Abstract
Low-dose aspirin therapy has become a standard in the treatment of cardiovascular diseases. Aspirin has been shown to inhibit atherosclerosis in mouse models. To determine the mechanisms by which aspirin might inhibit atherosclerosis, we incubated HEPG2 cells and rat primary hepatocytes with aspirin or salicylic acid and noted an increase in paraoxonase 1(PON1) activity in the medium, together with an induction of PON1 and apolipoprotein A-I (apoA-I) gene expression. Mice treated with aspirin also showed a 2-fold increase in plasma PON1 activity and a significant induction of both PON1 and apoA-I gene expression in the liver. The induction of the PON1 gene in cell culture was accompanied by an increase in arylhydrocarbon receptor (AhR) gene expression. Accordingly, aspirin treatment of AhR−/− animals failed to induce PON1 gene expression. We previously suggested that aspirin might be hydrolyzed by serum PON1, which could account for its short plasma half-life of 10 min. Taken together with the current studies, we suggest that the antiatherosclerotic effects of aspirin might be mediated by its hydrolytic product salicylate and that the induction of PON1 and apoA-I might be important in the cardioprotective effects of aspirin.
Keywords: atherosclerosis, arylhydrocarbon receptor, salicylate, HDL, paraoxon
Serum paraoxonase 1(PON1) is synthesized mainly by the liver and circulates in association with apolipoprotein A-I (apoA-I) and HDL (1). PON1 is known to hydrolyze various types of substrates, such as arylesters, phosphate esters, and lactones (2), including thiolactones (3). More significantly, PON1 is known to inactivate lipid peroxides and hydrogen peroxide and therefore is known to offer protection against oxidative stress (4–6). PON1-deficient mice have been found to be more susceptible to lipoprotein oxidation and atherosclerosis (7, 8), and transgenic mice overexpressing PON1 have been found to have decreased atherosclerotic lesions (9). Other paraoxonases have also been noted to contribute significantly to protection against cardiovascular diseases (10).
ApoA-I is the primary protein associated with HDL, and mice overexpressing apoA-I have been found to have reduced atherosclerosis (11). There have been discrepancies regarding the correlation between PON1 levels and apoA-I or HDL levels; however, a recent study using apoA-I−/− mice crossed with LDL receptor-null (LDLr−/−) mice showed that lack of apoA-I severely affected PON1 levels (12).
Both serum PON1 activity and PON1 gene expression have been found to be modulated by nutritional, pharmacological, and environmental factors. Flavonoids such as quercetin and catechin increase PON1 activity in both humans and mice (13, 14). Resveratrol, a phytoalexin and a major biologically active component of red wine, has been known to induce PON1 gene expression in primary hepatocyte culture and in the HuH7 human hepatoma cell line, and this effect was partially mediated by the arylhydrocarbon receptor (AhR) (15). Modulation of PON1 activity and gene expression by pharmaceutical drugs, including aspirin, has been considered to be a useful tool for the prevention of cardiovascular disease (15, 16, 31)
Aspirin therapy has become essential, in combination with other treatment modalities, for those with established risk factors for cardiovascular diseases. Aspirin is analgesic, antiinflammatory, and antipyretic (17). It also inhibits the cyclooxygenase (COX) enzyme by acetylation of the active site, and the pharmacological effects of aspirin are due to the inhibition of the formation of COX products, including prostaglandins, thromboxanes, and prostacyclin (18, 19). The beneficial effects of aspirin on atherosclerosis cannot be solely attributed to its platelet-inhibitory function, because the aggregation of platelets contributes little to experimental atherosclerosis (20, 21). Moreover, aspirin predominantly exists in the form of salicylate in the plasma (22). The plasma half-life of aspirin is approximately 10 min, and the half-life for salicylate increases with the dose of aspirin (23), suggesting that the antioxidant and antiinflammatory effects of salicylate might play a role in mediating its antiatherosclerotic effects, rather than the acetylating ability of aspirin (22, 23).
In our previous studies, we have shown that aspirin competes for the hydrolysis of ρ-nitrophenylacetate (ρNPA) and paraoxon. We have also shown that nitroaspirin is effectively hydrolyzed by human plasma and isolated HDL preparations (24). In this study, we report the in vitro induction of the PON1 and apoA-I genes by aspirin and salicylate and the in vivo induction by aspirin. We have also established the in vitro and in vivo induction of the AhR gene by aspirin. The induction of PON1 and apoA-I by aspirin and salicylate provides elucidation of the additional mechanisms by which aspirin may protect against atherosclerosis.
MATERIALS AND METHODS
Chemicals
All chemicals, such as aspirin, paraoxon, ρ-NPA, and salicylate were purchased from Sigma-Aldrich Chemical Co., Saint Louis, MO. Oligofectamine, control RNA, and primers were purchased from Invitrogen. PON1 short interfering RNA (siRNA) was purchased from Sigma-Aldrich Chemical Co. HEPG2 cells were purchased from the American Type Culture Collection, Manassas, VA.
PON1 enzymatic activity
The cell-associated and plasma PON1 arylesterase activity was measured using 1 mM ρNPA as substrate, as assessed from the formation of ρ-nitrophenol at 410 nm at 37°C (26). Typically, aliquots of 10 μl of medium or plasma were placed in microtiter plate wells in triplicate; the reaction was initiated by the addition of the substrate ρNPA, to yield a final concentration of 1 mM in PBS buffer containing calcium and magnesium. After mixing, the plate was read immediately to establish 0 time values, and the reactions were incubated at 37°C. Readings were recorded at the end of 30 min. PON activity was expressed as percent of control, where 100% corresponds to the PON1 activity in the control alcohol-treated cells.
