Abstract
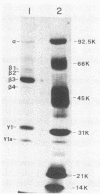
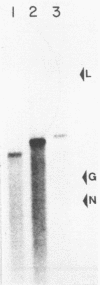
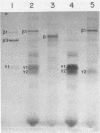
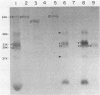
The two segments of double-stranded RNA from infectious pancreatic necrosis virus Sp were cloned into the plasmid vector pUC8. Two sets of overlapping clones were identified by restriction enzyme and Southern blot analyses. Each of these sets was shown by Northern blot analysis to be exclusively related to either segment A or B of the genomic RNA. The entire lengths of the cloned segments were estimated to be 2.9 and 2.6 kilobases, respectively. Sequences from the two segments of viral cDNA were subcloned into the bacteriophage T7 RNA polymerase vectors pT71 and pT72. The activity of the single-stranded RNAs transcribed from these subclones in a rabbit reticulocyte lysate translation system provided information on the polarity of and the protein products coded for by each subclone. The four proteins encoded by the genome of infectious pancreatic necrosis virus were identified among the translation products of the individual cloned segments by immunoprecipitation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. By constructing plasmids containing deletions in the sequences from either the 5' or 3' end of segment A, we were able to construct a physical map for the larger segment of double-stranded RNA. The proteins derived from these plasmids indicated that the linear gene order for viral proteins encoded in segment A is beta, gamma 2, and gamma 1.
Full text
PDF

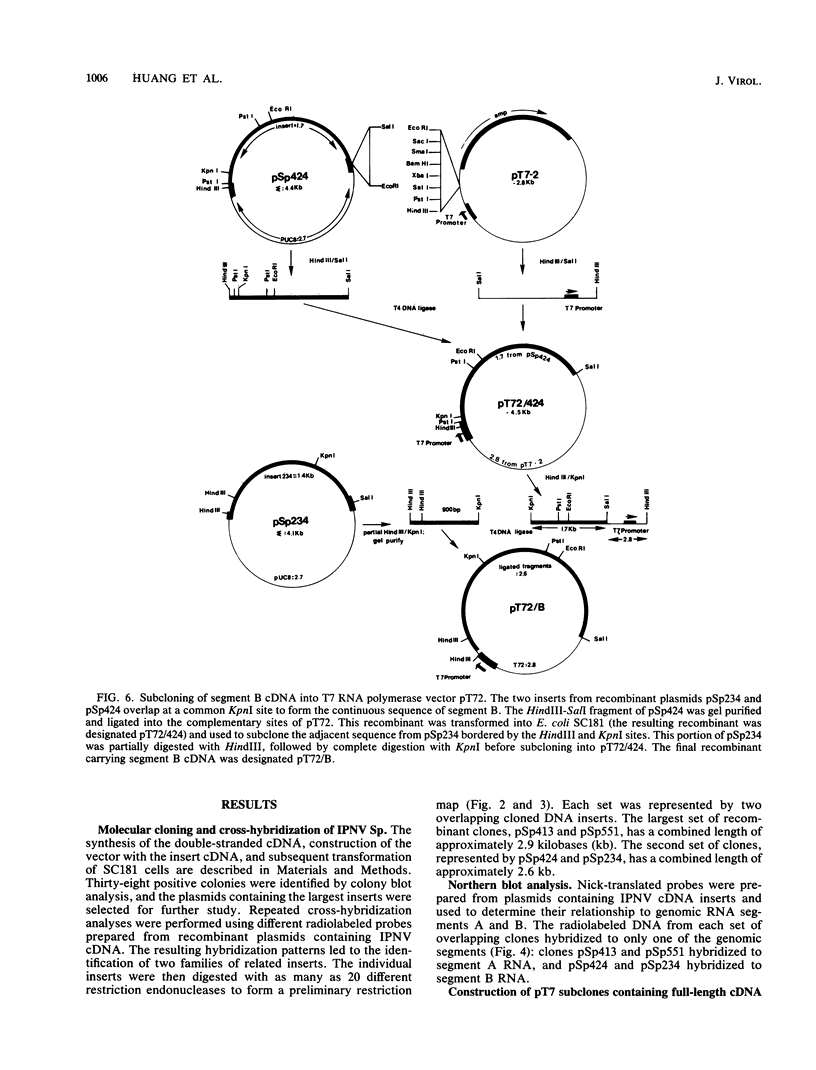
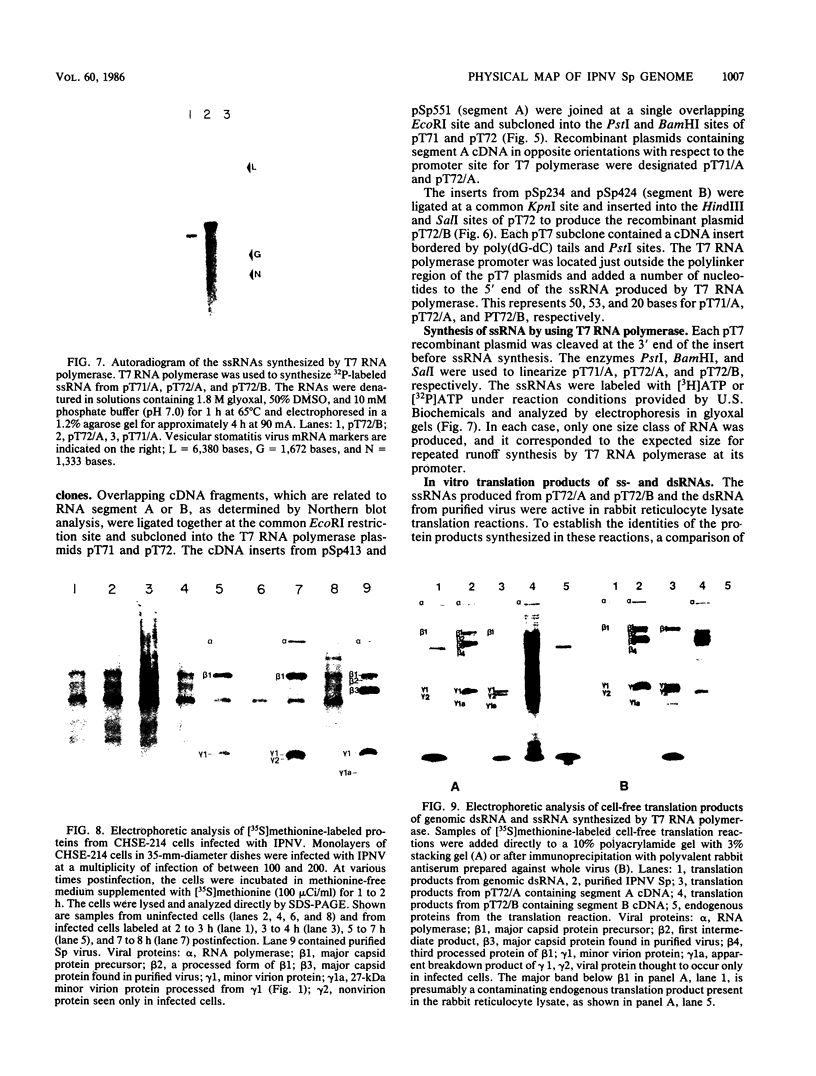
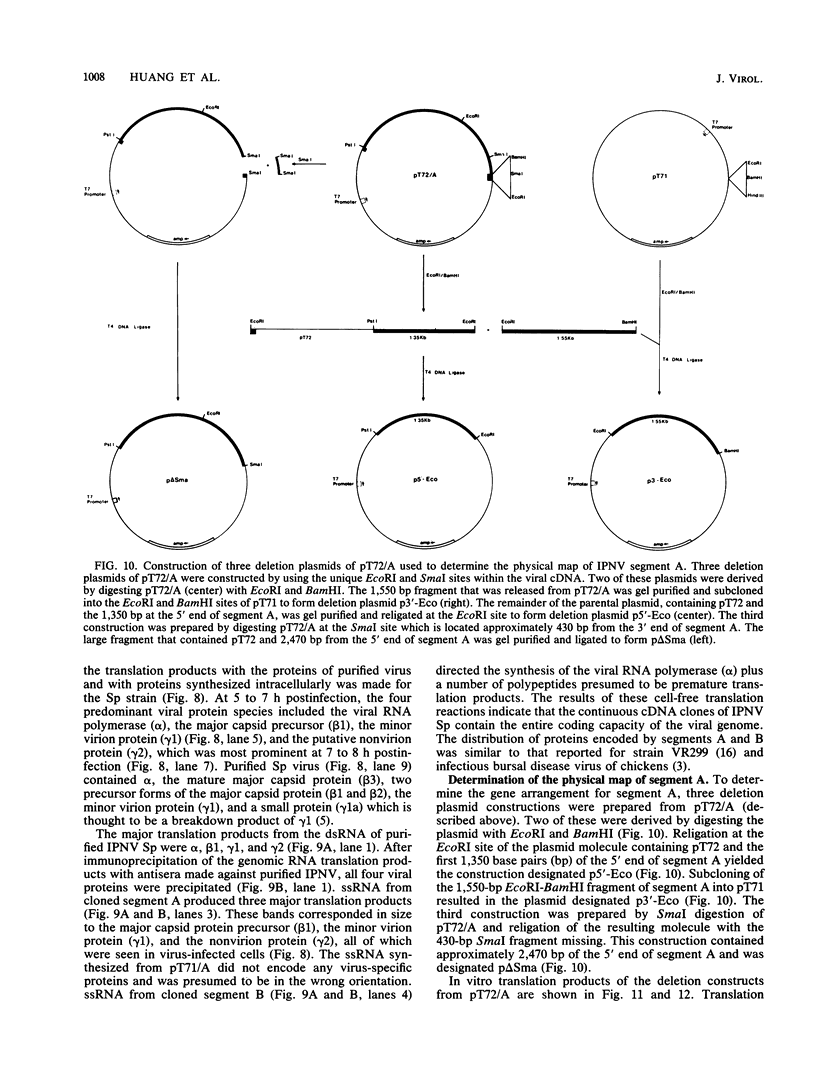
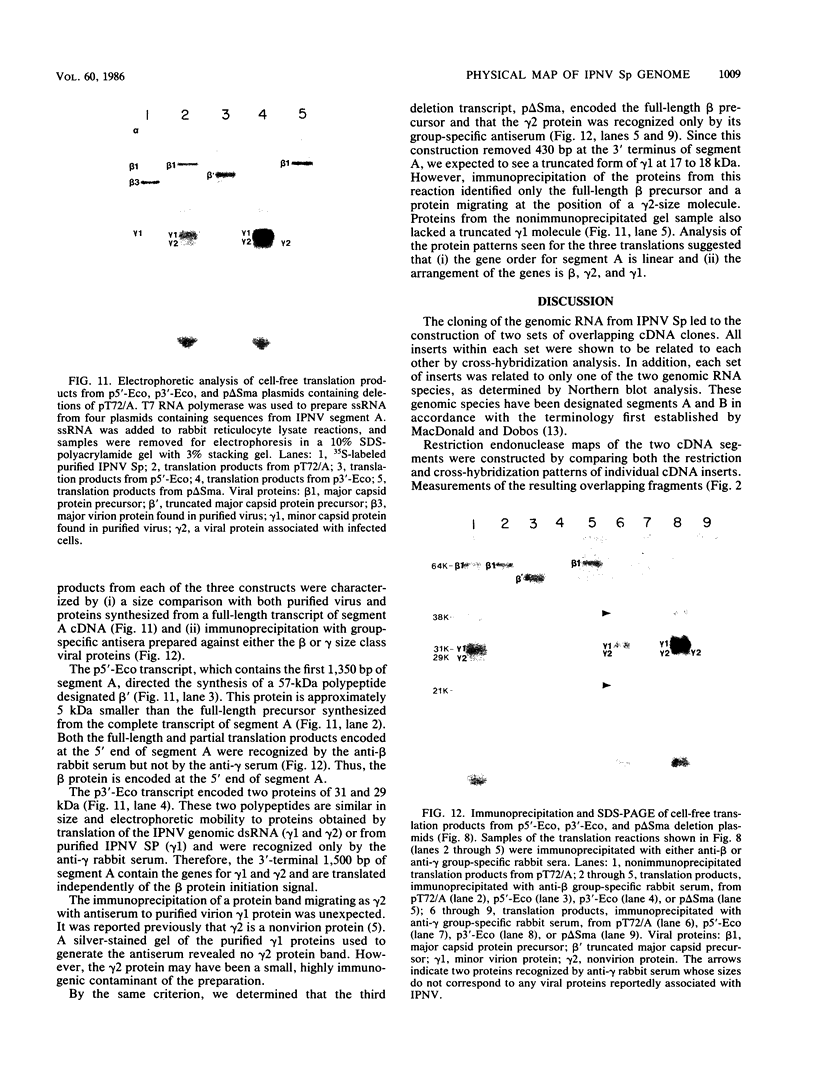


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad A. A., Barrett S. A., Fahey K. J. The characterization and molecular cloning of the double-stranded RNA genome of an Australian strain of infectious bursal disease virus. Virology. 1985 May;143(1):35–44. doi: 10.1016/0042-6822(85)90094-7. [DOI] [PubMed] [Google Scholar]
