Abstract
BACKGROUND
Adjunctive direct peritoneal resuscitation (DPR) from hemorrhagic shock (HS) improves intestinal blood flow and abrogates postresuscitation edema. HS causes water shifts as a result of sodium redistribution and changes in transcapillary Starling forces. Conventional resuscitation (CR) with crystalloid aggravates water sequestration. We examined the compartment pattern of organ tissue water after HS and CR, and modulation of tissue edema by adjunctive DPR.
STUDY DESIGN
Rats were hemorrhaged (40% mean arterial pressure for 60 minutes) and assigned to four groups (n = 7): sham, no HS; HS no resuscitation; HS+CR (shed blood plus 2 volumes Ringer’s lactate); and HS+CR+DPR (20 mL clinical intraperitoneal (IP) dialysis fluid). Isotopic markers determined equilibrium distribution volumes [VD] in gut, liver, lung, and muscle by quantitative autoradiography (2-hour postresuscitation). Total tissue water (TTW) was determined by wet-dry weights. Extracellular water was measured from 14C-mannitol VD, and intravascular volume (IVV) from 131I-labeled IgG VD. Cellular and interstitial water volumes were calculated.
RESULTS
HS alone decreased IVV in all tissues and TTW in gut, lung, and muscle, but not liver, compared with shams. IVV remained decreased with all resuscitations despite restoration of central hemodynamics. CR caused interstitial edema in gut, liver, and muscle, and cellular edema in lung. DPR reduced (liver, muscle) or prevented (gut, lung) these volume shifts.
CONCLUSIONS
HS decreases IVV. HS-induced water shifts are organ-specific and prominent in gut, lung, and muscle. CR restores central hemodynamics, does not restore IVV, and alters organ-specific TTW distribution. Adjunctive DPR with IP dialysis fluid normalizes TTW and water compartment distribution and prevents edema. Combined effect of DPR and intravascular fluid replacement appears to prevent global tissue edema and improve outcomes from HS.
Conventional IV fluid replacement targets the deficits caused by trauma and blood loss. In clinical practice resuscitation, fluid therapy is administered in volumes far exceeding estimated blood loss. This is done for a presumable extracellular fluid sequestration into the intracellular compartment or the interstitial volume.1 Replacement of fluid loss to the interstitial space is also a recognized practice during surgical operative procedures.2,3 Data from studies showing improved survival after blood and crystalloid fluid resuscitation in excess of the blood lost lend support for the concept of an intracellular fluid sequestration during hemorrhagic shock.4,5 Excessive crystalloid resuscitation was associated with development of wet-lung syndrome in otherwise healthy soldiers resuscitated with large volumes after traumatic injury.6-8 Others have noted that adult respiratory distress syndrome correlates with long intervals of shock before definitive care, and typically occurs after underresuscitation rather than overresuscitation.9 Despite the clinical appreciation for the need to replace fluid lost to both extracellular and intracellular spaces during resuscitation from hemorrhage, the magnitude, mechanisms, and implications of these fluid shifts are poorly described. This is in large part because of the difficulty getting accurate experimental assessments of fluid shifts during hemorrhagic shock and resuscitation.10
During hemorrhagic shock resuscitation, fluid shifts occur at the cell–interstitium interface and at the vascular, primarily capillary–interstitium interface. The shift of fluid at the cell–interstitium interface is an adaptive cell volume regulatory mechanism, and capillary–interstitium fluid shifts are precipitated by perturbation of the transcapillary Starling forces. Morphometric studies by Mazzoni and colleagues11-13 have shown that hemorrhagic shock reduces the patent capillary cross-sectional area by > 20% because of capillary vascular endothelium swelling, which is mediated by activation of the Na+/H+ exchanger. The Na+/H+ exchanger plays an active role in regulation of intracellular volume and pH during low-flow states. Accumulated intracellular H+from hypoxia-induced anaerobic metabolism activates the Na+/H+ exchanger. This activation results in cellular H+efflux and Na+ influx associated with an osmotically driven solvent-drag (water shift), which causes endothelial cell swelling. Hemorrhage alters the ability of all cell membranes to regulate the interchange of ions between the cell and its immediate microenvironment. This phenomenon is global and not restricted to the vascular endothelium. Sentinel studies by Shires and colleagues14-16 demonstrated doubling of skeletal muscle intracellular Na+ ions after hemorrhagic shock by ion exchange mechanisms other than the Na+/H+ exchanger. This sodium pump dysfunction was the postulated mechanism for a decrease in the extracellular volume (ECV) after shock.17,18 Hemorrhagic shock and resuscitation also compromise the basic capillary function of fluid exchange because of perturbations of transcapillary Starling forces.19,20 Because transcapillary fluid inflow and lymph outflow determine interstitial fluid volume (IFV), alteration of this inflow exchange mechanism could influence total IFV.
Our resent studies suggest that conventional blood and IV crystalloid resuscitation from hemorrhagic shock is associated with a persistent increase in total tissue water (TTW) (edema).21 We have shown that this edema formation is inhibited by adjunctive direct peritoneal resuscitation (DPR), which uses a clinical glucose-based peritoneal dialysis solution.21 The present studies were conducted to measure fluid shifts as a result of hemorrhage and resuscitation, to quantitatively describe the patterns of partitioning of TTW, and modulation of these patterns with DPR using dual-label quantitative autoradiography (QAR).
METHODS
Animals
All experiments were performed in 200- to 350-g male Sprague-Dawley rats (Charles River Laboratories). Animals had free access to water and standard rat chow until the morning of the experiment. All procedures were approved by the Louisville Veterans Medical Center, the University of Louisville, and the University of Mississippi Committee on Animal Resources.
Materials
Immunoglobulin G (anti-goat IgG, no. G-6638; Sigma) was labeled with 131I (Amersham microvial, type P15; Amersham Life Science). Iodination was performed using Iodo-Beads (Pierce). The isotope was purified by passing the solution along an ion-exchange column (1-XP; Bio-Rad). Before each experiment the isotope was checked for degradation and free 131I by trichloroacetic acid. If free 131I was >1%, dilution and concentration were repeated until free 131I was < 1% by trichloroacetic acid precipitation. [14C]mannitol was purchased from Amersham Life Science.
Operation
Anesthesia was induced by IM injection of pentobarbital sodium (60 mg/kg) to the hind leg and maintained with subsequent subcutaneous injections. Operation was initiated on loss of blink reflex. A tracheostomy was performed to reduce airway resistance. Two arterial lines were established using PE-50 catheters. The left carotid artery was cannulated to allow for continuous blood pressure measurements on a pressure measurement system (model PE-10z Statham pressure transducer; Window Graf; Gould Valley Instruments), and a left femoral artery catheter was used for induction of hemorrhage. A venous catheter was secured into the left femoral vein for tracer injection and for resuscitation with an infusion pump (model 22; Harvard Apparatus). The animal’s rectal temperature was monitored and maintained between 36.5°C and 38°C with a servo-controlled warming blanket (Harvard Apparatus) and an overhead heating lamp. The peritoneal cavity was exposed through a midline abdominal incision ~1.5 cm, and the renal pedicles were ligated bilaterally. The slit-like abdominal incision was closed using a continuous suture after careful inspection to ensure there was no bleeding. Before closure a multihole catheter was placed with the aid of a trocar through the abdominal wall into the peritoneal cavity and secured with a purse stitch.
