Abstract
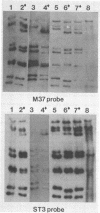
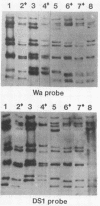
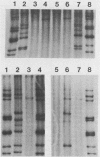
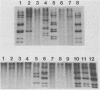
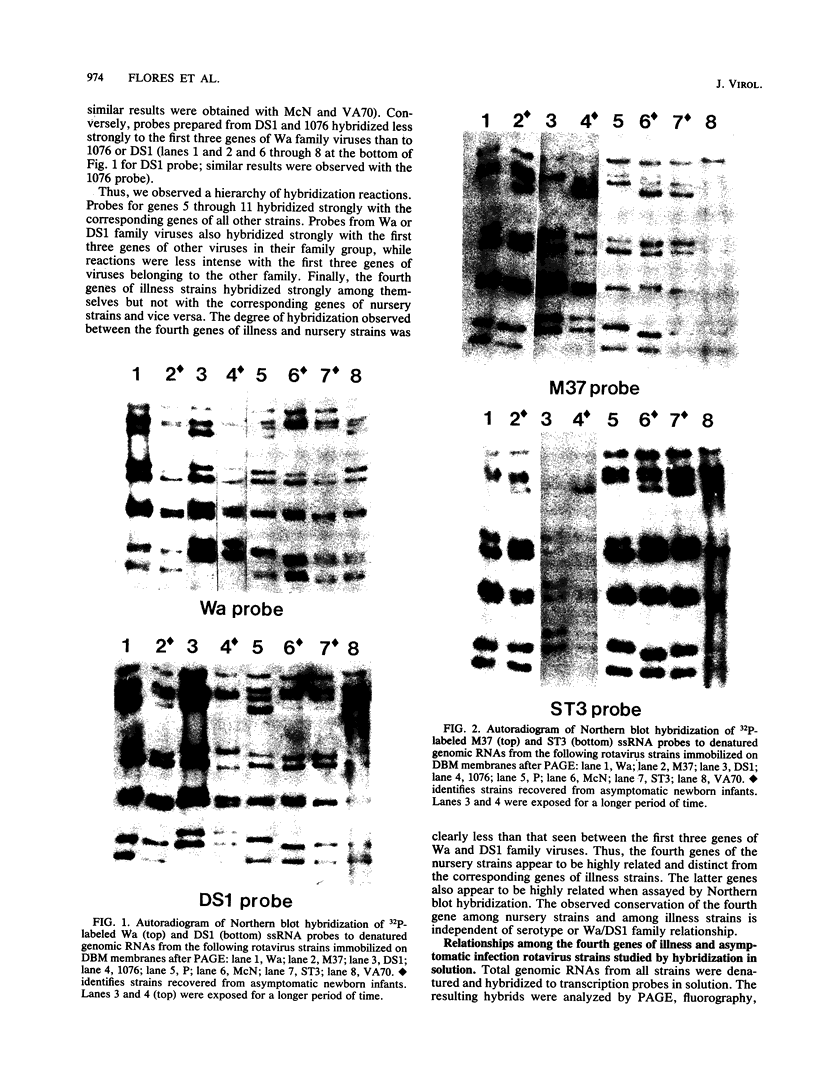
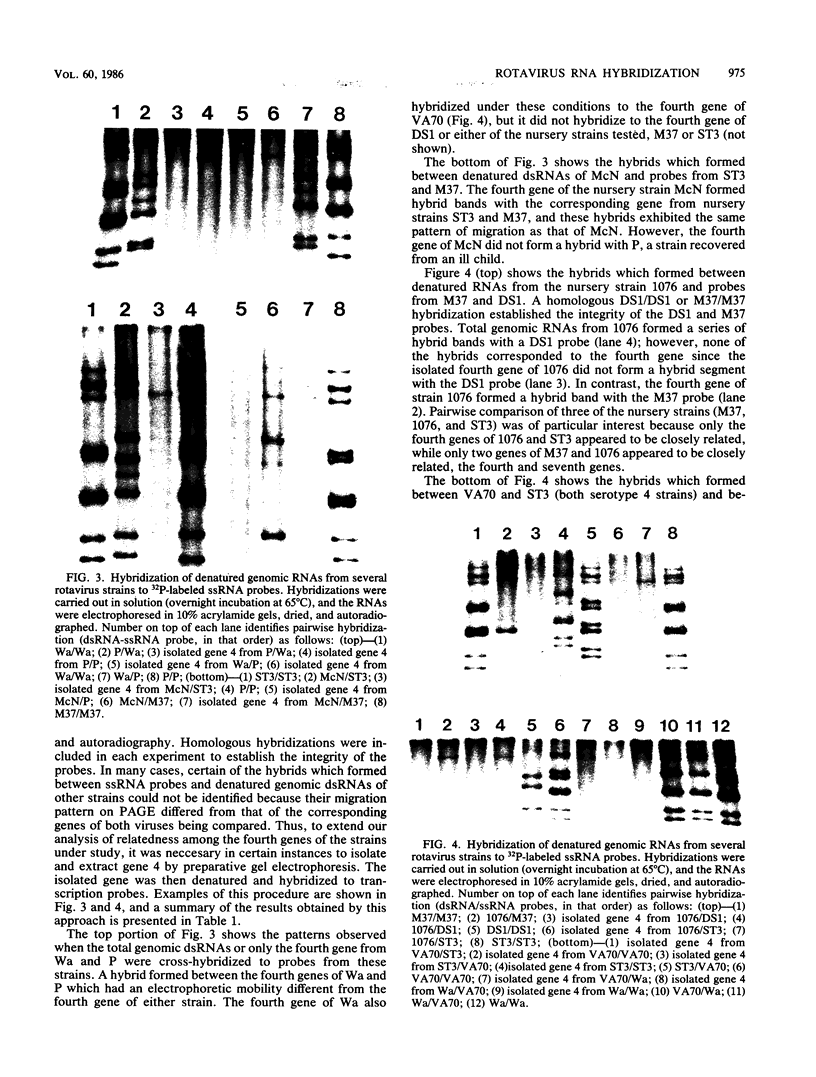
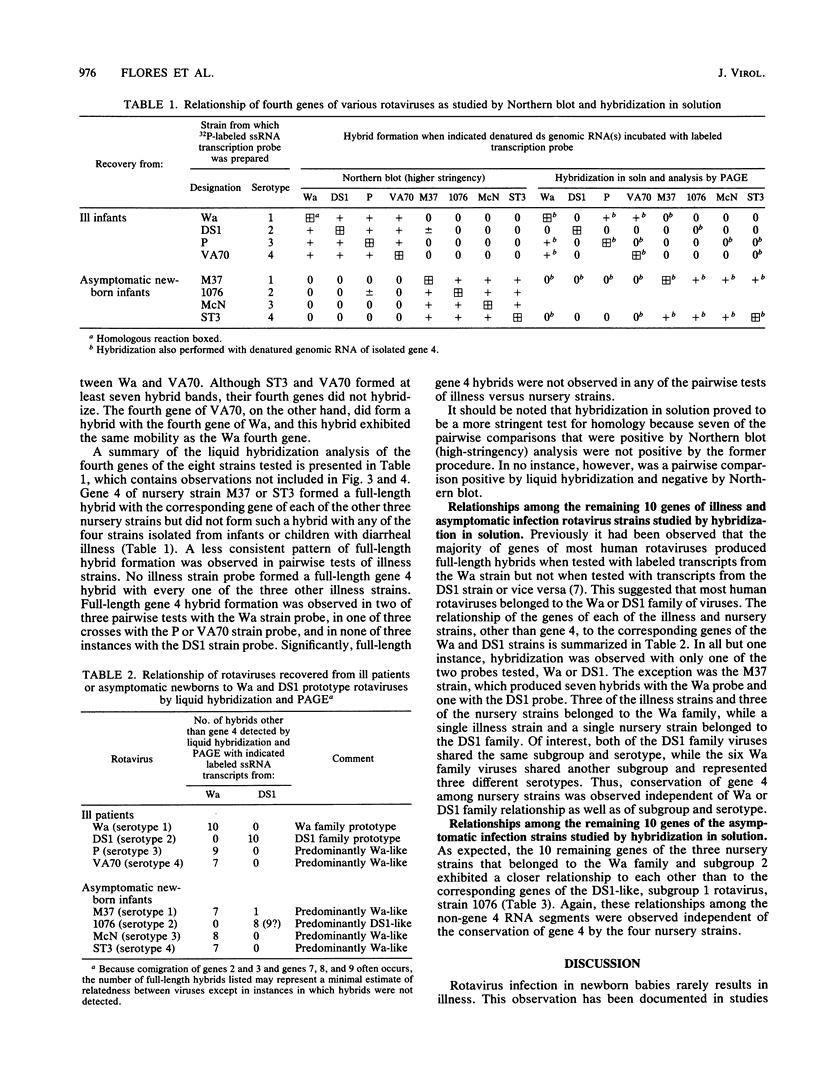
RNA-RNA hybridization was performed to assess the extent of genetic relatedness among human rotaviruses isolated from children with gastroenteritis and from asymptomatic newborn infants. 32P-labeled single-stranded RNAs produced by in vitro transcription from viral cores of the different strains tested were used as probes in two different hybridization assays: undenatured genomic RNAs were resolved by polyacrylamide gel electrophoresis, denatured in situ, electrophoretically transferred to diazobenzyloxymethyl-paper (Northern blots), and then hybridized to the probes under two different conditions of stringency; and denatured genomic double-stranded RNAs were hybridized to the probes in solution and the hybrids which formed were identified by polyacrylamide gel electrophoresis. When analyzed by Northern blot hybridization at a low level of stringency, all genes from the strains tested cross-hybridized, providing evidence for some sequence homology in each of the corresponding genes. However, when hybridization stringency was increased, a difference in gene 4 sequence was detected between strains recovered from asymptomatic newborn infants ("nursery strains") and strains recovered from infants and young children with diarrhea. Although the nursery strains exhibited serotypic diversity (i.e., each of the four strains tested belonged to a different serotype), the fourth gene appeared to be highly conserved. Similarly, each of the virulent strains tested belonged to a different serotype; nonetheless, there was significant conservation of sequence among the fourth genes of three of these viruses. Significantly, the conserved fourth genes of the nursery strains were distinct from the fourth gene of each of the virulent viruses. These results were confirmed and extended during experiments in which the RNA-RNA