Abstract
Hepatic overproduction of apolipoprotein B (apoB)-containing lipoproteins is characteristic of the dyslipidemia associated with insulin resistance. Recently, we demonstrated that the flavonoid naringenin, like insulin, decreased apoB secretion from HepG2 cells by activation of both the phosphoinositide-3-kinase (PI3-K) pathway and the mitogen-activated protein kinase/extracellular-regulated kinase (MAPKerk) pathway. In the present study, we determined whether naringenin-induced signaling required the insulin receptor (IR) and sensitized the cell to the effects of insulin, and whether the kinetics of apoB assembly and secretion in cells exposed to naringenin were similar to those of insulin. Immunoblot analysis revealed that insulin stimulated maximal phosphorylation of IR and IR substrate-1 after 10 min, whereas naringenin did not affect either at any time point up to 60 min. The combination of naringenin and submaximal concentrations of insulin potentiated extracellular-regulated kinase 1/2 activation and enhanced upregulation of the LDL receptor, downregulation of microsomal triglyceride transfer protein expression, and inhibition of apoB-100 secretion. Multicompartmental modeling of apoB pulse-chase studies revealed that attenuation of secreted radiolabeled apoB in naringenin- or insulin-treated cells was similar under lipoprotein-deficient or oleate-stimulated conditions. Naringenin and insulin both stimulated intracellular apoB degradation via a kinetically defined rapid pathway. Therefore, naringenin, like insulin, inhibits apoB secretion through activation of both PI3-K and MAPKerk signaling, resulting in similar kinetics of apoB secretion. However, the mechanism for naringenin-induced signaling is independent of the IR. Naringenin represents a possible strategy for reduction of hepatic apoB secretion, particularly in the setting of insulin resistance.
Keywords: apolipoprotein B, citrus flavonoid:, oleate, multicompartmental modeling, kinetics, degradation, microsomal triglyceride transfer protein, LDL receptor, phosphoinositide-3-kinase, mitogen-activated protein kinase/extracellular-regulated kinase
Naringenin, like insulin, modulates hepatic VLDL apolipoprotein B-100 (apoB-100) production through activation of intracellular signaling cascades, namely the phosphoinositide-3-kinase (PI3-K) and mitogen-activated protein kinase/extracellular-regulated kinase (MAPKerk) cascades (1, 2). Activation of these two pathways results in upregulation of the LDL receptor (LDLr) and inhibition of microsomal triglyceride transfer protein (MTP), leading to the inhibition of apoB-100 secretion (1–4). Exposure of cells to insulin rapidly activates intracellular signaling by tyrosine phosphorylation of the insulin receptor (IR) and insulin receptor substrate-1 (IRS-1) within 10 min (5). However, the involvement of the IR and IRS-1 in mediating naringenin-induced cell signaling has not been clearly established. Preliminary experiments demonstrated that exposure of HepG2 cells to naringenin for 6 h did not increase IRS-1/2 tyrosine phosphorylation, suggesting that naringenin may activate these pathways independent of signaling through IRS-1/2 (2). Therefore, we hypothesized that rapid activation of MAPKerk signaling by naringenin is independent of IR and IRS phosphorylation. Because insulin and naringenin inhibit apoB-100 secretion through activation of the same signaling mechanisms, we postulated that naringenin has the ability to potentiate the effects of insulin in HepG2 cells and that naringenin sensitizes cells to insulin. Thus, examination of the molecular events in hepatocytes exposed to naringenin may provide insight into novel mechanisms for reduction of hepatic overproduction of apoB-100-containing lipoproteins, a characteristic of the dyslipidemia associated with insulin resistance (6–8).
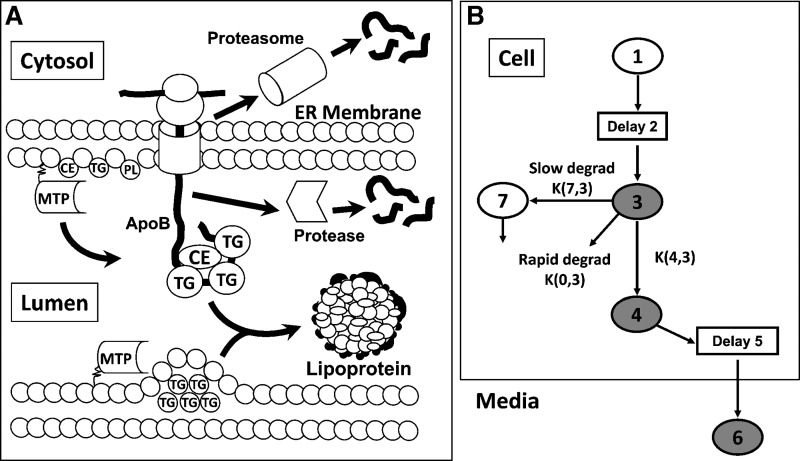
Secretion of apoB-100-containing lipoproteins into the circulation is a complex process that requires the coordinated assembly of apoB, triglyceride (TG), free cholesterol, cholesteryl ester (CE), and phospholipids (PLs) within the endoplasmic reticulum (ER) (as reviewed in Ref. 9) (Fig. 1A). MTP is an absolute requirement for apoB secretion, inasmuch as it facilitates transfer of TG, CE, and PL to apoB as well as accretion of ER luminal TG for subsequent transfer to apoB (10). Assembly and secretion of apoB-100 involves a) APOB mRNA transcription and translation, b) translocation of apoB-100 across the ER membrane, and either c) association of apoB-100 with core and surface lipid via MTP and transport through the secretory pathway into plasma, or d) intracellular apoB-100 degradation. Although the proportion of apoB secreted from hepatocytes is predominantly regulated by an intracellular pool of secretion-coupled lipid, and by the transfer of these lipids to the nascent lipoprotein (11), each step may be subject to regulation. Pulse-chase experiments have provided a detailed examination of the kinetic movement of apoB-100 throughout the synthesis and secretory pathways and have revealed that in HepG2 cells, degradation of apoB-100 is mainly via a rapid proteasomal pathway, and to a lesser extent by ER luminal proteases or in post-ER compartments (3, 11). Previous pulse-chase studies in HepG2 cells analyzed by multicompartmental modeling have shown that naringenin inhibits apoB-100 secretion via increased intracellular degradation of apoB that is partially mediated through inhibition of MTP activity (12). Insulin has also been shown to inhibit the secretion of apoB-100 from hepatocytes (1, 2, 4, 13–16), mediated in part through activation of PI3-K activity (1, 2, 4) and inhibition of MTP expression (1, 2). However, an extensive pulse-chase protocol coupled with multicompartmental modeling has not been used to characterize how insulin affects the kinetic movement of apoB-100 within hepatocytes.
Fig. 1.
Multicompartmental modeling of apolipoprotein B-100 (apoB-100) synthesis and secretion from HepG2 cells. A schematic representation of the assembly and secretion of apoB-100-containing lipoproteins from hepatocytes (A). Secretion of apoB-100-containing lipoproteins requires the assembly of apoB, triglyceride (TG), free cholesterol, cholesteryl ester (CE), and phospholipid (PL) within the endoplasmic reticulum (ER). Microsomal triglyceride transfer protein (MTP) is an absolute requirement for apoB secretion, facilitating the transfer of TG, CE, and PL to apoB as well as accretion of ER luminal TG for subsequent transfer to apoB. Assembly and secretion of apoB-100 involves a) apoB mRNA transcription and translation, b) translocation of apoB-100 across the ER membrane, and either c) association of apoB-100 with core and surface lipid via MTP and transport through the secretory pathway into plasma, or d) intracellular degradation via the cytoplasmic proteasome or luminal proteases. A diagram of the multicompartmental kinetic model used for analysis of apoB secretion and intracellular degradation (B). Compartments 1 to 5 and 7 are within the HepG2 cell. Compartment 6 represents apoB in the culture media. Compartments 1 and 2 represent an intracellular pool of tracer and a delay compartment to allow for apoB synthesis after introduction of the tracer, respectively. Compartment 3 represents newly synthesized apoB. A parameter termed “Init” calculated by the model represents the amount of radioactive apoB-100 entering the system (initially compartment 3) required to achieve the tracer curve for total apoB (cell plus media) measured experimentally. ApoB-100 from compartment 3 may be transferred to compartment 4 and subsequently secreted. From compartment 4, apoB passes through a delay, compartment 5, before secretion into the media, compartment 6. ApoB may be degraded directly from compartment 3 by a rapid degradation pathway. A second, more slowly turning over pool of apoB destined for degradation is represented by compartment 7. The shaded compartments represent the compartments containing apoB radioactivity that were determined experimentally.
