amount of pure EtOH (kg) consumed over lifetime; onset of heavy drinking, age, when alcohol consumption exceeded 100 drinks per month.
sALC and nsALC were not significantly different on average number of drinks per month consumed over 1 and 3 years before enrollment (p > 0.8). However, sALC tended to have a greater average number of alcoholic drinks consumed per month over lifetime than nsALC (p = 0.06) and tended to begin drinking at levels higher than 100 drinks per month at younger age (p = 0.06). sALC did not differ from nsALC on measures of self-reported depressive and anxiety symptomatology, γ-glutamyltransferase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum albumin, or prealbumin. Average GGT and AST levels in both sALC and nsALC were elevated beyond normal range (see Table 2). Average AST and ALT levels in nsALC were higher than in nsLD (all p < 0.05). The measures of withdrawal symptomatology for sALC and nsALC were not clinically elevated. For sRA, RBC count and hematocrit were slightly lower than normal. sALC, compared with nsALC, showed 9% lower RBC (p = 0.04) levels and 8% lower concentration of hemoglobin (p = 0.05). nsALC did not differ from nsLD on hematologic variables; however, sALC demonstrated significantly lower RBC and hematocrit values than nsLD (both p < 0.02; see Table 2).
Table 2.
Laboratory Variables (Mean ± Standard Deviation)
| Parameter | nsLD N = 19
(3F) |
nsALC N = 10
(0F) |
sALC N = 19
(1F) |
|---|---|---|---|
| GGT (IU) | 21 ± 10# | 143 ± 196# | 88 ± 59 |
| AST (IU) | 22.9 ± 7.1# | 54 ± 50# | 36 ± 19 |
| ALT (IU) | 27.1 ± 15.4# | 66 ± 56# | 41 ± 29 |
| Albumin (g/dL) | 4.08 ± 0.29 | 3.86 ± 0.38 | 4.05 ± 0.31 |
| Prealbumin (mg/dL) | 31.1 ± 5.8 | 30.1 ± 6.2 | 28.4 ± 5.7 |
| CO2 | 27.8 ± 2.2 | 27.6 ± 1.5 | 28.8 ± 2.1 |
| WBC | 6.4 ± 1.5 | 7.8 ± 0.9 | 7.3 ± 1.7 |
| RBC | 4.92 ± 0.33$$ | 4.76 ± 0.38* | 4.35 ± 0.39*,$$ |
| Hemoglobin (g/dL) | 14.4 ± 2.9 | 15.4 ± 1.0* | 14.1 ± 1.3* |
| Hematocrit (%) | 44.1 ± 3.0$ | 44.1 ± 2.5* | 41.1 ± 3.3*,$ |
| MCV | 88.8 ± 2.5$$ | 90.7 ± 7.4* | 94.9 ± 3.0*,$$ |
| Hep-C (number of participants) | 0 | 3 | 2 |
GGT, γ-glutamyltransferase; local normal range = 7–64 institutional units (IU); AST, aspartate aminotransferase; local normal range = 5–35 IU; ALT, alanine aminotransferase; local normal range = 7–56 IU; albumin local normal range = 3.3–5.2 g/dL; prealbumin local normal range, 18–45 mg/dL; NA, not available; WBC, white blood cell count; local normal range = 4.8–10.8 K/cmm; RBC = red blood cell count; local normal range = 4.7–6.1 M/cmm; hemoglobin local normal range = 14–18 g/dL; hematocrit local normal range = 42–52%.
Contrast between nsALC and nsLD: p<0.05.
Contrast between sALC and nsALC: p<0.05.
Contrast between sALC and nsLD: p<0.05.
Contrast between sALC and nsALC: p<0.01.
sALC smoked 21 ± 9 cigarettes per day (minimum = 5, maximum = 35) and smoked at this level for 22 ± 13 years (minimum = 2, maximum = 44), and cigarette pack-years was 24 ± 18 (minimum = 1, maximum = 61). The sALC Fagerstrom score was 5.4 ± 2.2 (minimum = 2, maximum = 10), indicating a medium to high level of nicotine dependence.
The groups did not differ on time elapsed after intake of last caffeinated beverage [F(2, 26) = 0.48, p = 0.62], which was consumed on average over 8.4 ± 3.0 hours before the perfusion scan in all groups (range between 2 and 10 hours). sALC smoked their last cigarette on average 2.2 ± 1.0 hours before the measurement of cerebral perfusion: 18 sALC smoked more than 90 minutes before perfusion measurement, whereas 1 sALC smoked 5 minutes before the acquisition (after a break); however, his perfusion measures were within the range of sALC perfusion.
