Abstract
Sniffing, a rhythmic inhalation and exhalation of air through the nose, is a behavior thought to play a critical role in shaping how odor information is represented and processed by the nervous system. Although the mouse has become a prominent model for studying olfaction, little is known about sniffing behavior in mice. Here, we characterized mouse sniffing behavior by measuring intranasal pressure transients in behaving mice. Sniffing was monitored during unstructured exploratory behavior and during performance of 3 commonly used olfactory paradigms: a habituation/dishabituation task, a sand digging–based discrimination task, and a nose poke–based discrimination task. We found that respiration frequencies in quiescent mice ranged from 3 to 5 Hz—higher than that reported for rats. During exploration, sniff frequency increased up to ∼12 Hz and was highly dynamic, with rapid changes in frequency, amplitude, and waveform. Sniffing behavior varied strongly between tasks as well as for different behavioral epochs of each task. For example, mice performing the digging-based task showed little increase in sniff frequency prior to digging, whereas mice performing a nose poke–based task showed robust increases. Mice showed large increases in sniff frequency prior to reward delivery in all tasks. Mice also showed increases in sniff frequency when nose poking in a nonodor-guided task. These results show that mouse sniffing behavior is highly dynamic, varies with behavioral context, and is strongly modulated by olfactory as well as nonolfactory events.
Keywords: digging, discrimination, go/no-go, habituation, nose poke, respiration
Introduction
The sense of smell in terrestrial vertebrates is first initiated by the inhalation of odorant molecules into the nasal cavity. This process can occur in the course of resting respiration but is also mediated by the voluntary inhalation of air for the purpose of odorant sampling—a behavior commonly referred to as sniffing. As first demonstrated by the pioneering work of Welker and others, sniffing behavior is highly dynamic and precisely coordinated with other motor systems (Welker 1964; Macrides et al. 1982; Youngentob et al. 1987). Sniffing behavior is particularly dynamic during odor-guided behaviors. For example, rats and hamsters increase their frequency of sniffing from “resting” frequencies near 2 Hz to 4–12 Hz when investigating novel odor sources or sampling odorants during operant tasks (Welker 1964; Macrides et al. 1982; Youngentob et al. 1987; Uchida and Mainen 2003; Kepecs et al. 2007; Verhagen et al. 2007; Wesson et al. 2008). Animals also alter other parameters of sniffing during odor-guided behavior, such as amplitude, inhalation–exhalation waveform, and duration (Youngentob et al. 1987; Thesen et al. 1993; Kepecs et al. 2007). Importantly, behavioral context and additional sensory information also influence sniffing behavior during odor-guided tasks. For example, dogs show different sniffing behaviors when tracking a scent in air versus on the ground (Thesen et al. 1993), and water shrews hunting for prey change their sniffing behavior depending on the shape of the object being investigated (Catania et al. 2008).
The strong modulation of sniffing behavior during odor sampling has led to the general idea that sniffing plays a critical role in odor information processing by shaping spatial and temporal patterns of afferent input to the olfactory bulb (OB) as well as patterns of higher level neural activity. As first demonstrated by Adrian (1953), the bursting of OB neurons is strongly coupled with odorant inhalation patterns (Macrides and Chorover 1972). Further work has emphasized the importance of inhalation of odorants on initiating olfactory receptor neuron (ORN) responses (Mozell 1964, 1970; Scott 2006; Scott et al. 2006). The control of ORN responses by sniffing in an awake-behaving rat has also recently been demonstrated (Verhagen et al. 2007). This respiratory-based modulation of olfactory neuron responses also occurs in the second-order mitral/tufted cells of the OB (Macrides and Chorover 1972; Kay and Laurent 1999). Finally, even cortical neurons in higher order olfactory centers are strongly coupled with respiration (Rennaker et al. 2007).