hybridization was carried out in solution and the resulting hybrids were analyzed by polyacrylamide gel electrophoresis. Under these conditions, the fourth genes of the nursery strains were closely related to each other but not to the fourth genes of the virulent viruses. Full-length hybrids did not form between the fourth genes from the nursery strains and the corresponding genes from the strains recovered from symptomatic infants and young children.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop R. F., Barnes G. L., Cipriani E., Lund J. S. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Bodkin D. K., Knudson D. L. Sequence relatedness of Palyam virus genes to cognates of the Palyam serogroup viruses by RNA-RNA blot hybridization. Virology. 1985 May;143(1):55–62. doi: 10.1016/0042-6822(85)90096-0. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Greenberg H. B., Myslinski J., Kalica A. R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Use of transcription probes for genotyping rotavirus reassortants. Virology. 1982 Sep;121(2):288–295. doi: 10.1016/0042-6822(82)90168-4. [DOI] [PubMed] [Google Scholar]
- Flores J., Myslinski J., Kalica A. R., Greenberg H. B., Wyatt R. G., Kapikian A. Z., Chanock R. M. In vitro transcription of two human rotaviruses. J Virol. 1982 Sep;43(3):1032–1037. doi: 10.1128/jvi.43.3.1032-1037.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Boeggeman E., White L., Perez M., Purcell R., Hoshino Y., Midthun K., Chanock R. M., Kapikian A. Z. Genetic relatedness among human rotaviruses. J Med Virol. 1985 Oct;17(2):135–143. doi: 10.1002/jmv.1890170206. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez I., White L., Perez M., Kalica A. R., Marquina R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Genetic relatedness among human rotaviruses as determined by RNA hybridization. Infect Immun. 1982 Aug;37(2):648–655. doi: 10.1128/iai.37.2.648-655.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Passarani N., Battaglía M., Percivalle E. Rapid serotyping of human rotavirus strains by solid-phase immune electron microscopy. J Clin Microbiol. 