The aim of this study is to compare the ability of naringenin and insulin to inhibit the secretion of apoB-100 in HepG2 cells and to determine whether naringenin activates rapid IR and IRS-1 phosphorylation. Furthermore, because naringenin and insulin both inhibit apoB-100 secretion via activation of the PI3-K and MAPKerk pathways, we hypothesize that exposure of cells to naringenin will potentiate or sensitize cells to the effects of insulin and that naringenin and insulin will induce similar kinetics for the attenuation of apoB-100 assembly and secretion from HepG2 cells.
RESEARCH DESIGN AND METHODS
Cell culture and chemicals
HepG2 cells were obtained from the American Type Culture Collection (Rockville, MD) and grown as described previously (17). For experiments, HepG2 cells were plated in 6-well (35 mm) culture dishes from Falcon Scientific (VWR, Mississauga, ON) and cultured in MEM containing 5% human lipoprotein-deficient serum (LPDS) or serum-free media [0.5% insulin-free, fatty acid-free (FAF) BSA (Sigma, St. Louis, MO)]. Naringenin (Sigma) was solubilized in DMSO (concentration in cell cultures did not exceed 0.5% for any of the treatments). Bovine pancreatic insulin (Sigma) was solubilized in 0.01 N HCl.
ApoB and extracellular-regulated kinase 1/2 immunoblotting
ApoB secretion into the media was measured by immunoblot analysis as previously described (2). HepG2 cells grown in 6-well (35 mm) culture dishes were incubated for 24 h with either insulin (25 nM or 100 nM), naringenin (25 μM or 100 μM), or the combination of insulin and naringenin at various concentrations. Media apoB-100 was determined following 4.5% SDS-PAGE, transfer to PVDF membranes, and immunoblot analyses (2). Where indicated, HepG2 cells were preincubated for 30 min in the presence or absence of UO126 (10 μM) or UO124 (10 μM) (Calbiochem, San Diego, CA) followed by a further 23.5 h with either insulin, naringenin, or naringenin plus insulin. For extracellular-regulated kinase 1/2 (ERK1/2) phosphorylation experiments, HepG2 cells were grown in 6-well (35 mm) culture dishes and were incubated overnight in serum-free media to induce quiescence. A dose-response was established in cells incubated with increasing doses of naringenin for 30 min or insulin for 15 or 30 min. The time-course was determined in cells incubated with DMSO alone, naringenin (25 μM or 100 μM), insulin (25 nM or 100 nM), or the combination of naringenin and insulin at various concentrations for up to 60 min. For sensitizing experiments, cells were preincubated with naringenin (100 μM) for 30 min, followed by the addition of insulin (25 nM) for another 30 min. ERK1/2 phosphorylation and total ERK1/2 protein levels were measured following 12% SDS-PAGE, transfer to PVDF membranes, and immunoblot analyses using a phospho-specific ERK1/2 antibody and an ERK1/2 antibody (all from Cell Signaling Technology, Beverly, MA) as previously described (1). ERK1/2 phosphorylation was expressed as a ratio of the phosphorylated band to the protein band relative to control.
IR and IRS-1 immunoprecipitation and immunoblotting
HepG2 cells were grown in 6-well (35 mm) culture dishes and were incubated overnight in serum-free media to induce quiescence. Cells were incubated with DMSO alone, naringenin (100 μM), or insulin (100 nM) for up to 60 min. IR activation was measured in cell lysates by immunoprecipitating the β-subunit of the IR or IRS-1 using polyclonal antibodies (Upstate Biotechnology, Charlottesville, VA) and protein G plus agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). The immunoprecipitant was then run on a 10% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. IR or IRS-1 activation was measured by immunoblotting with a phospho-tyrosine antibody (Upstate Biotechnology), and the amount of IR or IRS-1 was determined using the same antibody as was used to immunoprecipitate. Activation was determined as tyrosine-phosphorylated IR or IRS-1 expressed relative to total IR or IRS-1 protein levels, and the ratio was normalized to control.
Gene expression by quantitative real-time RT-PCR
The expression of genes was determined in HepG2 cells grown in 6-well culture dishes and incubated in LPDS-MEM ± treatments for 6 h. Briefly, RNA was extracted using Trizol reagent (Invitrogen, Burlington, ON). Target gene RNA quantitation was performed by quantitative real-time RT-PCR (qRT-PCR) on an ABI Prism (model 7900HT) Sequence Detection System (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Total RNA (10 μg) was reverse transcribed using the Applied Biosystems High-capacity cDNA archive kit according to the manufacturer's protocol. cDNA (20–30 ng) was assayed in 20 μl reactions using the Taqman Assays-on-demand qRT-PCR protocol from Applied Biosystems. The primer probe sets for MTP, the LDLr, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were obtained from Applied Biosystems (Hs00165177_m1, Hs00181192_m1, Hs99999905_m1). The standard curve method was used to determine mRNA abundance. Expression of MTP and expression of LDLr were normalized to GAPDH expression.
Pulse-chase studies
Secreted and cellular apoB-100, synthesized in the absence or presence of 0.1 mM oleic acid (OA) (Sigma) (complexed to FAF-BSA) was measured following preincubation of cells for 24 h in the absence or presence of insulin (100 nM) or naringenin (100 μM). OA (0.1 mM) was added to the media for 20 min prior to the pulse. Cells were pulsed for 10 min with 100 μCi/ml Tran 35S-label (1,000 Ci/mmol, l-[35S]methionine and l-[35S]cysteine; ICN, Costa Mesa, CA) and chased for a further 120 min (18). Media and cellular apoB-100 were immunoprecipitated using a polyclonal anti-human apoB antibody obtained from Boehringer Mannheim (Montreal, Canada) for the pulse-chase studies in LPDS media or from Midland Bioproducts Corporation (Boone, IA) for the pulse-chase studies with OA supplementation. ApoB-100 was resolved and quantitated as described previously (18).
Multicompartmental modeling
Data obtained from pulse-chase experiments, which included time points from 0 to 130 min postpulse, were analyzed by multicompartmental modeling using the SAAM II program (SAAM Institute, Seattle, WA) as previously described (12). We previously reported a model describing apoB synthesis, secretion, and degradation, which was developed using full-length apoB (apoB-100) radioactivity data obtained from pulse-chase experiments (18). Figure 1B shows the compartments and pathways between compartments included in the model. The model includes intracellular compartments and a single extracellular compartment. The shaded compartments within the cell represent apoB-100 radioactivity measured in cell lysates. The media compartment (compartment 6) represents apoB-100 radioactivity in media samples. Compartment 1 is a dosing compartment for the 35S label. The transport of tracer into cells from the media was assumed to be essentially instantaneous. The second compartment (Delay 2) accounts for the time from the initial pulse until cellular radioactivity in immunoprecipitable apoB-100 was detected. Compartments 3, 4, and 7 describe the kinetics of intracellular apoB-100 radioactivity. A parameter termed “Init,” determined during modeling, represents the amount of radioactive apoB-100 entering the system (initially compartment 3) required to achieve the tracer curve for total apoB (cell plus media) measured experimentally. Rate constants calculated by the model allow for quantitation of the movement of radioactivity incorporated into apoB-100 between compartments, as well as the loss (secretion and degradation) of newly synthesized apoB from the cell.
Statistical analysis
Values are presented as mean ± SEM of at least three experiments performed in duplicate. Means were compared by a one-way ANOVA with posthoc Tukey's tests to determine statistical significance using SPSS 16.0. P < 0.05 was considered significant.
RESULTS
Naringenin does not activate the IR or IRS-1 in cultured hepatocytes
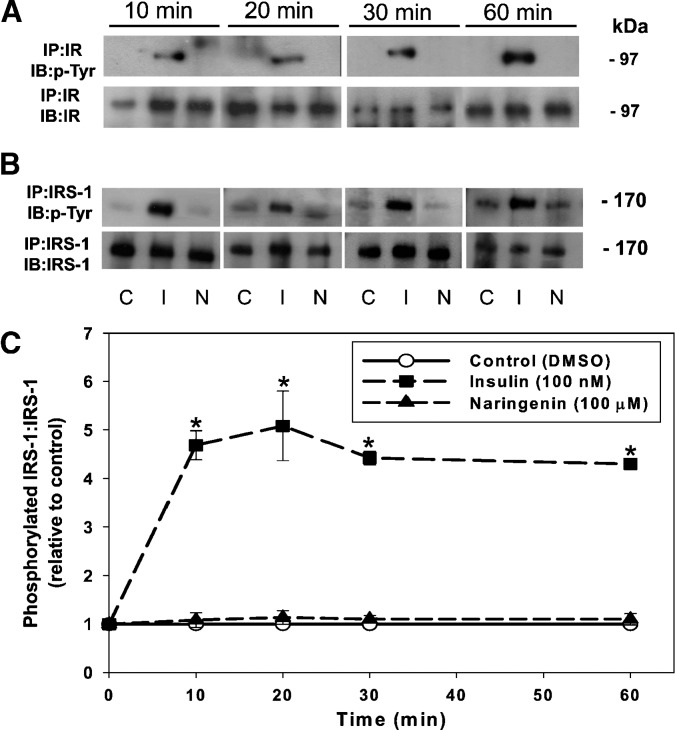
Naringenin, like insulin, activates the MAPKerk pathway to inhibit MTP expression and apoB secretion (1); however, it is unknown whether naringenin does so via rapid activation of the IR and IRS-1. HepG2 cells were incubated with insulin (100 nM) and naringenin (100 μM), concentrations that decreased apoB-100 secretion by approximately 50% (2, 12). Control (DMSO-treated) HepG2 cells displayed no tyrosine phosphorylation of the immunoprecipitated β-subunit of the IR at any time point from 0 to 60 min (Fig. 2A). Insulin caused rapid tyrosine phosphorylation of the IR at 10 min, which remained activated up to 60 min. In contrast, naringenin did not cause tyrosine phosphorylation of the IR at any time point up to 60 min.