Hemorrhage and resuscitation model
Hemorrhagic shock was achieved with blood withdrawal (1 mL/min) from the femoral artery in a syringe prerinsed with 0.02 mL heparin (1,000 U/mL). This was continued until 40% of mean arterial pressure was attained. This nominal mean arterial pressure was maintained for 60 minutes, with additional blood withdrawal or reinfusion as required. Total volume of blood withdrawn averaged 5.13 ± 0.18 mL. Conventional resuscitation (CR) was initiated with the IV return of the shed blood during 5 minutes. This was followed by infusion of lactated Ringer’s solution (equal to 2× volume of shed blood) during the next 25 minutes. Adjunctive DPR was initiated simultaneously at the time of CR with IP instillation of 20 mL 2.5% glucose-based clinical peritoneal dialysis solution through the peritoneal catheter.
Experimental groups
Animals were randomized according to the groups as follows (n = 7 animals/group):
group I: hemorrhagic shock (HS)
group II: HS+CR
group III: HS+CR+DPR
group IV: Sham operation
Experimental protocol
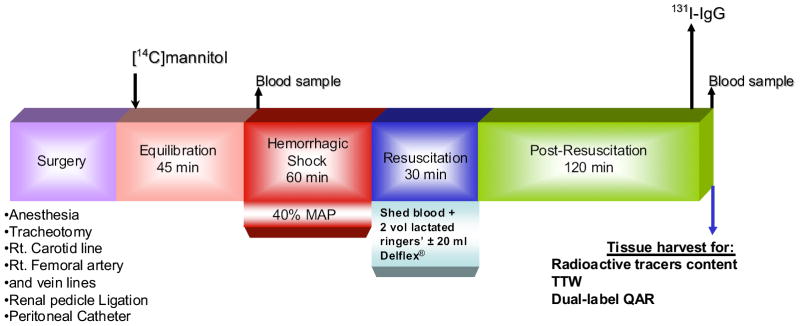
Timeline experimental protocol is depicted in Figure 1. After surgical preparation, 30 minutes was allowed for the animal to recover from the surgical stress. Then, 30 μCi [14C]mannitol was injected through a short venous catheter. Sixty minutes after tracer injection, a 100-μL blood sample was obtained to determine plasma concentration of [14C]mannitol. After this, HS was induced according to protocol, except for sham animals. After 60 minutes of hypovolemia, animals were randomly assigned for CR (return of shed blood plus 2 volumes of Ringers’ lactate), or CR and adjunctive DPR (IP instillation of 20 mL 2.5% glucose-based clinical peritoneal dialysis solution). Ten minutes before termination of the experiment, 50 μCi 131I-IgG was injected IV. At 2 hours after resuscitation, 100 μL blood was collected in microcaps to determine plasma concentrations of 131I-IgG and [14C]mannitol, after which animals were euthanized with anesthetic overdose at 120 minutes after resuscitation. Tissue samples from the terminal ileum, liver, lung, and abdominal muscle were harvested in triplicate in preweighed vials and their wet weight immediately determined. Tissue harvest in the HS group (group I) occurred at the end of the shock period. Plasma and tissue contents of the radioactive tracers were immediately determined. Tissues were also processed for dual-label QAR on termination of each experiment.
Figure 1.
Time-line experimental protocol. MAP, mean arterial pressure; QAR, quantitative autoradiography; TTW, total tissue water; [14C]mannitol, tracer marker for the local extracellular fluid volume; 131I-IgG, tracer marker for the local intravascular fluid volume.
Dual-label QAR
QAR was used to determine the local concentration of each tracer in the tissue at the time of harvest. Tissue samples were imbedded in paraffin and sectioned by microtome into 20-μm–thick pieces. The general assumption for determination of a particular space within the tissue was that the tracer was equilibrated between the plasma and the volume of distribution of the tracer within the tissue. The volume within tissue, i, equals the ratio of the tissue concentration to the plasma concentration, Ctissuei/Cplasmai. Autoradiograms for the tissue contents of the 131I-labeled IgG were first processed with the use of proper shielding to prevent emission of β-particles on the film. After 10 to 12 half-lives of 131I-labeled IgG, tissue slides and 14C standards were placed against x-ray film to produce autoradiograms of the tissue containing the [14C]mannitol. After the films were developed, tissue slides were stained with hematoxylin and eosin. Each slide was examined by light microscopy to determine the tissue boundaries to ensure that tracer concentration is determined within the tissue proper. The films were analyzed with a computerized densitometer (MCID; Imaging Research) that measures optical density versus position in the tissue. The isotopic standards were used to construct a calibration curve (concentration versus optical density) to convert the unknown optical density values from the tissue samples into concentrations. By superimposing the tissue histology over the autoradiogram, we determined the location of the reading and obtained multiple tracer concentrations from the tissue proper. The electronic grid used to obtain tissue tracer concentration has a pixel size of 50 × 50 μm. This allows for multiple determinations of tissue tracer concentration from each tissue section. Dividing these concentrations by the plasma concentration provided the volume of distribution of the tracer.
Extracellular volume
ECV is defined as a unit of volume within the tissue that is not occupied by cells or solid material. ECV was determined by IV bolus injection of 30 μCi [14C]mannitol after ligation of the renal pedicles to prevent renal excretion and then maintain a constant plasma concentration of the labeled mannitol. Labeled mannitol equilibrates within the extracellular space and its tissue concentration divided by the plasma concentration provides the estimate of extracellular fluid volume. Sixty minutes after [14C]mannitol injection, HS was induced according to protocol.
Intravascular and interstitial volumes
Intravascular volume (IVV) is defined as the volume within the blood vessels that is not occupied by cells. Ten minutes before termination of the experiment, a bolus injection of 131I-IgG (50 to 100 μCi) was given IV to mark the plasma space, IVV. Blood was sampled just before termination of the experiment for both 131I and [14C]mannitol. The transvascular escape rate of the labeled macromolecular tracer from the vascular space in 10 minutes is negligible. Tissue concentrations were determined by single-label (131I-IgG) QAR. IVV was calculated from the ratio of tissue concentration and plasma concentration. IFV in the tissue was calculated from the difference between the ECV and IVV (IFV = ECV − IVV).
TTW volume
TTW volume is equal to the sum of the volumes of water in the extracellular and intracellular spaces and is calculated from the wet-to-dry ratio [TTW = (tissue wet weight)/(tissue dry weight) to yield (mL/g dry tissue, where 1 g water = 1 mL)]. The intracellular water volume (ICV) was calculated by subtraction: ICV = TTW − ECV.
Calculations
There is no specific marker for interstitial space. IFV can be calculated from the difference between the extracellular space (ECV) and intravascular space (IVV)
Small molecules such as [14C]mannitol are not taken up or metabolized by mammalian cells and can act as extracellular markers. Proteins such as 131I-labeled IgG mark the intravascular space. Tissue ECV and ICV were calculated from:
And,
where Vec is extracellular fluid volume, Mtot is total tissue mass, Ctissue is local tissue tracer concentration, and Cplasma is plasma tracer concentration. Measurement of ECV as the extracellular distribution of [14C]mannitol is based on the assumption that the concentration of the tracer in the interstitial fluid is equal to that in the plasma at the time of tissue sampling. Because it is not possible to directly sample the interstitial fluid, we maintained a constant plasma concentration by elimination of renal excretion and assume a steady state after 180 minutes of tracer equilibration.