- Dobos P., Hill B. J., Hallett R., Kells D. T., Becht H., Teninges D. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J Virol. 1979 Nov;32(2):593–605. doi: 10.1128/jvi.32.2.593-605.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Rowe D. Peptide map comparison of infectious pancreatic necrosis virus-specific polypeptides. J Virol. 1977 Dec;24(3):805–820. doi: 10.1128/jvi.24.3.805-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer J. L., Yusha A., Pilcher K. S. The in vitro cultivation of tissue and cells of Pacific salmon and steelhead trout. Ann N Y Acad Sci. 1965 Aug 10;126(1):566–586. doi: 10.1111/j.1749-6632.1965.tb14303.x. [DOI] [PubMed] [Google Scholar]
- Hedrick R. P., Okamoto N., Sano T., Fryer J. L. Biochemical characterization of eel virus european. J Gen Virol. 1983 Jun;64(Pt 6):1421–1426. doi: 10.1099/0022-1317-64-6-1421. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kurath G., Ahern K. G., Pearson G. D., Leong J. C. Molecular cloning of the six mRNA species of infectious hematopoietic necrosis virus, a fish rhabdovirus, and gene order determination by R-loop mapping. J Virol. 1985 Feb;53(2):469–476. doi: 10.1128/jvi.53.2.469-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macdonald R. D., Dobos P. Identification of the proteins encoded by each genome segment of infectious pancreatic necrosis virus. Virology. 1981 Oct 30;114(2):414–422. doi: 10.1016/0042-6822(81)90222-1. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens P. P., Dobos P. Messenger RNA of infectious pancreatic necrosis virus is polycistronic. Nature. 1982 May 20;297(5863):243–246. doi: 10.1038/297243a0. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher K. S., Fryer J. L. The viral diseases of fish: a review through 1978. Part 1: Diseases of proven viral etiology. Crit Rev Microbiol. 1980;7(4):287–363. doi: 10.3109/10408418009077984. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Franke C. A., Strauss J. H., Hruby D. E. Expression of Sindbis virus structural proteins via recombinant vaccinia virus: synthesis, processing, and incorporation into mature Sindbis virions. J Virol. 1985 Oct;56(1):227–239. doi: 10.1128/jvi.56.1.227-239.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]