Cell culture
The human hepatoma cell line HEPG2 was maintained at 37°C in an atmosphere containing 5% CO2 and cultivated in advanced DMEM, which was supplemented with 10% FBS (Invitrogen), 1% l-glutamine, and 1% penicillin-streptomycin (Invitrogen). In experiments to monitor the change in PON1 activity and gene expression, HEPG2 cells (6 × 106 cells per 6-well plates) were pretreated for 4 h with minimum essential medium, which was serum free and phenol free. Cells were then treated with 0–1 mM aspirin, 0–1 mM salicylate, and 0–250 μM paraoxon for 48 h in the same minimum essential medium. Aspirin and paraoxon were dissolved in alcohol. The control cells were treated with alcohol alone. After the complete period of treatment, the medium was collected to assay PON1 enzyme activity, and the cells were washed with PBS (Invitrogen) to eliminate serum traces that might have contained PON1. Cells were harvested in Trizol (Invitrogen) for RNA isolation. Total RNA preparation from the cells was performed as previously described (25).
Primary rat hepatocyte culture
Male Sprague Dawley rats of mature age and weighing 200–250 g at the time of euthanization were obtained from Charles River Laboratories. For isolation of primary hepatocytes, rats (n = 3) were fasted overnight and anesthetized with sodium pentobarbital solution (50 mg/kg) intraperitoneally. The inferior vena cava was cannulated, and the liver was perfused in situ with oxygenated HBSS containing 100 U/ml penicillin-streptomycin, pH 7.4 (8 ml/min, 37°C for 10 min), followed by perfusion with oxygenated HBSS containing 1 mM CaCl2 and MgCl2, penicillin-streptomycin (100 U/ml), and 0.04% collagenase type IV, pH 7.4, for 10 min. The liver was removed and then gently minced in HBSS containing 1 mM CaCl2 and MgCl2, penicillin-streptomycin (100 U/ml), and 1 × 10−7 M insulin, pH 7.4. The liver cell suspension was then filtered with 70 μm Falcon cell strainers and centrifuged at 50 g for 2 min. Cells were plated on 24-well plates (8 × 104) (Biocoat Collagen I Cellware plates) in Williams's Medium E containing 10% FBS, 100 U/ml penicillin-streptomycin, and 1 × 10−7 M insulin and cultured at 37°C with 5% CO2. After an initial 4 h attachment period, cultures were washed gently with PBS and set up with serum-free and phenol-free minimum essential culture medium for 4 h as pretreatment. Hepatocytes were then treated with 0–0.5 mM aspirin in the same culture medium. Aspirin was prepared in alcohol. Control cells were treated with alcohol alone. Experiments indicated that concentrations of 0.01 mM–0.5 mM aspirin caused no cell injury in these cells. All experiments with animals were performed in accordance with the requirements of the Institutional Animal Ethics Committee.
Mice tissue samples
Ten to twelve week-old LDLr−/− mice (n = 6), and eight week-old normal C57BL6 mice (n = 6) and AhR−/− mice (n = 6) were obtained from Jackson Laboratory, Bar Harbor, ME. The AhR−/− mice are homozygotes and do not respond to aryl-hydrocarbon receptor agonists. They show reduced liver weight (25% decrease), delayed extramedullary hematopoiesis, and transient hepatic microvesicular steatosis, according to the supplier. These mice are also known to have decreased body weight and altered growth characteristics. The strain was developed using a construct that removed exon 2 of the targeted gene. The original mutation was crossed 12 times to C57BL/6 mice, and the generation obtained was N12.
Aspirin was fed orally at a concentration of 2 mg/day dissolved in 50 μl alcohol. The animals were fed an atherogenic diet in the presence and absence of aspirin. LDLr−/− mice (n = 3) were fed aspirin for 6 days, and the C57BL6 mice (n = 3) and AhR−/− mice (n = 3) were fed aspirin for 9 days. The animals were also supplied with water ad libitum during the time of feeding. The control animals of each group (n = 3) were fed 50 μl alcohol, the solvent vehicle, per day. At the end of feeding, the animals were fasted overnight prior to euthanization. Blood was collected over heparin, and plasma was prepared and assayed for PON1 activity. Liver was flushed with ice-cold PBS and collected for extraction of total RNA.
Lipid analysis
Fasting plasma total cholesterol, triglycerides, HDL, and LDL measurements were determined using the Cholestech l*D*X analyzer (Cholestech Corporation, Hayward, CA).
Effect of siRNA-mediated PON1 gene silencing in HEPG2 cells
We tested whether silencing the PON1 gene would reduce PON1 activity in the medium and gene expression in the cells. HEPG2 cells were grown in 6-well plates. When approximately 50% to 60% confluent, the cells were transfected with PON1-targeted siRNA and control (nonsilencing) siRNA. Both PON1 siRNA duplexes and control siRNA were transfected using oligofectamine reagent to give a final concentration of 140 nM. Oligofectamine was mixed gently, diluted in serum-free medium, and incubated for 5–10 min at room temperature. PON1 siRNA/control RNA was mixed with the diluted oligofectamine reagent. The mixture was incubated for 15–20 min at room temperature. While complexes were formed, the growth medium was removed from the cells and the cells were washed once with serum-free medium. Following this, the cells were incubated with PON1 siRNA/control RNA and serum-free medium. Forty-eight hours after transfection, cells were washed and incubated with fresh medium. Cells were then treated with 0.5 mM aspirin and the solvent vehicle alone (ethanol). Medium was collected and PON1 activity was assayed. Cell lysates were stored for PON1 mRNA determination and real-time PCR analysis.