Fig. 2.
Naringenin does not signal through the insulin receptor (IR) or insulin receptor substrate-1 (IRS-1). HepG2 cells were incubated with DMSO (C), insulin (I) (100 nM) or naringenin (N) (100 μM) for 10, 20, 30, or 60 min in serum-free media. Cell lysates were immunoprecipitated with an anti-IR (β-subunit) (A) or anti-IRS-1 (B) antibody. Half of each sample was run on one of two separate 10% gels, transferred to a membrane, and then probed with either an anti-phospho-tyrosine antibody (p-Tyr) (A and B), an anti-IR (β-subunit) (A), or anti-IRS-1 (B) antibody. C: For IRS-1, the level of activation was determined by expressing the amount of phosphorylated protein relative to total protein. Values are the mean ± SEM and are presented as the ratio of the phosphorylated form to total protein (relative to DMSO control). *P < 0.05 compared with control.
We observed basal tyrosine phosphorylation of IRS-1 in HepG2 cells, which was not stimulated in the presence of naringenin (100 μM) at 10, 20, 30, or 60 min (Fig. 2B). However, as expected, insulin (100 nM) maximally increased the tyrosine phosphorylation of IRS-1 at 10 min, which remained significantly elevated over the 60 min time-course. The role of IRS-2 was not pursued, inasmuch as its expression in HepG2 cells is very low compared with IRS-1 (data not shown).
Naringenin amplifies the ability of insulin to activate ERK1/2
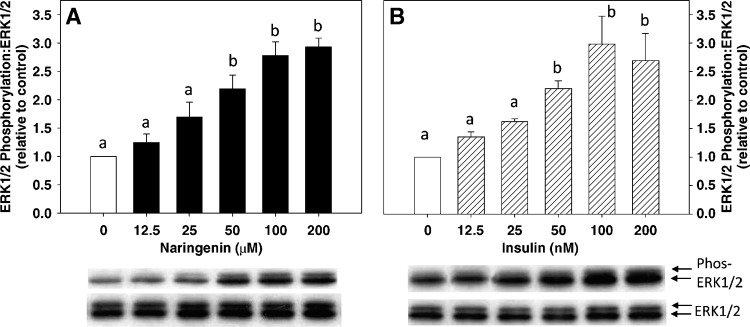
Previously, we reported that in HepG2 cells, MAPKerk is activated by insulin (100 nM) and naringenin (100 μM), resulting in maximal ERK1/2 phosphorylation at 15 min and 30 min, respectively (1). Furthermore, inhibition of ERK1/2 phosphorylation blocked the inhibitory effect of both insulin and naringenin on apoB-100 secretion (1). In the present study, we tested the hypothesis that the addition of insulin to naringenin would enhance ERK1/2 phosphorylation. Initially, a dose-response for ERK1/2 activation by both insulin and naringenin was determined. HepG2 cells were incubated with increasing doses of naringenin for 30 min or insulin for 15 min, and the activation of ERK1/2 was determined. Naringenin dose-dependently increased ERK1/2 phosphorylation up to 2.8-fold at 100 μM, which did not increase further at 200 μM (Fig. 3A). Insulin induced a dose-dependent increase in ERK1/2 phosphorylation, reaching a maximum of 3-fold at 100 nM. Increasing the insulin concentration to 200 nM did not produce any further phosphorylation of ERK1/2 (Fig. 3B). Although quantitatively lower, the pattern for ratios of ERK1/2 phosphorylation to total ERK1/2 in cells incubated with insulin for 30 min was similar to that observed for 15 min of insulin exposure (data not shown).
Fig. 3.
Naringenin and insulin dose-dependently increase extracellular-regulated kinase 1/2 (ERK1/2) phosphorylation. HepG2 cells in serum-free media were treated with DMSO (control) and 12.5, 25, 50, 100, or 200 μM naringenin for 30 min (A) or DMSO and 12.5, 25, 50, 100, or 200 nM insulin for 15 min (B). Total and phosphorylated ERK1/2 were measured by immunoblot analysis and presented as a ratio of the phosphorylated form to total ERK1/2 protein (relative to DMSO control). Values are the mean ± SEM for six experiments. Values with different letters are significantly different at P < 0.05.
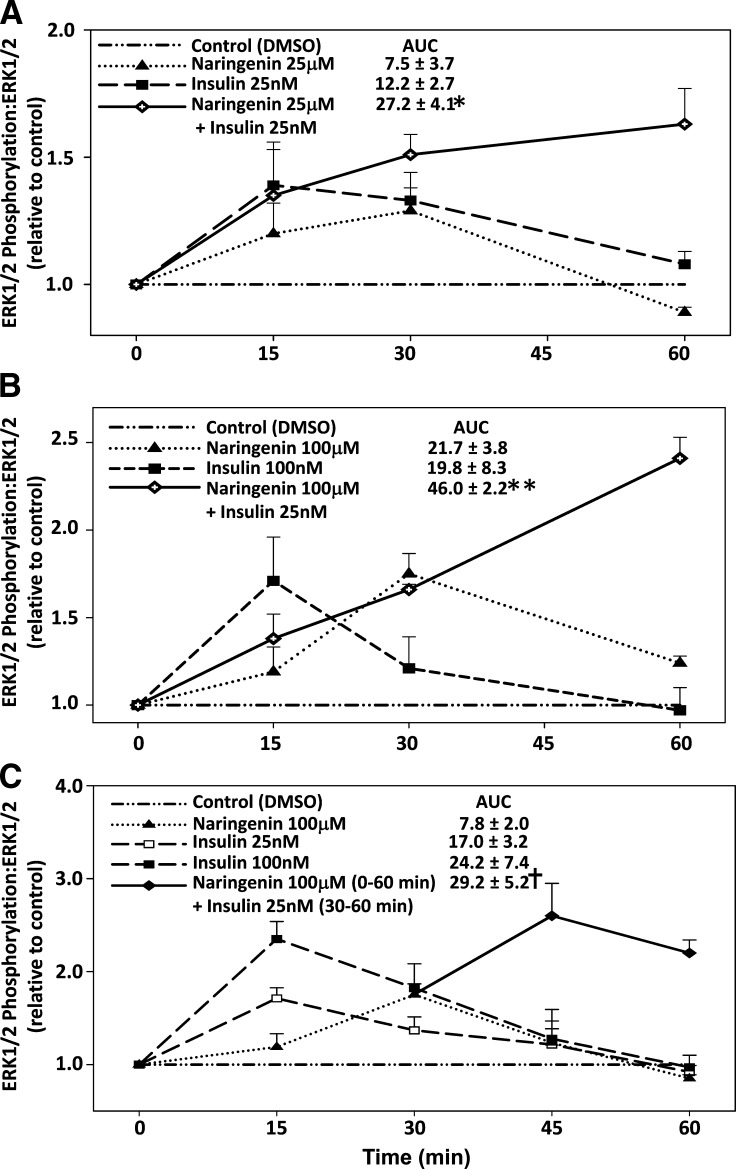
This observation, together with our finding that naringenin and insulin activate the MAPKerk pathway via different mechanisms, suggested that the addition of naringenin to insulin would potentiate ERK1/2 phosphorylation. To test this, HepG2 cells were initially incubated with naringenin (25 μM) plus insulin (25 nM), concentrations that induced submaximal ERK1/2 phosphorylation when used alone (Fig. 3A, B). Naringenin (25 μM) plus insulin (25 nM) resulted in a 1.5-fold and a 1.7-fold increase in ERK1/2 phosphorylation at 30 and 60 min, respectively (Fig. 4A). These increases were greater than the maximal increase for ERK1/2 phosphorylation observed for insulin (25 nM) alone or naringenin (25 μM) alone. Furthermore, the increase in ERK1/2 phosphorylation was sustained for up to 60 min, when the phospho-ERK1/2 response to either compound alone had returned to baseline. The maximal increase in ERK1/2 phosphorylation for naringenin (25 μM) plus insulin (25 nM) was similar to the maximal values observed for 100 μM naringenin (1.75-fold at 30 min) and for 100 nM insulin (1.7-fold at 15 min) when used alone (Fig. 4B). Incremental area under the curve for ERK1/2 phosphorylation from 0–60 min for naringenin (25 μM) plus insulin (25 nM) (27.2 ± 4.1) was significantly greater (P < 0.05) than either 25 μM naringenin (7.5 ± 3.7) or 25 nM insulin (12.2 ± 2.7) when used alone. A similar pattern of ERK1/2 phosphorylation was observed when naringenin (100 μM) was combined with insulin (25 nM), although the response was amplified further (Fig. 4B). With this combination, ERK1/2 phosphoryation increased 1.7-fold at 30 min and 2.4-fold at 60 min, which was similar to or greater than values observed for naringenin alone (100 μM) or insulin (100 nM) at any of the time points examined. Area under the curve analysis for ERK1/2 phosphorylation from 0–60 min demonstrated that naringenin (100 μM) plus insulin (25 nM) (46.0 ± 2.2) was significantly greater (P < 0.05) than either naringenin alone at both 25 μM (7.5 ± 3.7) and 100 μM (21.7 ± 3.8) or insulin alone at both 25 nM (12.2 ± 2.7) and 100 nM (19.8 ± 8.3), suggesting that the effects are synergistic.