Analysis 1: ALC Versus LD
Multivariate analyses of variance comparing perfusion in all regions between nsLD and the combined ALC group (i.e., sALC and nsALC) yielded a significant group (alcohol) effect [F(2, 91) = 3.44, p = 0.04] for perfusion measured at PLD = 1,500 ms and a corresponding trend at PLD = 1,200 ms [F(2, 91) = 2.95, p = 0.06]. No main effects for hemisphere or group-by-hemisphere interactions were observed; therefore, all findings reported represent average perfusion values of both hemispheres. Follow-up analyses for the 1,500-ms sequence indicated 13% lower frontal GM perfusion (p = 0.005) and 8% lower parietal GM perfusion (p = 0.03) in ALC compared with nsLD (see Fig. 4). The follow-up analyses for the 1,200-ms sequence yielded results similar to those with the 1,500-ms sequence. No main effects (group, hemisphere) or interactions for WM perfusion were detected with either sequence. These results were essentially unchanged after all left-handed participants, females, participants who smoked immediately before their perfusion scans, or participants with recurrent major depression and substance-induced mood disorders (including those on anti-depressive medications) were excluded from analyses. Also, correction of GM perfusion for cerebrospinal fluid (CSF) content within voxels did not change the results.
Fig. 4.
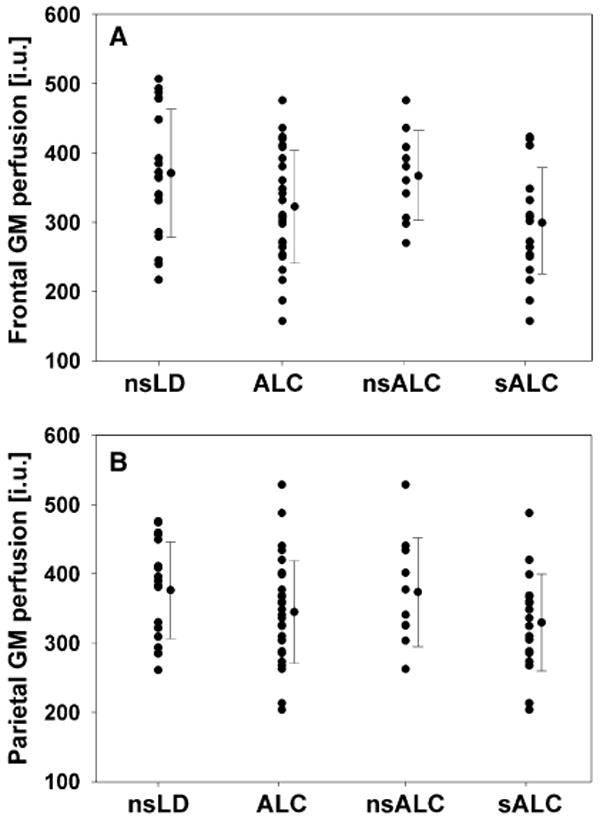
Frontal (A) and parietal (B) gray matter perfusion in nsLD, combined ALC, nsALC, and sALC with the 1,500-ms sequence. Means and standard deviations are displayed in institutional units (IU).
Analysis 2: sALC Versus nsALC versus nsLD (Compare Table 3)
Table 3.
Regional Gray Matter Perfusion (in Institutional Units As Described in Methods)
| PLD (ms) | Region | nsLD N = 19 | ALC N = 29 | nsALC N = 10 | sALC N = 19 | Group contrast |
|---|---|---|---|---|---|---|
| 1,500 | Left-frontal | 360 ± 82 | 319 ± 74 | 360 ± 55 | 297 ± 75 | sALC ≪ nsALC, nsLD |
| Right-frontal | 381 ± 107 | 326 ± 90 | 374 ± 82 | 301 ± 86 | sALC ≪ nsALC, nsLD | |
| Left-parietal | 371 ± 64 | 336 ± 77 | 362 ± 90 | 322 ± 67 | sALC ≪ nsLD; sALC<nsALC | |
| Right-parietal | 381 ± 81 | 353 ± 79 | 385 ± 84 | 337 ± 74 | sALC ≪ nsLD; sALC<nsALC | |
| 1,200 | Left-frontal | 440 ± 72 | 397 ± 103 | 429 ± 98 | 380 ± 104 | sALC ≪ nsLD; sALC<nsALC |
| Right-frontal | 457 ± 99 | 408 ± 111 | 439 ± 120 | 392 ± 106 | sALC ≪ nsLD; sALC<nsALC | |
| Left-parietal | 445 ± 81 | 399 ± 97 | 387 ± 113 | 405 ± 90 | sALC, nsALC<nsLD | |
| Right-parietal | 442 ± 88 | 406 ± 83 | 407 ± 97 | 406 ± 78 | sALC, nsALC<nsLD |
PLD, postlabeling delay (time between tagging and excitation pulse); nsLD, nonsmoking light drinker, ALC, smoking or nonsmoking 1-week-abstinent alcohol-dependent individuals.