Data reduction and statistical analysis
All data are presented as mean ± standard error of the mean. Local intravascular and extracellular volume fractions were assessed from 20-μm tissue thin sections using computerized densitometer. On average, 15 measurements of each of the volume fractions were made from each tissue section per animal. TTW was measured from: [(wet tissue weight/tissue dry weight) − 1] to yield mL H2O/g dry tissue. All local tissue volume fractions were normalized to the TTW. To assess patterns of distribution of TTW in the experimental groups, local volume fractions were normalized and expressed as percentage change from sham. Differences in patterns of TTW or partitioning of TTW between groups or tissues were determined by two-way ANOVA followed by Bonferroni multiple comparison posttests when the ANOVA indicated major differences in fractional volume change, resuscitation type, or groups. Statistical significance was set a priori for the probability of a type-one error at p < 0.05.
RESULTS
There were no substantial differences in baseline hemodynamics between the four groups. HS decreased mean arterial pressure by 60% in all hemorrhage animals. Resuscitation with the return of shed blood plus 2 volumes of lactated Ringer’s solution restored and maintained mean arterial pressure to prehemorrhage level in all hemorrhaged animals.
Tissue water distribution in hemorrhage and resuscitation
TTW in the present studies was assessed from the tissue wet weight to tissue dry weight ratio, which allowed for only one determination per tissue at the termination of the experiment. This method produced results similar to previous QAR determinations of the equilibrium volume of distribution of [14C]urea, a surrogate for the volume of tissue that is made up of water.22 Because TTW and its redistribution differed according to tissue type and resuscitation modality, results from each tissue will be presented separately. Absolute values for TTW and its distribution are shown in Table 1.
Table 1.
Absolute Values of Water Distribution Within the Gut, Liver, Lung, and Abdominal Muscle
| Tissue | Compartment | Sham | HS alone | HS + CR | HS+CR+DPR |
|---|---|---|---|---|---|
| Gut | TTW | 3.304 ± 0.269 | 2.783 ± 0.215* | 3.653 ± 0.214 | 3.495 ± 0.014 |
| IFV | 0.436 ± 0.004 | 0.405 ± 0.005* | 0.640 ± 0.004* | 0.458 ± 0.004* | |
| ICV | 2.837 ± 0.003 | 2.361 ± 0.004* | 2.997 ± 0.001 | 3.025 ± 0.001 | |
| IVV | 0.030 ± 0.002 | 0.017 ± 0.003* | 0.016 ± 0.001* | 0.012 ± 0.001* | |
|
| |||||
| Liver | TTW | 2.527 ± 0.169 | 2.368 ± 0.419 | 2.455 ± 0.177 | 2.544 ± 0.157 |
| IFV | 0.477 ± 0.117 | 0.722 ± 0.141* | 0.795 ± 0.034* | 0.671 ± 0.046* | |
| ICV | 1.730 ± 0.120 | 1.500 ± 0.061 | 1.509 ± 0.020 | 1.741 ± 0.041 | |
| IVV | 0.320 ± 0.006 | 0.145 ± 0.024* | 0.151 ± 0.022* | 0.132 ± 0.019* | |
|
| |||||
| Lung | TTW | 3.508 ± 0.136 | 2.813 ± 0.407* | 4.032 ± 0.383* | 3.597 ± 0.182† |
| IFV | 0.627 ± 0.063 | 0.415 ± 0.118 | 0.452 ± 0.049 | 0.651 ± 0.070 | |
| ICV | 2.661 ± 0.032 | 2.253 ± 0.087* | 3.434 ± 0.152* | 2.801 ± 0.160*† | |
| IVV | 0.219 ± 0.006 | 0.142 ± 0.004* | 0.146 ± 0.003* | 0.132 ± 0.004* | |
|
| |||||
| Muscle | TTW | 3.179 ± 0.161 | 3.141 ± 0.146 | 3.555 ± 0.461* | 3.777 ± 0.412* |
| IFV | 0.244 ± 0.024 | 0.321 ± 0.061* | 0.292 ± 0.039* | 0.387 ± 0.064*† | |
| ICV | 2.920 ± 0.003 | 2.812 ± 0.059 | 3.254 ± 0.037* | 3.383 ± 0.063* | |
| IVV | 0.015 ± 0.002 | 0.008 ± 0.002* | 0.009 ± 0.002* | 0.007 ± 0.001* | |
All values are expressed in units of mL/g.
CR, conventional resuscitation, return of shed blood plus 2 volumes of lactated Ringers’ solution; DPR, direct peritoneal resuscitation with 20 mL glucose-based clinical peritoneal dialysis solution intraperitoneally; HS, hemorrhagic shock; ICV, local intracellular fluid volume fraction; IFV, local interstitial fluid volume fraction; IVV, intravascular volume; Sham, sham operation, no hemorrhage; TTW, total tissue water.
p < 0.01 versus sham by two-way ANOVA and Bonferroni posttest.
p < 0.01 versus group III (HS+CR) by two-way ANOVA and Bonferroni posttest.
Gut
TTW of the gut averaged 3.304 ± 0.269 mL/g dry tissue (n = 7) distributed as: IVV 0.92% ± 0.03%, IFV 13.19% ± 0.24%, and ICV 85.89%. HS, as depicted in the upper panel of Figure 2, caused a substantial decrease in TTW as reflected by a substantial decrease of all local volume fractions making up the TTW. Intravenous resuscitation alone or combined with DPR restored TTW and substantially expanded IFV at the expense of the IVV, which remained depleted. In the lower panel of Figure 2, patterns of distribution of TTW for the three experimental groups are compared with sham control animals. HS, as seen in the figure, decreased TTW and expanded IFV at the expense of the IVV and ICV. CR restored TTW and substantially expanded the IFV, but failed to restore the IVV. Adjunctive DPR restored to near-normal TTW distribution, with the exception of continued depletion of the local IVV.
Figure 2.
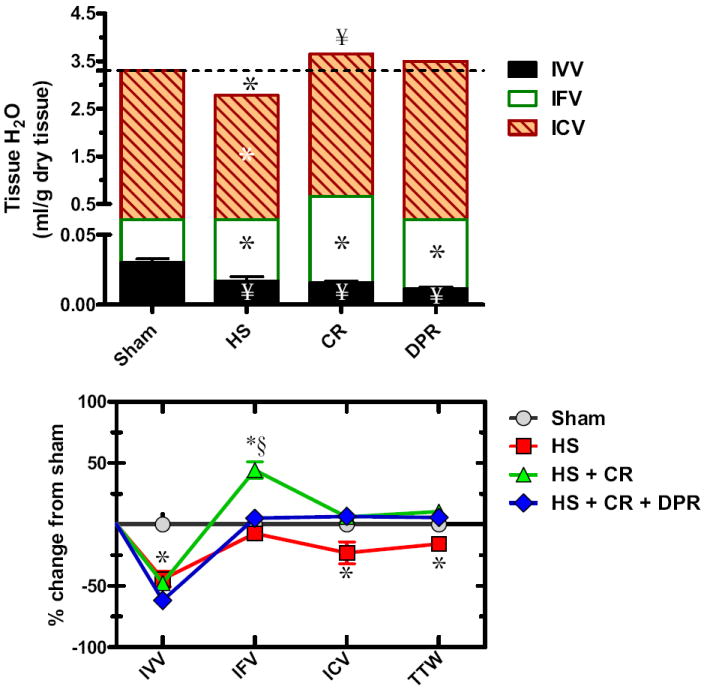
Water distribution within the gut (upper panel); and water redistribution relative to sham control (lower panel). Y axis in the upper panel was divided into two segments to visualize the small intravascular volume (IVV). IFV, local interstitial fluid volume fraction; ICV, local intracellular fluid volume fraction; hatched line represents total tissue water in the sham control group; Sham, Sham operation, no hemorrhage; HS, hemorrhagic shock; CR, conventional resuscitation with the return of shed blood plus 2 volumes of lactated Ringers’ solution; DPR, direct peritoneal resuscitation with 20 mL glucose-based clinical peritoneal dialysis solution IP. *p < 0.01 versus sham by two-way ANOVA and Bonferroni posttest. §p < 0.05 versus sham control by two-way ANOVA and Bonferroni posttest.