Sniffing behavior has been relatively well studied in humans (Laing 1982; Sobel et al. 1998; Johnson et al. 2003; Mainland and Sobel 2006; Porter et al. 2007), canines (Thesen et al. 1993; Steen et al. 1996), and rats (Welker 1964; Macrides et al. 1982; Youngentob et al. 1987). Surprisingly, the sniffing behavior of mice remains almost completely undescribed, despite them being a primary model system for behavioral studies of odor perception and neurophysiological and molecular studies of olfactory coding and information processing. Indeed, to our knowledge, of the 2 published studies that measured mouse sniffing behavior, one monitored sniffing in wild-type and genetically modified mice via whole-body plethysmography and reported spontaneous (i.e., untrained) changes in sniffing behavior in response to odorants but did not provide an absolute characterization of sniffing behavior (e.g., ranges of sniff frequencies) (Youngentob 2005). A second study reported effects of estrogen treatment on sniff frequencies of mice measured during a digging-based operant odorant detection task (Sorwell et al. 2008). Neither study though characterized sniffing across different behavioral paradigms or as a continuous function of time.
We therefore set out to provide a quantitative and qualitative description of mouse sniffing behavior during a range of odor-guided behaviors, including those behavioral paradigms most commonly used in assessing olfactory function. These paradigms included a measure of spontaneous odor discrimination (odorant habituation/dishabituation) (Sundberg et al. 1982; Baum and Keverne 2002; Pankevich et al. 2004) and 2 types of operant odor-discrimination tasks—a 2-choice sand-digging task (Birrell and Brown 2000; Mihalick et al. 2000; Fortin et al. 2002; Wei et al. 2006; Tait et al. 2007) and a go/no-go nose poke task (Slotnick and Nigroshi 1974; Macrides et al. 1982; Bodyak and Slotnick 1999; Uchida and Mainen 2003; Abraham et al. 2004; Rinberg et al. 2006b; Wesson et al. 2006). We found that sniffing behavior differed dramatically between behavioral tasks and that sniffing was strongly and stereotypically modulated during particular epochs within each task. Interestingly, sniffing was equally modulated by nonolfactory aspects of task performance as it was by the act of odor detection or discrimination. These results suggest that olfactory information processing is highly dynamic in the behaving animal and may vary due to differing sniffing strategies depending on behavioral context.
Materials and methods
Animals
Adult male C57BL/6 mice (n = 14) from Charles River Laboratories (Wilmington, MA) were used. All animals were less than 6 months of age by completion of data collection. Mice were housed up to 3 per cage and were kept on a 12:12 h light:dark cycle, with lights on at 8:00 h. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Boston University Institutional Animal Care and Use Committee.
Surgery
Each mouse was implanted with an intranasal (sniff) cannula using aseptic techniques under general anesthesia induced by ketamine (70 mg/kg; Henry Schein Inc., Melville, NY) and medetomidine (Domitor, 1 mg/kg; Pfizer Inc., New York, NY). Additionally, bupivacaine (150 μl of a 1% solution; Sigma-Aldrich, St Louis, MO) was injected into the epidermis overlying the frontonasal bone for local anesthesia. A midline incision was made and the skull cleaned using 3% H202. A hole was then drilled unilaterally through the right nasal bone for the cannula (position: 1 mm ant frontal/nasal fissure and 1 mm lat). A hollow cannula (#C313G; Plastics One Inc., Roanoke, VA) was cut to extend 0.8 mm from the pedestal, lowered into the hole, and fixed in place with dental cement. The entire cranial implant, including cement and cannula weighed approximately 0.3 g. Mice received atipamezole (S.C., Antisedan, 1 mg/kg; Pfizer Inc.) at the end of surgery to antagonize the medetomidine-induced effects and accelerate recovery from anesthesia. Mice were given carprofen (Rimadyl, 5 mg/kg; Pfizer Inc.) as an analgesic immediately prior to surgery and for 4 days following. Mice were allowed to recover for a minimum of 7 days before behavioral testing began.
Recordings of sniffing behavior
Sniffing behavior was monitored by measuring intranasal respiratory transients in the nose via the sniff cannula. A piece of polyethylene tubing (0.1 mm ID × 0.15 mm OD) was connected from the sniff cannula to an airtight swivel (model 375/22PS; Instech Laboratories, Plymouth Meeting, PA), which allowed the animal to freely move within the testing chamber. The swivel was then connected to a pressure transducer (model CPXL04GF; Honeywell International, Morristown, NJ) to convert pressure transients into voltage. This method has been previously verified to correlate strongly with airflow (measured via intranasal thermocouple) within the nose of the rat (Verhagen et al. 2007). The voltage was then amplified 100×, low-pass filtered at 100 Hz, and digitized at 500 Hz using custom software written in LabVIEW (Austin, TX).