Fig. 4.
Addition of naringenin to insulin enhances and prolongs ERK1/2 activation in HepG2 cells. HepG2 cells in serum-free media were incubated with DMSO, naringenin (closed triangle), insulin (closed square), or insulin and naringenin added simultaneously (open diamond) from 0 to 60 min. Total and phosphorylated ERK1/2 for incubations with 25 μM naringenin alone, 25 nM insulin alone, and insulin (25 nM) plus naringenin (25 μM) (A) and 100 μM naringenin alone, 100 nM insulin alone, and 100 μM naringenin plus 25 nM insulin (B) were measured by immunoblotting. C: HepG2 cells in serum-free media were pretreated for 30 min with DMSO or naringenin (100 μM) followed by the addition of 25 nM insulin for a subsequent 30 min (closed diamond). Cells were also treated with insulin alone (at both 25 nM, open squares and 100 nM, closed squares) or 100 μM naringenin alone (closed triangles) from 0 to 60 min. Total and phosphorylated ERK1/2 were measured by immunoblot analysis and presented as a ratio of phosphorylated form to total ERK1/2 protein (relative to DMSO control). Mean incremental area under the curve (AUC) ± SEM are presented for four experiments. *P < 0.05 for simultaneous addition of naringenin (25 μM) plus insulin (25 nM) compared with naringenin alone (25 μM) and insulin alone (25 nM). **P < 0.05 for simultaneous addition of 100 μM naringenin plus 25 nM insulin compared with naringenin alone (25 and 100 μM), insulin alone (25 and 100 nM), and the simultaneous addition of naringenin (25 μM) and insulin (25 nM). †P < 0.05 for naringenin (100 μM) (0–60 min) plus insulin (25 nM) (30–60 min) compared with insulin alone (25 nM) and naringenin alone (100 μM).
We next examined the potential for naringenin to sensitize HepG2 cells to insulin-stimulated ERK1/2 phosphorylation. HepG2 cells were preincubated for 30 min with 100 μM naringenin before adding 25 nM insulin. A time of 30 min was used because this was the time at which maximal ERK1/2 phosphorylation was observed for naringenin alone (Fig. 4B). ERK1/2 phosphorylation peaked 15 min following the addition of insulin, reaching values 2.6-fold greater than baseline (Fig. 4C). This was greater than peak values for insulin alone at 15 min (1.7-fold for 25 nM insulin, and 2.3-fold for 100 nM insulin) or naringenin alone at 30 min (1.8-fold at 100 μM). At 30 min following the addition of insulin (25 nM) to cells preincubated for 30 min with naringenin (100 μM), ERK1/2 phosphorylation was substantially greater than values for insulin or naringenin alone at the same time point. Area under the curve for ERK1/2 phosphorylation from 0–60 min for cells preincubated with naringenin (100 μM) for 30 min followed by the addition of insulin (25 nM) was significantly greater (P < 0.05) than values obtained over the same time-course by naringenin (100 μM) alone or insulin (25 nM) alone.
Naringenin amplifies the ability of insulin to regulate the expression of MTP and the LDLr
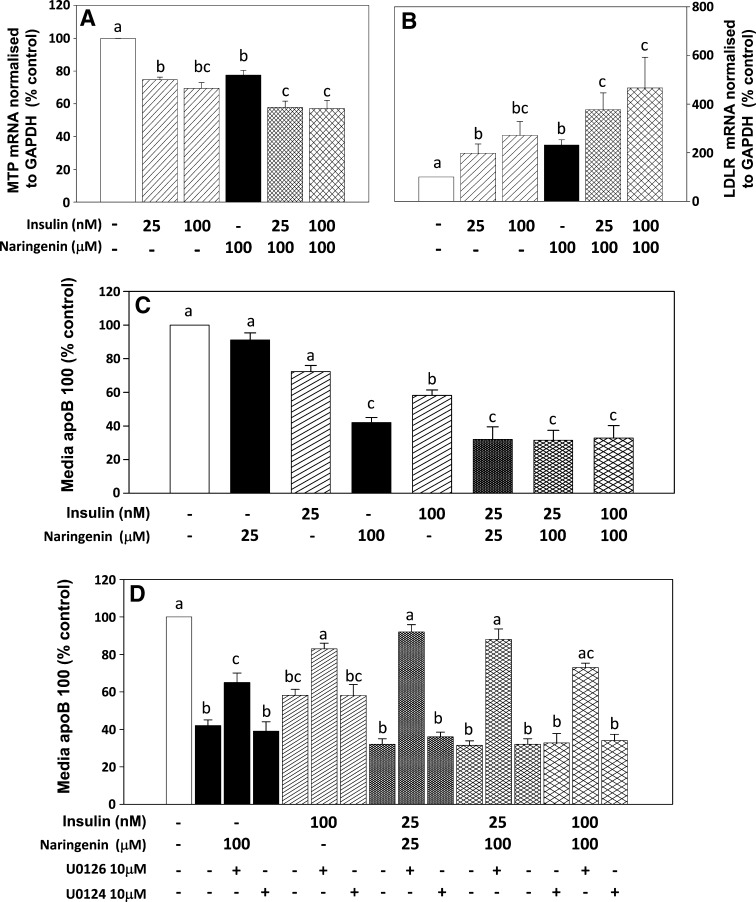
The expression of MTP and the LDLr in HepG2 cells is regulated by both naringenin and insulin through activation of the MAPKerk and PI3-K pathways (1, 2). To determine whether the ability of naringenin to amplify insulin-stimulated ERK1/2 phosphorylation extends to the regulation of MTP and LDLr expression, HepG2 cells were incubated for 6 h with naringenin (100 μM) alone or insulin (25 nM or 100 nM) alone, or were preincubated with naringenin (100 μM) followed by addition of insulin (25 nM). Insulin dose-dependently decreased MTP expression by 25% at 25 nM and 35% at 100 nM (P < 0.05 for both) relative to control. Preincubation with naringenin (100 μM) for 30 min followed by addition of insulin (25 nM) for a subsequent 5.5 h decreased MTP mRNA by 40% (P < 0.05), which was a greater reduction than that observed for 25 nM insulin alone or 100 μM naringenin alone (P < 0.05), and similar to that observed for 100 nM insulin alone (Fig. 5A). Preincubation with naringenin (100 μM) for 30 min followed by addition of insulin (100 nM) for a subsequent 5.5 h resulted in no further decrease in MTP mRNA expression. Incubation of cells with insulin for 6 h dose-dependently increased LDLr expression 2.5-fold in the presence of 25 nM insulin and 3-fold with 100 nM insulin (P < 0.05 for both). Preincubation of cells with naringenin for 30 min prior to the addition of insulin (25 nM) for an additional 5.5 h increased LDLr expression 5-fold (P < 0.05), which was greater than that observed for 25 nM insulin alone (P < 0.05), 100 nM insulin alone, or 100 μM naringenin alone (P < 0.05) (Fig. 5B). Preincubation with naringenin (100 μM) for 30 min followed by addition of insulin (100 nM) for a subsequent 5.5 h resulted in no further increase in LDLr mRNA expression. These observations suggest that naringenin potentiates the effects of insulin on expression of MTP and expression of LDLr in HepG2 cells, both of which are determinants of net apoB-100 secretion.