Contrasts between sALC, nsALC, and nsALC: “<”: p<0.05; “ ≪ ”: p<0.01.
Multivariate analyses of variance comparing perfusion in right and left hemispheric frontal and parietal GM perfusion showed a significant group main effect for both the PLD = 1,500 ms [F(4, 178) = 3.76, p = 0.006] and the PLD = 1,200 ms [F(4, 178) = 3.31, p = 0.01] sequences. No main effects for hemisphere or group by hemisphere interactions were observed; therefore, all findings reported below represent perfusion averaged over both hemispheres. Follow-up ANOVAs for the 1,500-ms sequence revealed significant group effects for frontal [F(2, 90) = 7.90, p = 0.001] and parietal [F(2, 90) = 4.25, p = 0.02] GM perfusion. Frontal GM perfusion in sALC was 19% lower than in nsLD (p = 0.001) and 18% lower than in nsALC (p = 0.003). Parietal GM perfusion in sALC was 12% lower than in nsLD (p = 0.004) and 11% lower than in nsALC (p = 0.02; see Fig. 4). Notably, nsALC and nsLD were not significantly different on frontal or parietal GM perfusion. The 1,200-ms MANOVA generally confirmed the results presented above. Follow-up ANOVAs showed a significant group effect for frontal GM [F(2, 90) = 4.03, p = 0.02] and a trend for group differences for parietal GM [F(2, 90) = 2.52, p = 0.09]. sALC showed 11% lower frontal GM perfusion than nsALC (p = 0.04) and 14% lower frontal GM perfusion than nsLD (p = 0.004). Again, nsALC and nsLD were not significantly different on this perfusion measure. For the parietal GM, sALC showed 11% lower and nsALC 9% lower perfusion than nsLD (both p = 0.03). These results were essentially unchanged when perfusion values were corrected for CSF content within GM voxels. Finally, a 3-way MANOVA including all groups, hemisphere, and PLD as factors confirmed Analysis 2 findings by showing a significant effect of PLD [F(2, 201) = 4.14, p = 0.017]. The follow-up analyses indicated that PLD affected the results for parietal GM perfusion only (p = 0.02). As in Analysis 1, these results were not affected by mood disorders in the ALC, sex, smoking immediately before perfusion scans, or left-handed participants. No main effects (group, hemisphere) or interactions were detected for WM perfusion with either sequence.
Analysis 3: sALC Versus nsALC
This analysis tested for effects of concurrent chronic cigarette smoking on regional perfusion in alcoholics by directly comparing sALC and nsALC. sALC tended to consume more alcoholic drinks per month over lifetime than nsALC and tended to start drinking heavily at a younger age. Therefore, these measures of drinking were used as covariates.
For the 1,500-ms sequence, the MANCOVA comparing frontal and parietal GM perfusion with average number of drinks per month over lifetime as a covariate yielded a significant group effect [F(2, 52) = 3.16, p = 0.05]. Follow-up t tests indicated that sALC compared with nsALC had lower frontal GM perfusion (p = 0.007) and tended to demonstrate lower parietal GM perfusion (p = 0.07). The MANCOVA for the 1,200-ms sequence with average number of drinks per month over lifetime used as a covariate was not significant [F(2, 52) = 2.09, p = 0.13].
When the age of onset of heavy drinking was used as a covariate, MANCOVA for the 1,500-ms sequence yielded a significant group effect [F(2, 52) = 3.29, p = 0.05]. Follow-up t tests indicated that sALC had lower perfusion than nsALC in frontal GM (p = 0.01) but no significant group differences for parietal GM. For the 1,200-ms sequence, MANCOVA with average number of drinks per month over lifetime as a covariate yielded a significant group effect [F(2, 52) = 3.33, p = 0.04]; however, the follow-up t tests showed no group differences (p > 0.09).
Correlations Among Outcome Measures
In the sALC group, more cigarettes smoked per day correlated with lower frontal GM perfusion (r = −0.57, p = 0.005) and tended to correlate with lower parietal GM perfusion (r = −0.51, p = 0.01). In the combined ALC group, lower frontal GM perfusion tended to inversely relate to greater average number of drinks per month over lifetime (r = −0.38, p = 0.02). These effects were not lateralized and the other measures of smoking and drinking severity were not related to regional perfusion (measured with PLD = 1,500 ms). No significant correlations between regional perfusion and measures of neurocognition were observed for sALC or nsALC. Depressive and anxiety symptomatology scores did not correlate with perfusion measured by either sequence, in any region, for both sALC and nsALC. Time after last cigarette (for sALC) and time after last caffeinated beverage were not related to perfusion levels in any region in any group.