Food deprivation
In all experiments, mice were placed on 24-hr food deprivation beginning several days prior to training and no less than 1 week post surgery. In addition to the rewards received during operant testing, the mice were fed one-half of a food pellet (∼2 g) each day. With this protocol, mice were maintained at ∼80% of baseline body weight and remained healthy and active. Subjects were weighed daily and water was available ad libitum except during testing.
Olfactometry
Odor control in the habituation/dishabituation and nose-poke odor tasks was achieved using a custom, computer-controlled flow-dilution olfactometer that allowed precise control of odorant concentration, identity, and onset timing in concert with the behavioral paradigm (Bozza et al. 2004; Verhagen et al. 2007). In this design, odorant was continuously flowing to the odor port but was removed by a vacuum before entering the testing chamber or the port entrance; turning off this vacuum via a solenoid valve allowed rapid entry of odorant into the chamber. Saturated vapor of pure liquid odorant stock was generated in a nitrogen stream and then diluted in air for a final flow rate of 2 l/min (habituation/dishabituation task) or 1 l/min (nose poke task). Both air and nitrogen were medical grade and filtered through a hydrocarbon filter cartridge before use. Onset timing and the removal of odorant from the behavioral chambers between trials were verified with a photoionization detector (MiniRae 2000, RAE Systems Inc., San Jose, CA). All odors used for data collection and analysis were single monomolecular hydrocarbon compounds (Sigma-Aldrich).
Behavioral paradigms
Freely expressed exploratory sniffing
Mice (n = 3) were placed into a plastic testing chamber (12 × 29 × 15 cm) which was filled with several “novel items” to encourage active exploration. Novel items included clean bedding, a 3″ piece of PVC pipe, and several plastic bottle caps. Mice were allowed to explore for 20–25 min, during which sniffing behavior was continuously monitored. Every ∼5 min an item was removed or a novel item added to encourage constant exploration. The testing chamber was cleaned with 70% ethanol between each mouse.
Odor habituation/dishabituation task
This paradigm was adapted from previously published reports (Sundberg et al. 1982; Baum and Keverne 2002). Mice (n = 6) were acclimated to a chamber consisting of an open-top plastic box (12 × 12 × 26 cm) with a recessed odor port on one side to provide odorant delivery. An exhaust fan on the opposite side from the odor port provided constant air removal from the chamber. Each animal was tested once per day within a single session lasting from 15–70 min (mean, 45 min; 2–3 sessions per animal). The structure of the session is outlined in Figure 1A. Each of the 3 odorants was presented 4 consecutive times for a duration of 5 s, followed by a 1- to 2-min intertrial interval (ITI). Each odorant was presented 4 times to ensure robust habituation to the test stimulus. To establish a baseline for sniffing behavior and to control for airflow changes in the chamber, a “blank” odorant was presented in the same manner at the start of each session. Odorants used included ethyl butyrate, hexanone, methyl valerate, valeric acid, hexanal, and isoamyl acetate. All odorants were presented at 0.5% saturated vapor. The dishabituation test relied upon an animal's investigation of a novel odor stimulus (i.e., presentation 1). In accordance with our previous work in rats characterizing novelty responses (Verhagen et al. 2007; Wesson et al. 2008), we only used odorants to which the animal had not been exposed within the last 48 h. The testing chamber was cleaned with 70% ethanol between each mouse.
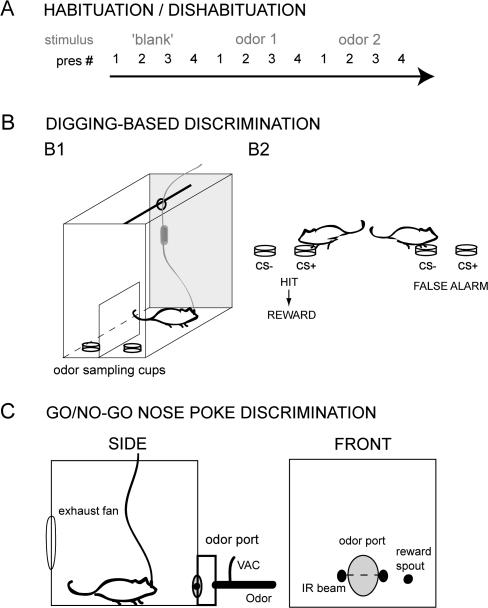
Figure 1.
