Abstract
Platelet-activating factor (PAF), the potent phospholipid mediator of inflammation, is involved in atherosclerosis. Platelet-activating factor-acetylhydrolase (PAF-AH), the enzyme that inactivates PAF bioactivity, possesses both acetylhydrolase and transacetylase activities. In the present study, we measured acetylhydrolase and transacetylase activities in human atherogenic aorta and nonatherogenic mammary arteries. Immunohistochemistry analysis showed PAF-AH expression in the intima and the media of the aorta and in the media of mammary arteries. Acetylhydrolase and transacetylase activities were (mean ± SE, n = 38): acetylhydrolase of aorta, 2.8 ± 0.5 pmol/min/mg of tissue; transacetylase of aorta, 3.3 ± 0.7 pmol/min/mg of tissue; acetylhydrolase of mammary artery, 1.4 ± 0.3 pmol/min/mg of tissue (P < 0.004 as compared with acetylhydrolase of aorta); transacetylase of mammary artery, 0.8 ± 0.2 pmol/min/mg of tissue (P < 0.03 as compared with acetylhydrolase of mammary artery). Lyso-PAF accumulation and an increase in PAF bioactivity were observed in the aorta of some patients. Reverse-phase HPLC and electrospray ionization mass spectrometry analysis revealed that 1-O-hexadecyl-2 acetyl-sn glycero-3-phosphocholine accounted for 60% of the PAF bioactivity and 1-O-hexadecyl-2-butanoyl-sn-glycerol-3-phosphocholine for 40% of the PAF bioactivity. The nonatherogenic properties of mammary arteries may in part be due to low PAF formation regulated by PAF-AH activity. In atherogenic aortas, an imbalance between PAF-AH and transacetylase activity, as well as lyso-PAF accumulation, may lead to unregulated PAF formation and to progression of atherosclerosis.
Keywords: atherosclerosis, macrophages, smooth muscle cells, lipoproteins, atherogenesis, oxidized LDL, macrophages
The platelet-activating factor (PAF) (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) belongs to a family of potent phospholipid (PL) mediators for numerous inflammatory and thrombotic responses (1). PAF exhibits a wide spectrum of actions on major pro-inflammatory cells, including monocytes/macrophages, endothelial cells, and smooth muscle cells (SMCs) (as reviewed in Ref. 2). PAF bioactivity is mediated by the PAF receptor (PAF-R) in a wide range of cellular types; it is a seven-transmembrane receptor coupled to G-proteins (3, 4). For expression of PAF bioactivity, the structural requirements of the molecules are: an ether linkage at the sn-1 position, a short acyl chain (shorter than five carbons) at the sn-2 position, and a choline head group (5, 6). Atherosclerosis is a chronic inflammatory disease (7), and several studies suggest that PAF may play a role in atherogenesis and atherosclerosis (as reviewed in Ref. 8).
PAF is synthesized by the majority of pro-inflammatory cells upon stimulation via enzymatic pathways that are tightly regulated and are related to eicosanoid metabolism (9). PAF is degraded by specific intracellular PAF acetylhydrolases or those circulating in plasma (as reviewed in Ref. 10).
Additionally, uncontrolled free radical-catalyzed oxidation of PUFAs attached to the sn-2 position of PLs can produce several break-down products that structurally resemble PAF. Oxidized species of phosphatidylcholine have been shown to act in vitro as potent activators of both vascular and blood cells (11). The bioactivity of some of these may be due to their structural analogy with PAF (12).
Plasma PAF- acetylhydrolase (PAF-AH) is a secretory Ca2+-independent phospholipase A2 (PLA2) belonging to the group VIIA (13) that inactivates PAF and its analogs (oxidized phosphatidylcholine containing either up to five carbons in native form or up to nine carbons in oxidized form) by hydrolyzing their sn-2 acyl chains and thus converting them to lyso-PAF and lysophosphatidylcholine (lyso-PC) (14–17). PAF-AH in plasma is associated mainly with LDL and HDL (16).
Diffusion of LDLs into the vessel wall and their oxidative modification, together with monocyte transmigration along with their maturation into macrophages and foam cell formation, are key events in the pathogenesis of atherosclerosis (7, 18). PAF bioactivity is formed upon Cu2+-induced oxidation of LDL, in which PAF-AH has been chemically inactivated by serine esterase inhibitors (19). In physiological conditions, PAF is formed in lipoproteins lacking PAF-AH activity (20), and it is also detected in oxidized LDL subfractions possessing low levels of PAF-AH activity, i.e., LDL particles of intermediate size (21). PAF is involved in the monocyte adhesion to endothelium activated by LDL and oxidized LDL (22), and for this reason, LDL-associated PAF-AH may possess anti-inflammatory properties, protecting lipoprotein particles against formation of PAF and oxidized PLs. The anti-inflammatory activities of PAF-AH in numerous animal models (23) strongly suggest an anti-atherogenic role for this enzyme in vascular physiology. Indeed, the overexpression of PAF-AH shows marked anti-atherogenic properties in animal models (24). However, epidemiological data in the Caucasian population have shown that its level might be a risk factor for cardiovascular disease (as reviewed in Ref. 10). Thus, the question about the pro- or anti-atherogenic role of PAF-AH remains to be explored. Of interest, in a recent study by Vadas et al. (25), the proportion of patients with low PAF-AH values increased with the severity of anaphylaxis, and its level was lower in patients with fatal peanut anaphylaxis, underlying the importance of PAF-AH in human physiopathology.
Two studies have shown that PAF-AH is responsible for both acetylhydrolase and transacetylase activities (26, 27). Transacetylase transfers short-chain fatty acids from PAF and its close ether- and ester-linked analogs to ether/ester-linked lyso-PLs. Such transacetylase activity may exceed the acetylhydrolase activity in the presence of exogenously added lyso-PLs) or in the presence of oxidative conditions. Due to the LDL-associated PAF-AH, a PAF-like bioactivity can be formed transiently during the first hour of LDL oxidation. PAF bioactivity occurs in the presence of exogenous lyso-PAF, without chemical inactivation of PAF-AH, especially in the pro-atherogenic small, dense LDL subfraction (27). Enzymatic transfer of acetate from the inactive ester analogs to lyso-PAF may also be responsible for extracellular PAF bioactivity formation (6). This happens in addition to the extracellular PAF bioactivity formation by chemical peroxidation of PLs with PUFAs esterified at the sn-2 position of the molecules (6).
The aim of the present study was to investigate the role of the PAF system in the progression of atherosclerosis. For this purpose, in human nonatherogenic mammary arteries (28) and diseased aortas of the same patients, we measured: i) PAF-AH, transacetylase, and acetylhydrolase activities; ii) PAF bioactivity; iii) lyso-PAF accumulation; and iv) the expression of PAF-R.