Liver
TTW of the liver averaged 2.527 ± 0.169 mL/g dry tissue (n = 7) distributed as: IVV 13.37% ± 0.01%, IFV 19.93% ± 0.34%, and ICV 66.70% ± 0.56% (n = 7). HS, as shown in the upper panel of Figure 3, caused a substantial decrease in TTW, as reflected by a substantial decrease of all local volume fractions making up the TTW. Intravenous resuscitation alone or combined with DPR restored TTW, substantially expanded IFV at the expense of the IVV, which remained depleted. Compared with the sham’s pattern of TTW distribution, HS alone resulted in considerable expansion of the IFV at the expense of the local IVV. This pattern was not changed by CR alone or after addition of DPR.
Figure 3.
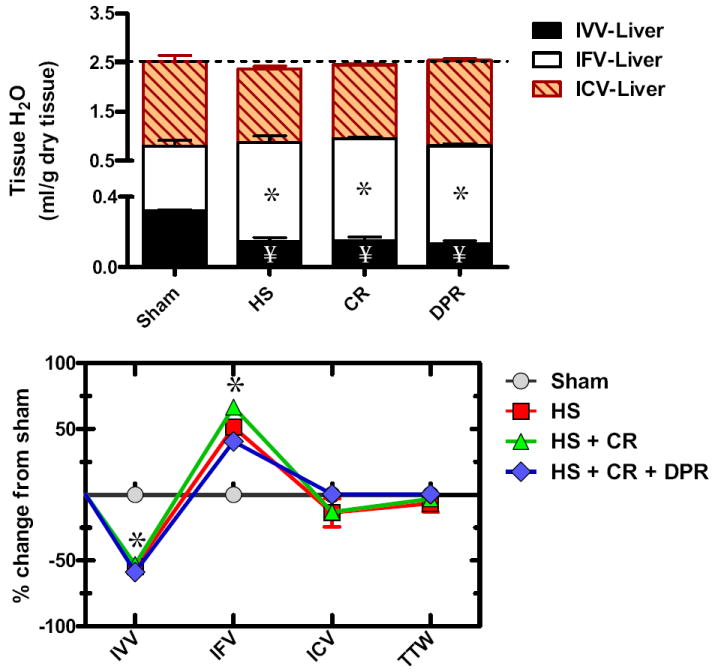
Water distribution within the liver (upper panel); and water redistribution relative to sham control (lower panel). Y axis in the upper panel was divided into two segments to visualize the small intravascular volume (IVV). IFV, local interstitial fluid volume fraction; ICV, local intracellular fluid volume fraction; hatched line represents total tissue water in the sham control group; Sham, sham operation, no hemorrhage; HS, hemorrhagic shock; CR, conventional resuscitation with the return of shed blood plus 2 volumes of lactated Ringers’ solution; DPR, direct peritoneal resuscitation with 20 mL glucose-based clinical peritoneal dialysis solution IP. *p < 0.01 versus sham by two-way ANOVA and Bonferroni posttest. §p < 0.05 versus sham control by two-way ANOVA and Bonferroni posttest.
Lung
TTW of the lung averaged 3.508 ± 0.136 mL/g dry tissue (n = 7) distributed as: IVV 6.24% ± 0.20%, IFV 17.88% ± 0.09%, and ICV 75.88% ± 0.03%. HS, as shown in the upper panel of Figure 4, decreased TTW, as reflected by a substantial decrease of all volume fractions. CR resulted in a substantial increase in TTW, and lung IVV remained depleted. Adjunctive DPR restored lung TTW and prevented expansion of the ICV. Compared with the sham’s lung pattern of TTW distribution, HS caused a considerable decrease of all local volume fractions. CR markedly increased lung TTW mainly because of expansion of the ICV, and IVV remained depleted at the shock level. In contrast, adjunctive DPR restored TTW along with IFV and ICV, and IVV remained depleted.
Figure 4.
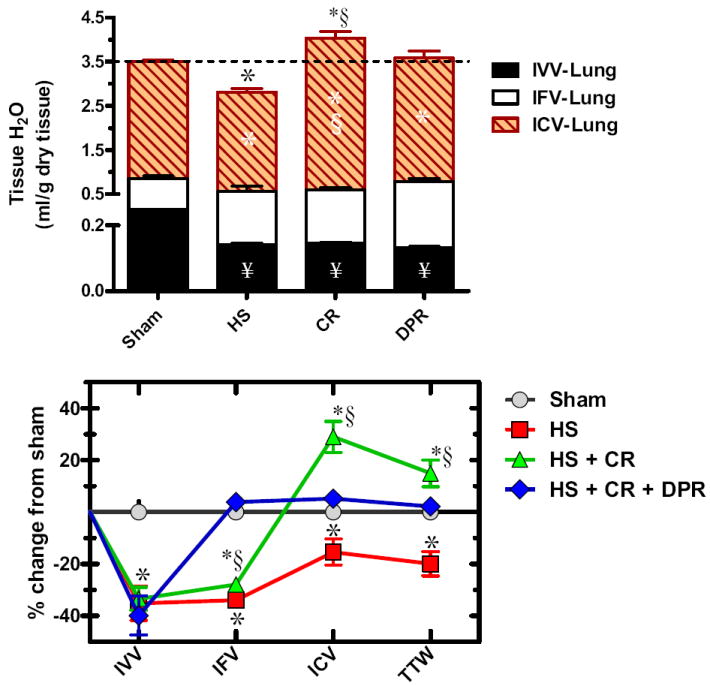
Water distribution within the gut (upper panel); and water redistribution relative to sham control (lower panel). Y axis in the upper panel was divided into two segments to visualize the small intravascular volume (IVV). IFV, local interstitial fluid volume fraction; ICV, local intracellular fluid volume fraction; hatched line represents total tissue water in the sham control group; Sham, sham operation, no hemorrhage; HS, hemorrhagic shock; CR, conventional resuscitation with return of shed blood plus 2 volumes of lactated Ringers’ solution; DPR, direct peritoneal resuscitation with 20 mL glucose-based clinical peritoneal dialysis solution IP. *p < 0.01 versus sham by two-way ANOVA and Bonferroni posttest. §p < 0.05 versus sham control by two-way ANOVA and Bonferroni posttest. §p < 0.01 versus group IV (HS+CR+DPR) by two-way ANOVA and Bonferroni posttest.
Abdominal muscle
TTW of the anterior abdominal muscle averaged 3.179 ± 0.161 mL/g dry tissue (n = 7) distributed as: IVV 0.5% ± 0.004%, IFV 7.7% ± 0.026%, and ICV 91.8% ± 0.016%. HS, as shown in the upper panel of Figure 5, had no effect on TTW of the abdominal muscle, but caused substantial expansion of the IFV, presumably at the expense of the local IVV. CR alone or when combined with adjunctive DPR modestly increased TTW of the abdominal muscle, mainly because of slight increases of both the ICV and IFV fractions.
