Abstract
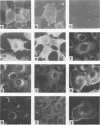
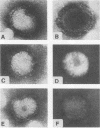
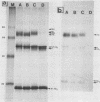
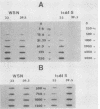
We investigated the intracellular block in the transport of hemagglutinin (HA) and the role of HA in virus particle formation by using temperature-sensitive (ts) mutants (ts134 and ts61S) of influenza virus A/WSN/33. We found that at the nonpermissive temperature (39.5 degrees C), the exit of ts HA from the rough endoplasmic reticulum to the Golgi complex was blocked and that no additional block was apparent in either the exit from the Golgi complex or post-Golgi complex transport. When MDBK cells were infected with these mutant viruses, they produced noninfectious virus particles at 39.5 degrees C. The efficiency of particle formation at 39.5 degrees C was essentially the same for both wild-type (wt) and ts virus-infected cells. When compared with the wt virus produced at either 33 or 39.5 degrees C or the ts virus formed at 33 degrees C, these noninfectious virus particles were lighter in density and lacked spikes on the envelope. However, they contained the full complement of genomic RNA as well as all of the structural polypeptides of influenza virus with the exception of HA. In these spikeless particles, HA could not be detected at the limit of 0.2% of the HA present in wt virions. In contrast, neuraminidase appeared to be present in a twofold excess over the amount present in ts virus formed at 33 degrees C. These observations suggest that the presence of HA is not an obligatory requirement for the assembly and budding of influenza virus particles from infected cells. The implications of these results and the possible role of other viral proteins in influenza virus morphogenesis are discussed.
Full text
PDF


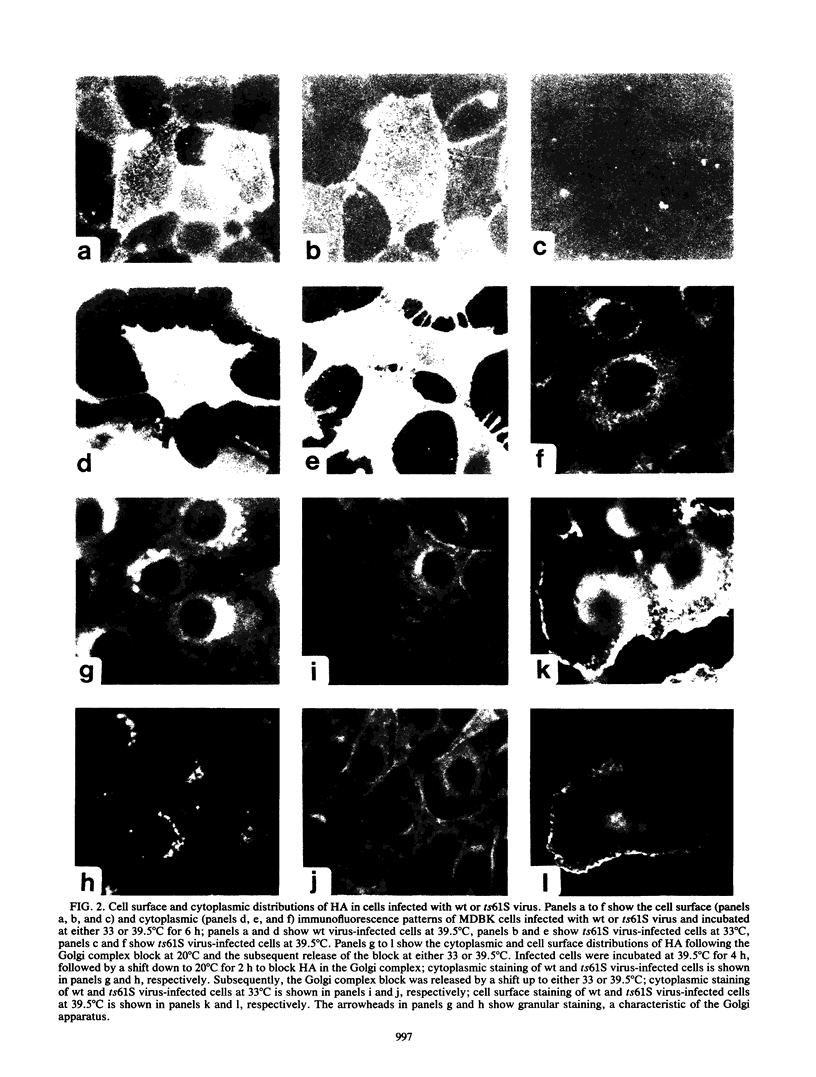
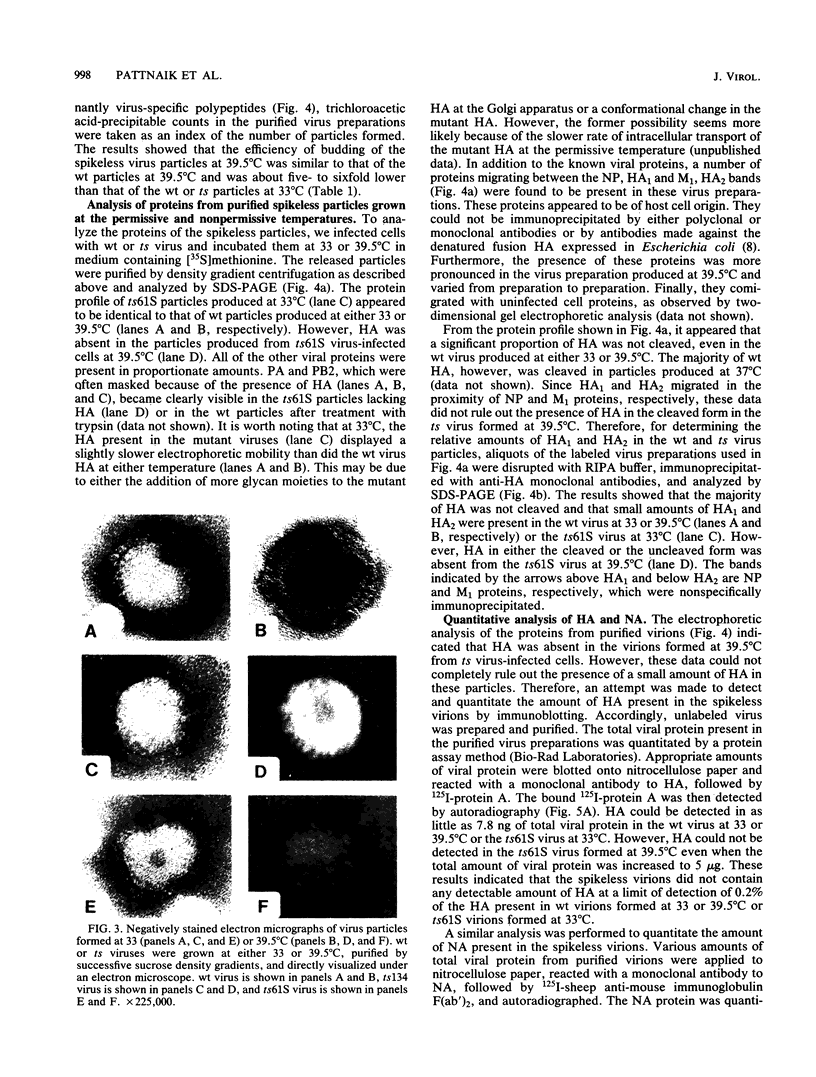



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond J. W., Felsenreich V. Phosphorylation of the nucleoprotein of an avian influenza virus. J Gen Virol. 1982 Jun;60(Pt 2):295–305. doi: 10.1099/0022-1317-60-2-295. [DOI] [PubMed] [Google Scholar]
- Bos T. J., Nayak D. P. Identification of defects in the neuraminidase gene of four temperature-sensitive mutants of A/WSN/33 influenza virus. Virology. 1986 Oct 15;154(1):85–96. doi: 10.1016/0042-6822(86)90432-0. [DOI] [PubMed] [Google Scholar]