MATERIALS AND METHODS
Materials
C16:0 PAF, C16:0 lyso-PAF, C16:0 lyso-PC, fatty acid-free BSA, creatine phosphate, creatine phosphokinase were from Sigma, acetic anhydride was from Fluka, and Pefablok SC (4-[2-aminoethyl benzenesulfonyl fluoride, Pefablok) was from Pierce. Liquid scintillation fluid (Optiphase Hi-Safe 3) was supplied by E.G.G. BN 52021 was kindly provided by Dr. P. Braquet (Institut Henri Beaufour). Lipase from Rhizopus arrhizus was supplied by Boehringer Mannheim. Solvents were from Lab-Scan. 1-O-hexadecyl-2-[3H-acetyl]sn-glycero-phosphocholine (10 Ci/mmol), 1-O-[3H]hexadecyl-sn-glycero-phosphocholine (58.3 mCi/mmol), and 1-[palmitoyl-1-14C]phosphatidylcholine (50.5 mCi/mol) and were from DuPont-New England Nuclear.
Patients and sample collection
The study group included 38 patients, 30 male and 8 female, age 63 ± 13 years, with symptomatic coronary artery disease in which at least two vessels would be grafted. The epidemiologic characteristics of the patients were recorded. Full-thickness aortic tissue samples 4–5 mm in diameter from the ascending aorta, two for each patient, were obtained with a No. 11 knife during the construction of the proximal anastomosis in bypass surgery with extracorporeal circulation after cardiac arrest was induced with cold (10°C) blood cardioplegia (29).
As a control, two samples of internal mammary artery free of atherosclerotic lesions were obtained from the same patients.
The study was approved by the ethics committee of our hospital, inasmuch as the method of sample collection did not affect the patients' health; to the contrary, it is included in the routine practice of heart surgery (29).
The samples were freed of adipose tissue with fine scissors and washed with saline to remove any blood elements. Then one sample from each origin (aorta and internal mammary artery) was quickly frozen in liquid nitrogen and stored at −80°C until the biochemical analyses, and one sample of each origin was put in formaldehyde for histology, totaling four samples from each patient.
Antibodies
For immunohistochemistry, the following monoclonal antibodies were used: CD68 (dil. 1/500; clone: KP1, Dako), CD3 (dil. 1/100; clone: F7.238, Dako), PAF-R (dil. 1/10; clone: 21, Alexis Biochemicals), PAF-AH (dil. 1/20, Cayman).
Histological studies
After microwave treatment (5 min at 900 W in citrate buffer), deparaffinized and rehydrated sections were incubated for 30 min at room temperature with primary antibodies, washed, and incubated for 30 min with a Multilink kit (Biosys) for polyclonal antibodies and an ABC Vector kit (Biosys) for monoclonal antibodies. After washing, the alkaline phosphatase/anti-alkaline phosphatase complexes (Dako) were added. Fast Red™ substrate system (Dako), gave a red precipitate on positive cells. Slides were counterstained with aqueous hematoxylin and mounted with Immunomount (Shandon). Negative controls were obtained by replacing primary antibodies with either mouse IgG1 or an irrelevant antibody.
Transacetylase and acetylhydrolase activities
To measure enzymatic activities, the samples of the arteries were homogenized at 4°C in 400 μl of a homogenizing buffer (50 mM Tris, pH 8, 10 mM CHAPS, 2 mM EGTA and EDTA, 1 μg/ml leupeptin, and 1 μg/ml antipain) using sonication. The homogenate was centrifuged (12,000 rpm for 2 min at 4°C). Supernatants contained the PAF-AH activity. The activity was also measured in the supernatant of a further centrifugation (100,000 g for 1 h) in two patients. PAF-AH activity (84% and 91%) was recovered in the supernatant of the second centrifugation.
Both activities were measured under the incubation conditions described in (27). Transacetylase assay was performed by incubating 100 μl of the supernatant in 10 mM Tris + 0.05% EDTA, pH 7.4, with PAF and [14C]lyso-PC dissolved in 10 mM BSA/Tris + 2.5 mg/ml 0.05% EDTA. Reactions were performed in polypropylene tubes for 60 min at 37°C. The final concentrations were 80 μM PAF, 30 μM [14C]lyso-PC (0.1 μCi), and 250 μg/ml BSA, in a reaction mixture of 0.4 ml. The reaction was stopped by extracting the lipids according to Bligh and Dyer (30). Total lipids were then subjected to TLC on silica gel G plates by using chloroform-methanol-water (65:35:6; v/v/v) as a solvent system. Lipids were identified after brief exposure to iodine. The band corresponding to the relative mobility (Rf) of standard PAF was scraped off the plate and the radioactivity was measured by liquid scintillation counting. In some experiments, the supernatants were preincubated with 1 mM Pefablok for 30 min at 37°C.
Acetylhydrolase assay was performed by incubating 30 μl of the supernatant in 10 mM Tris and 0.05% EDTA, pH 7.4, with [3H-acetyl]PAF and lyso-PAF, dissolved in 10 mM BSA/Tris and 1.25 mg/ml 0.05% EDTA, in an Eppendorf polypropylene tube for 60 min at 37°C. The final concentrations were 80 μM [3H-acetyl]PAF (0.1 μCi), 30 μM lyso-PAF, and 250 μg/ml BSA in a reaction mixture of 0.1 ml. The reaction was stopped in an ice bath. Unreacted [3H-acetyl]PAF was bound to an excess of BSA (final concentration, 16.7 mg/ml) for 10 min and precipitated by the addition of trichloroacetic acid (final concentration, 8% v/v) as previously described (31). The samples were then centrifuged in an Eppendorf centrifuge for 5 min and the [3H]acetate released into the aqueous phase was measured by liquid scintillation counting. In some experiments, the supernatants were preincubated with 1 mM Pefablok for 30 min at 37°C.
Extraction and quantification of PAF bioactivity: an estimate of lyso-PAF accumulation
The remaining samples were subjected to extraction with chlorofom-methanol-water (1:1:0.9; v/v/v) (30) and brought to dryness under a nitrogen stream. Samples containing lipids and PAF were kept at −20°C for further purification and analysis.
Samples containing crude lipid extracts were subjected to TLC on silica gel G plates and developed in a mixture of chloroform-methanol-water (65:35:6; v/v/v) as mobile phase. The bands corresponding to the Rf of synthetic standard PAF were scraped off, extracted, and dried. The samples containing lipids with the Rf of PAF were redissolved in a small volume of ethanol (60%, v/v) for quantitation of PAF bioactivity by the thromboxane A2- and ADP-independent aggregation of washed rabbit platelets, as previously described (32). The aggregating activity of the samples was measured over the linear portion of the calibration curve established with 0.5 to 20 pg synthetic PAF C16:0. Aggregation was characterized as PAF-like by its inhibition by the specific PAF receptor antagonist BN 52021 and its resistance in the treatment of lipase from Rhizopus arrhizus, as described previously (33). The results are expressed as equivalent pmol of PAF per mg of wet tissue.