PON1, apoA-I, and AhR gene expression by quantitative real-time PCR
Total RNA from cells and mice liver tissues was extracted with Trizol, and total RNA preparation was performed as previously described (25). cDNA was generated from 1 μg of total RNA. Reverse transcriptase products were subjected to PCR amplification with Ready Mix PCR Master Mix (Invitrogen). All primers were purchased from Invitrogen. At least three independent trials were performed. Real-time PCR was performed using the iCycler from BioRad. Melting curves were established for the reactions. Fold induction was calculated according to established formulas using reference gene and target gene changes. GAPDH was used as the reference gene.
Statistical analysis
All data are expressed as mean ± SD. Statistical analysis used ANOVA for group comparison and Student's t-test for pairs, with significance at P < 0.05.
RESULTS AND DISCUSSION
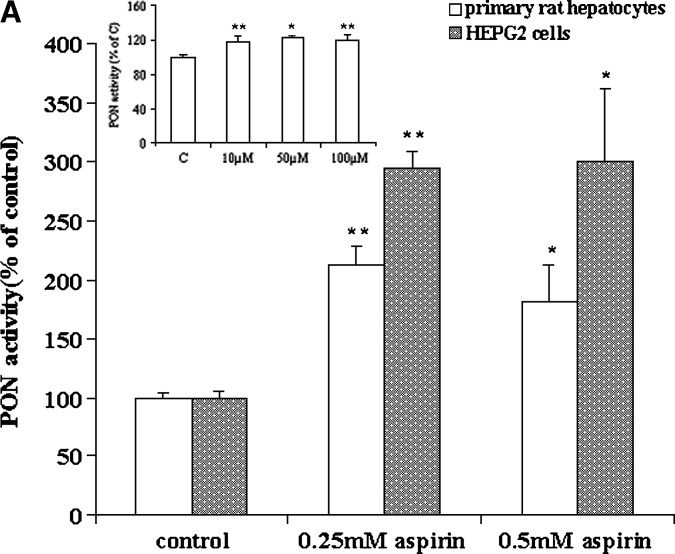
We evaluated HEPG2 cells and primary rat hepatocytes as in vitro models of hepatic cells, because liver is the major source for circulatory PON1. The secreted PON1 arylesterase activity was measured in the medium from HEPG2 cells and primary rat hepatocytes treated with increasing concentrations of aspirin (Fig. 1A). A significant 3-fold increase in PON1 activity was observed in HEPG2 cells treated under these conditions, and as little as 10 μM aspirin significantly (P < 0.01) increased PON1 activity in the medium in primary rat hepatocytes treated with aspirin (insert in Fig. 1A). However, no clear-cut dose response could be seen. This could be due to a variety of reasons, such as hydrolysis of aspirin in the medium and increased permeability of fresh primary hepatocytes. Incubation of HEPG2 cells with paraoxon and salicylate under similar conditions also showed an increase in PON1 activity (data not shown).
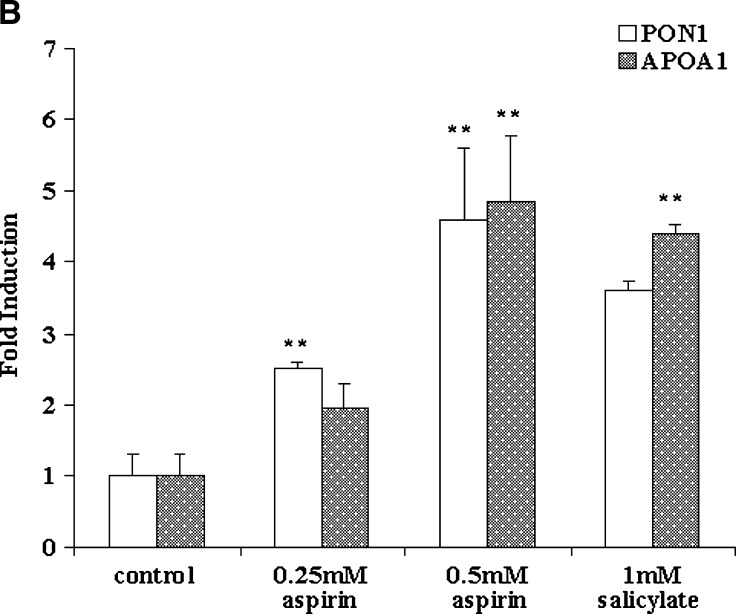
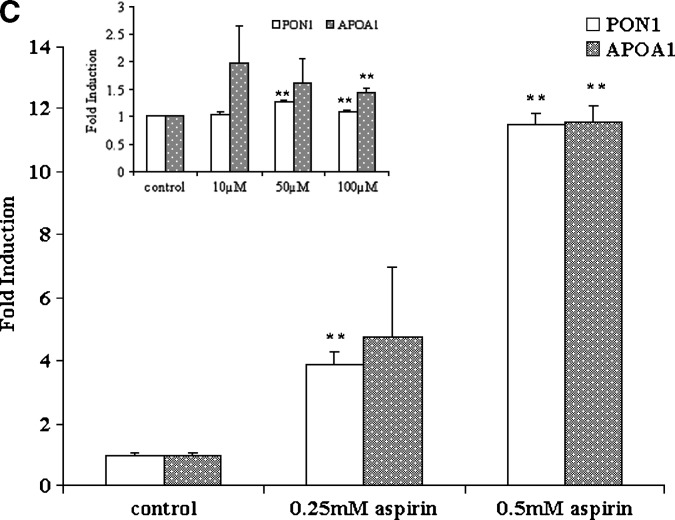
Fig. 1.