Discussion
To our knowledge, this is the first quantitative report of the effects of alcohol dependence and comorbid chronic cigarette smoking on cerebral perfusion. Our methodology noninvasively quantitated perfusion by altering magnetic properties of endogenous water molecules in arterial blood (arterial spin labeling), rather than by employing exogenous contrast agents.
Our results suggest that concurrent alcohol dependence and chronic cigarette smoking, but not alcohol dependence alone, is associated with significant regional GM hypoperfusion in our sample of 1-week-abstinent alcohol-dependent individuals. The major findings in this cohort are as follows: (1) as a group, alcoholics (smokers and nonsmokers combined) demonstrated decreased frontal and parietal GM perfusion relative to nonsmoking light drinkers; (2) smoking alcoholics demonstrated frontal and parietal GM hypoperfusion, while the regional GM perfusion of nonsmoking alcohol-dependent participants was generally similar to that of nonsmoking light drinkers; (3) no hemispheric differences in regional perfusion were observed; and (4) in smoking alcoholics, lower frontal and parietal perfusion was associated with a higher number of cigarettes smoked per day. These findings were not affected by sex, smoking immediately before perfusion scans, left-handedness, or ALC participants with mood disorders. Also, our results do not appear to be driven by acute effects of smoking, because the time after last cigarette in our study averaged more than 2 hours, similar to other studies on chronic effects of smoking (Rogers et al., 1983; Yamashita et al., 1988). Also, Brody (2005) points out that the acute effects of smoking on absolute perfusion in prefrontal cortex, which is part of our frontal GM region, are controversial.
The regional hypoperfusion pattern in alcoholics as a group (Analysis 1) was largely consistent with previous research reporting bilateral frontal and parietal perfusion deficits in alcoholics in treatment (Demir et al., 2002; Mampunza et al., 1995; Nicolas et al., 1993), which support the validity of our experimental methods. In contrast to some previous studies (Berglund et al., 1987; Erbas et al., 1992; Tutus et al., 1998), we did not detect any hemispheric differences in cerebral hypoperfusion in alcoholics.
When the smoking status of the alcoholic cohort was taken into account (Analysis 2), the largest difference in frontal and parietal GM perfusion was found between sALC and nsLD. This effect was not dependent on the PLD, suggesting that our results accurately represent perfusion deficits in smoking alcoholics, not simply differences in arterial blood transit time. Thus, the differences of frontal and parietal GM perfusion in the ALC (sALC and nsALC combined) versus nsLD appear to be largely driven by lower perfusion in sALC.
At the shorter PLD (1,200 ms) nsALC showed significantly lower parietal GM perfusion than nsLD; this was not apparent at PLD = 1,500 ms (Analysis 2). Studies using transcranial Doppler sonography demonstrated decreased cerebral blood flow velocities in carotid arteries in some alcohol-dependent individuals (Gdovinova, 2001) and increased cerebral blood flow velocities in chronic cigarette smokers (e.g., Terborg et al., 2002, and references therein). This suggests that potential group differences in arterial blood flow velocities might confound magnetic resonance perfusion studies. This notion is further supported by Analysis 3, which demonstrated that average number of drinks per month over lifetime accounted for some of the variance of perfusion measured with the 1,500-ms but not the 1,200-ms sequence, suggesting some influence of drinking history on arterial blood transit times.
Contrary to some previous studies (Demir et al., 2002; Nicolas et al., 1993, 2001; Noel et al., 2002), we did not find any associations between regional perfusion and neurocognition. This may be due to the limited number of abilities assessed by our brief cognitive battery, relatively large regions of interests, and/or significantly shorter abstinence from alcohol than in the other studies. Our cognitive measures could have been affected by the generalized effects of detoxification (e.g., Fein et al., 1990) but not likely by clinically significant withdrawal symptoms (see Table 1).
