Abstract
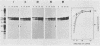
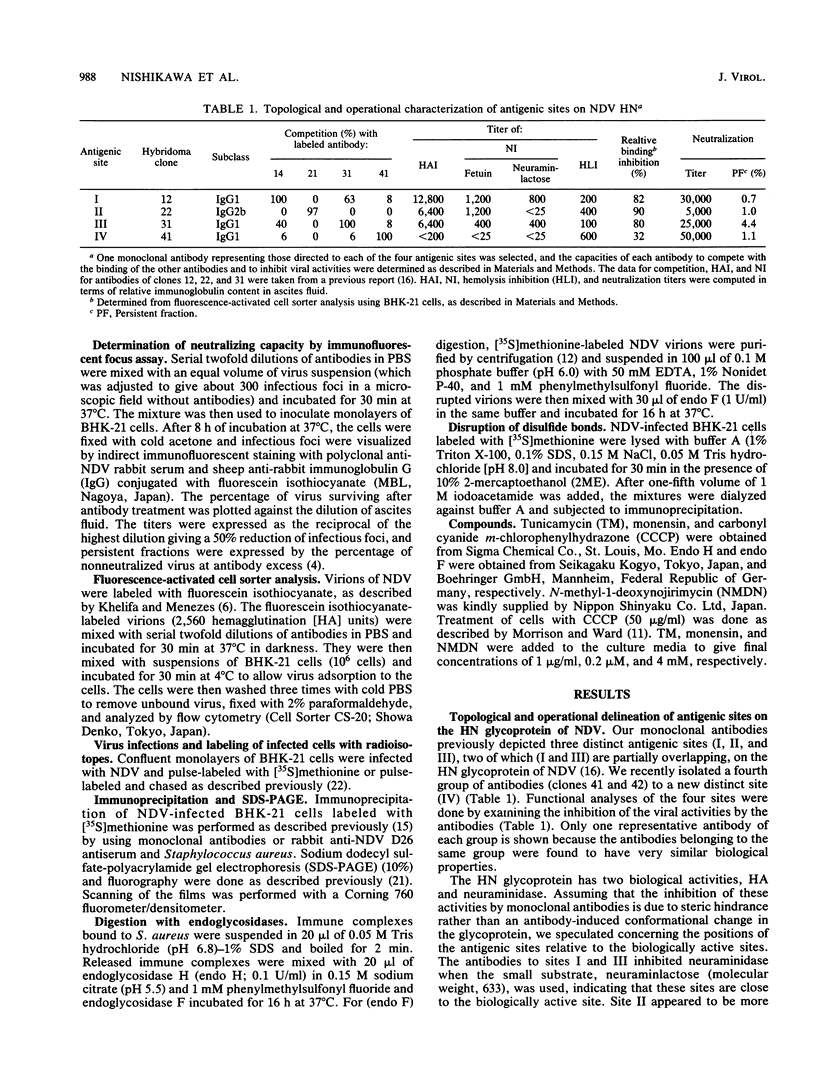
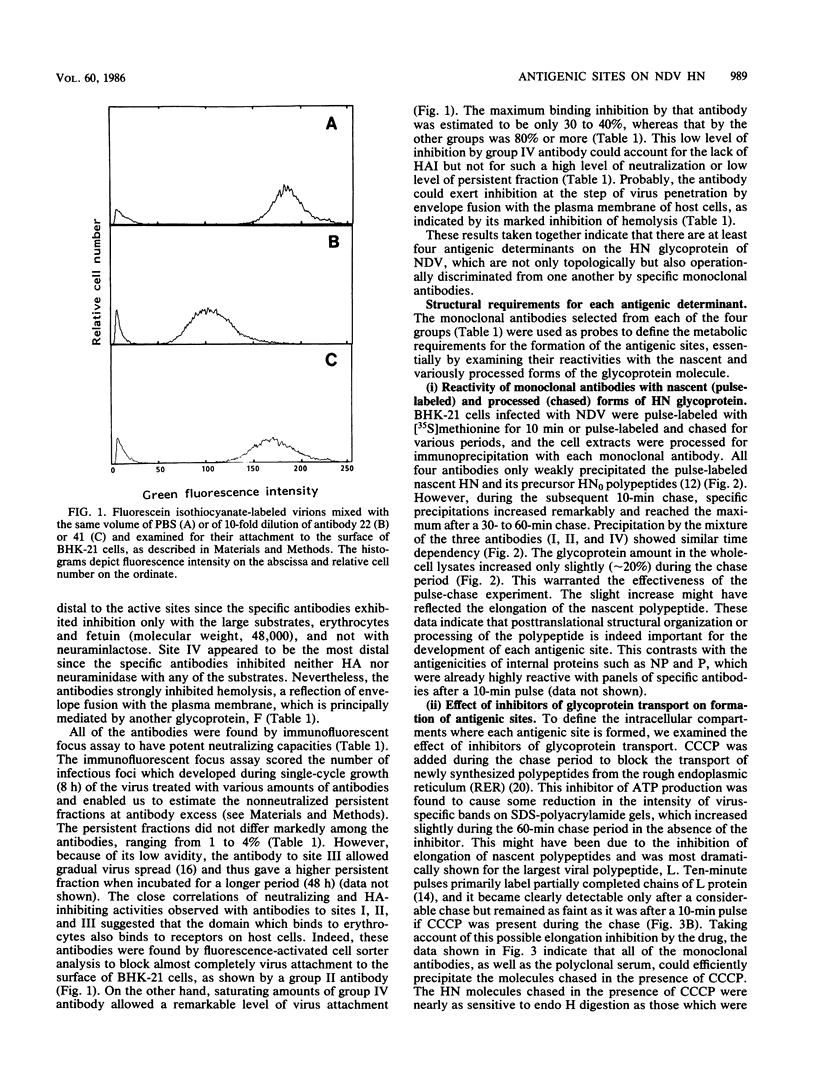
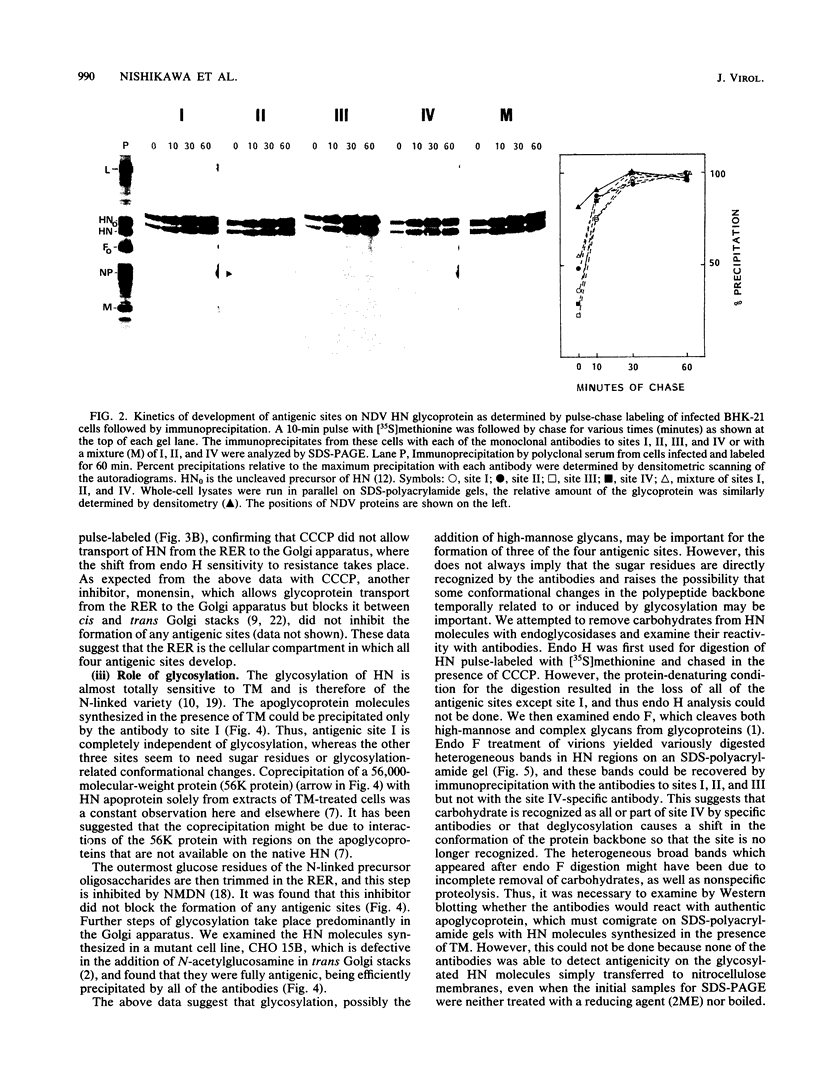
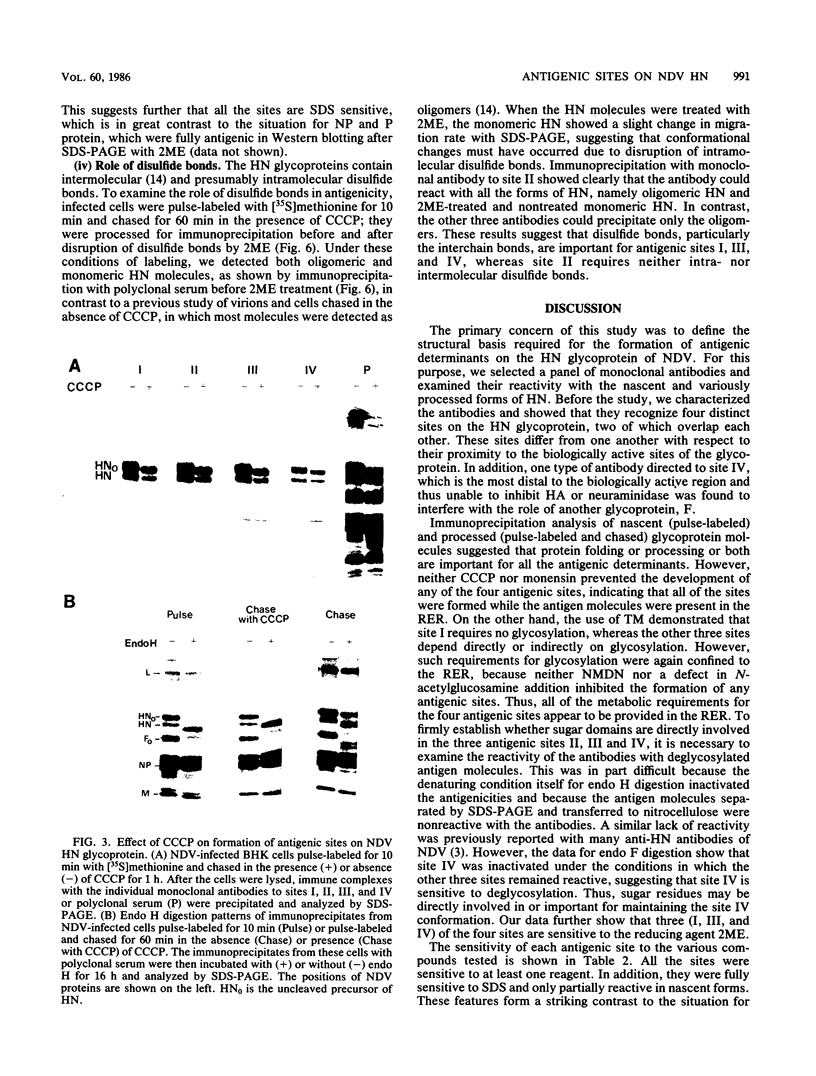
Monoclonal antibodies to the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus have identified four antigenic sites on the glycoprotein, which are topologically and operationally discriminated from one another. To define the metabolisms and cellular compartments required for formation of the individual antigenic sites, a panel of monoclonal antibodies were examined for their reactivity with the nascent and variously processed forms of the antigen molecules in combination with the use of inhibitors of glycosylation (tunicamycin and N-methyl-1-deoxynojirimycin) and glycoprotein transport (carbonyl cyanide m-chlorophenylhydrazone and monensin). Reactivity was also examined with the antigen molecules deglycosylated by endoglycosidase F and with the antigen molecules reduced by 2-mercaptoethanol. The results taken together suggest that posttranslational organization of the glycoprotein is important for all four of the antigenic sites. At the same time, there appeared to be marked site-specific requirements with respect to glycosylation and disulfide bond formation. However, all of these metabolic requirements were found to be provided within the rough endoplasmic reticulum, and no further processing of the antigen molecules appeared to be necessary for the formation of any of the antigenic sites.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elder J. H., McGee J. S., Alexander S. Carbohydrate side chains of Rauscher leukemia virus envelope glycoproteins are not required to elicit a neutralizing antibody response. J Virol. 