Fig. 5.
Naringenin sensitizes HepG2 cells to the effects of insulin on mRNA expression of both MTP and the LDLr and apoB-100 secretion. Cells were pretreated with or without naringenin (100 μM) for 30 min before being treated with 25 nM or 100 nM insulin for a combined time of 6 h (cross-hatched bars). Insulin alone (25 nM or 100 nM, striped bars) for 6 h and naringenin alone (100 μM, solid bars) are also shown. MTP (A) or LDLr (B) expression was determined by quantitative real-time RT-PCR and normalized to GAPDH. Values are the mean ± SEM for five experiments. C: HepG2 cells were incubated in LPDS media for 24 h in the presence of DMSO, naringenin alone, insulin alone, or the combination of naringenin plus insulin at the indicated concentrations. Media was collected and apoB-100 measured by immunoblotting. D: HepG2 cells in LPDS media were preincubated for 30 min with DMSO, the MEK1/2 inhibitor UO126 (10 μM), or its inactive isoform UO124 (10 μM) followed by a 23.5 h incubation with naringenin alone, insulin alone, or the combination of naringenin plus insulin at the indicated concentrations. Media apoB-100 was measured by immunoblotting. Values are the mean ± SEM for five experiments. Values with different letters are significantly different at P < 0.05.
Naringenin amplifies insulin's ability to inhibit apoB secretion
The impact of combining naringenin and insulin on apoB secretion was determined in HepG2 cells incubated for 24 h with either compound alone or in combination (Fig. 5C). ApoB in the media was significantly decreased, by 68% (P < 0.05), by the combination of naringenin (25 μM) plus insulin (25 nM). This was a significantly greater reduction than for either naringenin alone at 25 μM (−9%) or insulin alone at 25 nM (−28%), suggesting a synergistic reduction of apoB secretion with this combination. This 68% decrease was greater than insulin alone at 100 nM (−41%) and similar to that observed for naringenin alone at 100 μM (−58%). Addition of 25 nM insulin to naringenin (100 μM) decreased apoB secretion by 68%, which was greater than that observed for naringenin alone (at both 25 μM and 100 μM) and insulin alone (at both 25 nM and 100 nM), and the same as the combination of naringenin (25 μM) plus insulin (25 nM). The combination of 100 μM naringenin plus 100 nM insulin also decreased apoB secretion by 68%, suggesting that maximal reduction by the combination was achieved by 25 μM naringenin plus 25 nM insulin.
ApoB secretion is inhibited by both naringenin alone (100 μM) (−58%; P < 0.05) and insulin alone (100 nM) (−42%; P < 0.05) in a MAPKerk-dependent manner (Fig. 5D). Addition of UO126, a specific MEK1/2 inhibitor, resulted in significant attenuation of the effects of both naringenin and insulin, whereas the inactive isoform UO124 had no effect. These results confirm our previous observations (1). Figure 5D also demonstrates that the reduction of apoB secretion by the combination of naringenin plus insulin is dependent on MAPKerk. Addition of UO126 significantly blocked the majority of the effect on apoB secretion for all combinations, whereas UO124 had little or no effect. Therefore, inhibition of apoB secretion by the combination of naringenin plus insulin is primarily regulated by activation of the MAPKerk pathway.
Naringenin and insulin both increase the degradation of apoB-100 through a kinetically defined, rapid pathway
Activation of the PI3-K and MAPKerk signaling pathways by naringenin or insulin leads to the inhibition of apoB-100 secretion from HepG2 cells (1, 2), suggesting that the kinetics of assembly and secretion of apoB-containing lipoproteins would be modulated through similar mechanisms. Although we have previously conducted detailed pulse-chase studies in HepG2 cells treated with naringenin, in which the kinetic parameters for the intracellular trafficking and secretion of apoB were quantitated using multicompartmental modeling (12), similar quantitative studies have not been reported for insulin. Therefore, pulse-chase studies were conducted in HepG2 cells incubated with naringenin (100 μM) and insulin (100 nM) under basal (LPDS) conditions or following OA treatment, which stimulates apoB secretion. These concentrations of naringenin and insulin inhibit the accumulation of apoB-100 in the media of HepG2 cells by approximately 50% over 24 h [(1) and Fig. 5C]. Prior to the start of each pulse-chase, cells were preincubated for 24 h with or without naringenin or insulin in LPDS media alone. In some experiments, OA (0.1 mM) was added to the media for 20 min prior to the pulse. Media and cell lysates were collected at 10 time points throughout the pulse and the chase up to 2 h.
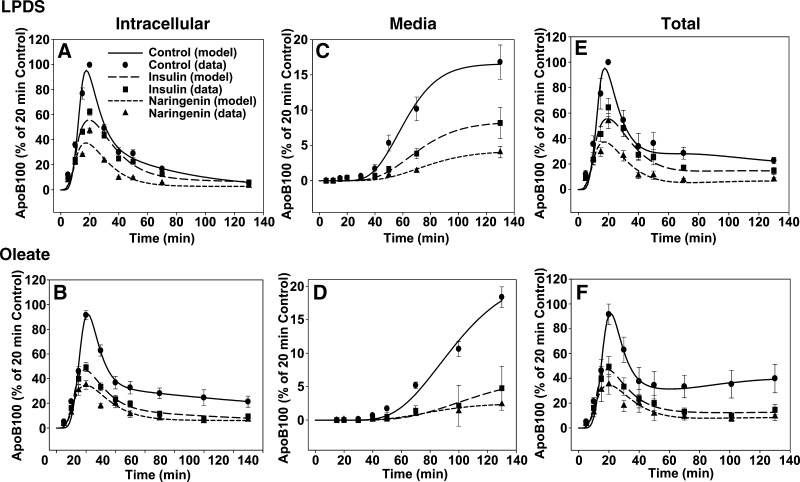
The data points shown in Fig. 6A–D represent radioactivity in full-length apoB-100 measured experimentally. The curves in each graph are fits to the experimental data obtained from analyses using the multicompartmental model shown in Fig. 1B. Under basal conditions, naringenin and insulin significantly decreased the amount of intracellular and secreted apoB-100 (Fig. 6A, C). Even under oleate-stimulated conditions, in which apoB-100 secretion is substantially increased in control cells, naringenin and insulin significantly decreased the intracellular accumulation and secretion of newly synthesized apoB-100 (Fig. 6B, D). Inspection of the curves indicated that under both conditions, naringenin and insulin decrease the peak of radioactivity in apoB-100 during the pulse and early chase period, prior to the appearance of any radiolabeled apoB-100 in the media. Although this is normally interpreted as a decrease in the synthesis of a protein, it has been well established that within hepatocytes, apoB-100 degradation can occur cotranslationally as well as post-translationally (3, 19). Cotranslational degradation is observed experimentally as a decrease in radiolabeled full-length apoB-100 appearing in the cell. Detailed pulse-chase protocols for apoB-100 cannot distinguish between apoB-100 synthesis and cotranslational degradation. In previous studies, we demonstrated, using the same protocol and multicompartmental modeling analyses, that in HepG2 cells incubated with lactacystin or ALLN to inhibit proteasomal degradation, peak intracellular apoB-100 radioactivity was substantially higher than in control cells (12). This increased peak radioactivity was interpreted as inhibition of cotranslational degradation, permitting increased availability of apoB-100 for particle formation. In the present studies peak intracellular apoB-100 radioactivity in insulin- or naringenin-treated cells was substantially lower than that of control cells (Fig. 6A, B). Taken together with our observations and those of others that neither naringenin nor insulin affects apoB mRNA (1, 14, 20–22), our results are consistent with the interpretation that both insulin and naringenin stimulate cotranslational degradation of apoB. However, an effect of insulin or naringenin on apoB-100 synthesis cannot be completely ruled out. The curves for total apoB-100 radioactivity (Fig. 6E, F), which is determined as the sum of intracellular (Fig. 6A, B) and media apoB-100 (Fig. 6C, D), show that naringenin and insulin increase the degradation of apoB-100 in a similar manner.
Fig. 6.