Possible Mechanisms of Perfusion Deficits in Smoking Alcoholics
The potential effects of perfusion deficits in alcohol dependence were previously associated with loss of neurons in frontal cortex (e.g., Nicolas et al., 1993) and decreased brain metabolism (e.g., Volkow et al., 1992). However, cigarette smoking may promote further neuronal damage and metabolic derangement (Durazzo et al., 2004). The gas and particulate phases of cigarette smoke contain many noxious compounds [e.g., free radicals, carbon monoxide (CO), nitrosamines, aldehydes; Fowles et al., 2000] that may directly or indirectly compromise central nervous system tissue. Chronic cigarette smoking significantly increases the risk of atherosclerosis (Bolego et al., 2002) and exposure to nicotine is associated with alterations of vascular endothelial function (see Hawkins et al., 2002). Also, CO levels are significantly higher in cigarette smokers (Deveci et al., 2004), which may promote both a reduction of effective hemoglobin concentrations, leading to diminished oxygen carrying capacity of the blood (Macdonald et al., 2004) and decreased efficiency of the mitochondrial respiratory chain (Alonso et al., 2004). Chronic cigarette smoking has also been equated to a type of repeated acute (mild) CO poisoning (Alonso et al., 2004), and is associated with increased oxidative stress (Panda et al., 2000) and nocturnal hypoxia (Casasola et al., 2002), as well as to respiratory risks such as chronic obstructive pulmonary disease and other conditions that can compromise lung function (Bartal, 2001). Therefore, a combination of chronically increased CO levels and oxidative stress, compromised vascular function, and potentially undiagnosed pulmonary disease may result in structural and/or metabolic injury of brain tissue of sALC, reflected in diminished metabolic demand and lower cerebral perfusion.
Study Limitations
The lack of a smoking LD group prevented us from a direct assessment of the effects of alcohol and smoking and their interaction on cerebral perfusion. All but one of our 19 sALC participants had their last cigarette more than 90 minutes before the perfusion scan. Previous studies in nonalcoholic adults found 4 to 12% lower global perfusion in chronic smokers after at least 2 hours of abstinence from smoking compared with nonsmokers (Rogers et al., 1983; Yamashita et al., 1988). This appears less than the perfusion deficits in frontal and parietal GM found in our study (19 and 12%, respectively), which suggests that the perfusion deficits in smoking alcoholics may not be explained by acute cigarette smoking alone. Moreover, we cannot exclude effects of caffeinated beverages (Field et al., 2003) or craving for cigarettes on our cerebral perfusion measures (see Brody, 2005; Brody et al., 2002; Domino et al., 2004).
The retrospective assignment of ALC to smoking and nonsmoking groups and the resulting unbalanced group membership could have lead to underestimation of the effects of alcohol dependence per se on cerebral perfusion. This study consisted mostly of male participants, so that sex effects of concurrent alcohol dependence and cigarette smoking could not be assessed. The method we used to acquire perfusion weighted images did not allow to control for potential differences in arterial transit times as well as relaxation rates of blood and brain tissue leading to possible confounds in quantization. A numerically larger proportion of nsALC (3/10) than sALC (2/17) took Librium® at the time of study, which may have contributed to underestimating the effects of comorbid smoking on perfusion in alcoholics. Both ALC groups did not demonstrate clinically significant withdrawal symptomatology at the time of assessment (CIWA-Ar; see Table 1) suggesting that withdrawal symptoms were not a major study confound. Neurocognitive assessment of the 1-week-abstinent ALC was brief by necessity and did not evaluate all of the functions that were related to regional perfusion in previous studies. Also, as our method was optimized to measure GM perfusion, quantization of WM perfusion is likely less accurate. Finally, potential unrecorded group differences in nutrition, exercise, overall physical health, and genetic predispositions may contribute to the perfusion deficits in smoking alcoholics described in this study.
Conclusions
Our results replicated findings of previous SPECT and PET perfusion studies in recovering alcoholics. Most notably, this study provides preliminary evidence that chronic cigarette smoking adversely affects cerebral perfusion in 1-week-abstinent alcohol-dependent individuals. It is not clear if the reported GM perfusion deficits in smoking alcoholics reflect neuronal injury and/or metabolic dysfunction or premorbid conditions or if the perfusion abnormalities are reversible with abstinence from alcohol and/or smoking. Thus, longitudinal cerebral perfusion studies evaluating the effects of abstinence from alcohol and/or cigarettes will assist in answering these questions. Moreover, larger prospective studies with a smoking control group matched to the ALC groups on measures of smoking severity and recency of last cigarette use are necessary for rigorous assessments of the specific effects of alcohol dependence and chronic smoking on cerebral perfusion, cognitive function, and their interrelationships.
Finally, these results and our other studies (Durazzo et al., 2004, 2006; Gazdzinski et al., 2005) indicate that consideration of the effects of chronic cigarette smoking is warranted in studies investigating the neurobiological consequences of alcohol dependence and other conditions in which smoking is a prevalent comorbid factor.