1986 Jan;57(1):340–342. doi: 10.1128/jvi.57.1.340-342.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger J., Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975 May 10;250(9):3303–3309. [PubMed] [Google Scholar]
- Iorio R. M., Bratt M. A. Monoclonal antibodies as functional probes of the HN glycoprotein of Newcastle disease virus: antigenic separation of the hemagglutinating and neuraminidase sites. J Immunol. 1984 Oct;133(4):2215–2219. [PubMed] [Google Scholar]
- Iorio R. M., Bratt M. A. Monoclonal antibodies to newcastle disease virus: delineation of four epitopes on the HN glycoprotein. J Virol. 1983 Nov;48(2):440–450. doi: 10.1128/jvi.48.2.440-450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio R. M., Bratt M. A. Neutralization of Newcastle disease virus by monoclonal antibodies to the hemagglutinin-neuraminidase glycoprotein: requirement for antibodies to four sites for complete neutralization. J Virol. 1984 Aug;51(2):445–451. doi: 10.1128/jvi.51.2.445-451.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelifa R., Menezes J. Use of fluoresceinated Epstein-Barr virus to study Epstein-Barr virus-lymphoid cell interactions. J Virol. 1982 Feb;41(2):649–656. doi: 10.1128/jvi.41.2.649-656.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L., Portetelle D., Ghysdael J., Gonze M., Burny A., Meulemans G. Monoclonal antibodies to hemagglutinin-neuraminidase and fusion glycoproteins of Newcastle disease virus: relationship between glycosylation and reactivity. J Virol. 