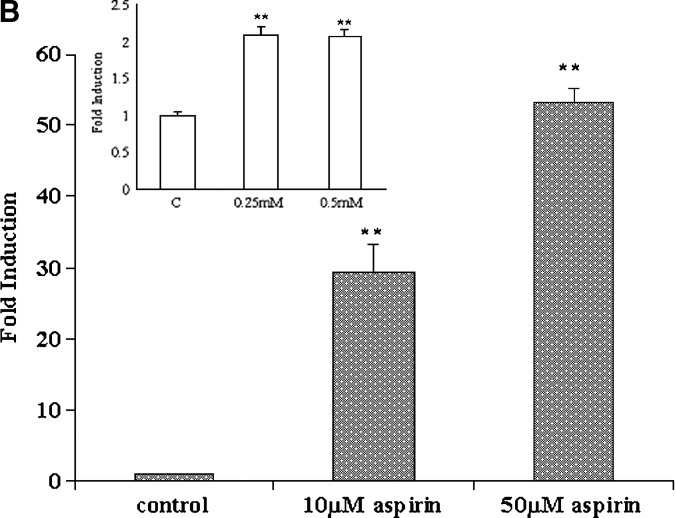
Aspirin increases paraoxonase 1 (PON1) activity in the medium and PON1 and apolipoprotein A-I (apoA-I) gene expression in HEPG2 cells and primary rat hepatocytes. The cell-associated PON1 arylesterase activity was measured using 1 mM ρ-nitrophenyl acetate (ρNPA) as substrate, as assessed from the formation of ρ-nitrophenol (ρNP) at 410 nm. A: HEPG2 cells and primary rat hepatocytes cultured in 6-well plates were treated with increasing concentrations of aspirin (0–0.5 mM). Insert shows increase in PON1 activity in the medium in primary rat hepatocyes treated with low concentrations of aspirin (0–100 μM). PON1 enzyme activity is expressed as percent of control; 100% corresponds to the activity in control cells treated with alcohol alone. B: Real-time PCR analysis showing induction of PON1 and apoA-I gene expression in HEPG2 cells treated with increasing concentrations of aspirin (0–0.5 mM) and 1 mM salicylate. C: Real-time PCR analysis of PON1 and apoA-I gene expression in rat primary hepatocytes treated with increasing concentrations of aspirin (0–0.5 mM). Insert shows induction of the PON1 and apoA-I genes with low concentrations of aspirin (0–100 μM). The experimental setup, RNA extraction, and real-time PCR analysis were carried out as described in Materials and Methods. Values are expressed as mean ± SD (n = 3). * P < 0.05; ** P < 0.01.
PON1 gene expression was determined in cells treated with aspirin, and as seen in Fig. 1B, a 5-fold induction was observed. HEPG2 cells treated with salicylate also showed a 4-fold induction. We chose also to determine the expression of the apoA-I gene, because many studies have shown a potential correlation between HDL levels and PON1 activity (26). We observed that under the conditions used, PON1 gene expression was induced by aspirin and salicylate; there was also an induction of apoA-I gene expression (Fig. 1B). Primary rat hepatocytes were also treated with different concentrations of aspirin and PON1, and apoA-I gene expression was measured. As seen in Fig. 1C, as little as 10 μM aspirin (insert) increased the gene expression levels of both PON1 and apoA-I. However, at concentrations above 100 μM, the induction was more pronounced and significant.
The apoA-I promoter sequence contains motifs for peroxisome proliferator-activated receptor-α (PPAR-α) (27). We and others have shown that PPAR-α ligands, including oxidized fatty acids, induce the expression of apoA-I (28, 29) in cultured cells. However, in light of the reported species differences in apoA-I target gene PPAR response (28), it is unlikely that aspirin is directly involved in the induction of the apoA-I gene via PPAR-α. On the other hand, products derived from aspirin metabolism, for example salicylate or dihydroxy benzoate, could have indirect effects by decreasing NFκB activation, thereby affecting apoA-I transcription (30). Modulation of cytochrome P450, an AhR-dependent oxidase (CYP1A-I), by PPAR-α has also been reported (31, 32). Thus, the induction of apoA-I seen in our studies could very well be mediated by the xenobiotic effects of aspirin.