Acknowledgments
We thank Mary Rebecca Young, Bill Clift, and Dr. Donald Tusel of the San Francisco VA Substance Abuse Day Hospital and Dr. David Pating, Karen Moise, and their colleagues at the San Francisco Kaiser Permanente Chemical Dependency Recovery Program for their valuable assistance in recruiting research participants and Kasia Gazdzinska for editing the manuscript. Last but not least, we wish to extend our appreciation to all study participants who made this research possible.
This project was supported by AA10788 (D. J. M.)
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Alonso JR, Cardellach F, Casademont J, Miro O. Reversible inhibition of mitochondrial complex IV activity in PBMC following acute smoking. Eur Respir J. 2004;23:214–218. doi: 10.1183/09031936.03.00038203. [DOI] [PubMed] [Google Scholar]
- Bartal M. Health effects of tobacco use and exposure. Monaldi Arch Chest Dis. 2001;56:545–554. [PubMed] [Google Scholar]
- Beck AT. Depression Inventory. Center for Cognitive Therapy; Philadelphia: 1978. [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test—Revised. Psychological Assessment Resources Inc.; Odessa, FL: 1997. [Google Scholar]
- Berglund M, Hagstadius S, Risberg J, Johanson TM, Bliding A, Mubrin Z. Normalization of regional cerebral blood flow in alcoholics during the first 7 weeks of abstinence. Acta Psychiatr Scand. 1987;75:202–208. doi: 10.1111/j.1600-0447.1987.tb02775.x. [DOI] [PubMed] [Google Scholar]
- Bolego C, Poli A, Paoletti R. Smoking and gender. Cardiovasc Res. 2002;53:568–576. doi: 10.1016/s0008-6363(01)00520-x. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Part 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2005 doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Casasola GG, Alvarez-Sala JL, Marques JA, Sanchez-Alarcos JM, Tashkin DP, Espinos D. Cigarette smoking behavior and respiratory alterations during sleep in a healthy population. Sleep Breath. 2002;6:19–24. doi: 10.1007/s11325-002-0019-y. [DOI] [PubMed] [Google Scholar]
- Daeppen JB, Smith TL, Danko GP, Gordon L, Landi NA, Nurnberger JI, Jr, Bucholz KK, Raimo E, Schuckit MA. Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. The Collaborative Study Group on the Genetics of Alcoholism. Alcohol Alcohol. 2000;35:171–175. doi: 10.1093/alcalc/35.2.171. [DOI] [PubMed] [Google Scholar]
- Demir B, Ulug B, Lay Ergun E, Erbas B. Regional cerebral blood flow and neuropsychological functioning in early and late onset alcoholism. Psychiatry Res. 2002;115:115–125. doi: 10.1016/s0925-4927(02)00071-9. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Deveci SE, Deveci F, Acik Y, Ozan AT. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir Med. 2004;98:551–556. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie SK, Ohl L, Ni L, Koeppe RA, Cross DJ, Zubieta J. Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience. 2000;101:277–282. doi: 10.1016/s0306-4522(00)00357-2. [DOI] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:319–327. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: preliminary evidence of the effects of the effects of concurrent chronic cigarette smoking. Alcohal Clin Exp Res. 30:539–51. doi: 10.1111/j.1530-0277.2006.00060.x. in press. [DOI] [PubMed] [Google Scholar]
- Dursun S, Kutcher S. Smoking, nicotine and psychiatric disorders: evidence for therapeutic role, controversies and implications for future research. Medical Hypotheses. 1999;52:101–109. doi: 10.1054/mehy.1997.0623. [DOI] [PubMed] [Google Scholar]
- Erbas B, Bekdik C, Erbengi G, Enunlu T, Aytac S, Kumbasar H, Dogan Y. Regional cerebral blood flow changes in chronic alcoholism using Tc-99m HMPAO SPECT. Comparison with CT parameters. Clin Nucl Med. 1992;17:123–127. doi: 10.1097/00003072-199202000-00012. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1991;69:763–765. [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. Western J Med. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol Med. 2003;33:1357–1367. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Field AS, Laurienti PJ, Yen YF, Burdette JH, Moody DM. Dietary caffeine consumption and withdrawal: confounding variables in quantitative cerebral perfusion studies? Radiology. 2003;227:129–135. doi: 10.1148/radiol.2271012173. [DOI] [PubMed] [Google Scholar]