Paradigms used to characterize mouse sniffing behavior. (A) Schematic of the odor habituation/dishabituation task design. Sniffing behavior was monitored from mice while they were given 4 consecutive presentations of an odorant, followed by 4 presentations of a different odorant, and so on. The first presentation served as the “dishabituation” trial. Each session began with an acclimation phase consisting of 4 presentations of a blank (clean air) stimulus. (B) Behavioral apparatus (B1) and task design (B2) of the digging-based odor-discrimination paradigm. Odorants were presented by placing a drop of liquid odorant onto cups of sand and lifting a barrier to allow the mouse access to the cups. Subjects were conditioned to dig at the cup containing the rewarded (CS+) odorant in order to receive a reward form the experimenter. (C) Schematic of the apparatus used for the go/no-go nose poke–based discrimination task. Mice were trained to nose poke into an odor delivery port, breaking an infrared photobeam (IR beam). After 100 ms of IR beam break, 1 of 2 odorants was delivered and mice were free to sample the odorant for up to 2 s. Mice moved to a separate reward spout and licked to receive a liquid food reward in response to the CS+ odorant but received no reward for the CS− odorant. A separate cohort of mice was also trained to nose poke and hold their snout in the port in exchange for a reward, with no stimulus discrimination (or delivery). All trials in both the odor-discrimination nose poke task and the nonolfactory nose poke task were separated by 5-s ITIs. Sniffing was recorded continuously throughout each session for all paradigms. See Materials and methods for additional details on all paradigms.
Odor-cup sand-digging task
The sand-digging task was adapted from work by Mihalick et al. (2000). Following 1 week of food deprivation (one-half food pellet per day), mice (n = 6) were acclimated to the testing chamber which consisted of a 12- × 29- × 15-cm plastic box with a divider at one end, splitting half the chamber into 2 sections. Mice were randomly assigned to 1 of 2 cohorts (n = 3 per cohort).
A schematic of the testing apparatus and task structure are shown in Figures 1B1,B2. Odorants were presented by lowering 2 small cups (1″ diameter, 0.5″ tall) into the test chamber (one on each side of the divider). Each cup contained approximately 10 g of fresh sand onto the surface of which 25 μl of liquid odorant had been applied. Mice were initially trained on a 2-choice odor-detection task requiring them to dig in a cup laced with a 1:100 dilution (in mineral oil) of isoamyl acetate (CS+) but not to dig in a cup with mineral oil alone (CS−). Stimuli were presented on random sides of the divider, with not more than 3 consecutive presentations of the CS+ on the same side. As shown in Figure 1B2, correct responses (“hits,” digging in the CS+ cup) resulted in a food reward (one-eighth of a Cheerio) being lowered to the mouse by the experimenter using forceps. Incorrect responses (“false alarms,” digging in the CS− cup) did not result in a food reward. Animals were given 20 trials per day until a criterion of 85% accuracy (3 or fewer errors in 20 trials) was met. A failure to dig within 15 min, for 2 trials in a row, resulted in termination of the behavioral session. The moment of digging was recorded into the data by a manual button press by the experimenter.
Animals were then trained to perform a 2-choice discrimination between the rewarded odorant (CS+, isoamyl acetate, for cohort I, or methyl valerate, for cohort II) and a nonrewarded odorant (CS−, methyl valerate, for cohort I, or isoamyl acetate, for cohort II). Each odorant was diluted 1:100 v/v in light mineral oil (Sigma-Aldrich).
For the “difficult” test of odor discrimination, 4 mice previously tested on the above discrimination task were trained on a “binary ratio” discrimination. In this test, the same procedures were used except that the CS+ odorant was composed of 68% isoamyl acetate: 32% methyl valerate for cohort I (vice versa for cohort II); and the CS− odorant was composed of 32% isoamyl acetate: 68% methyl valerate for cohort I (vice versa for cohort II). Odorants were at a 1:100 concentration prior to mixture. The testing chamber was cleaned with 70% ethanol between each mouse.