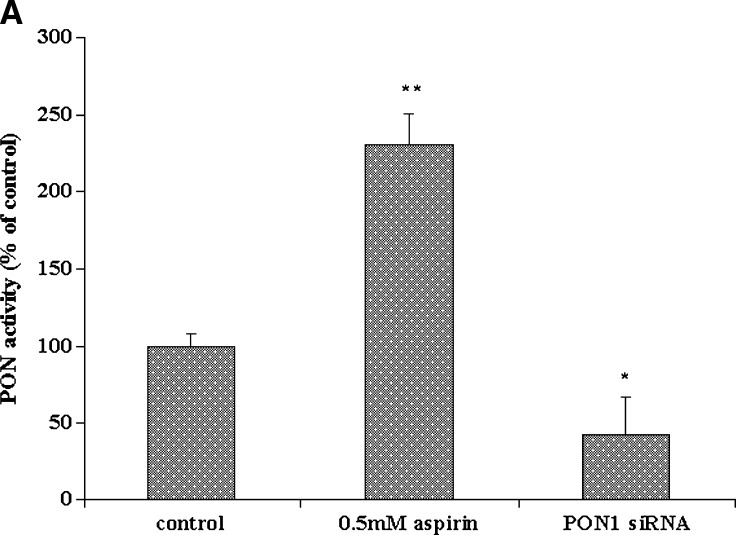
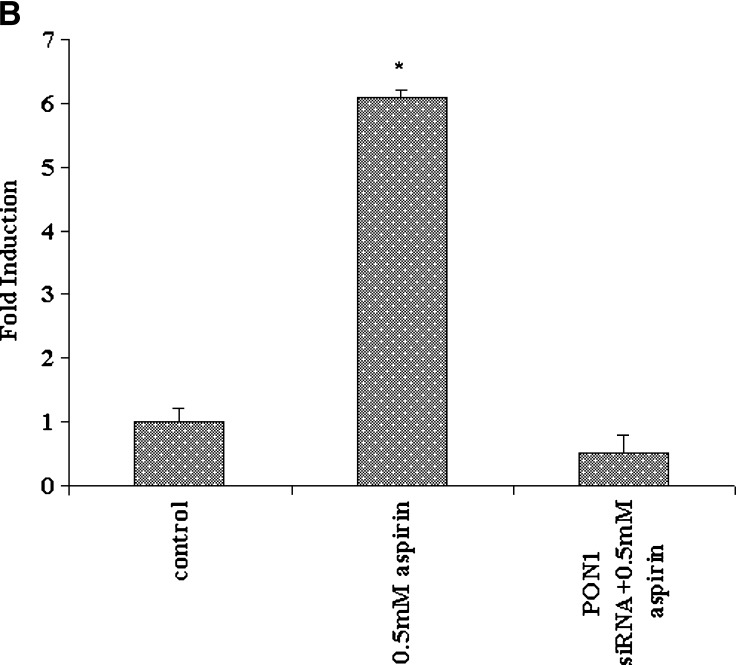
We also used targeted gene silencing. Endogenous PON1 gene expression was inhibited with a specific PON1-targeted siRNA. In these experiments, cells were treated for 48 h, because preliminary experiments showed that siRNA-mediated gene silencing was most efficient at about 40 h after transfection. PON1 activity in the medium was reduced in the siRNA-treated cells as compared with nontransfected cells (Fig. 2A). The inducing effect of aspirin on PON1 gene expression under these conditions was completely abolished, as compared with control nontransfected cells, as seen in Fig. 2B, confirming the involvement of aspirin in the regulation of the PON1 gene.
Fig. 2.
PON1 gene expression in HEPG2 cells treated with PON1 short interfering RNA (siRNA) and aspirin. A: PON1 enzyme activity in the medium from nontransfected control HEPG2 cells and PON1 siRNA-treated cells. The cell-associated PON1 arylesterase activity was measured using 1 mM ρNPA as substrate, as assessed from the formation of ρNP at 410 nm. PON1 enzyme activity is expressed as percent of control; 100% corresponds to the activity in control nontransfected cells. B: Real-time PCR analysis shows that upon silencing the PON1 gene using PON1 siRNA in HEPG2 cells, 0.5 mM aspirin treatment fails to induce the expression of PON1. Cells treated with control siRNA were also maintained. The experimental set up, RNA extraction, and real-time PCR analysis are as described in Materials and Methods. Values are expressed as mean ± SD (n = 3). * P < 0.05; ** P < 0.01.
It has been reported that the AhR ligand resveratrol, a phytochemical, induced the PON1 gene by mechanisms involving AhR (15). It is possible that the ability of aspirin to induce PON1 also could be mediated by AhR. Aspirin and salicylate might activate AhR-mediated signaling via the hydroxylation of salicylate to dihydroxybenzoate. Therefore, we determined the effect of aspirin treatment on AhR gene expression in both HEPG2 and rat primary hepatocytes.
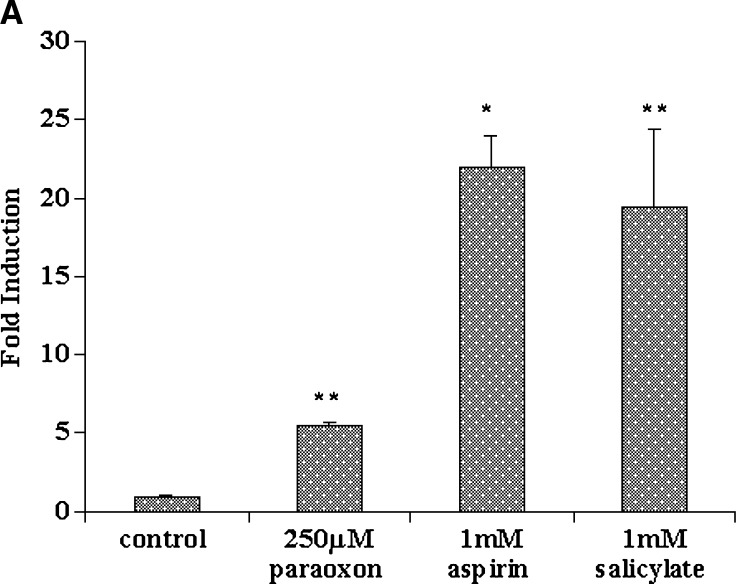
Different levels of induction of AhR gene expression were seen with aspirin, salicylate, and paraoxon treatment. A significant 20-fold induction of AhR was seen in HEPG2 cells treated with 1 mM aspirin, and a 5-fold induction was seen in HEPG2 cells treated with 250 μM paraoxon, as shown in Fig. 3A. Treatment of primary rat hepatocytes with as little as 10 μM aspirin caused a significant and dramatic 50-fold induction (Fig. 3B). The “membrane leakiness” of fresh hepatocytes could be a factor in such a high induction. PPAR-α has been implicated in the response of AhR to its ligands, and AhR is known to have cross-talk with PPAR-α (32). The AhR-mediated events that affect PPAR-α could be one of the potential mechanisms by which aspirin could induce both PON1 and apoA-I. The ability of PPAR-α agonists to induce PON1 gene expression remains to be identified.
Fig. 3.