- Fowles J, Bates M, Noiton D. The Chemical Constituents in Cigarettes and Cigarette Smoke: Priorities for Harm Reduction. Epidemiology and Toxicology Group; New Zealand: 2000. pp. 1–65. [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gdovinova Z. Blood flow velocity in the middle cerebral artery in heavy alcohol drinkers. Alcohol Alcohol. 2001;36:346–348. doi: 10.1093/alcalc/36.4.346. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alcohol Depend. 2001;63:277–286. doi: 10.1016/s0376-8716(00)00216-7. [DOI] [PubMed] [Google Scholar]
- Gilman S, Adams K, Koeppe RA, Berent S, Kluin KJ, Modell JG, Kroll P, Brunberg JA. Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with positron emission tomography. Ann Neurol. 1990;28:775–785. doi: 10.1002/ana.410280608. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hasin D, Grant B. Major depression in 6050 former drinkers: association with past alcohol dependence. Arch Gen Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Haustein KO, Krause J, Haustein H, Rasmussen T, Cort N. Changes in hemorheological and biochemical parameters following short-term and long-term smoking cessation induced by nicotine replacement therapy (NRT) Int J Clin Pharmacol Ther. 2004;42:83–92. doi: 10.5414/cpp42083. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Brown RC, Davis TP. Smoking and ischemic stroke: a role for nicotine. Trends Pharmacol Sci. 2002;23:78–82. doi: 10.1016/s0165-6147(02)01893-x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr, Morse RM, Palmen MA, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol Clin Exp Res. 1994;18:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Jahng GH, Zhu XP, Matson GB, Weiner MW, Schuff N. Improved perfusion-weighted MRI by a novel double inversion with proximal labeling of both tagged and control acquisitions. Magn Reson Med. 2003;49:307–314. doi: 10.1002/mrm.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003;38:606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KL, Zhu X, Hylton N, Jahng GH, Weiner MW, Schuff N. Four-phase single-capillary stepwise model for kinetics in arterial spin labeling MRI. Magn Reson Med. 2005;53:511–518. doi: 10.1002/mrm.20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald G, Kondor N, Yousefi V, Green A, Wong F, Aquino-Parsons C. Reduction of carboxyhaemoglobin levels in the venous blood of cigarette smokers following the administration of carbogen. Radiother Oncol. 2004;73:367–371. doi: 10.1016/j.radonc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Mampunza S, Verbanck P, Verhas M, Martin P, Paternot J, Le Bon O, Kornreich C, Den Bulk A, Pelc I. Cerebral blood flow in just detoxified alcohol dependent patients. A 99m Tc-HMPAO-SPECT study. Acta Neurol Belg. 1995;95:164–169. [PubMed] [Google Scholar]
- Marks JL, Hill EM, Pomerleau CS, Mudd SA, Blow FC. Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever-smokers. J Subst Abuse Treat. 1997;14:521–527. doi: 10.1016/s0740-5472(97)00049-4. [DOI] [PubMed] [Google Scholar]
- Nicolas JM, Catafau AM, Estruch R, Lomena FJ, Salamero M, Herranz R, Monforte R, Cardenal C, Urbano-Marquez A. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J Nucl Med. 1993;34:1452–1459. [PubMed] [Google Scholar]
- Noel X, Paternot J, Van der Linden M, Sferrazza R, Verhas M, Hanak C, Kornreich C, Martin P, De Mol J, Pelc I, Verbanck P. Correlation between inhibition, working memory and delimited frontal area blood flow measure by 99mTc-Bicisate SPECT in alcohol-dependent patients. Alcohol Alcohol. 2001;36:556–563. doi: 10.1093/alcalc/36.6.556. [DOI] [PubMed] [Google Scholar]
- Noel X, Sferrazza R, Van Der Linden M, Paternot J, Verhas M, Hanak C, Pelc I, Verbanck P. Contribution of frontal cerebral blood flow measured by (99m)Tc-Bicisate SPECT and executive function deficits to predicting treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2002;37:347–354. doi: 10.1093/alcalc/37.4.347. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Eberling JL, Schuff N, Jagust W, Reed B, Soto G, Ezekiel F, Klein G, Weiner MW. Method to correlate 1H MRSI and 18FDG-PET. Magn Reson Med. 2000;43:244–250. doi: 10.1002/(sici)1522-2594(200002)43:2<244::aid-mrm11>3.0.co;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M, Mochizuki Y, Shikata E. Corpus callosum atrophy and cerebral blood flow in chronic alcoholics. J Neurol Sci. 1999;162:51–55. doi: 10.1016/s0022-510x(98)00279-2. [DOI] [PubMed] [Google Scholar]
- Panda K, Chattopadhyay R, Chattopadhyay DJ, Chatterjee IB. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic Biol Med. 2000;29:115–124. doi: 10.1016/s0891-5849(00)00297-5. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14(Part 1):7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Aubin HJ, Pomerleau OF. Self-reported alcohol use patterns in a sample of male and female heavy smokers. J Addict Dis. 1997;16:19–24. doi: 10.1300/J069v16n03_02. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Grubb RL, Jr, Gado MH, Eichling JO, Ter-Pogossian MM. Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Arch Neurol. 1976;33:523–526. doi: 10.1001/archneur.1976.00500080001001. [DOI] [PubMed] [Google Scholar]