Go/no-go nose poke odor discrimination
The Go/no-go nose poke task was adapted from work by Slotnick and Nigroshi (1974). Mice (n = 6) were food deprived for 1 week (one-half food pellet per day) and acclimated to the testing chamber (12 × 12 × 26 cm) over several days. On one wall of the testing chamber was a recessed teflon odor port (11 × 8 mm) and 15 mm to the right was a stainless steel lick spout for reward delivery (Figure 1C). On the opposite wall was an exhaust fan to remove odorant from the testing chamber. The floor of the chamber was stainless steel, which was connected to the lick spout through a contact lickometer circuit (ENV-250; Med Associates, St Albans, VT). Upon licking the reward spout, the lickometer output triggered the brief opening of a pinch valve, supplying a small amount (∼3 μl) of liquid food reward (Pediasure; Ross pediatrics, Columbus, OH). Nose poke into the odor port was monitored with infrared photodiodes.
Training for this task occurred in several phases. First, mice were taught to nose poke into the odor port and then lick from the lick spout for a reward. Second, after ∼100 completed poke-reward sequences (generally 1–2 sessions), the required poke duration was gradually increased to 500 ms in 100-ms intervals to encourage the animal to maintain nose poke. Third, the CS+ odorant (1% isoamyl acetate) was presented after 100 ms of continuous nose poke. During this phase of training, a reward was only presented if the mouse sampled the odorant for 500 ms (total time in odor port, 600 ms). After 2 sessions of conditioning on the CS+, unrewarded blank (no odorant) CS− trials were added, with CS+ and CS− trials occurring in pseudorandom order (no more than 3 consecutive presentations of the same odorant). Once mice reached 85% criterion for licking only in CS+ trials, the CS− was switched to methyl valerate (1.5% s.v.). Mice were then trained to criterion on the 2-odor discrimination task. The final trial structure used for data collection consisted of a 5-s ITI, a required 100-ms poke duration before odorant delivery, a minimum 200 ms odorant sampling time, and a 3-s lick time-out window (time following poke in which reward is available). Importantly, because the reward port was located away from the sampling port, requiring the animal to withdraw in order to receive reward, this design allowed for measures of odorant sampling duration for both CS+ and CS− trials (defined as the duration from odorant onset to nose withdrawal with a 50-ms time resolution), as has been done in previous studies (Uchida and Mainen 2003; Abraham et al. 2004; Rinberg et al. 2006b; Slotnick 2007). This design also separated the time of odorant sampling from the time of reward delivery. Each subject was tested for 112–448 trials over several daily sessions. The testing chamber was cleaned with 70% ethanol between each mouse.
Data analysis
Sniffing and behavioral performance data were extracted using custom software written in LabVIEW. We restricted our quantitative analysis of sniffing behavior to measures of sniff frequency, which was determined by detecting the peaks of each inhalation off-line. The intranasal pressure signal was first band-pass filtered (0.1–100 Hz) and the threshold for peak detection set manually by visual inspection of each sniff trace. Robustness and accuracy of the peak detection was verified by visual inspection for each recording (for example, see Figure 2A). The time point of each detected sniff peak was recorded relative to either odorant onset (for habituation/dishabituation and go/no-go nose poke tasks) or to the moment of digging in the stimulus cup (for the sand-digging task) for each trial. Further data analysis was carried out in MatLAB (MathWorks, Natick, MA). To analyze sniff frequency within and across trials, each trial was divided into 50- or 100-ms time bins, and the instantaneous sniff frequency was calculated for each sniff based on the interval between a sniff and the one preceding it, then this value was assigned to the time bin corresponding to that of each sniff. To ensure that all time bins were equally weighted from each trial, any empty time bins within a trial were filled with the value of the subsequent instantaneous sniff frequency. Thus, all time bins had one value per trial. Unless otherwise specified, for display and for statistical analyses, all data for a given paradigm were collapsed across trials (i.e., each trial was counted as an independent observation without regard to session or animal number. The number of trials per animal and per session was roughly equal for each mouse within a particular paradigm.