Naringenin and insulin similarly inhibit the secretion of apoB-100 from HepG2 cells through a kinetically defined rapid intracellular degradation pathway. HepG2 cells were preincubated for 24 h in lipoprotein-deficient serum (LPDS) with naringenin (100 μM, triangles), insulin (100 nM, squares), or DMSO (control, circles). Cells were then incubated for a further 20 min in the absence (A, C, E) or presence (B, D, F) of 0.1 mM oleic acid prior to pulse labeling (10 min). Cells were then chased for a further 120 min in the presence of their respective treatments in LPDS or oleate-enriched media. Intracellular apoB-100 radioactivity is shown in panels A and B, apoB-100 radioactivity secreted into the media is shown in panels C and D, and total apoB-100 radioactivity (determined as the sum of apoB-100 in the media and in the cell) is shown in panels E and F. The symbols in each graph represent apoB-100 radioactivity measured experimentally and are expressed as the mean ± SEM for four experiments (LPDS) and five experiments (oleate). The curves in each graph are fits to the experimental data obtained from analyses using the multicompartmental model shown in Fig. 1.
Mathematical analysis of the cellular apoB-100 kinetics by multicompartmental modeling allowed us to define and quantitate the relative contributions of each pathway for degradation and secretion for each pulse-chase experiment. The major kinetic parameters are listed in Table 1. The Init parameter indicates the amount of radioactive full-length apoB-100 entering the system required to achieve the tracer curve for total apoB-100 (cell plus media) measured experimentally. This parameter is directly related to the peak apoB-100 radioactivity achieved, and thus provides a quantitative estimate of the amount of apoB-100 available for particle assembly, and does not include partially synthesized apoB that is degraded cotranslationally. Compared with control cells, the Init parameter decreased significantly in both naringenin- (−64%) and insulin- (−51%) treated cells cultured under basal conditions. This parameter increased +65% (P < 0.05) with the addition of OA, and was significantly decreased by the addition of either naringenin (−56%) or insulin (−49%). In cells cultured under basal conditions, the percent of apoB secreted was significantly reduced by both naringenin (−65%) and insulin (−53%), whereas total degradation was increased similarly. This degradation of apoB-100 is independent of the Init parameter and therefore is independent of cotranslational apoB degradation. The decreased secretion for both treatments was reflected in a decrease in the rate constant for secretion, k(4,3), and was due primarily to increases in the rate constant for rapid degradation, k(0,3), although increases in the slow degradation pathway, k(7,3), which represents less than 5% of total degradation, were observed. However, the changes in k(7,3) did not reach statistical significance. The addition of OA prior to and during the pulse and chase period increased the percent of radiolabeled apoB-100 secreted over 2-fold in control cells compared with LPDS media, resulting in a proportional reduction in apoB-100 degradation. The addition of OA exerts its stimulation of secretion by decreasing the rapid component of intracellular degradation, k(0,3). In OA-treated cells, the percent of apoB secreted was also significantly decreased by both naringenin (−77%) and insulin (−71%), whereas the total degradation of apoB-100 increased proportionally. The decreased secretion for both treatments was reflected by a decrease in the rate constant for secretion, k(4,3), and was due to significant increases in the rate constant for rapid degradation, k(0,3), although nonsignificant increases in the slow degradation pathway, k(7,3), were observed. Previously, we reported that degradation from the rapidly turning over pool was largely proteasomal, inasmuch as it was selectively inhibited by both ALLN and lactacystin (12). This suggests that the increased rapid degradation of apoB-100 induced by either naringenin or insulin is similar and occurs via the cytoplasmic proteasome.
TABLE 1.
Naringenin and insulin similarly stimulate the degradation and inhibit the secretion of apoB-100 from HepG2 cells
| Control | Naringenin | Insulin | |
|---|---|---|---|
| LPDS | 100 μM | 100 nM | |
| Init (arbitrary units)c | 52.23 ± 7.61a | 18.98 ± 2.88b | 25.94 ± 8.18b |
| Total degraded (%)d | 93.43 ± 1.25a | 97.67 ± 0.34b | 96.89 ± 0.47b |
| Total secreted (%)e | 6.57 ± 1.25a | 2.33 ± 0.34b | 3.11 ± 0.47b |
| k(4,3) (pools/min)f | 0.008 ± 0.001a | 0.004 ± 0.001b | 0.004 ± 0.001b |
| k(0,3) (pools/min)f | 0.117 ± 0.022a | 0.152 ± 0.014b | 0.122 ± 0.006a |
| k(7,3) (pools/min)f | 0.003 ± 0.003a | 0.007 ± 0.003a | 0.010 ± 0.003a |
| Portion degraded via rapid pathway (%)g | 91.4 ± 1.6a | 93.9 ± 1.4a | 89.3 ± 2.4a |
| Oleate | |||
| Init (arbitrary units)c | 86.50 ± 12.8a | 30.08 ± 8.48b | 43.64 ± 9.31b |
| Total degraded (%)d | 84.81 ± 4.05a | 96.53 ± 0.87b | 95.66 ± 1.11b |
| Total secreted (%)e | 15.19 ± 4.05a | 3.47 ± 0.87b | 4.34 ± 1.11b |
| k(4,3) (pools/min)f | 0.008 ± 0.002a | 0.0026 ± 0.0004b | 0.0030 ± 0.0007b |
| k(0,3) (pools/min)f | 0.055 ± 0.014a | 0.081 ± 0.010b | 0.070 ± 0.009a |
| k(7,3) (pools/min)f | 0.0007 ± 0.0002a | 0.0011 ± 0.0002a | 0.0008 ± 0.0001a |
| Portion degraded via rapid pathway (%)g | 83.78 ± 4.04a | 95.29 ± 0.85b | 94.51 ± 1.09b |
Apolipoprotein B-100 (apoB-100) pulse-chase data were analyzed by multicompartmental modeling using SAAM II. The percent of newly synthesized apoB-100 secreted and degraded was determined using the kinetic model. Values are mean ± SEM for lipoprotein-deficient serum (LPDS) (n = 4) and oleate (n = 5).
Means with different letters indicate statistical difference of at least P < 0.05.
Means with different letters indicate statistical difference of at least P < 0.05.
The Init parameter is calculated by the model and represents the amount of radioactive apoB-100 entering the system (initially compartment 3) required to achieve the tracer curve for total apoB (cell plus media) measured experimentally.
Calculated using the formula [k(0,3) + k(7,3) / k(4,3) + k(0,3) + k(7,3)] × 100.
Calculated using the formula [k(4,3) / k(4,3) + k(0,3) + k(7,3)] × 100.
k(4,3) is the rate constant for apoB-100 transfer from compartment 3 to compartment 4, or the rate constant for secretion. k(0,3) is the rate constant for apoB-100 degradation directly from compartment 3, or the rate constant for rapid degradation. k(7,3) is the rate constant for apoB-100 transfer from compartment 3 to compartment 7, or the rate constant for slow degradation.
The percent of apoB-100 degraded directly from compartment 3 is calculated using the formula [k(0,3) / k(4,3) + k(0,3) + k(7,3)] × 100.
DISCUSSION
A major metabolic abnormality associated with insulin resistance is dyslipidemia characterized by the overproduction of hepatic VLDL-apoB-100, which contributes to the increased risk of cardiovascular disease in this population (6–8). The failure of insulin to suppress hepatic de novo lipogenesis, increased free fatty acid (FFA) flux, TG synthesis, and decreased fatty acid oxidation collectively lead to increased VLDL overproduction. Although the mechanisms regulating the assembly and secretion of hepatic apoB-100-containing lipoproteins have been described (3, 9, 11, 23), potential treatments to correct this imbalance in VLDL production have not been identified. We have previously shown that naringenin, like insulin, inhibits apoB-100 secretion via activation of two signaling pathways, namely PI3-K and MAPKerk (1, 2). In this study, we show that in contrast to insulin, the ability of naringenin to decrease apoB secretion in HepG2 cells does not involve signaling through the IR. Naringenin activates MAPKerk and PI3-K through a mechanism that does not require phosphorylation of the IR or IRS-1. Furthermore, we demonstrate that naringenin is able to sensitize HepG2 cells to insulin and/or potentiate the effects of insulin on ERK1/2 signaling, the mRNA expression of both MTP and the LDLr, and apoB secretion. Even though naringenin and insulin utilize different mechanisms to initiate intracellular signaling, they inhibit the assembly and secretion of apoB-100 with similar kinetics.
The rapid increase in ERK1/2 phosphorylation [Fig. 4 and (1)] and PI3-K activity (2) by naringenin parallels that of insulin. However, we show for the first time that naringenin activation of both pathways occurs independent of IR phosphorylation. Furthermore, we demonstrate that naringenin does not activate the downstream effector of IR activation, IRS-1, within 1 h of exposure. This is consistent with our previous observations that naringenin fails to phosphorylate IRS-1 in HepG2 cells at 6 h (2). Nevertheless, the mechanism for the naringenin-induced activation of PI3-K and MAPKerk, which is linked to the regulation of apoB-100 secretion, is unknown. The activation of the MAPKerk pathway in hepatocytes by insulin has been shown to require activation of Raf-1, the most upstream member of the MAPKerk signaling cascade, whereas the activation of Ras proteins, another important upstream mediator of MAPKerk signaling, is not required (24). Ras activation requires farnesylation (25). In preliminary experiments in HepG2 cells, we found that the phosphorylation of ERK1/2 by naringenin, but not insulin, involves farnesylation (unpublished observations), supporting the concept that initiation of MAPKerk signaling by naringenin occurs through a mechanism distinct from insulin. However, the identity of the cellular receptor and upstream mediators responsible for the activation of Ras by naringenin remains to be elucidated.