- Bächi T., Gerhard W., Yewdell J. W. Monoclonal antibodies detect different forms of influenza virus hemagglutinin during viral penetration and biosynthesis. J Virol. 1985 Aug;55(2):307–313. doi: 10.1128/jvi.55.2.307-313.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chanda P. K., Chambers T. M., Nayak D. P. In vitro transcription of defective interfering particles of influenza virus produces polyadenylic acid-containing complementary RNAs. J Virol. 1983 Jan;45(1):55–61. doi: 10.1128/jvi.45.1.55-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Dimmock N. J. An electron microscopic study of single-cycle infection of chick embryo fibroblasts by influenza virus. Virology. 1969 Nov;39(3):499–515. doi: 10.1016/0042-6822(69)90098-1. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Bos T., Ueda M., Nayak D. P., Dowbenko D., Compans R. W. Immune response to human influenza virus hemagglutinin expressed in Escherichia coli. Gene. 1983 Mar;21(3):273–284. doi: 10.1016/0378-1119(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Gerhard W., Yewdell J., Frankel M. E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981 Apr 23;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Hewitt J. A. On the influence of polyvalent ligands on membrane curvature. J Theor Biol. 1977 Feb 7;64(3):455–472. doi: 10.1016/0022-5193(77)90282-x. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Davis A. R., Nayak D. P., De B. K. Diversity and generation of defective interfering influenza virus particles. Virology. 1979 May;95(1):48–58. doi: 10.1016/0042-6822(79)90400-8. [DOI] [PubMed] [Google Scholar]
- Jones L. V., Compans R. W., Davis A. R., Bos T. J., Nayak D. P. Surface expression of influenza virus neuraminidase, an amino-terminally anchored viral membrane glycoprotein, in polarized epithelial cells. Mol Cell Biol. 1985 Sep;5(9):2181–2189. doi: 10.1128/mcb.5.9.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. Cotranslational and posttranslational processing of viral glycoproteins. Curr Top Microbiol Immunol. 1980;90:19–48. doi: 10.1007/978-3-642-67717-5_2. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lohmeyer J., Talens L. T., Klenk H. D. Biosynthesis of the influenza virus envelope in abortive infection. J Gen Virol. 1979 Jan;42(1):73–88. doi: 10.1099/0022-1317-42-1-73. [DOI] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY J. S., BANG F. B. Observations with the electron microscope on cells of the chick chorio-allantoic membrane infected with influenza virus. J Exp Med. 1952 Mar;95(3):259–268. doi: 10.1084/jem.95.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983 Aug;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Murti K. G., Webster R. G. Distribution of hemagglutinin and neuraminidase on influenza virions as revealed by immunoelectron microscopy. Virology. 1986 Feb;149(1):36–43. doi: 10.1016/0042-6822(86)90084-x. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Tobita K., Janda J. M., Davis A. R., De B. K. Homologous interference mediated by defective interfering influenza virus derived from a temperature-sensitive mutant of influenza virus. J Virol. 1978 Oct;28(1):375–386. doi: 10.1128/jvi.28.1.375-386.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Marx P. S., Kingsbury D. W. A temperature-sensitive mutant of Sendai virus with an altered hemagglutinin-neuraminidase polypeptide: consequences for virus assembly and cytopathology. Virology. 1975 Sep;67(1):179–187. doi: 10.1016/0042-6822(75)90415-8. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Ivanov I. E., Plesken H., Sabatini D. D. Polarized delivery of viral glycoproteins to the apical and basolateral plasma membranes of Madin-Darby canine kidney cells infected with temperature-sensitive viruses. J Cell Biol. 1985 Jan;100(1):136–151. doi: 10.1083/jcb.100.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Paskiet K. T., Salas P. J., Bard E. Intracellular transport of influenza virus hemagglutinin to the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1984 Jan;98(1):308–319. doi: 10.1083/jcb.98.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W., Giusti L., Davis A. R., Nayak D. P., Gething M. J., Sambrook J. Influenza virus hemagglutinin expression is polarized in cells infected with recombinant SV40 viruses carrying cloned hemagglutinin DNA. Cell. 1983 Jun;33(2):435–443. doi: 10.1016/0092-8674(83)90425-7. [DOI] [PubMed] [Google Scholar]
- Schnitzer T. J., Dickson C., Weiss R. A. Morphological and biochemical characterization of viral particles produced by the tsO45 mutant of vesicular stomatitis virus at restrictive temperature. J Virol. 1979 Jan;29(1):185–195. doi: 10.1128/jvi.29.1.185-195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Ueda M., Kilbourne E. D. Temperature-sensitive mutants of influenza virus: a mutation in the hemagglutinin gene. Virology. 1976 Apr;70(2):425–431. doi: 10.1016/0042-6822(76)90283-x. [DOI] [PubMed] [Google Scholar]
- Ueda M., Sugiura A. Physiological characterization of influenza virus temperature-sensitive mutants defective in the haemagglutinin gene. J Gen Virol. 1984 Nov;65(Pt 11):1889–1897. doi: 10.1099/0022-1317-65-11-1889. [DOI] [PubMed] [Google Scholar]
- Yeo K. T., Parent J. B., Yeo T. K., Olden K. Variability in transport rates of secretory glycoproteins through the endoplasmic reticulum and Golgi in human hepatoma cells. J Biol Chem. 1985 Jul 5;260(13):7896–7902. [PubMed] [Google Scholar]
- Zhirnov O. P., Bukrinskaya A. G. Two forms of influenza virus nucleoprotein in infected cells and virions. Virology. 1981 Feb;109(1):174–179. doi: 10.1016/0042-6822(81)90482-7. [DOI] [PubMed] [Google Scholar]