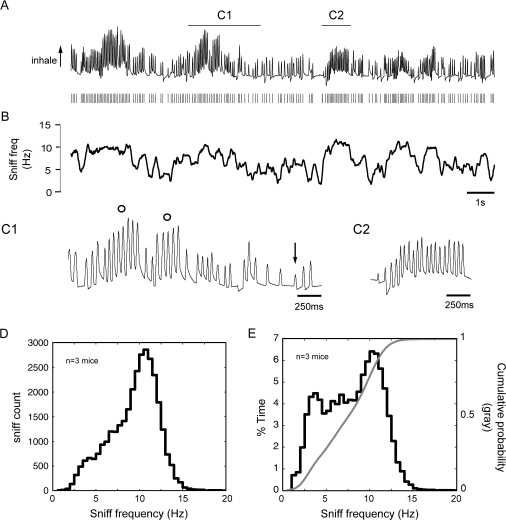
Figure 2.
Intranasal pressure transients (sniffing) recorded from freely moving mice. (A) Trace of intranasal pressure and detected sniff peaks (ticks, below trace) from a mouse freely exploring a novel environment. Upward deflections represent inhalation. Trace duration, 15 s. The trace is filtered between 0.001 and 100 Hz. (B) Trace showing sniff frequency as a function of time for the sniffing record in (A). Plot shows a smoothed moving average of sniff frequency (500-ms time window for averaging, followed by smoothing with a 250-ms sliding window) to highlight sustained sniff frequencies. Sniff frequency varied widely and rapidly, reaching peak sustained frequency of approximately 10 Hz and minimum frequencies of 3–5 Hz. (C) Epochs of sniffing magnified from (A), displaying bouts of high-frequency sniffing (C1 circles and C2) and changes in amplitude (C1 arrow) and (to a lesser extent) duration across individual sniffs. (D) Histogram of sniff frequency recorded from 3 mice, while exploring the novel testing chamber, displaying the distribution of all sniffing recorded across frequencies, within 0.5-Hz bins. (E) % time sniffing distribution (bars) and cumulative probability distribution (line) from the same data as in (D) but normalized to the cumulative amount of time mice sniffed at each frequency (viz., % time). Frequency bins = 0.5 Hz.
For comparisons of sniff frequencies at different time points, several consecutive time bins (specified in the Results) were averaged together. Comparisons were made using analysis of variance (ANOVA) or t-tests (see Results for specific tests). One-way or 1-way repeated measures ANOVA was used for analyses of habituation/dishabituation and sand-digging odor-discrimination data. Due to the subject sample size in the nose poke odor discrimination and the nose poke–only tasks (n = 3), either within-groups or between-groups t-tests were used for analyses. Statistics were carried out in MatLAB or StatVIEW (SAS Institute Inc., Cary, NC). All values are reported as mean ± standard deviation (SD) unless otherwise stated.
Results
Sniffing behavior during free exploration
We began our investigation into mouse sniffing behavior by first recording intranasal pressure transients from 3 mice while they explored a novel environment (see Materials and methods). Consistent with previous literature, herein we refer to all pressure transients associated with inhalation as “sniffs” without attempting to differentiate between “active” sniffing and “passive” respiration (Welker 1964; Macrides 1975; Youngentob et al. 1987; Kepecs et al. 2007). Monitoring sniffing from each mouse for approximately 20 min yielded >32,500 individual sniffs. Mouse sniffing behavior was qualitatively similar to that described previously in rats (Welker 1964; Macrides et al. 1982; Youngentob et al. 1987), in that it was highly complex and dynamic, varying in amplitude, waveform, and frequency on a cycle-by-cycle basis. A continuous sniffing trace from one mouse is shown in Figure 2A with the time points of the corresponding inhalation peaks displayed below (black ticks). The trace shows bouts of high-frequency (9–13 Hz) sniffing interspersed with epochs of lower frequency (3–5 Hz) sniffing, but no obvious dichotomy between different modes of sniffing behavior. A smoothed plot of the instantaneous sniff frequency for these data is shown in Figure 2B, illustrating the high degree of variability and lack of obvious structure in sniff frequency over time. High-frequency sniffing bouts (see horizontal bars in Figure 2A, displayed as magnified in Figure 2C1 “open circles” and 2C2) were elicited by both external and internal events such as a sudden noise in the testing room or initiating locomotion (data not shown). These bouts rarely lasted for longer than 2 s and generally involved sustained frequencies ∼10 Hz (Figure 2B). Figures 2C1 and 2C2 also illustrate the variation in amplitude and (to a lesser degree) duration of individual sniffs. Thus, sniffing in freely behaving mice is highly dynamic across multiple parameters.