Effect of aspirin, salicylate, and paraoxon on arylhydrocarbon receptor (AhR) gene expression in HEPG2 cells and primary rat hepatocytes. HEPG2 cells and primary rat hepatocytes were cultured in six well plates as described in the methods section. AhR gene expression in (A) HEPG2 cells treated with 1 mM aspirin, 1 mM salicylate, and 250 μM paraoxon, and (B) primary rat hepatocytes treated with 0–100 μM aspirin and 0–0.5 mM aspirin treatment (insert). RNA extraction and real-time PCR analysis were carried out as described in Materials and Methods. Values are expressed as mean ± SD (n = 3). * P < 0.05; ** P < 0.01.
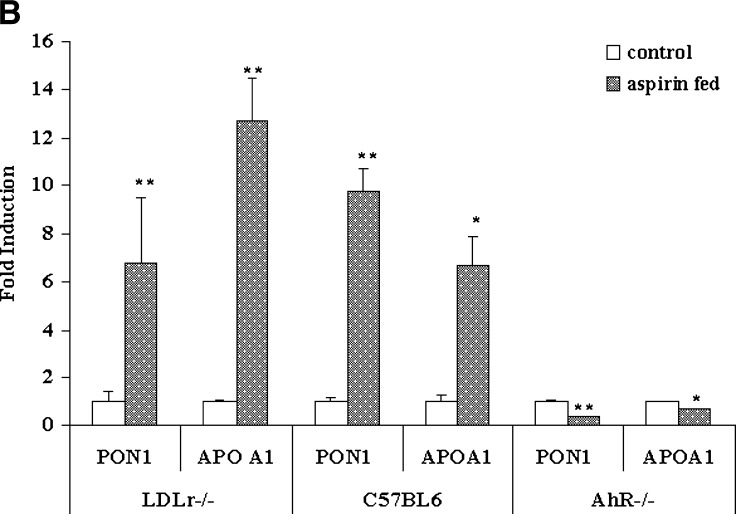
To determine the in vivo effects of aspirin on PON1 activity and gene expression, we treated LDLr−/− mice with an atherogenic diet in the presence and absence of aspirin at a concentration of 2 mg/day for 6 days. Plasma lipid analysis showed a 1.5-fold increase in HDL levels (data not shown) and a 2-fold increase in plasma PON1 arylesterase activity in aspirin-fed mice as compared with control mice (Fig. 4A). The results indicate that aspirin or its hydrolytic product, salicylate, induce metabolic pathways leading to PON1 synthesis. The liver was harvested to determine PON1 and apoA-I gene induction by aspirin. As shown in Fig. 4B, a 7-fold increase in PON1 and a 12-fold induction of apoA-I gene expression were observed in LDLr−/− mice fed aspirin.
Fig. 4.
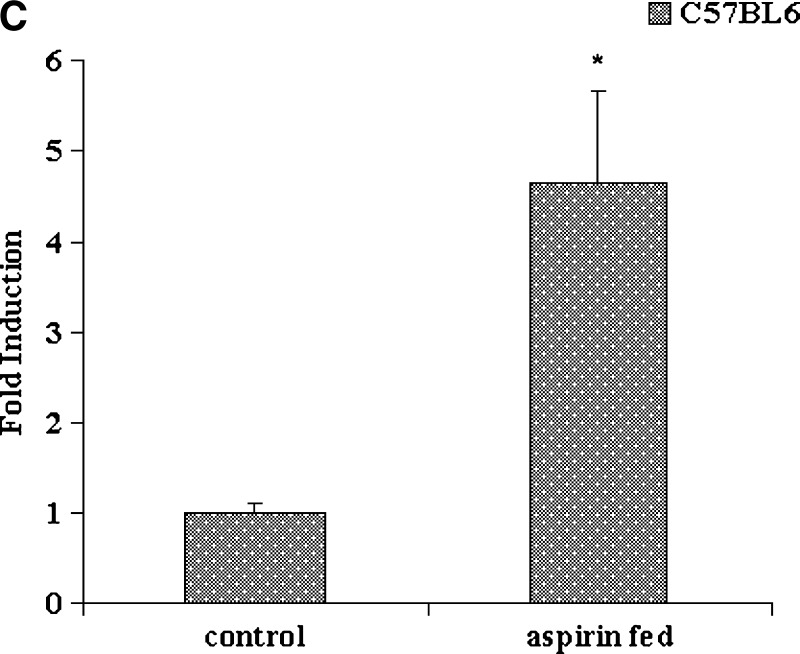
Plasma PON1 activity and liver PON1, apoA-I, and AhR gene expression in LDL receptor (LDLr)−/−, normal C57BL6, and AhR−/− mice fed aspirin. A: LDLr−/−mice (n = 6) were fed an atherogenic diet in the presence and absence of aspirin at a concentration of 2 mg/day for 6 days. Normal C57BL6 mice (n = 6) and AhR−/−mice (n = 6) were fed an atherogenic diet in the presence and absence of aspirin at a concentration of 2 mg/day for 9 days. Plasma PON1 arylesterase activity was measured using 1 mM ρNPA acetate as substrate, as assessed from the formation of ρNP at 410 nm. PON1 enzyme activity is expressed as percent of control; 100% corresponds to the activity in control mice treated with alcohol alone. B: PON1, apoA-I, and AhR gene expression in liver tissues of LDLr−/−, normal C57BL6, and AhR−/− mice fed an atherogenic diet in the presence and absence of aspirin. The filled bars indicate gene expression levels in aspirin-fed mice, and the open bars indicate the control mice that were fed alcohol, the solvent vehicle, alone. C: Induction of AhR gene expression in normal C57BL6 mice fed aspirin as compared with control alcohol-fed mice. RNA extraction and real-time PCR analysis were carried out as described in Materials and Methods. Values are expressed as mean ± SD (n = 3). * P < 0.05; ** P < 0.01.