In a previous report, using multicompartmental analysis of pulse-chase experiments, we demonstrated that naringenin inhibits apoB-100 secretion and stimulates apoB-100 degradation, in both the absence of lipoproteins and the presence of OA (12). However, this is the first report of multicompartmental analyses of pulse-chase data to define the ability of insulin to modulate the kinetics of apoB-100 assembly and secretion. Analysis revealed that naringenin and insulin modulated the intracellular kinetics of apoB-100 in a very similar manner, in both the absence and presence of OA. Both compounds decrease the availability of apoB for particle formation, with very similar kinetics. Furthermore, insulin, like naringenin, increases intracellular degradation of newly formed apoB-100, and this degradation is from a kinetically defined, rapidly turning over cellular apoB pool. The decrease in apoB availability could be due to decreased synthesis or enhanced cotranslational degradation of apoB; however, pulse-chase studies cannot distinguish between the effects of naringenin or insulin on synthesis versus cotranslational degradation. Although it has been reported that insulin inhibits hepatocyte apoB synthesis in a cell-free translation system (16), this has not been a consistent finding in other studies. In rat hepatocytes, insulin was shown to inhibit the maturation phase of VLDL assembly by preventing lipid transfer to pre-VLDL particles, resulting in preferential degradation of apoB-100 from these pre-VLDL particles (26). Furthermore, Chirieac et al. (27) examined apoB-100 secretion by pulse-chase in primary mouse hepatocytes and concluded that blocking the effect of insulin with wortmannin increased the amount of apoB-100 available for secretion. It was not possible to distinguish decreased synthesis from increased cotranslational degradation. The availability of apoB-100 is greatly enhanced by proteasomal inhibitors, suggesting that this parameter largely reflects cotranslational degradation (12). However, an effect of insulin or naringenin on apoB-100 synthesis cannot be completely ruled out. Nevertheless, the kinetics for apoB-100 availability are similar in cells exposed to either insulin or naringenin.
The naringenin- and insulin-induced inhibition of secretion results in enhanced degradation of apoB-100, mainly via a kinetically defined rapid degradation pathway. Previously, we demonstrated that this rapid pathway is proteasomal, inasmuch as inhibitors of the cytosolic proteasome selectively blocked this degradation pathway (12). Increased proteasomal degradation occurs when apoB is inadequately lipidated during assembly (as reviewed in Ref. 9). This is consistent with our findings that a) naringenin and an exogenous inhibitor of MTP selectively decrease the accumulation of newly synthesized TG within the ER lumen (28), b) an MTP inhibitor enhances the rapid apoB-100 degradation pathway (28), and c) naringenin and insulin both decrease the expression of MTP via activation of MAPKerk (1). The slow degradation pathway for apoB, which accounts for less than 5% of total apoB-100 degradation, was affected by naringenin, and, to a lesser extent, by insulin. The nature of the slow degradation pathway has not been fully characterized, but may reflect ER luminal degradation (3) or lysosomal degradation mediated by the LDL receptor (25). In isolated LDLr−/− mouse hepatocytes, modeling of pulse-chase experiments demonstrated that the slow apoB-100 degradation pathway was significantly decreased compared with wild-type hepatocytes (29). Our finding that both naringenin and insulin induce minor increases in the slow apoB-100 degradation pathway is consistent with the increase in LDLr expression resulting from the activation of the PI3-K and MAPKerk pathways by both naringenin and insulin (2).
Insulin resistance is often characterized by an elevation of circulating FFAs, and it is proposed that these FFAs exacerbate the insulin signaling defects, increase TG synthesis and apoB-100 secretion, and cause pancreatic β-cell toxicity, ultimately leading to decreased insulin secretion and type 2 diabetes (30). It is well established that OA increases the secretion of apoB from hepatocytes, because it increases the availability of substrate for the synthesis of TG, thereby providing more neutral lipid for apoB-containing lipoprotein formation, thus protecting apoB from cotranslational degradation or degradation of full-length apoB-100 (as reviewed in Ref. 20). Consistent with this observation, multicompartmental analysis of apoB-100 pulse-chase kinetic experiments revealed that OA treatment resulted in considerably more apoB-100 availability and significantly less apoB degradation, resulting in more apoB-100 secretion from HepG2 cells, compared with LPDS media (12). The kinetics of intracellular apoB-100 trafficking in naringenin- and insulin-treated cells exposed to OA are very similar. Naringenin and insulin substantially decrease apoB-100 secretion by greater than 70%, indicating that exposure of cells to either treatment completely overcomes the stimulatory effects of OA.
The ability of naringenin to activate intracellular signaling pathways independent of the IR and IRS-1 allows it to act as an insulin sensitizer. Initially, we established a dose-dependent relationship for the activation of ERK1/2 by naringenin or insulin. Naringenin- and insulin-induced ERK1/2 phosphorylation in HepG2 cells reached a maximal increase by 30 min at 100 μM of naringenin and by 15 min at 100 nM of insulin, a normal physiologic in vitro insulin concentration. One quarter of this insulin concentration (25 nM) increased ERK1/2 phosphorylation to a maximum of 1.5-fold at 15 min. Coincubation of insulin at 25 nM with naringenin (25 or 100 μM) significantly increased the peak of ERK1/2 activation, compared with either insulin or naringenin used alone. Furthermore, the time of ERK1/2 activation was substantially prolonged. Preincubation of HepG2 cells with naringenin (100 μM) for 30 min prior to the addition of 25 nM insulin for an additional 30 min increased ERK1/2 phosphorylation to levels similar to those achieved by 100 nM insulin alone. In cells exposed to 25 nM or 100 nM insulin alone for 60 min, ERK1/2 phosphorylation had returned to baseline levels, suggesting that preexposure to naringenin sensitizes cells to insulin, resulting in enhanced and prolonged cellular signaling.
The ability of naringenin to sensitize HepG2 cells to stimulation by insulin extended to the expression of MTP and the LDLr, both of which are important determinants of hepatocyte apoB-100 secretion (23, 29). We previously reported that expression of both genes is regulated in hepatocytes by naringenin and insulin through activation of the MAPKerk and PI3-K pathways (1, 2). We show that there is a dose-dependent decrease in MTP expression and increase in LDLr expression when cells are incubated with 25 or 100 nM insulin. Preincubation of cells with 100 μM naringenin prior to addition of 25 nM insulin reduced MTP expression and increased LDLr expression to levels similar to those achieved by 4-fold higher concentrations of insulin alone (100 nM). The combination of naringenin (25 μM) plus insulin (25 nM), at one-quarter of their IC50 concentrations, induced a significantly greater reduction in apoB-100 secretion than for either naringenin alone at 25 μM or insulin alone at 25 nM, suggesting a synergistic reduction of apoB secretion with this combination. Furthermore, this combination resulted in similar reductions in apoB-100, compared with either compound alone, at four times the concentration. Importantly, these observations solidify the concept that naringenin sensitizes HepG2 cells to a low dose of insulin. In insulin-resistant subjects, a given concentration of insulin fails to elicit a normal biological response. Therefore, naringenin may be able to restore sensitivity to insulin by priming the signaling pathways via a mechanism that does not involve activation of the IR.
In summary, naringenin inhibits the assembly and secretion of apoB-100-containing lipoproteins from HepG2 cells in a manner very similar to insulin. This is supported by detailed kinetic analysis of pulse-chase experiments, which revealed that the kinetics of apoB-100 secretion were similar for naringenin and insulin. One of the mechanisms involved includes activation by naringenin of PI3-K- and MAPKerk signaling, two pathways that are also activated by insulin, to mediate the decrease in apoB-100 secretion. Of significance, we demonstrate that activation of these two cell signaling pathways by naringenin, in contrast to insulin, does not involve rapid activation of the IR or IRS-1. The exact mechanism of how naringenin activates PI3K and MAPKerk signaling has not been fully elucidated. However, the difference in activation of these two pathways probably underlies the ability of naringenin to either sensitize cells to insulin or potentiate the effect of insulin in mediating the inhibition of apoB-100 secretion. It will be imperative to test whether naringenin can ameliorate the hepatic VLDL-apoB-100 overproduction characteristic of insulin resistance.