1986 Mar;57(3):1198–1202. doi: 10.1128/jvi.57.3.1198-1202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura N., Uchida T., Okada Y. HVJ (Sendai virus)-induced envelope fusion and cell fusion are blocked by monoclonal anti-HN protein antibody that does not inhibit hemagglutination activity of HVJ. Exp Cell Res. 1982 Oct;141(2):409–420. doi: 10.1016/0014-4827(82)90229-4. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Simpson D. Synthesis, stability, and cleavage of Newcastle disease virus glycoproteins in the absence of glycosylation. J Virol. 1980 Oct;36(1):171–180. doi: 10.1128/jvi.36.1.171-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Ward L. J. Intracellular processing of the vesicular stomatitis virus glycoprotein and the Newcastle disease virus hemagglutinin-neuraminidase glycoprotein. Virus Res. 1984;1(3):225–239. doi: 10.1016/0168-1702(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Morrison T., Ward L. J., Semerjian A. Intracellular processing of the Newcastle disease virus fusion glycoprotein. J Virol. 1985 Mar;53(3):851–857. doi: 10.1128/jvi.53.3.851-857.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Maeno K., Iinuma M., Yoshida T., Matsumoto T. Inhibition of virus growth by ouabain: effect of ouabain on the growth of HVJ in chick embryo cells. J Virol. 1972 Feb;9(2):234–243. doi: 10.1128/jvi.9.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Ogura H., Klenk H. Studies on the assembly of the envelope of Newcastle disease virus. Virology. 1976 Feb;69(2):523–538. doi: 10.1016/0042-6822(76)90482-7. [DOI] [PubMed] [Google Scholar]