There was also an increase in plasma PON1 activity in normal C57BL6 mice fed aspirin at a concentration of 2 mg/day with an atherogenic diet for 9 days, as compared with control mice fed alcohol (Fig. 4A). These mice were fed an atherogenic diet for such a short time to mimic the conditions employed in using the LDLr−/− mice. These mice were compared with AhR−/− mice fed similar doses of aspirin. As seen in Fig. 4A, AhR−/− mice fed aspirin at the same concentration did not show any increase or decrease in plasma PON1 enzyme activity, as compared with control mice. Liver gene expression in the normal C57BL6 aspirin-fed mice showed a 10-fold induction in PON1 and a 6-fold induction in apoA-I gene expression, as compared with controls (Fig. 4B). A 5-fold induction in AhR gene expression was also observed (Fig. 4C), whereas the AhR−/− mice that were aspirin fed did not show an induction of either PON1 or apoA-I (Fig. 4B.)
The antiatherosclerotic nature of nitroaspirin has been described in the literature (33), and nitrated aspirin is one of the most common analogs of aspirin. Nitroaspirin significantly induced PON1 activity by 1.5-fold in HEPG2 cells (P < 0.01), and PON1 gene expression by 14-fold and apoA-I by 2.5-fold (data not shown) under the same experimental conditions as aspirin.
Our studies not only reveal the role of PON1 in the metabolism of aspirin but also elucidate when aspirin therapy would be effective. If PON1-mediated hydrolysis is essential for the activity of aspirin, then patients with higher PON1 levels might respond better to aspirin treatment, and strategies to induce PON1 levels would be extremely important. On the other hand, if the hydrolysis of aspirin is wasteful, then the inhibition of the enzyme needs to be considered. It is unlikely that such would be the case, because salicylate is the natural plant medicine and aspirin was synthetically acetylated to “buffer” and reduce its acidity.
Studies have shown that people who consume aspirin have increased PON1 activity in the serum. Low HDL levels correlate positively with low PON1 (34). It is tempting to speculate whether people with low HDL are more likely to have aspirin resistance. Our initial discovery that PON1 could hydrolyze aspirin, coupled with the fact that aspirin has a short half-life in the plasma, would suggest that one has to think beyond COX. These studies would pave the way for designing a better class of PON1 activators that may serve as important deterrents not only to atherosclerosis but also to diabetes and other diseases in which deficiencies in PON1 have been noted.
Acknowledgments
The authors thank Dr. Sheik Wisel and Larissa Brophy for assistance.
Abbreviations
AhR, arylhydrocarbon receptor
apoA-I, apolipoprotein A-I
COX, cyclooxygenase
ρNPA, ρ-nitrophenylacetate
PON1, paraoxonase 1
PPAR-α, peroxisome proliferator-activated receptor-alpha
Published, JLR Papers in Press, June 2, 2008.
Footnotes
This study was supported by National Institutes of Health Grants RO1 HL-56353 and RO1 HL-069038.
References
- 1.Teiber J. F., D. I. Draganov, and B. N. La Du. 2004. Purified human serum PON1 does not protect LDL against oxidation in the in vitro assays initiated with copper or AAPH. J. Lipid Res. 45 2260–2268. [DOI] [PubMed] [Google Scholar]
- 2.Teiber J. F., D. I. Draganov, and B. N. La Du. 2003. Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3. Biochem. Pharmacol. 66 887–896. [DOI] [PubMed] [Google Scholar]
- 3.Jakubowski H. 2000. Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J. Biol. Chem. 275 3957–3962. [DOI] [PubMed] [Google Scholar]
- 4.Shamir R., C. Hartman, R. Karry, E. Pavlotzky, R. Eliakim, J. Lachter, A. Suissa, and M. Aviram. 2005. Paraoxonases (PONs) 1, 2, and 3 are expressed in human and mouse gastrointestinal tract and in Caco-2 cell line: selective secretion of PON1 and PON2. Free Radic. Biol. Med. 39 336–344. [DOI] [PubMed] [Google Scholar]
- 5.Deakin S., X. Moren, and R. W. James. 2007. HDL oxidation compromises its influence on paraoxonase-1 secretion and its capacity to modulate enzyme activity. Arterioscler Thromb Vasc Biol. 27 1146–1152. [DOI] [PubMed] [Google Scholar]
- 6.Aviram M., M. Rosenblat, C. L. Bisgaier, R. S. Newton, S. L. Primo-Parmo, and B. N. La Du. 1998. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Invest. 101 1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karabina S. A., A. N. Lehner, E. Frank, S. Parthasarathy, and N. Santanam. 2005. Oxidative inactivation of paraoxonase—implications in diabetes mellitus and atherosclerosis. Biochim. Biophys. Acta. 1725 213–221. [DOI] [PubMed] [Google Scholar]
- 8.Shih D. M., L. Gu, Y. R. Xia, M. Navab, W. F. Li, S. Hama, L. W. Castellani, C. E. Furlong, L. G. Costa, A. M. Fogelman, et al. 1998. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 394 284–287. [DOI] [PubMed] [Google Scholar]
- 9.Tward A., Y. R. Xia, X. P. Wang, Y. S. Shi, C. Park, L. W. Castellani, A. J. Lusis, and D. M. Shih. 2002. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 106 484–490. [DOI] [PubMed] [Google Scholar]