Figure 5.
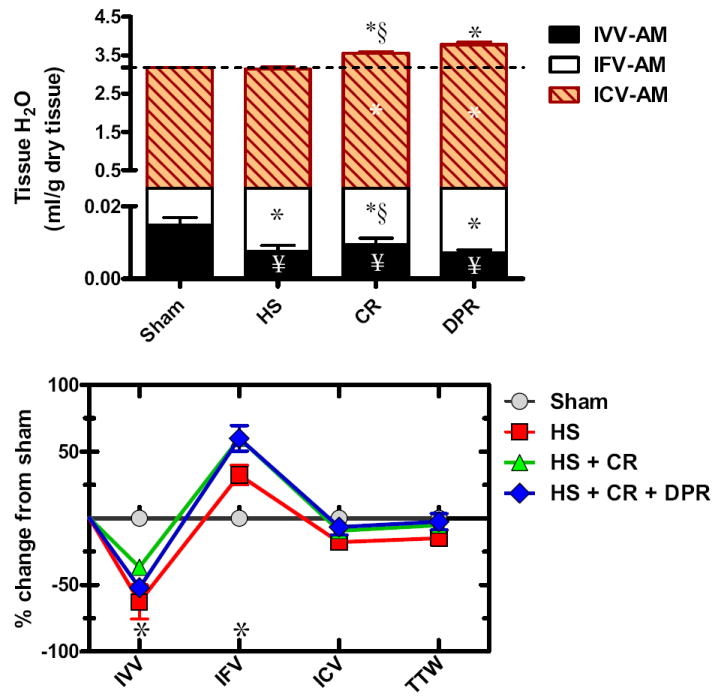
Water distribution within the abdominal muscle (upper panel); and water redistribution relative to sham control (lower panel). Y axis in the upper panel was divided into two segments to visualize the small intravascular volume (IVV). IFV, local interstitial fluid volume fraction; ICV, local intracellular fluid volume fraction; hatched line represents total tissue water in the sham control group; Sham, sham operation, no hemorrhage; HS, hemorrhagic shock; CR, conventional resuscitation with the return of shed blood plus 2 volumes of lactated Ringers’ solution; DPR, direct peritoneal resuscitation with 20 mL glucose-based clinical peritoneal dialysis solution IP. * p < 0.01 versus sham by two-way ANOVA and Bonferroni posttest. § p < 0.05 versus sham control by two-way ANOVA and Bonferroni posttest. §p < 0.01 versus group IV (HS+CR+DPR) by two-way ANOVA and Bonferroni posttest.
DISCUSSION
These studies examined patterns of distribution of tissue water that result from HS, CR from shock, and modulations of these patterns with adjunctive DPR. Salient findings of this study include: the level of tissue hydration and pattern of distribution of TTW in the resting state vary depending on tissue type; HS that decreases total blood volume is associated with a parallel decrease in local IVV of splanchnic and distant organs; hemorrhage-induced IVV depletion causes tissue water shifts that are organ-specific and prominent in gut, lung, and muscle; conventional IV volume resuscitation from HS, which restores central hemodynamics, does not restore local IVV, and alters organ-specific TTW distribution; adjunctive DPR with IP dialysis fluid normalizes TTW and water compartment distribution and prevents edema; and transcapillary escape of crystalloid resuscitation fluid is large, and accounts for persistent depletion of the local IVV, despite a presumable replacement of volume deficit.
Technique and assumptions
Dual-label QAR allows for simultaneous determination of the fractions of tissue space making up the ECV and IVV.22-24 Unlike measuring these fractions with traditional whole tissue count of radioactive tracers, which requires corrections for tracer spillover and radioactive decay, dual-label QAR provides for specific measurements of tissue tracer concentrations from thin tissue sections. Because there is no specific marker for the interstitium, IFV can only be determined indirectly using labeled tracers that separately mark the ECV and IVV spaces. Markers used to measure these tissue fractions and should have a long half-life and no access to the cellular water compartment during either control or experimental conditions. Previously used ECV markers, such as 22Na, 82Br, or 35SO4, can readily transport into the cellular compartment, be taken up by blood cellular elements, or bind to plasma components, and provide desperate results on the status of the ECV during shock and resuscitation.10 In addition, for the tracer’s distribution volume to be valid, the concentration of the tracer in the volume to be measured should equal its concentration in plasma at the time of sampling. To approximate a steady-state of equilibrium, investigators have maintained constant tracer concentration in plasma by bolus dose injection and continuous infusion,25 or by tracer infusion after bilateral nephrectomy.26 Larsson and colleagues25 found that in naïve rats, the volume of distribution of 51Cr-ethylene diamine tetraacetic acid (molecular mass = 341 kDa) increased with duration of infusion, both in whole animals and tissues, but in anephric rats, a more reliable volume of distribution was obtained because of elimination of renal clearance.27
Reed and Wiig26 reported that extracellular spaces measured with 51Cr-ethylene diamine tetraacetic acid in anephric cats were similar 2 and 6 to 7 hours after tracer injection. Our previous studies determined the volume of distribution for [14C]mannitol (molecular mass = 180 kDa) to be 0.174 ± 0.006 L/kg,28 which is similar to our mathematic model predictions for mannitol.29 In previous control experiments, ECV of the abdominal muscle as measured after a steady-state of [14C]mannitol in the plasma and peritoneal fluid was obtained.22 These bidirectional transport studies showed no substantial difference between equilibration times of 60 or 120 minutes.22 We are confident that the 180-minute equilibration time used in this study is sufficient for [14C]mannitol in the plasma to equilibrate throughout the extracellular space. TTW in the present studies was determined from the difference between the tissue wet weight and tissue dry weight. This method produced results similar to our previous QAR determination of the equilibrium volume of distribution of [14C]urea, a surrogate for volume of tissue that is made up of water.22 Also, we have shown that the volume of distribution of [14C]urea and tritiated water [3H]2O are comparable and equally predicted total body water of 626 ± 8 mL/kg rat and 658 ± 14 mL/kg rat, for [14C]urea and [3H]2O, respectively.