To estimate the lyso-PAF accumulation, the TLC bands corresponding to the Rf of standard lyso-PC were scraped off the plate and extracted according to Bligh and Dyer (30). Dry samples containing lipids with the Rf of lyso-PC were dissolved in 200 μl pyridine and chemically acetylated to PAF with 200 μl acetic anhydride (34). The lyso-PAF was quantified as C16:0 PAF equivalents using the bioassay, as described above.
Characterization of the molecular species responsible for the PAF bioactivity: molecular characterization of C16:0 lyso-PAF
The dry residues containing PAF bioactivity were suspended in 50 μl of methanol before separation of the molecular species of PAF on a reverse-phase Spherisorb C6 column (Waters). The HPLC mobile phase consisted of 55% methanol-ammonium acetate (10 mM) (1:3; v/v) and 45% acetonitrile, and the flow rate was 1 ml/min. The retention times of PAF-like molecules were determined using 3H-labeled lyso-PAF, 3H-labeled C16:0 or C18:0 PAF, with their radiolabeled ester and ether analogs used as standards. Fractions were collected; part of each fraction was extracted with chloroform, dried, and assayed for PAF biological activity. The remaining part was extracted with chlorofom-methanol-water (1:1:0.9; v/v/v) added with 40 μl formic acid and was further analyzed by electrospray ionization mass spectrometry (ESI-MS) (Plotform LS, Micromass, UK). The curtain gas flow was 4.5 l/min nitrogen. PLs were introduced into the mass spectrometer by flow injection analysis (FIA). FIA solvent consisted of methanol-ammonium acetate (10 mM) (70:30). PLs suspended in FIA solvent were injected at a rate of 50 μl/min. The orifice potential was maintained at 75 V, and the electrospray ionization potential at +3.5 kV for detection of positive ions.
For the molecular characterization of C16:0 lyso-PAF, dry residues containing the lipids of the TLC bands corresponding to the Rf of standard lyso-PC were subjected to reverse-phase HPLC, as described above. Fractions corresponding to the retention time of C16:0 lyso-PAF were extracted and analyzed by ESI-MS, exactly as was done for PAF molecular characterization.
In two patients, lyso-PAF was assayed by the routine assay described above but was also assayed in the reverse-phase HPLC fraction with the retention time of C16:0 lyso-PAF, by the washed rabbit platelet bioassay after acetylation.
Statistical analysis
Results are expressed as mean ± SE of the individual specimen measurements. Values of P < 0.05 were considered statistically significant. Student's t-test for dependent variables and the nonparametric Mann-Whitney U test for non-Gaussian distributions tests were used, when appropriate, for comparison between groups, and Pearson correlation coefficient was used for correlation analysis. Statistical analysis was done using Statistica 4.3 for Windows.
RESULTS
Immunohistochemical analysis of aortas and internal mammary arteries
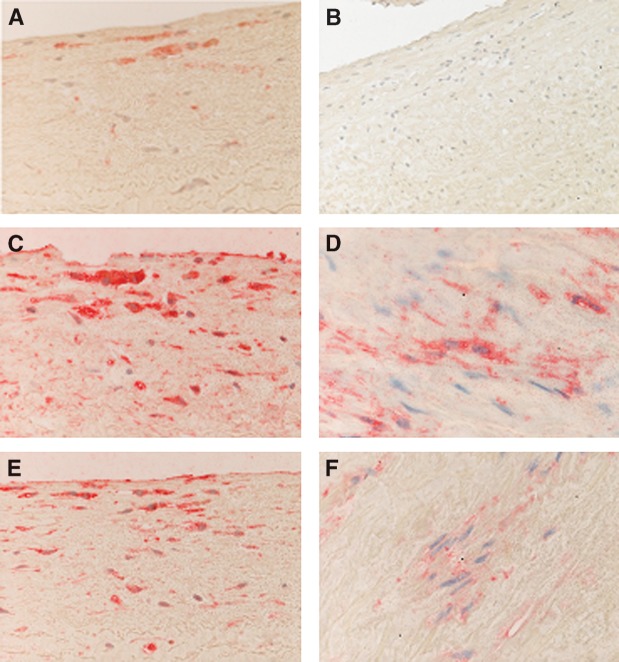
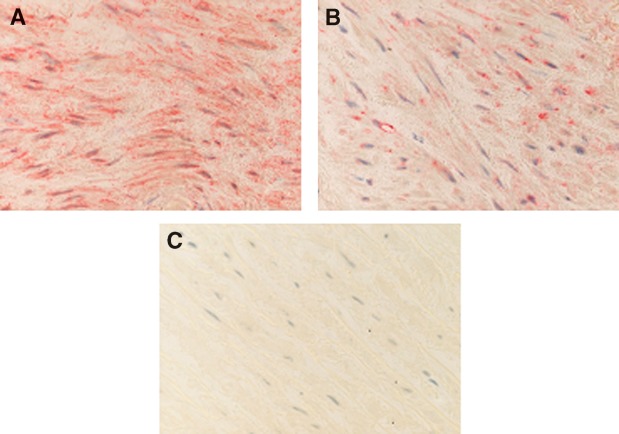
The aim of this study was twofold: a) to investigate both PAF acetylhydrolase and transacetylase activities of PAF-AH in human atherosclerotic and nonatherosclerotic arteries and b) to determine whether these activities were correlated with the accumulation of lyso-PAF and PAF formation, leading to progression of atherosclerosis. For this purpose, full-thickness resections of aortas and internal mammary arteries were obtained from patients undergoing bypass surgery. Specimens were subjected to immunohistochemical analysis, and only those composed of well-defined intima, media, and adventitia layers were characterized as intact vessels. All internal mammary specimens studied showed a healthy intima without inflammatory infiltrate, with only a few scattered monocytes located close to the lumen and adjacent to endothelial cells (data not shown). In contrast, an intimal hyperplasia was observed in 20 out of 28 aortic specimens, and initial lesions with intimal fibrosis in 8 out of 28 patients; thus, all specimens were classified as initial atherosclerotic lesions. Representative results of the immunohistochemical analysis of aortas are presented in Fig. 1 and of mammary arteries in Fig. 2. Fatty streaks with thickened intima and macrophage foam cell accumulation without lymphocytes were observed in 20 out of 28 arteries (Fig. 1A, B). Monocyte-macrophages in the subendothelium and foam cells within the fatty streak expressed diffuse and heterogeneous cytoplasmic staining with PAF-AH (Fig. 1C). SMCs located in the media were strongly stained with the monoclonal antibodies against PAF-AH (Fig. 1D). Monocytes located in the lumen and adjacent to endothelial cells, as well as macrophages, were strongly stained with the monoclonal antibodies directed against PAF-R (Fig. 1E). The signal was also located in the cytoplasm of a few SMCs, and the extracellular matrix was negative (Fig. 1F). An important finding of the present work was the expression of PAF-AH in the media of healthy internal mammary arteries (Fig. 2A). A weak expression of PAF-R was also observed in the media of mammary arteries (Fig. 2B).