1984 Feb;19(2):273–278. doi: 10.1128/jcm.19.2.273-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Hoshino Y., Buckler-White A., Blumentals I., Glass R., Flores J., Kapikian A. Z., Chanock R. M. Conservation of amino acid sequence of VP8 and cleavage region of 84-kDa outer capsid protein among rotaviruses recovered from asymptomatic neonatal infection. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7039–7043. doi: 10.1073/pnas.83.18.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Flores J., Midthun K., Kapikian A. Z. Serotypic characterization of rotaviruses derived from asymptomatic human neonatal infections. J Clin Microbiol. 1985 Mar;21(3):425–430. doi: 10.1128/jcm.21.3.425-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- McLean B. S., Holmes I. H. Effects of antibodies, trypsin, and trypsin inhibitors on susceptibility of neonates to rotavirus infection. J Clin Microbiol. 1981 Jan;13(1):22–29. doi: 10.1128/jcm.13.1.22-29.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I., Daoud G., White L., Urbina G., Daoud N., Perez M., Flores J. Rotavirus shedding by newborn children. J Med Virol. 1984;14(2):127–136. doi: 10.1002/jmv.1890140206. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Birch C., McLean B., Holmes I. H. Molecular epidemiology of human rotaviruses in Melbourne, Australia, from 1973 to 1979, as determined by electrophoresis of genome ribonucleic acid. J Clin Microbiol. 1981 Feb;13(2):272–278. doi: 10.1128/jcm.13.2.272-278.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street J. E., Croxson M. C., Chadderton W. F., Bellamy A. R. Sequence diversity of human rotavirus strains investigated by northern blot hybridization analysis. J Virol. 1982 Aug;43(2):369–378. doi: 10.1128/jvi.43.2.369-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totterdell B. M., Nicholson K. G., MacLeod J., Chrystie I. L., Banatvala J. E. Neonatal rotavirus infection: role of lacteal neutralising alpha1-anti-trypsin and nonimmunoglobulin antiviral activity in protection. J Med Virol. 1982;10(1):37–44. doi: 10.1002/jmv.1890100106. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]