Tissue fluid shifts in hemorrhagic hypovolemia
Fluid shifts during HS occur at the cell membrane level and at the capillary wall level, each by a different mechanism. HS shock alters the ability of the cell membrane to regulate the interchange of ions between the cell and its immediate microenvironment. The studies by Shires and colleagues15 demonstrated doubling of skeletal muscle intracellular Na+ ions after HS by ion-exchange mechanisms other than the Na+/H+ exchanger, whereas activation of this exchanger during HS was linked to Na+ and water shift into the vascular endothelium.11-13 The low-flow state of hemorrhagic hypovolemia undermines one of the most basic functions of the capillaries, that of maintaining fluid balance between the blood and tissue compartments. Unbalanced transcapillary fluid exchange during HS results from alterations of the transcapillary Starling forces, much in accordance with the severity of shock as reviewed elsewhere.19 Studies on patterns and distribution of TTW as a result of HS are scant. In one study, whole animal plasma water and regional ECV in liver, spleen, and subcutaneous fat were decreased after hemorrhage, and whole animal ECV did not change.30 It was proposed that during hemorrhagic hypovolemia, microvascular and interstitial compensatory events are triggered to rapidly restore IVV.31 This is not supported by our studies, as both the ECV and ICV fractions making up TTW are decreased after HS. Assuming that tissue fluid shifts do not occur with HS, blood loss should only deplete the IVV. The decrease in local IVV, IFV, and ICV fractions in the present studies suggest that a fraction of the intravascular water must have had access to a compartment, such as the vascular endothelium not marked with either of the dual-label tracers (131I-IgG, [14C]mannitol). Previous morphometric studies demonstrated substantial vascular endothelium swelling caused by hemorrhage-induced activation of the membrane-bound Na+/H+ exchanger.11-13 This was supported by our recent intravital microscopy studies showing substantial endothelial cell swelling as a result of hemorrhage-induced cellular acidosis-mediated Na+/H+ exchanger activation.32 Because of the size of the endothelial cell mass, this fraction of the intracellular compartment provides a substantial sink for Na+ and water during conditions that favor access to this specific cellular compartment, such as in HS. This concept of endothelial cell swelling could be a plausible explanation for the Na+ deficit observed in shock and trauma patients and in surgical patients who require fluid therapy.14,16
Postresuscitation tissue fluid shifts
Redistribution of tissue water as a result of resuscitation from HS appears to be dependent on resuscitation solution and tissue type.33-35 Measurements of whole animal fluid compartments, such as whole animal plasma water and ECV,33-35 are not sensitive methods to assess fluid shifts at the tissue level. Alternatively, postresuscitation redistribution of tissue water should be determined from simultaneous measurements of tissue volume fractions making up the TTW because of variations in both IVV and IFV between tissue types as demonstrated in this and other studies.36-40 Overhydration with isotonic crystalloid solution in otherwise normovolemic rats selectively expanded ECV of compliant organ tissues, and whole animal plasma water remained unchanged,41 suggesting substantial expansion of IFV, as a result, in part, of extravasation of the isotonic crystalloid solution. This reasoning is consistent with the continued postresuscitation depletion of the IVV in all tissues investigated in the present studies. Previously, we demonstrated that CR from HS that restores and maintains central hemodynamics is associated with a persistent and progressive splanchnic vasoconstriction and hypoper-fusion.42,43 Although this end-organ microvascular dysfunction can compromise local blood volume, it is unlikely that such end-organ microvascular dysfunction is an explanation for postresuscitation IVV depletion. This is because IVV depletion was also observed after adjunctive DPR, which is recognized for reversal of the postresuscitation end-organ microvascular dysfunction and for markedly increasing end-organ blood flow.44,45 Persistent IVV depletion in the present studies can be explained by transcapillary escape of crystalloid resuscitation solution secondary to changes in the transcapillary Starling forces,19,20 as evident by expansion of the IFV at the expense of the IVV. In addition, the idea of fluid and Na+ uptake by the vascular endothelium during hemorrhagic hypovolemia might be a contributing factor for persistent IVV depletion.
Theoretically, resuscitation with a small volume of hypertonic saline or colloid-supplemented hypertonic saline should restore the local IVV by exerting an osmotic effect on the vascular endothelium.13 A sustained restoration of the IVV by such a mechanism is unlikely to occur because of rapid dissipation of the osmotic load. This lends support for the finding that hypertonic saline resuscitation only transiently restores central hemodynamics, and with the theoretic dissipation of the osmotic load, hemodynamics returns readily to hemorrhage levels.46 In this study, redistribution of the crystalloid resuscitation fluid was manifested by a substantial expansion of the interstitial space (IFV) of all tissues studied, with the exception of the lung, where a marked increase in total lung water, mainly from expansion of the ICV, was observed. This pattern of lung water redistribution is related to the unique mechanisms that control lung fluid balance.47-49 In particular, compliance of the lung interstitium is at least 20-fold lower compared with other tissues47; implying that, in the early phase of interstitial edema, the associated increase in interstitial hydrostatic pressure is not necessarily accompanied by expansion of the IFV, as in other tissues with high compliance. This low interstitial lung compliance is attributed to the structure of the interstitial ground matrix, and represents an important “tissue safety factor” to counteract a rapid progression of pulmonary edema. With crystalloid resuscitation, the hydraulic permeability of the vascular endothelium increases, causing the rapid transcapillary escape of the crystalloid solution leading to edema as a result, in part, of loss of tissue safety factor, which corresponds to an increase in lung interstitial compliance.48
Interpretation of our abdominal muscle data should account for the change in forces that control fluid homeostasis in this organ under the conditions of the present studies. Unlike the lung, compliance of the interstitium of the abdominal muscle is high, implying that a change in interstitial hydrostatic pressure is accompanied by a change in the IFV. The pressure to volume relationship is typically nonlinear.23 In the present studies, opening the abdominal cavity for ligation of the renal pedicles changed the interstitial hydrostatic pressure by a few cmH2O from slightly subatmospheric pressure.23 This accounted for the modest expansion of the IFV during HS, and together with the fast transcapillary escape rate of the crystalloid resuscitation solution, caused the additional incremental expansion of the IFV. This reasoning also applies to both the liver and gut, where the obligatory fluid sequestration from the fast transcapillary escape rate of crystalloid resuscitation solution expanded the IFV. Adjunctive DPR caused a near-normal pattern of tissue water distribution in all organs studies 2 hours postresuscitation.
Clinical correlation and summary
CR from trauma and hypovolemic shock that targets restoration and maintenance of central hemodynamics is associated with life-threatening complications, such as adult respiratory distress syndrome and abdominal compartment syndrome. A common theme in the pathogenesis of these syndromes is tissue fluid sequestration and edema formation, which compromise tissue perfusion.50,51 Therapeutic-assisted mobilization of edema with loop diuretics, as reviewed elsewhere, has limited effects on fluid sequestration and no effect on tissue perfusion, and it increases incidence of renal failure, presumably by decreasing IVV.31 In contrast, adjunctive DPR improves splanchnic end-organ perfusion, downregulates the gut-derived systemic inflammatory response, prevents fluid sequestration, and promotes early fluid mobilization to improve survival and resuscitation outcomes.21 Based on these considerations, adequacy and end points of resuscitation should be redefined on the basis of the ability of the resuscitation regimen to restore and maintain end-organ perfusion and not be based on the clinical target of restoring central hemodynamics.
The present studies are the first to demonstrate a persistent depletion of the local IVV, despite attempted correction of the volume deficit as measured by restoration and maintenance of hemodynamics. The importance of this continued IVV depletion to the overall pathophysiology of shock and resuscitation cannot be discerned from the present data. Because of the high transcapillary escape of the crystalloid resuscitation solution, increasing the resuscitation volume probably would not restore local tissue IVV. Instead, colloid supplementation of a relatively low volume crystalloid resuscitation solution to enhance plasma oncotic pressure would theoretically be a more viable strategy to restore local IVV and maintain an operational tissue safety factor in the lung. Colloid-supplemented resuscitation is a controversial issue as its use has been associated with higher incidence of renal failure, prolonged number of days on a ventilator, impaired oxygenation and increased mortality.31,52,53 We think that these events would not necessarily occur when colloid-supplemented resuscitation is combined with adjunctive DPR, as DPR restores and maintains splanchnic and distant organ perfusion and normalizes tissue water distribution. The primary effect of colloid would be an improvement in local IVV.