- 10.Ng C. J., N. Bourquard, S. Y. Hama, D. Shih, V. R. Grijalva, M. Navab, A. M. Fogelman, and S. T. Reddy. 2007. Adenovirus-mediated expression of human paraoxonase 3 protects against the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 27 1368–1374. [DOI] [PubMed] [Google Scholar]
- 11.Rader D. J. 2002. High-density lipoproteins and atherosclerosis. Am. J. Cardiol. 90 62–70. [DOI] [PubMed] [Google Scholar]
- 12.Moore R. E., M. Navab, J. S. Millar, F. Zimetti, S. Hama, G. H. Rothblat, and D. J. Rader. 2005. Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circ. Res. 97 763–771. [DOI] [PubMed] [Google Scholar]
- 13.Gusman J., H. Malonne, and G. Atassi. 2001. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 22 1111–1117. [DOI] [PubMed] [Google Scholar]
- 14.Pervaiz S. 2003. Resveratrol: from grapevines to mammalian biology. FASEB J. 17 1975–1985. [DOI] [PubMed] [Google Scholar]
- 15.Gouedard C., R. Barouki, and Y. Morel. 2004. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol. Cell. Biol. 24 5209–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciolino H. P., P. J. Daschner, and G. C. Yeh. 1999. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A-I transcription differentially. Biochem. J. 340 715–722. [PMC free article] [PubMed] [Google Scholar]
- 17.Vane J. R., and R. M. Botting. 2003. The mechanism of action of aspirin. Thromb. Res. 110 255–258. [DOI] [PubMed] [Google Scholar]
- 18.Wu K. K. 2003. Aspirin and other cyclooxygenase inhibitors: new therapeutic insights. Semin. Vasc. Med. 3 107–112. [DOI] [PubMed] [Google Scholar]
- 19.Vane J. R. 1971. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 231 232–235. [DOI] [PubMed] [Google Scholar]
- 20.Breddin H. K. 2004. Aspirin and platelet function. J. Thromb. Haemost. 2 1216–1218. [DOI] [PubMed] [Google Scholar]
- 21.Cyrus T., Y. Yao, L. X. Tung, and D. Pratico. 2006. Stabilization of advanced atherosclerosis in low-density lipoprotein receptor-deficient mice by aspirin. Atherosclerosis. 184 8–14. [DOI] [PubMed] [Google Scholar]
- 22.Fu C. J., S. Melethil, and W. D. Mason. 1991. The pharmacokinetics of aspirin in rats and the effect of buffer. J. Pharmacokinet. Biopharm. 19 157–173. [DOI] [PubMed] [Google Scholar]
- 23.Petersen T., S. E. Husted, A. K. Pedersen, and E. Geday. 1982. Systemic availability of acetylsalicylic acid in human subjects after oral ingestion of three different formulations. Acta Pharmacol. Toxicol. (Copenh.). 51 285–291. [DOI] [PubMed] [Google Scholar]
- 24.Santanam N., and S. Parthasarathy. 2007. Aspirin is a substrate for paraoxonase-like activity: implications in atherosclerosis. Atherosclerosis. 191 272–275. [DOI] [PubMed] [Google Scholar]
- 25.Gouedard C., N. Koum-Besson, R. Barouki, and Y. Morel. 2003. Opposite regulation of the human paraoxonase-1 gene PON-1 by fenofibrate and statins. Mol. Pharmacol. 63 945–956. [DOI] [PubMed] [Google Scholar]
- 26.Abbott C. A., M. I. Mackness, S. Kumar, A. J. Boulton, and P. N. Durrington. 1995. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler. Thromb. Vasc. Biol. 15 1812–1818. [DOI] [PubMed] [Google Scholar]
- 27.Januzzi J. L., N. Azrolan, A. O'Connell, K. Aalto-Setala, and J. L. Breslow. 1992. Characterization of the mouse apolipoprotein Apoa-1/Apoc-3 gene locus: genomic, mRNA, and protein sequences with comparisons to other species. Genomics. 14 1081–1088. [DOI] [PubMed] [Google Scholar]
- 28.Vu-Dac N., S. Chopin-Delannoy, P. Gervois, E. Bonnelye, G. Martin, J. C. Fruchart, V. Laudet, and B. Staels. 1998. The nuclear receptors peroxisome proliferator-activated receptor alpha and Rev-erb alpha mediate the species-specific regulation of apolipoprotein A-I expression by fibrates. J. Biol. Chem. 273 25713–25720. [DOI] [PubMed] [Google Scholar]
- 29.Rong R., S. Ramachandran, M. Penumetcha, N. Khan, and S. Parthasarathy. 2002. Dietary oxidized fatty acids may enhance intestinal apolipoprotein A-I production. J. Lipid Res. 43 557–564. [PubMed] [Google Scholar]
- 30.Kopp E., and S. Ghosh. 1994. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 265 956–959. [DOI] [PubMed] [Google Scholar]
- 31.Mohan W. S., Z. Q. Chen, X. Zhang, K. Kahlili, T. Honjo, R. G. Deeley, and S.P Tam. 1998. Human S mu binding protein-2 binds to the drug response element and transactivates the human apoA-I promoter: role of gemfibrozil. J. Lipid Res. 39 255–267. [PubMed] [Google Scholar]
- 32.Fallone F., P. H. Villard, L. Decome, E. Seree, M. Meo, C. Chacon, A. Durand, Y. Barra, and B. Lacarelle. 2005. PPAR alpha activation potentiates AhR-induced CYP1A-I expression. Toxicology. 216 122–128. [DOI] [PubMed] [Google Scholar]
- 33.Wallace J. L., L. J. Ignarro, and S. Fiorucci. 2002. Potential cardioprotective actions of no-releasing aspirin. Nat. Rev. Drug Discov. 5 375–382. [DOI] [PubMed] [Google Scholar]
- 34.Blatter-Garin M. C., B. Kalix, S. De Pree, and R. W. James. 2003. Aspirin use is associated with higher serum concentrations of the anti-oxidant enzyme, paraoxonase-1. Diabetologia. 46 593–594. [DOI] [PubMed] [Google Scholar]