Fig. 1.
Immunohistochemical analysis of atherogenic aortas. A: CD68 expression in macrophages located in the intima; original magnification × 200. B: Staining with anti-CD3 showing the absence of T cells and serving as a blank; original magnification × 200. C: PAF-acetylhydrolase (PAF-AH) cytoplasmic expression in macrophages; original magnification × 200. D: PAF-AH expression in smooth muscle cells (SMCs); original magnification × 400. E: PAF receptor (PAF-R) expression in monocyte-macrophages in the intima; original magnification × 200. F: PAF-R expression in SMCs; original magnification × 400.
Fig. 2.
Immunohistochemical analysis of nonatherogenic mammary arteries. A: Strong expression of PAF-AH in the media; original magnification × 400. B: Weak expression of PAF-R in the media; original magnification × 400. C: Staining with anti-CD68 showing the absence of macrophages and serving as a blank; original magnification × 200.
Measurement of enzymatic PAF acetylhydrolase and transacetylase activities in aortas and mammary arteries
PAF acetylhydrolase and transacetylase activities were detected in the lysates of aortas and mammary arteries (Table 1). Both PAF acetylhydrolase and transacetylase activities were significantly higher in aortas than in mammary arteries and were inhibited by low doses (1 mM) of the serine esterase inhibitor Pefablok. In aortas, the transacetylase activity was slightly higher than the acetylhydrolase activity; however, this difference did not reach statistical significance. In internal mammary arteries, the transacetylase activity was significantly lower than the acetylhydrolase activity (Table 1).
TABLE 1.
PAF acetylhydrolase and transacetylase activities in aortic and mammary arteries
| AH Activity | AH Activity plus Pefablok | TA Activity | TA Activity plus Pefablok | |
|---|---|---|---|---|
| pmol/min/mg tissue | ||||
| Aorta | 2.8 ± 0.5 | 0.3 ± 0.1 | 3.3 ± 0.7 | 0.5 ± 0.3 |
| Mammary artery | 1.4 ± 0.3a | 0.1 ± 0.1 | 0.8 ± 0.2b | 0.2 ± 0.1 |
AH, acetylhydrolase; PAF, platelet-activating factor; TA, transacetylase. Results are given as mean ± SE (n = 38).
P < 0.004 as compared with AH of aorta.
P < 0.03 as compared with AH of mammary artery, t-test for dependent samples.
Measurement of PAF bioactivity and lyso-PAF accumulation in aortas and mammary arteries
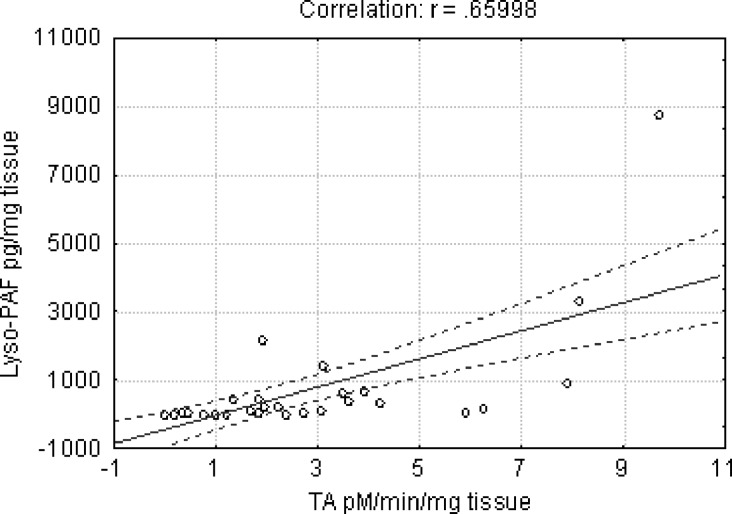
PAF bioactivity in the arterial specimens was extracted, purified, and quantified by the washed rabbit platelet bioassay. Lyso-PAF accumulation in the arteries was estimated after TLC purification and chemical acetylation as C16:0 PAF equivalents (see Materials and Methods). Both aortas and mammary arteries of the patients showed comparable amounts of PAF bioactivity; however, a large variability was observed in aortas (Table 2). For instance, the amount of PAF was less than 10 pg/mg tissue in 56% of aortas and in 53% of mammary arteries and was 50 to 200 times higher in aortas of only a few patients (Fig. 3A). Lyso-PAF accumulation showed a distribution pattern similar to that of PAF-bioactivity (Fig. 3B); however, it was statistically higher in aortas as compared with the internal mammary arteries (Table 2). A positive correlation was detected solely between the transacetylase activity and lyso-PAF accumulation in the specimens (Pearson coefficient, 0.66; P < 0.001) (Fig. 4).
TABLE 2.
PAF and lyso-PAF in aortic and mammary arteries
| PAF | Lyso-PAF | |
|---|---|---|
| pg/mg tissue | ||
| Aortic arteries | 135 ± 65 | 928 ± 475 |
| Mammary arteries | 21 ± 5 | 110 ± 72a |
Results are given as mean ± SE (n = 34).
P < 0.02, nonparametric Mann Whitney U test.
Fig. 3.
A: Bar graph showing the distribution of PAF bioactivity in arteries of patients. B: Bar graph showing the distribution of lyso-PAF in arteries of patients.
Fig. 4.
Correlation between transacetylase activity and lyso-PAF in human arteries. The 95% confidence interval is shown by the dashed line. Transacetylase activity was expressed in pmol/min/mg of tissue and lyso-PAF in pg/mg tissue. r, Pearson correlation coefficient.
