Abstract
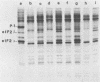
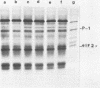
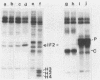
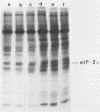
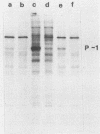
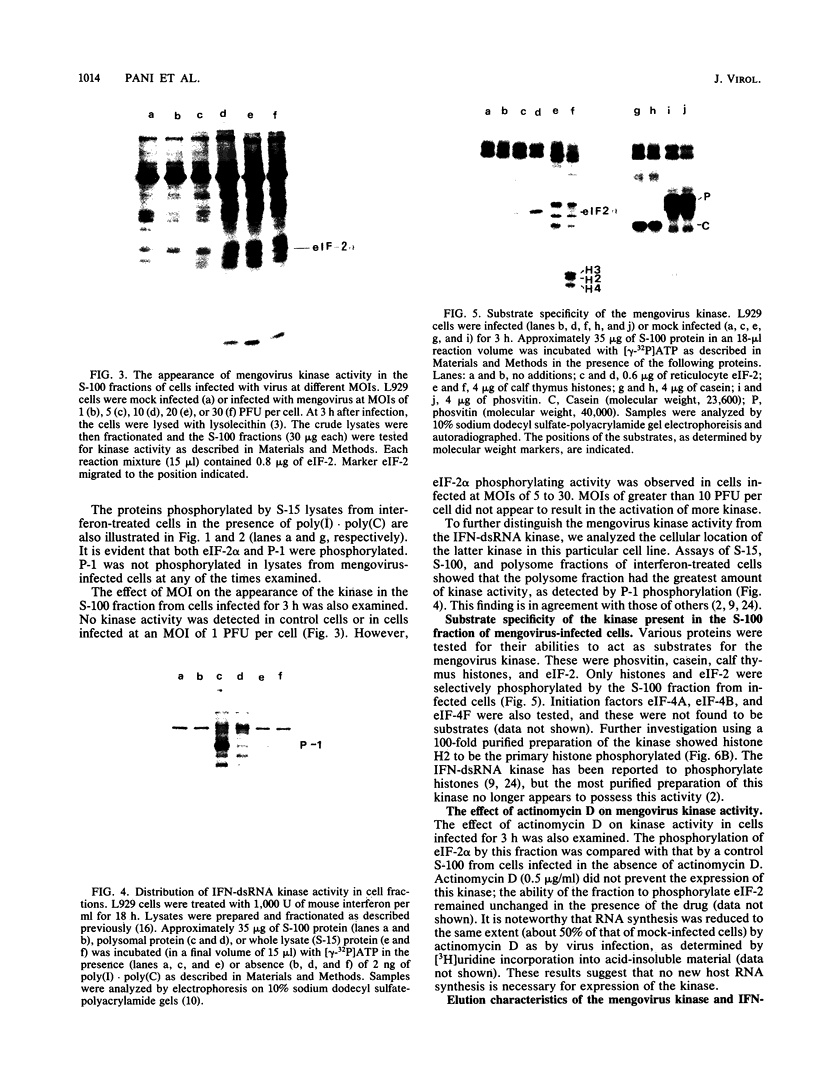
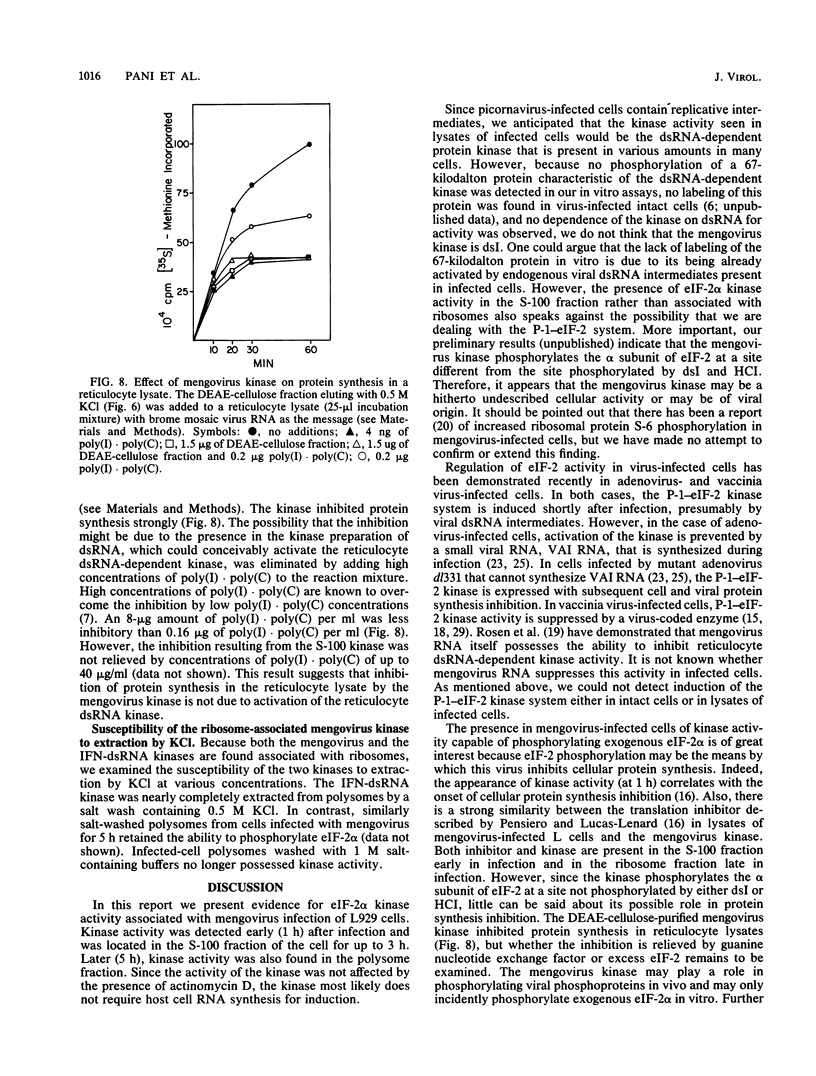
Infection of mouse L929 cells by mengovirus resulted in the expression of a kinase activity that selectively phosphorylated the small, 38,000-molecular-weight subunit of eucaryotic initiation factor 2 and histone H2. This kinase activity was independent of host cell RNA synthesis and was located in the postribosomal supernatant (S-100 fraction) early after infection (up to 3 h). At later times after infection (5 h), kinase activity was also associated with the polysome fraction. The kinase present in the S-100 fraction bound strongly to DEAE-cellulose, its peak activity eluting at 0.5 M KCl. Kinase activity was independent of the presence of exogenous double-stranded RNA, and KCl at concentrations greater than 0.1 M inhibited eucaryotic initiation factor 2 phosphorylation. The 67,000-molecular-weight phosphoprotein activated in interferon-treated cells by double-stranded RNA was not detected by standard phosphorylation assays in lysates from mengovirus-infected cells. Labeling of this protein in vivo during 5 h of infection was also not detected. The DEAE-cellulose-purified mengovirus kinase inhibited protein synthesis in reticulocyte lysates, and the inhibition was not reversible by high concentrations of poly(I).poly(C).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu S. L., Lucas-Lenard J. Cellular protein synthesis shutoff by mengovirus: translation of nonviral and viral mRNA's in extracts from uninfected and infected Ehrlich ascites tumor cells. J Virol. 1976 Apr;18(1):182–194. doi: 10.1128/jvi.18.1.182-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Knutson G. S., Lasky S. R., Munemitsu S. M., Samuel C. E. Mechanism of interferon action. Purification and substrate specificities of the double-stranded RNA-dependent protein kinase from untreated and interferon-treated mouse fibroblasts. J Biol Chem. 1985 Sep 15;260(20):11240–11247. [PubMed] [Google Scholar]
- Brown G. D., Peluso R. W., Moyer S. A., Moyer R. W. A simple method for the preparation of extracts from animal cells which catalyze efficient in vitro protein synthesis. J Biol Chem. 1983 Dec 10;258(23):14309–14314. [PubMed] [Google Scholar]