Other than the clinical observation that edema and fluid overload in critically ill trauma patients is associated with poor outcomes, the role of this fluid overload in the pathogenesis of such an outcome is not well-studied.This present study suggests a pivotal role for the hemorrhage and resuscitation-mediated changes in both composition and volume of the fluid compartments making up TTW in the pathogenesis of end-organ damage. Our previous studies indicated that endothelial cell swelling that results from the hemorrhage-stimulated Na+/H+ exchanger injures the endothelial cell and results in end-organ tissue hypoperfusion.32 Similarly, the change in the composition of the IFV that results from extravasation of the resuscitation solution, contributes to both microvascular dysfunction and an exaggerated systemic inflammatory response observed with crystalloid resuscitation from HS.
It is concluded from the present studies that HS decreases IVV; HS-induced water-shifts are organ-specific and prominent in gut, lung, and muscle; CR restores central hemodynamics, but does not restore IVV, and CR alters organ-specific TTW distribution; adjunctive DPR with IP dialysis fluid is a technique that normalizes TTW and water compartment distribution and prevents edema in vital organs; use of DPR as an adjunctive technique to intravascular fluid replacement with blood and crystalloid resuscitation from HS prevents edema and has been shown to improve organ function. It remains to be determined whether resuscitation methods that would restore IVV to baseline values results in additional favorable outcomes.
Acknowledgments
Supported in part by VA Merit Review funding and in part by National Institutes of Health research grant no. R01 HL076160–03, funded by the National Heart, Lung, and Blood Institute and the United States Army Medical Resources and Material Command.
Abbreviations and Acronyms
- CR
conventional resuscitation
- DPR
direct peritoneal resuscitation
- ECV
extracellular volume
- HS
hemorrhagic shock
- ICV
intracellular water volume
- IP
intraperitoneal
- IFV
interstitial fluid volume
- IVV
intravascular volume
- QAR
quantitative autoradiography
- TTW
total tissue water
Footnotes
Competing Interests Declared: None.
Presented at the Southern Surgical Association 119th Annual Meeting, Hot Springs, VA, December 2007.
Author Contributions
Study conception and design: Zakaria, Matheson, Garrison
Acquisition of data: Zakaria, Matheson, Flessner
Analysis and interpretation of data: Zakaria, Matheson, Garrison
Drafting of manuscript: Zakaria, Matheson, Garrison
Critical revision: Zakaria, Matheson, Garrison
References
- 1.Shires GT. Shock and metabolism. Surg Gynecol Obstet. 1967;124:284–287. [PubMed] [Google Scholar]
- 2.Barash PG, Cullen BF, Stoelting RK. Handbook of clinical anesthesia. Philadelphia: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 3.Kaye AD, Grogono AW. Fluid and electrolyte physiology. Philadelphia: Churchill Livingstone; 2000. [Google Scholar]
- 4.Brand ED, Suh TK, Avery MC. Reversal of postoligemic shock in the cat by hypervenobaric massive fluid therapy. Am J Physiol. 1966;211:1232–1240. doi: 10.1152/ajplegacy.1966.211.5.1232. [DOI] [PubMed] [Google Scholar]
- 5.Wolfman EF, Jr, Neill SA, Heaps DK, et al. Donor blood and isotonic salt solution. Effect on survival after hemorrhagic shock and operation. Arch Surg. 1963;86:869–873. doi: 10.1001/archsurg.1963.01310110179023. [DOI] [PubMed] [Google Scholar]
- 6.Mills M. Pulmonary effects of nonthoracic trauma. The clinical syndrome. J Trauma. 1968;8:651–655. [PubMed] [Google Scholar]
- 7.Simmons RL, Heisterkamp CA, III, Collins JA, et al. Acute pulmonary edema in battle casualties. J Trauma. 1969;9:760–775. doi: 10.1097/00005373-196909000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Simmons RL, Heisterkamp CA, III, Moseley RV, et al. Postresuscitative blood volumes in combat casualties. Surg Gynecol Obstet. 1969;128:1193–1201. [PubMed] [Google Scholar]
- 9.Flint L. Invited commentary: hemorrhage and surgery cause a contraction of the extra cellular space needing replacement—evidence and implications. Surgery. 2006;139:432–434. doi: 10.1016/j.surg.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Brandstrup B, Svensen C, Engquist A. Hemorrhage and operation cause a contraction of the extracellular space needing replacement—evidence and implications? A systematic review. Surgery. 2006;139:419–432. doi: 10.1016/j.surg.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Mazzoni MC, Intaglietta M, Cragoe EJ, Jr, et al. Amiloride-sensitive Na+ pathways in capillary endothelial cell swelling during hemorrhagic shock. J Appl Physiol. 1992;73:1467–1473. doi: 10.1152/jappl.1992.73.4.1467. [DOI] [PubMed] [Google Scholar]
- 12.Mazzoni MC, Borgstrom P, Intaglietta M, et al. Lumenal narrowing and endothelial cell swelling in skeletal muscle capillaries during hemorrhagic shock. Circ Shock. 1989;29:27–39. [PubMed] [Google Scholar]
- 13.Mazzoni MC, Borgstrom P, Intaglietta M, et al. Capillary narrowing in hemorrhagic shock is rectified by hyperosmotic saline-dextran reinfusion. Circ Shock. 1990;31:407–418. [PubMed] [Google Scholar]
- 14.Carrico CJ, Canizaro PC, Shires GT. Fluid resuscitation following injury: rationale for the use of balanced salt solutions. Crit Care Med. 1976;4:46–54. [PubMed] [Google Scholar]
- 15.Shires GT, Cunningham JN, Backer CR, et al. Alterations in cellular membrane function during hemorrhagic shock in primates. Ann Surg. 1972;176:288–295. doi: 10.1097/00000658-197209000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shires GT. Pathophysiology and fluid replacement in hypovolemic shock. Ann Clin Res. 1977;9:144–150. [PubMed] [Google Scholar]
- 17.Shires GT, Carrico CJ, Coln D. The role of the extracellular fluid in shock. Int Anesthesiol Clin. 1964;97:435–454. doi: 10.1097/00004311-196402000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Shires GT, Canizaro P. Fluid and electrolyte therapy. Surg Annu. 1971;3:63–95. [PubMed] [Google Scholar]
- 19.Zweifach BW. Mechanisms of blood flow and fluid exchange in microvessels: hemorrhagic hypotension model. Anesthesiology. 1974;41:157–168. doi: 10.1097/00000542-197408000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lucas CE, Benishek DJ, Ledgerwood AM. Reduced oncotic pressure after shock: a proposed mechanism. Arch Surg. 1982;117:675–679. doi: 10.1001/archsurg.1982.01380290121021. [DOI] [PubMed] [Google Scholar]
- 21.Garrison RN, Conn AA, Harris PD, et al. Direct peritoneal resuscitation as adjunct to conventional resuscitation from hemorrhagic shock: a better outcome. Surgery. 2004;136:900–908. doi: 10.1016/j.surg.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Zakaria ER, Lofthouse J, Flessner MF. Effect of intraperitoneal pressures on tissue water of the abdominal muscle. Am J Physiol Renal Physiol. 2000;278:875–885. doi: 10.1152/ajprenal.2000.278.6.F875. [DOI] [PubMed] [Google Scholar]