Abbreviations
apoB, apolipoprotein B
CE, cholesteryl ester
ER, endoplasmic reticulum
ERK, extracellular-regulated kinase
FFA, free fatty acid
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
IR, insulin receptor
IRS, insulin receptor substrate
LDLr, low-density lipoprotein receptor
LPDS, lipoprotein-deficient serum
MAPK, mitogen-activated protein kinase
MTP, microsomal triglyceride transfer protein
OA, oleic acid
PI3-K, phosphoinositide-3-kinase
PL, phospholipid
TG, triglyceride
Published, JLR Papers in Press, June 27, 2008.
Footnotes
This research was supported by grants from the Heart and Stroke Foundation of Ontario (T-5603 and PRG-5967) to M.W.H., and the National Institutes of Health (NIBIB #P41 EB-001975) to P.H.R.B. P.H.R.B is a fellow of the National Health and Medical Research Council of Australia. E.M.A. received a post-doctoral fellowship from the Heart and Stroke Foundation of Canada, and E.E.M. is the recipient of a Canada Graduate Scholarship from the Canadian Institutes for Health Research.
References
- 1.Allister E. M., N. M. Borradaile, J. Y. Edwards, and M. W. Huff. 2005. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 54 1676–1683. [DOI] [PubMed] [Google Scholar]
- 2.Borradaile N. M., L. E. de Dreu, and M. W. Huff. 2003. Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes. 52 2554–2561. [DOI] [PubMed] [Google Scholar]
- 3.Fisher E. A., M. Pan, X. Chen, X. Wu, H. Wang, H. Jamil, J. D. Sparks, and K. J. Williams. 2001. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J. Biol. Chem. 276 27855–27863. [DOI] [PubMed] [Google Scholar]
- 4.Phung T. L., A. Roncone, K. L. Jensen, C. E. Sparks, and J. D. Sparks. 1997. Phosphoinositide 3-kinase activity is necessary for insulin-dependent inhibition of apolipoprotein B secretion by rat hepatocytes and localizes to the endoplasmic reticulum. J. Biol. Chem. 272 30693–30702. [DOI] [PubMed] [Google Scholar]
- 5.Sun X. J., M. Miralpeix, M. G. J. Myers, E. M. Glasheen, J. M. Backer, C. R. Kahn, and M. F. White. 1992. Expression and function of IRS-1 in insulin signal transmission. J. Biol. Chem. 267 22662–22672. [PubMed] [Google Scholar]
- 6.Chan D. C., G. F. Watts, P. H. Barrett, F. H. O'Neill, T. G. Redgrave, and G. R. Thompson. 2003. Relationships between cholesterol homoeostasis and triacylglycerol-rich lipoprotein remnant metabolism in the metabolic syndrome. Clin. Sci. (Lond.). 104 383–388. [DOI] [PubMed] [Google Scholar]
- 7.Chan D. C., G. F. Watts, T. G. Redgrave, T. A. Mori, and P. H. Barrett. 2002. Apolipoprotein B-100 kinetics in visceral obesity: associations with plasma apolipoprotein C-III concentration. Metabolism. 51 1041–1046. [DOI] [PubMed] [Google Scholar]
- 8.Watts G. F., P. H. Barrett, J. Ji, A. P. Serone, D. C. Chan, K. D. Croft, F. Loehrer, and A. G. Johnson. 2003. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 52 803–811. [DOI] [PubMed] [Google Scholar]
- 9.Fisher E. A., and H. N. Ginsberg. 2002. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 277 17377–17380. [DOI] [PubMed] [Google Scholar]
- 10.Gordon D. A., and H. Jamil. 2000. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein-B lipoprotein assembly. Biochim. Biophys. Acta. 1486 72–83. [DOI] [PubMed] [Google Scholar]
- 11.Shelness G. S., and J. A. Sellers. 2001. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 12 151–157. [DOI] [PubMed] [Google Scholar]
- 12.Borradaile N. M., L. E. de Dreu, P. H. Barrett, and M. W. Huff. 2002. Inhibition of hepatocyte apoB secretion by naringenin: enhanced rapid intracellular degradation independent of reduced microsomal cholesteryl esters. J. Lipid Res. 43 1544–1554. [DOI] [PubMed] [Google Scholar]
- 13.Dashti N., D. L. Williams, and P. Alaupovic. 1989. Effects of oleate and insulin on the production rates and cellular mRNA concentrations of apolipoproteins in HepG2 cells. J. Lipid Res. 30 1365–1373. [PubMed] [Google Scholar]
- 14.Pullinger C. R., J. D. North, B-B. Teng, V. A. Rifici, A. E. Ronhild de Brito, and J. Scott. 1989. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin and measurement of the mRNA half-life. J. Lipid Res. 30 1065–1077. [PubMed] [Google Scholar]
- 15.Theriault A., R. Cheung, and K. Adeli. 1992. Expression of apolipoprotein B in vitro in cell-free lysates of HepG2 cells: evidence that insulin modulates ApoB synthesis at the translational level. Clin. Biochem. 25 321–323. [DOI] [PubMed] [Google Scholar]
- 16.Adeli K., and A. Theriault. 1992. Insulin modulation of human apolipoprotein B mRNA translation: studies in an in vitro cell-free system from HepG2 cells. Biochem. Cell Biol. 70 1301–1312. [DOI] [PubMed] [Google Scholar]
- 17.Evans A. J., C. G. Sawyez, B. M. Wolfe, and M. W. Huff. 1992. Lipolysis is a prerequisite for lipid accumulation in HepG2 cells induced by large hypertriglyceridemic very low density lipoproteins. J. Biol. Chem. 267 10743–10751. [PubMed] [Google Scholar]
- 18.Wilcox L. J., P. H. R. Barrett, R. S. Newton, and M. W. Huff. 1999. ApoB-100 secretion from HepG2 cells is decreased by the ACAT inhibitor CI-1011: an effect associated with enhanced degradation of apoB. Arterioscler. Thromb. Vasc. Biol. 19 939–949. [DOI] [PubMed] [Google Scholar]
- 19.Yao Z., K. Tran, and R. S. McLeod. 1997. Intracellular degradation of newly synthesized apolipoprotein B. J. Lipid Res. 38 1937–1953. [PubMed] [Google Scholar]
- 20.Ginsberg H. N. 1995. Synthesis and secretion of apolipoprotein B from cultured liver cells. Curr. Opin. Lipidol. 6 275–280. [DOI] [PubMed] [Google Scholar]
- 21.White A. L., D. L. Graham, J. LeGros, R. J. Pease, and J. Scott. 1992. Oleate-mediated stimulation of apolipoprotein B secretion from rat hepatoma cells. A function of the ability of apolipoprotein B to direct lipoprotein assembly and escape presecretory degradation. J. Biol. Chem. 267 15657–15664. [PubMed] [Google Scholar]
- 22.Wu X., N. Sakata, K. M. Lele, M. Y. Zhou, H. S. Jiang, and H. N. Ginsberg. 1997. A two-site model for apoB degradation in HepG2 cells. J. Biol. Chem. 272 11575–11580. [DOI] [PubMed] [Google Scholar]
- 23.Shelness G. S., and A. S. Ledford. 2005. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr. Opin. Lipidol. 16 325–332. [DOI] [PubMed] [Google Scholar]
- 24.Au W. S., H. F. Kung, and M. C. Lin. 2003. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes. 52 1073–1080. [DOI] [PubMed] [Google Scholar]
- 25.Campbell S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene. 17 1395–1413. [DOI] [PubMed] [Google Scholar]
- 26.Brown A. M., and G. F. Gibbons. 2001. Insulin inhibits the maturation phase of VLDL assembly via a phosphoinositide 3-kinase-mediated event. Arterioscler. Thromb. Vasc. Biol. 21 1656–1661. [DOI] [PubMed] [Google Scholar]
- 27.Chirieac D. V., N. O. Davidson, C. E. Sparks, and J. D. Sparks. 2006. PI3-kinase activity modulates apo B available for hepatic VLDL production in apobec-1−/− mice. Am. J. Physiol. Gastrointest. Liver Physiol. 291 G382–G388. [DOI] [PubMed] [Google Scholar]
- 28.Borradaile N. M., L. E. de Dreu, P. H. Barrett, C. D. Behrsin, and M. W. Huff. 2003. Hepatocyte apoB-containing lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry. 42 1283–1291. [DOI] [PubMed] [Google Scholar]
- 29.Twisk J., D. L. Gillian-Daniel, A. Tebon, L. Wang, P. H. Barrett, and A. D. Attie. 2000. The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Invest. 105 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reaven G. M. 1988. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 37 1595–1607. [DOI] [PubMed] [Google Scholar]