- Das H. K., Das A., Ghosh-Dastidar P., Ralston R. O., Yaghmai B., Roy R., Gupta N. K. Protein synthesis in rabbit reticulocytes. Purification and characterization of a double-stranded RNA-dependent protein synthesis inhibitor from reticulocyte lysates. J Biol Chem. 1981 Jun 25;256(12):6491–6495. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Gupta S. L. Specific protein phosphorylation in interferon-treated uninfected and virus-infected mouse L929 cells: enhancement by double-stranded RNA. J Virol. 1979 Jan;29(1):301–311. doi: 10.1128/jvi.29.1.301-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hunt T., Jackson R. J., Robertson H. D. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975 Jan 25;250(2):409–417. [PubMed] [Google Scholar]
- Jaye M. C., Godchaux W., 3rd, Lucas-Lenard J. Further studies on the inhibition of cellular protein synthesis by vesicular stomatitis virus. Virology. 1982 Jan 15;116(1):148–162. doi: 10.1016/0042-6822(82)90410-x. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Zilberstein A., Schmidt A., Shulman L., Revel M. The interferon-induced protein kinase PK-i from mouse L cells. J Biol Chem. 1979 Oct 10;254(19):9846–9853. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Petryshyn R., London I. M. Characterization of double-stranded-RNA-activated kinase that phosphorylates alpha subunit of eukaryotic initiation factor 2 (eIF-2 alpha) in reticulocyte lysates. Proc Natl Acad Sci U S A. 1980 Feb;77(2):832–836. doi: 10.1073/pnas.77.2.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. H., Petryshyn R., London I. M. Characterization of purified double-stranded RNA-activated eIF-2 alpha kinase from rabbit reticulocytes. J Biol Chem. 1981 Jul 25;256(14):7638–7641. [PubMed] [Google Scholar]
- Marcus P. I., Guidon P. T., Jr, Sekellick M. J. Interferon induction by viruses. VII. Mengovirus: "interferon-sensitive" mutant phenotype attributed to interferon-inducing particle activity. J Interferon Res. 1981;1(4):601–611. doi: 10.1089/jir.1981.1.601. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Regulation of protein synthesis initiation in eucaryotes. Arch Biochem Biophys. 1983 Jun;223(2):325–349. doi: 10.1016/0003-9861(83)90598-2. [DOI] [PubMed] [Google Scholar]
- Paez E., Esteban M. Resistance of vaccinia virus to interferon is related to an interference phenomenon between the virus and the interferon system. Virology. 1984 Apr 15;134(1):12–28. doi: 10.1016/0042-6822(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Pensiero M. N., Lucas-Lenard J. M. Evidence for the presence of an inhibitor on ribosomes in mouse L cells infected with mengovirus. J Virol. 1985 Oct;56(1):161–171. doi: 10.1128/jvi.56.1.161-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Kerr I. M. Interferon-mediated, double-stranded RNA-dependent protein kinase is inhibited in extracts from vaccinia virus-infected cells. J Virol. 