- 23.Zakaria ER, Lofthouse J, Flessner MF. In vivo effects of hydrostatic pressure on interstitium of abdominal wall muscle. Am J Physiol. 1999;276:517–529. doi: 10.1152/ajpheart.1999.276.2.H517. [DOI] [PubMed] [Google Scholar]
- 24.Zakaria ER, Lofthouse J, Flessner MF. Hydrostatic and osmotic pressures modulate partitioning of tissue water in abdominal muscle during dialysis. Perit Dial Int. 1999;19:208–211. [PubMed] [Google Scholar]
- 25.Larsson M, Johnson L, Nylander G, et al. Plasma water and 51Cr EDTA equilibration volumes of different tissues in the rat. Acta Physiol Scand. 1980;110:53–57. doi: 10.1111/j.1748-1716.1980.tb06629.x. [DOI] [PubMed] [Google Scholar]
- 26.Reed RK, Wiig H. Compliance of the interstitial space in rats. I. Studies on hindlimb skeletal muscle. Acta Physiol Scand. 1981;113:297–305. doi: 10.1111/j.1748-1716.1981.tb06900.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson LK, Nylander G, Ohman U. Kinetics of 51CrEDTA in the rat. Acta Chir Scand. 1980;146:477–481. [PubMed] [Google Scholar]
- 28.Flessner MF, Dedrick RL, Schultz JS. A distributed model of peritoneal-plasma transport: analysis of experimental data in the rat. Am J Physiol. 1985;248:413–424. doi: 10.1152/ajprenal.1985.248.3.F413. [DOI] [PubMed] [Google Scholar]
- 29.Flessner MF, Fenstermacher JD, Dedrick RL, et al. A distributed model of peritoneal-plasma transport: tissue concentration gradients. Am J Physiol. 1985;248:425–435. doi: 10.1152/ajprenal.1985.248.3.F425. [DOI] [PubMed] [Google Scholar]
- 30.Larsson M, Nylander G, Ohman U. Posthemorrhagic changes in plasma water and extracellular fluid volumes in the rat. J Trauma. 1981;21:870–877. doi: 10.1097/00005373-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Lucas CE. The water of life: a century of confusion. J Am Coll Surg. 2001;192:86–93. doi: 10.1016/s1072-7515(00)00761-4. [DOI] [PubMed] [Google Scholar]
- 32.Zakaria ER, Li N, Matheson PJ, Garrison RN. Cellular edema regulates tissue capillary perfusion following hemorrhage resuscitation. Surg. 2007;142:487–496. doi: 10.1016/j.surg.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon PF, Hollyfield-Gilbert MA, Myers TL, et al. Effects of isotonic crystalloid resuscitation on fluid compartments in hemorrhaged rats. Shock. 1994;2:355–361. doi: 10.1097/00024382-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Moon PF, Hollyfield-Gilbert MA, Myers TL, et al. Fluid compartments in hemorrhaged rats after hyperosmotic crystalloid and hyperoncotic colloid resuscitation. Am J Physiol. 1996;270:F1–F8. doi: 10.1152/ajprenal.1996.270.1.F1. [DOI] [PubMed] [Google Scholar]
- 35.Ware J, Norberg KA, Norman M, et al. 51Cr EDTA determinations of the extracellular fluid volume in hemorrhage: a study with fed and starved rats. Acta Physiol Scand. 1982;116:235–238. doi: 10.1111/j.1748-1716.1982.tb07136.x. [DOI] [PubMed] [Google Scholar]
- 36.Reed RK, Wiig H. Compliance of the interstitial space in rats. III. Contribution of skin and skeletal muscle interstitial fluid volume to changes in total extracellular fluid volume. Acta Physiol Scand. 1984;121:57–63. doi: 10.1111/j.1748-1716.1984.tb10457.x. [DOI] [PubMed] [Google Scholar]
- 37.Reed RK, Bowen BD, Bert JL. Microvascular exchange and interstitial volume regulation in the rat: implications of the model. Am J Physiol. 1989;257:H2081–H2091. doi: 10.1152/ajpheart.1989.257.6.H2081. [DOI] [PubMed] [Google Scholar]
- 38.Wiig H, Reed RK. Interstitial compliance and transcapillary Starling pressures in cat skin and skeletal muscle. Am J Physiol. 1985;248:H666–H673. doi: 10.1152/ajpheart.1985.248.5.H666. [DOI] [PubMed] [Google Scholar]
- 39.Wiig H, Lund T. Relationship between interstitial fluid volume and pressure (compliance) in hypothyroid rats. Am J Physiol Heart Circ Physiol. 2001;281:H1085–H1092. doi: 10.1152/ajpheart.2001.281.3.H1085. [DOI] [PubMed] [Google Scholar]
- 40.Wiig H, Rubin K, Reed RK. New and active role of the interstitium in control of interstitial fluid pressure: potential therapeutic consequences. Acta Anaesthesiol Scand. 2003;47:111–121. doi: 10.1034/j.1399-6576.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 41.Larsson M, Ware J. Effects of isotonic fluid load on plasma water and extracellular fluid volumes in the rat. Eur Surg Res. 1983;15:262–267. doi: 10.1159/000128366. [DOI] [PubMed] [Google Scholar]
- 42.Fruchterman TM, Spain DA, Wilson MA, et al. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417–422. doi: 10.1097/00024382-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Zakaria ER, Spain DA, Harris PD, et al. Resuscitation regimens for hemorrhagic shock must contain blood. Shock. 2002;18:567–573. doi: 10.1097/00024382-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Zakaria ER, Garrison RN, Spain DA, et al. Intraperitoneal resuscitation improves intestinal blood flow following hemorrhagic shock. Ann Surg. 2003;237:704–711. doi: 10.1097/01.SLA.0000064660.10461.9D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zakaria ER, Hurt RT, Matheson PJ, et al. A novel method of peritoneal resuscitation improves organ perfusion after hemorrhagic shock. Am J Surg. 2003;186:443–448. doi: 10.1016/j.amjsurg.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Zakaria ER, Tsakadze NL, Garrison RN. Hypertonic saline resuscitation improves intestinal microcirculation in a rat model of hemorrhagic shock. Surgery. 2006;140:579–587. doi: 10.1016/j.surg.2006.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miserocchi G, Negrini D, Del FM, et al. Pulmonary interstitial pressure in intact in situ lung: transition to interstitial edema. J Appl Physiol. 1993;74:1171–1177. doi: 10.1152/jappl.1993.74.3.1171. [DOI] [PubMed] [Google Scholar]
- 48.Miserocchi G, Negrini D, Passi A, et al. Development of lung edema: interstitial fluid dynamics and molecular structure. News Physiol Sci. 2001;16:66–71. doi: 10.1152/physiologyonline.2001.16.2.66. [DOI] [PubMed] [Google Scholar]
- 49.Negrini D, Passi A. Interstitial matrix and transendothelial fluxes in normal lung. Respir Physiol Neurobiol. 2007;159:301–310. doi: 10.1016/j.resp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Balogh Z, McKinley BA, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848–859. doi: 10.1097/01.TA.0000070166.29649.F3. [DOI] [PubMed] [Google Scholar]
- 51.Czer LS, Appel P, Shoemaker WC. Pathogenesis of respiratory failure (ARDS) after hemorrhage and trauma: II. Cardiorespiratory patterns after development of ARDS. Crit Care Med. 1980;8:513–518. doi: 10.1097/00003246-198009000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Lucas CE, Ledgerwood AM, Higgins RF, et al. Impaired pulmonary function after albumin resuscitation from shock. J Trauma. 1980;20:446–451. doi: 10.1097/00005373-198006000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Lucas CE, Ledgerwood AM. Physiology of colloid-supplemented resuscitation from shock. J Trauma. 2003;54(suppl):S75–S81. doi: 10.1097/01.TA.0000064509.31860.7C. [DOI] [PubMed] [Google Scholar]