Molecular characterization of the PAF bioactivity and lyso-PAF accumulation
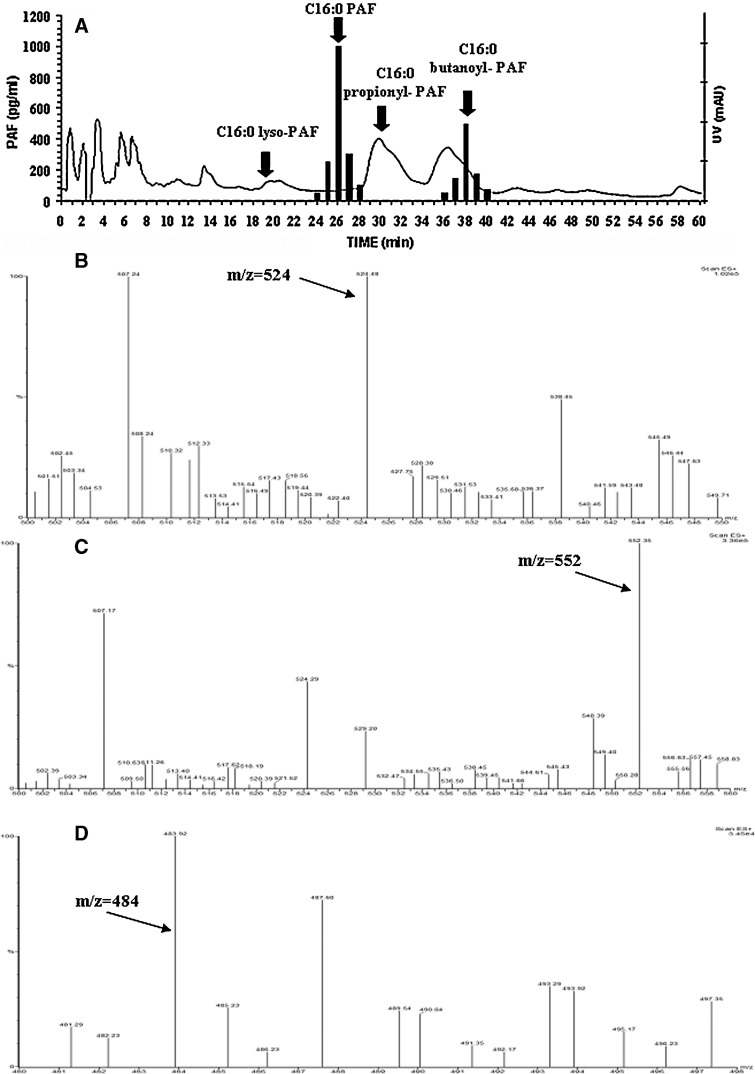
The TLC-purified PAF-like bioactivity found in aortas was analyzed by reverse-phase HPLC. Fractions were collected, and the C16:0 PAF equivalents were measured in each fraction by the bioassay. The retention times of different PAF analogs are indicated by arrows (Fig. 5A). After elution from the reverse-phase column, 60–70% of the bioactivity injected was recovered at the retention time of bioactive PAF analogs (Fig. 5A). A substantial amount of bioactivity (60%) was recovered from the reverse-phase HPLC with the retention time of C16:0 PAF. Moreover, as much as 40% of bioactivity was recovered from the reverse-phase HPLC column with the retention time of butanoyl-PAF (Fig. 5A). Moreover, the TLC band with the Rf of lyso-PLs of the aortas was analyzed by reverse-phase HPLC, and the lipids with the retention time of C16:0 lyso-PAF (Fig. 5A) were extracted and assayed for lyso-PAF by the bioassay, as described above.
Fig. 5.
A: Graphs show the PAF bioactivity in each HPLC fraction of aortic samples; arrows indicate the retention times of synthetic radiolabeled standards. B: PAF bioactive material, which was recovered from reverse-phase HPLC with the retention time of C16:0 PAF, was dissolved in methanol-ammonium acetate (10 mM; 70:30), introduced into the mass spectrometry ion source, and analyzed by positive-ion flow injection electrospray ionization mass spectrometry (ESI-MS). C: PAF bioactive material, which was recovered from reverse-phase HPLC with the retention time of butanoyl-PAF, was dissolved in methanol-ammonium acetate (10 mM; 70:30), introduced into the mass spectrometry ion source, and analyzed by the positive-ion flow injection ESI-MS. D: Lipids extracted from the reverse-phase HPLC fraction with the retention time of lyso-PAF were dissolved in methanol-ammonium acetate (10 mM; 70:30), introduced into the mass spectrometry ion source, and analyzed by positive-ion flow injection ESI-MS.
ESI-MS analysis in the positive-ion mode of the biologically active material that was recovered from the reverse-phase HPLC column is shown in Fig. 5B–D. The MS of the molecular species with the retention time of C16:0 PAF revealed a major diagnostic ion at m/z 524–525 corresponding to the protonated molecule of C16:0 PAF (Fig. 5B).
ESI-MS analysis of the biologically active material that was recovered from the reverse-phase HPLC column with the retention time of butanoyl-PAF is shown in Fig. 5C. The MS revealed a major ion at m/z 552, which corresponds to the [M+H]+ of C16:0 butanoyl-PAF. ESI-MS analysis of the lipids corresponding to the retention time of C16:0 lyso-PAF is shown in Fig. 5D. The MS revealed a diagnostic ion at m/z 484, which corresponds to the [M+H]+ of C16:0 lyso-PAF. Moreover, lyso-PAF quantified, after acetylation, by the bioassay in the fraction with the retention time of C16:0 lyso-PAF was 80% of that measured by the routine assay in the TLC fraction with the Rf of lyso-PC in the same patient.
The diagnostic ions observed in ESI-MS, and the retention time upon reverse-phase HPLC separation, allow us to suggest that C16:0 PAF and its butanoyl analog may be the PAF analogs responsible for the PAF-like bioactivity in the diseased aortas. Moreover, C16:0 lyso-PAF was the molecular species primarily responsible for the estimate of lyso-PAF accumulation observed in aortas. The formation of other lyso-PAF analogs in aorta cannot be excluded and needs further investigation.
DISCUSSION
In the present study, we show that several elements of the PAF system are potentially involved in the physiopathology of the human arterial wall. These factors are: a) formation of PAF, b) accumulation of lyso-PAF, c) PAF-AH and PAF-R expression, and d) PAF acetylhydrolase and transacetylase activities. The paired design of this study, in which aortas with atherosclerotic lesions and internal mammary arteries free of lesions from the same patients were used, provided appropriate specimens for investigating the contribution of PAF acetylhydrolase and transacetylase activities in the progression of atherosclerosis.
The immunohistochemical analysis confirmed PAF-AH expression in the arteries, as has already been shown in human aortic intima obtained from autopsies with several types of atherosclerotic lesions (35). In the present study, we also detected the expression of PAF-AH in the media of aortas and, importantly, in the media of the mammary arteries. Although the observed PAF-AH expression was higher in the aortic lesions than in the mammary arteries, the above finding challenges the strictly pro-atherogenic role of this enzyme in the arterial wall.
The biological actions of PAF are mediated by a specific cell-surface seven-transmembrane domain receptor, PAF-R, which couples to G-proteins (3, 4). Thus, we investigated the expression of PAF-R in all arterial specimens. The expression of PAF-R was observed in both the diseased aortic intima and the media. The above results are in accordance with our earlier study on human carotid plaque tissue (36), where we showed that the dedifferentiated SMCs were positive for PAF-R. The expression of PAF-R may be important for the migration of SMCs from the arterial media into the intima. Such migration in the context of PAF has been suggested in the development of human atherosclerotic lesions (37). The functionality of PAF-R in the media of mammary arteries is less clear.