- Naruse H., Nagai Y., Yoshida T., Hamaguchi M., Matsumoto T., Isomura S., Suzuki S. The polypeptides of mumps virus and their synthesis in infected chick embryo cells. Virology. 1981 Jul 15;112(1):119–130. doi: 10.1016/0042-6822(81)90618-8. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Isomura S., Suzuki S., Watanabe E., Hamaguchi M., Yoshida T., Nagai Y. Monoclonal antibodies to the HN glycoprotein of Newcastle disease virus. Biological characterization and use for strain comparisons. Virology. 1983 Oct 30;130(2):318–330. doi: 10.1016/0042-6822(83)90086-7. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Hiebert S. W., Lamb R. A. Expression at the cell surface of biologically active fusion and hemagglutinin/neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P. A., Datema R., Schwarz R. T. N-methyl-1-deoxynojirimycin, a novel inhibitor of glycoprotein processing, and its effect on fowl plague virus maturation. Virology. 1983 Oct 15;130(1):238–242. doi: 10.1016/0042-6822(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Schwalbe J. C., Hightower L. E. Maturation of the envelope glycoproteins of Newcastle disease virus on cellular membranes. J Virol. 1982 Mar;41(3):947–957. doi: 10.1128/jvi.41.3.947-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Hamaguchi M., Naruse H., Nagai Y. Persistent infection by a temperature-sensitive mutant isolated from a Sendai virus (HVJ) carrier culture: its initiation and maintenance without aid of defective interfering particles. Virology. 1982 Jul 30;120(2):329–339. doi: 10.1016/0042-6822(82)90034-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nakayama Y., Nagura H., Toyoda T., Nishikawa K., Hamaguchi M., Nagai Y. Inhibition of the assembly of Newcastle disease virus by monensin. Virus Res. 1986 Feb;4(2):179–195. doi: 10.1016/0168-1702(86)90040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]