1984 Apr;50(1):229–236. doi: 10.1128/jvi.50.1.229-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Knoller S., Kaempfer R. Messenger ribonucleic acid specificity in the inhibition of eukaryotic translation by double-stranded ribonucleic acid. Biochemistry. 1981 May 26;20(11):3011–3020. doi: 10.1021/bi00514a004. [DOI] [PubMed] [Google Scholar]
- Rosnitschek I., Traub U., Traub P. Enhanced phosphorylation of ribosomal protein s6 and other cytoplasmic proteins after mengovirus infection of Ehrlich ascites tumor cells. Hoppe Seylers Z Physiol Chem. 1978 May;359(5):593–600. doi: 10.1515/bchm.1978.359.1.593. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Duncan R., Knutson G. S., Hershey J. W. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2 alpha in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J Biol Chem. 1984 Nov 10;259(21):13451–13457. [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C., Taira H., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Characteristics of a double-stranded RNA-activated protein kinase system partially purified from interferon treated Ehrlich ascites tumor cells. J Biol Chem. 1978 Sep 10;253(17):5915–5921. [PubMed] [Google Scholar]
- Siekierka J., Mariano T. M., Reichel P. A., Mathews M. B. Translational control by adenovirus: lack of virus-associated RNAI during adenovirus infection results in phosphorylation of initiation factor eIF-2 and inhibition of protein synthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1959–1963. doi: 10.1073/pnas.82.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. H., Kung S., Koh T. T., Brandman P. Interferon-sensitive mutants of mengovirus. I. Isolation and biological characterization. Virology. 1976 Feb;69(2):727–736. doi: 10.1016/0042-6822(76)90501-8. [DOI] [PubMed] [Google Scholar]
- Tahara S. M., Traugh J. A., Sharp S. B., Lundak T. S., Safer B., Merrick W. C. Effect of hemin on site-specific phosphorylation of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Feb;75(2):789–793. doi: 10.1073/pnas.75.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon P. T., Merrick W. C., Traugh J. A. Site-specific phosphorylation if initiation factor 2 by three cyclic nucleotide-independent protein kinases. J Biol Chem. 1980 Nov 25;255(22):10954–10958. [PubMed] [Google Scholar]
- Whitaker-Dowling P., Youngner J. S. Characterization of a specific kinase inhibitory factor produced by vaccinia virus which inhibits the interferon-induced protein kinase. Virology. 1984 Aug;137(1):171–181. doi: 10.1016/0042-6822(84)90020-5. [DOI] [PubMed] [Google Scholar]