In agreement with the immunohistochemical analysis, PAF acetylhydrolase activity was detected in aortas and mammary arteries. In rabbit, PAF acetylhydrolase activity was measured in atherosclerotic arteries and was found to be higher than in healthy control arteries (35). In the present study with human aortas, PAFacetylhydrolase activity was equally higher than the activity in the internal mammary arteries. Two studies (26, 27) have shown that PAF-AH is responsible not only for acetylhydrolase activity but also for transacetylase activity. The latter is able to transfer short-chain fatty acids from PAF and its close ether- and ester-linked analogs to ether/ester-linked lyso-PLs. The transacetylase activity was described in LDL particles and may be considered as an enzymatic route for extracellular PAF formation (27). Interestingly, in the present study, we detected transacetylase activity in the arteries. In diseased aortas, the transacetylase activity was slightly higher than the acetylhydrolase activity; however, this difference did not reach statistical significance. In contrast, in internal mammary arteries, the transacetylase activity was significantly lower than the acetylhydrolase activity. The above results suggest that transacetylase activity may play a pro-atherogenic role in the arteries.
In the arterial wall, the enzyme PAF-AH is the main source of these activities, as suggested by: a) the enzymatic assay with EDTA and without Ca2+ in the incubation mixture, b) PL as the donor of acetate, and c) the inhibition of both acetylhydrolase and transacetylase activities by low doses of Pefablok. The results of the present study also demonstrate that PAF bioactivity is detectable in the lipid extracts of the arteries. PAF was detected in two other earlier studies using human arteries: the first, in the endarterectomy samples taken from coronary arteries with severe atherosclerosis (38); and the second, in the endarterectomy specimens of carotid plaques (39). In most patients in our study, the amount of PAF detected in aortas was comparable to that detected in the mammary arteries. The higher level of PAF reported in Table 2 for the aortas was the result of high PAF bioactivity, detected in the aortas of six patients. The PAF levels in the diseased samples in the study of Mueller et al. (38) also showed a high variability, and no statistically significant difference was seen between PAF levels in arteries with severe atherosclerosis and the control healthy arteries. Thus, the results of the present study, together with those of Mueller et al. (38), suggest that PAF bioactivity accumulates in the lesions of some patients.
The molecular characterization of PAF by MS in aorta showed that C16:0 PAF was the main component; this molecular species of PAF is the most bioactive member of PAF family. Additionally, an sn-2 C4 analog of PAF, C16:0 butanoyl-PAF, was also present in the diseased aorta. In oxidized LDL, the C16:0 PAF and its sn-2 C4 analogs (butanoyl- and butenoyl-PAF) are the main components of PAF bioactivity (6, 40). The sn-2 C4 analogs of PAF are the products of free radical peroxidation of arachidonoyl-PC (6, 40). In C16:0 PAF, its formation is due, in addition to peroxidation, to the enzymatic activity of the PAF transacetylase (6).
Oxidatively modified phosphatidylcholine, upon hydrolysis by PAF-AH, generates lyso-PC (41, 19). The arterial wall also contains other types of secreted PLA2 that may play a role in this process (42–44). Group II secretory PLA2 is highly expressed in SMCs, in both normal and atherosclerotic arteries (42), as well as the PLA2GX that is also present in macrophages and foam cells (44); thus, these activities may account for the accumulation of C16:0 lyso-PAF in the arteries observed in the present study. Lyso-PAF is a precursor and a degradation product of intracellular (9) and extracellular PAF metabolism (6, 27). At least some of the proinflammatory effects of oxidized LDL are mediated by lyso-PC (45, 46). The oxidative stress exerted, together with the recruitment and activation of pro-inflammatory cells in the media of atherosclerotic arteries, may explain the higher accumulation of lyso-PAF in the aortas. The positive correlation between transacetylase activity and lyso-PAF accumulation in the arteries may be explained by the presence of high amounts of lyso-PAF and the conditions in which transacetylase activity is favored and acetylhydrolase activity is inhibited (27). The noncorrelation between PAF formation and transacetylase activity, as well as that between lyso-PAF accumulation and acetylhydrolase activity, respectively, strongly suggests that other enzymes of the tissue, including PLA2 (42–44) and intracellular lyso-PAF acetyl-CoA acetyltransferases (9, 47), are also responsible for the formation of PAF and lyso-PAF.
Diffusion of LDL particles and their oxidative modification play an important role in vascular physiopathology (45, 48). Oxidized LDL is present in atherosclerotic lesions in vivo (48) and plays an important role in the pathogenesis of atherosclerosis (45). Moreover, oxidative modification of LDL, monocyte migration into the vessel wall, subsequent macrophage activation, and foam cell formation are key events in the pathogenesis of atherosclerosis (7, 45). Several characteristics of the PAF system described in the present study regarding human arteries express similarities with the characteristics of the PAF system in LDL. LDL is the main transporter of PAF-AH in human plasma (16) The LDL-associated PAF-AH possesses both acetylhydrolase and transacetylase activities (27), and upon LDL oxidation, PAF bioactivity and lyso-PAF accumulation are observed in LDL particles (19, 21, 40). C16:0 PAF and its sn-2 C4 analogs are the molecules responsible for PAF bioactivity in oxidized LDL (40, 6). Moreover, monocyte migration into the vessel wall, subsequent macrophage activation, and foam cell formation are also key events in the pathophysiology of the arterial wall (45, 48). Macrophages secrete PAF-AH activity in cell culture (49, 50), and PAF-AH is expressed by macrophages in human arterial lesions (35). Thus, LDL deposition and oxidation, monocyte recruitment and diffusion, macrophage activation, as well as SMCs of contractile phenotype are the factors assigning to the PAF system its main characteristics in the arterial wall (7).
As mentioned earlier (10), the question of the pro- or anti-atherogenic role of PAF-AH is still open. The in vivo results of the present study could suggest a dual role for PAF-AH. The nonatherogenic properties of mammary arteries may in part be due to low PAF formation regulated by PAF-acetylhydrolase activity. Regulated PAF formation may play an anti-atherogenic role via macrophage activation. This activation can then remove LDL oxidation and degradation products. In the diseased arterial intima of aortas, under oxidative stress and lyso-PAF accumulation, an imbalance between acetylhydrolase and transacetylase activity may contribute to unregulated PAF formation and to the progression of atherosclerosis.
Abbreviations
ESI-MS, electrospray ionization mass spectrometry
FIA, flow injection analysis
PAF, platelet-activating factor
PAF-AH, PAF-acetylhydrolase
PAF-R, PAF receptor
PC, phosphatidylcholine
PL, phospholipid
PLA2, phospholipase A2
SMC, smooth muscle cell
Published, JLR Papers in Press, June 27, 2008.
Footnotes
This work was co-funded by the European Union-European Social Fund and National Sources, in the framework of the program Pythagoras II of the Operational Program for Education and Initial Vocational Training of the 3rd Community Support Framework of the Hellenic Ministry of Education.