- Rogers RL, Meyer JS, Shaw TG, Mortel KF, Hardenberg JP, Zaid RR. Cigarette smoking decreases cerebral blood flow suggesting increased risk for stroke. JAMA. 1983;250:2796–2800. [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry. 2003;160:323–333. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Dupont RM, Grant I, Lehr PP, Lamoureux G, Halpern S, Yeung DW. Reduction in cortical IMP–SPET tracer uptake with recent cigarette consumption in a young group of healthy males. San Diego HIV Neurobehavioral Research Center. Eur J Nucl Med. 1997;24:422–427. doi: 10.1007/BF00881815. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W, Czernin J, Rapoport SI, Pietrini P, Alexander GE, Schapiro MB, Jagust WJ, Hoffman JM, Welsh-Bohmer KA, Alavi A, Clark CM, Salmon E, de Leon MJ, Mielke R, Cummings JL, Kowell AP, Gambhir SS, Hoh CK, Phelps ME. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The lifetime drinking history and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The functional neuroanatomy of mood disorders. J Psychiatr Res. 1997;31:393–432. doi: 10.1016/s0022-3956(97)00016-2. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. The Humana Press Inc.; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers' self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. published erratum appears in J Stud Alcohol 1989 Jan;50(1):92. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Self-evaluation Questionnaire. Consulting Psychologists Press, Inc.; Palo Alto, CA: 1977. [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF, Henningfield JE, London ED. Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology. 2003;28:765–772. doi: 10.1038/sj.npp.1300106. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. NIAAA Research Monograph No. 34: Human brain vulnerability to alcoholism: evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 473–508. [Google Scholar]
- Sullivan J, Sykora K, Schneiderman J, Naranjo C, Sellers E. Assesment of alcohol withdrawal: the revised clinical institute withdrawl assesment for alcohol scale. Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Terborg C, Bramer S, Weiller C, Rother J. Short-term effect of cigarette smoking on CO(2)-induced vasomotor reactivity in man: a study with near-infrared spectroscopy and tanscranial Doppler sonography. J Neurol Sci. 2002;205:15–20. doi: 10.1016/s0022-510x(02)00308-8. [DOI] [PubMed] [Google Scholar]
- Tutus A, Kugu N, Sofuoglu S, Nardali M, Simsek A, Karaaslan F, Gonul AS. Transient frontal hypoperfusion in Tc-99m hexamethylpropyleneamineoxime single photon emission computed tomography imaging during alcohol withdrawal. Biol Psychiatry. 1998;43:923–928. doi: 10.1016/s0006-3223(97)00322-3. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, Dewey SL, Wolf AP. Decreased brain metabolism in neurologically intact healthy alcoholics. Am J Psychiatry. 1992;149:1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, Wolf AP. Recovery of brain glucose metabolism in detoxified alcoholics. Am J Psychiatry. 1994;151:178–183. doi: 10.1176/ajp.151.2.178. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Weinrebe W, Graf-Gruss R, Schwabe R, Stippler D, Fusgen I. The two-factor method—a new approach to categorizing the clinical stages of malnutrition in geriatric patients. J Am Geriatr Soc. 2002;50:2105–2107. doi: 10.1046/j.1532-5415.2002.50637.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Hatazawa J, Kubota K, Abe Y, Fujiwara T, Matsuzawa T. Correlations between regional cerebral blood flow and age-related brain atrophy: a quantitative study with computed tomography and the xenon-133 inhalation method. J Am Geriatr Soc. 1983;31:412–416. doi: 10.1111/j.1532-5415.1983.tb03716.x. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Kobayashi S, Yamaguchi S, Kitani M, Tsunematsu T. Effect of smoking on regional cerebral blood flow in the normal aged volunteers. Gerontology. 1988;34:199–204. doi: 10.1159/000212953. [DOI] [PubMed] [Google Scholar]
- York JL, Hirsch JA. Drinking patterns and health status in smoking and nonsmoking alcoholics. Alcohol Clin Exp Res. 1995;19:666–673. doi: 10.1111/j.1530-0277.1995.tb01565.x. [DOI] [PubMed] [Google Scholar]