References
- 1.Prescott S. M., G. A. Zimmerman, D. M. Stafforini, and T. M. McIntyre. 2000. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 69 419–445. [DOI] [PubMed] [Google Scholar]
- 2.Montrucchio G., G. Alloatti, and G. Camussi. 2000. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol. Rev. 80 1669–1699. [DOI] [PubMed] [Google Scholar]
- 3.Honda Z., M. Nakamura, I. Miki, M. Minami, T. Watanabe, Y. Seyama, H. Okado, H. Toh, K. Ito, T. Miyamoto, et al. 1991. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 349 342–346. [DOI] [PubMed] [Google Scholar]
- 4.Izumi T., and T. Shimizu. 1995. Platelet-activating factor receptor: gene expression and signal transduction. Biochim. Biophys. Acta. 1259 317–333. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T., M. Iimori, H. Tsukatani, and A. Tokumura. 1994. Platelet-aggregating effects of platelet-activating factor-like phospholipids formed by oxidation of phosphatidylcholines containing an sn-2-polyunsaturated fatty acyl group. Biochim. Biophys. Acta. 1210 202–208. [DOI] [PubMed] [Google Scholar]
- 6.Androulakis N., H. Durand, E. Ninio, and D. C. Tsoukatos. 2005. Molecular and mechanistic characterization of platelet-activating factor-like bioactivity produced upon LDL oxidation. J. Lipid Res. 46 1923–1932. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. 1999. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 340 115–126. [DOI] [PubMed] [Google Scholar]
- 8.Ninio E. 2005. Phospholipid mediators in the wall: involvement in atherosclerosis. Curr. Opin. Clin. Nutr. Metab. Care. 8 123–131. [DOI] [PubMed] [Google Scholar]
- 9.Snyder F. 1995. Platelet-activating factor: the biosynthetic and catabolic enzymes. Biochem. J. 305 689–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karabina S. A., and E. Ninio. 2006. Plasma PAF-acetylhydrolase: an unfulfilled promise? Biochim. Biophys. Acta. 1761 1351–1358. [DOI] [PubMed] [Google Scholar]
- 11.Berliner J. A., G. Subbanagounder, N. Leitinger, A. D. Watson, and D. Vora. 2001. Evidence for a role of phospholipid oxidation products in atherogenesis. Trends Cardiovasc. Med. 11 142–147. [DOI] [PubMed] [Google Scholar]
- 12.Smiley P. L., K. E. Stremler, S. M. Prescott, G. A. Zimmerman, and T. M. McIntyre. 1991. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. J. Biol. Chem. 266 11104–11110. [PubMed] [Google Scholar]
- 13.Chakraborti S. 2003. Phospholipase A2 isoforms: a perspective. Cell. Signal. 15 637–665. [DOI] [PubMed] [Google Scholar]
- 14.Farr R. S., C. P. Cox, M. L. Wardlow, and R. Jorgensen. 1980. Preliminary studies of an acid labile factor (ALF) in human sera that inactivates platelet activating factor (PAF). Clin. Immunol. Immunopathol. 15 318–330. [DOI] [PubMed] [Google Scholar]
- 15.Blank M. L., T. Lee, V. Fitzgerald, and F. Snyder. 1981. A specific acetylhydrolase for 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (a hypotensive and platelet-activating lipid). J. Biol. Chem. 256 175–178. [PubMed] [Google Scholar]
- 16.Stafforini D. M., S. M. Prescott, and T. M. McIntyre. 1987. Human plasma platelet-activating factor acetylhydrolase. Purification and properties. J. Biol. Chem. 262 4223–4230. [PubMed] [Google Scholar]
- 17.Stremler K. E., D. M. Stafforini, S. M. Prescott, G. A. Zimmerman, and T. M. McIntyre. 1989. An oxidized derivative of phosphatidylcholine is a substrate for the platelet-activating factor acetylhydrolase from human plasma. J. Biol. Chem. 264 5331–5334. [PubMed] [Google Scholar]
- 18.Falk E., P. K. Shah, and V. Fuster. 1995. Coronary plaque disruption. Circulation. 92 657–671. [DOI] [PubMed] [Google Scholar]
- 19.Liapikos T. A., S. Antonopoulou, S-A. P. Karabina, D. C. Tsoukatos, C. A. Demopoulos, and A. D. Tselepis. 1994. Platelet-activating factor formation during oxidative modification of low-density lipoprotein when PAF-acetylhydrolase has been inactivated. Biochim. Biophys. Acta. 1212 353–360. [DOI] [PubMed] [Google Scholar]
- 20.Tokumura A., M. Toujima, Y. Yoshioka, and K. Fukuzawa. 1996. Lipid peroxidation in low density lipoproteins from human plasma and egg yolk promotes accumulation of 1-acyl analogues of platelet-activating factor-like lipid. Lipids. 31 1251–1258. [DOI] [PubMed] [Google Scholar]
- 21.Tsoukatos D. C., M. Arborati, T. Liapikos, K. L. Clay, R. C. Murphy, M. J. Chapman, and E. Ninio. 1997. Copper-catalyzed oxidation mediates PAF formation in human LDL subspecies. Protective role of PAF:acetylhydrolase in dense LDL. Arterioscler. Thromb. Vasc. Biol. 17 3505–3512. [DOI] [PubMed] [Google Scholar]
- 22.Lehr H. A., J. Seemuller, C. Hubner, M. D. Menger, and K. Messmer. 1993. Oxidized LDL-induced leukocyte/endothelium interaction in vivo involves the receptor for platelet-activating factor. Arterioscler. Thromb. 13 1013–1018. [DOI] [PubMed] [Google Scholar]
- 23.Tjoelker L. W., C. Wilder, C. Eberhardt, D. M. Stafforini, G. Dietsch, B. Schimpf, S. Hooper, H. Le Trong, L. S. Cousens, G. A. Zimmerman, et al. 1995. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature. 374 549–553. [DOI] [PubMed] [Google Scholar]
- 24.Quarck R., B. De Geest, D. Stengel, A. Mertens, M. Lox, G. Theilmeier, C. Michiels, M. Raes, H. Bult, D. Collen, et al. 2001. Adenovirus-mediated gene transfer of human platelet-activating factor-acetylhydrolase prevents injury-induced neointima formation and reduces spontaneous atherosclerosis in apolipoprotein E-deficient mice. Circulation. 103 2495–2500. [DOI] [PubMed] [Google Scholar]
- 25.Vadas P., M. Gold, B. Perelman, G. M. Liss, G. Lack, T. Blyth, F. E. Simons, K. J. Simons, D. Cass, and J. Yeung. 2008. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N. Engl. J. Med. 358 79–81. [DOI] [PubMed] [Google Scholar]
- 26.Liu M., and P. V. Subbaiah. 1994. Hydrolysis and transesterification of platelet-activating factor by lecithin-cholesterol acyltransferase. Proc. Natl. Acad. Sci. USA. 91 6035–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsoukatos D. C., T. A. Liapikos, A. D. Tselepis, M. J. Chapman, and E. Ninio. 2001. Platelet-activating factor acetylhydrolase and transacetylase activities in human plasma low-density lipoprotein. Biochem. J. 357 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox J. L., D. A. Chiasson, and A. I. Gotlieb. 1991. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog. Cardiovasc. Dis. 34 45–68. [DOI] [PubMed] [Google Scholar]
- 29.Mills, N. L., and C. Swayze Rigby. 1991. Techniques of coronary artery operations and reoperations. In Glenn's Thoracic and Cardiovascular Surgery. A. E. Bauer, A. S. Geha, G. L. Hammond, H. Laks, and K. S. Naunheim, editors. Churchill Livingston, Philadelphia. 1771–1789.
- 30.Bligh E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37 911–917. [DOI] [PubMed] [Google Scholar]
- 31.Tselepis A. D., C. Dentan, S. A. P. Karabina, M. J. Chapman, and E. Ninio. 1995. PAF-degrading acetylhydrolase is preferentially associated with dense LDL and VHDL-1 in human plasma. Catalytic characteristics and relation to the monocyte-derived enzyme. Arterioscler. Thromb. Vasc. Biol. 15 1764–1773. [DOI] [PubMed] [Google Scholar]
- 32.Bossant, M. J., E. Ninio, D. Delautier, and J. Benveniste. 1990. Bioassay of paf-acether by rabbit platelet aggregation. In Methods in Enzymology. R. C. Murphy and F. A. Fitzpatrick, editors. Academic Press, San Diego. 125–130. [DOI] [PubMed]
- 33.Benveniste J., J-P. Le Couedic, J. Polonsky, and M. Tence. 1977. Structural analysis of purified platelet-activating factor by lipases. Nature. 269 170–171. [DOI] [PubMed] [Google Scholar]
- 34.Lekka M. E., D. C. Tsoukatos, A. D. Tselepis, and V. M. Kapoulas. 1988. Semisynthetic preparation of 1-O-hexadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine (platelet-activating factor). Z Naturforsch. 43 665–670. [DOI] [PubMed] [Google Scholar]
- 35.Hakkinen T., J. S. Luoma, M. O. Hiltunen, C. H. Macphee, K. J. Milliner, L. Patel, S. Q. Rice, D. G. Tew, K. Karkola, and S. Yla-Herttuala. 1999. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 19 2909–2917. [DOI] [PubMed] [Google Scholar]
- 36.Brocheìriou I., D. Stengel, L. Mattsson-Hulteìn, J. Stankova, M. Rola-Pleszczynski, F. Koskas, O. Wiklund, Y. Le Charpentier, and E. Ninio. 2000. Expression of platelet-activating factor receptor in human carotid atherosclerotic plaques: relevance to progression of atherosclerosis. Circulation. 102 2569–2575. [DOI] [PubMed] [Google Scholar]
- 37.Stengel D., C. O'Neil, I. Brochériou, S. A. Karabina, H. Durand, N. M. Caplice, J. G. Pickering, and E. Ninio. 2006. PAF-receptor is preferentially expressed in a distinct synthetic phenotype of smooth muscle cells cloned from human internal thoracic artery: functional implications in cell migration. Biochem. Biophys. Res. Commun. 346 693–699. [DOI] [PubMed] [Google Scholar]
- 38.Mueller H. W., C. A. Haught, J. M. McNatt, K. Cui, S. J. Gaskell, D. A. Johnston, and J. T. Willerson. 1995. Measurement of platelet-activating factor in a canine model of coronary thrombosis and in endarterectomy samples from patients with advanced coronary artery disease. Circ. Res. 77 54–63. [DOI] [PubMed] [Google Scholar]
- 39.Lupia E., A. Pucci, P. Peasso, M. Merlo, P. Baron, C. Zanini, L. Del Sorbo, S. Rizea-Savu, L. Silvestro, M. Forni, et al. 2003. Intra-plaque production of platelet-activating factor correlates with neoangiogenesis in human carotid atherosclerotic lesions. Int. J. Mol. Med. 12 327–334. [PubMed] [Google Scholar]
- 40.Marathe G. K., S. S. Davies, K. A. Harrison, A. R. Silva, R. C. Murphy, H. Castro-Faria-Neto, S. M. Prescott, G. A. Zimmerman, and T. M. McIntyre. 1999. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J. Biol. Chem. 274 28395–28404. [DOI] [PubMed] [Google Scholar]
- 41.Quinn M. T., S. Parthasarathy, L. G. Fong, and D. Steinberg. 1987. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl. Acad. Sci. USA. 84 2995–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elinder L. S., A. Dumitrescu, P. Larsson, U. Hedin, J. Frostegard, and H. E. Claesson. 1997. Expression of phospholipase A2 isoforms in human normal and atherosclerotic arterial wall. Arterioscler. Thromb. Vasc. Biol. 17 2257–2263. [DOI] [PubMed] [Google Scholar]
- 43.Hurt-Camejo E., and G. Camejo. 1997. Potential involvement of type II phospholipase A2 in atherosclerosis. Atherosclerosis. 132 1–8. [DOI] [PubMed] [Google Scholar]
- 44.Karabina S. A., I. Brochériou, G. Le Naour, M. Agrapart, H. Durand, M. Gelb, G. Lambeau, and E. Ninio. 2006. Atherogenic properties of LDL particles modified by human group X secreted phospholipase A2 on human endothelial cell function. FASEB J. 20 2547–2549. [DOI] [PubMed] [Google Scholar]
- 45.Witztum J. L., and D. Steinberg. 1991. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Invest. 88 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parthasarathy S., U. P. Steinbrecher, J. Barnett, J. L. Witztum, and D. Steinberg. 1985. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc. Natl. Acad. Sci. USA. 82 3000–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harayama T., H. Shindou, R. Ogasavara, A. Suwabe, and T. Shimizu. 2008. Identification of a novel non-inflammatory biosynthetic pathway of platelet-activating factor. J. Biol. Chem. 283 11097–11106. [DOI] [PubMed] [Google Scholar]
- 48.Ylä-Herttuala S., W. Palinski, M. E. Rosenfeld, S. Parthasarathy, T. E. Carew, S. Butler, J. L. Witztum, and D. Steinberg. 1989. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Invest. 84 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmantier R., A. Dulioust, H. Maiza, J. Benveniste, and E. Ninio. 1989. Biosynthesis of paf-acether. XIV. Paf-acether output in murine peritoneal macrophages is regulated by the level of acetylhydrolase. Biochem. Biophys. Res. Commun. 162 475–482. [DOI] [PubMed] [Google Scholar]
- 50.Elstad M. R., D. M. Stafforini, T. M. McIntyre, S. M. Prescott, and G. A. Zimmerman. 1989. Platelet-activating factor acetylhydrolase increases during macrophage differentiation. A novel mechanism that regulates accumulation of platelet-activating factor. J. Biol. Chem. 264 8467–8470. [PubMed] [Google Scholar]