Frequency has been a commonly used parameter to characterize sniffing behavior in other animals (Macrides et al. 1982; Youngentob et al. 1987; Thesen et al. 1993; Steen et al. 1996; Uchida and Mainen 2003; Kepecs et al. 2007; Verhagen et al. 2007). Overall, we found that freely exploring mice sniffed at a broad range of frequencies. Excluding sniff frequencies greater than 3 SDs of the median (distribution range: 0.1–17.6 Hz, values outside of this range likely resulted from peak measurement errors) showed that 95% of all sniffs occurred above 3.3 Hz, with only 5% of sniffs occurring above 12.5 Hz (Figure 2D). Mice spent approximately half of their time sniffing above 7 Hz (Figure 2E).
Odor habituation/dishabituation
We next assessed how sniffing behavior changes during detection of a novel odorant and subsequent habituation in 6 mice. In this task, a novel odorant was presented 4 consecutive times, separated by 1- to 2-min ITIs, and followed by presentation of another novel odorant in the same manner (see Figure 1A and Materials and methods). Prior to odorant presentation, mice typically maintained a resting sniff frequency of 5–7 Hz (mean frequency: 2–3 s, preodor: 5.6 ± 3.1 Hz, n = 91 trials). The first presentation of a novel odorant evoked a significant increase in sniff frequency across all animals (Figure 3A) (F(1,8) = 39.556, P = 0.0002; 4–5 s after odorant onset vs. 2–3 s preodor; n = 25 trials). The average sniffing frequency during the last second of novel odorant presentation was 8.4 ± 1.0 Hz. Generally, the onset of the sniffing response to the odorant occurred 1–2 s after odorant presentation began (Figure 3A,B), likely reflecting the delay for the odorant to diffuse throughout the chamber and be detected by the animal. Sniff frequency returned to near-baseline levels within several seconds after the odorant offset (data not shown). Similar effects on sniff frequency were observed in a smaller subset of trials wherein animals were presented with “biologically relevant” odors (i.e., peanut butter or male mouse urine) (data not shown).
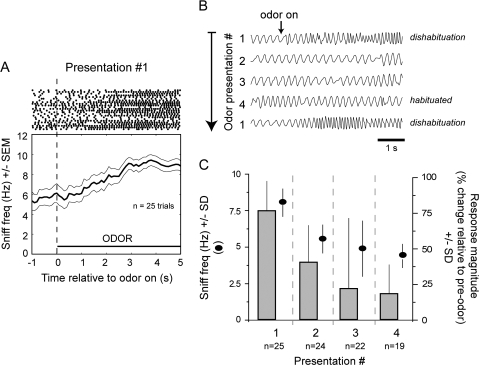
Figure 3.
Mouse sniffing behavior during odor habituation/dishabituation. (A) Raster and sniffing frequency plots relative to the moment of odorant onset, during dishabituation (viz., novel odorant) trials (n = 6 mice, 25 trials). Points in raster plots represent the moment of a peak in sniff inhalation, with each line representing sniffing within individual trials. Dishabituation trials are marked by an increase in sniffing during odorant presentation. Error lines indicate standard error of the mean. Time bins = 50 ms. (B) Consecutive sniffing traces recorded from one mouse throughout 4 presentations of the odorant hexanal and upon the first presentation of the odorant isoamyl acetate. Odorants were presented within each trial from the moment of “odor on” till the end of the trace. Note the increase in sniffing frequency during the first odorant presentation (i.e., ‘dishabituation’) of hexanal, which then decreased over subsequent presentations to show little or no modulation by the odorant upon the fourth presentation. On the subsequent trial presentation of the novel odorant isoamyl acetate evoked a robust dishabituation sniffing response. Different trials show different delays to onset of the sniffing response, presumably reflecting the time for the odorant to reach the mouse, which could be at different locations in the chamber. Traces are filtered between 0.05 and 50 Hz. (C) Average sniffing response magnitudes (bars) and average sniff frequencies (points) for the first through fourth presentation of all odorants. Response magnitude is expressed in % change in sniffing frequency during 4–5 s of odor on, relative to the 2–3 s preodor. Sniffing frequency (points) is reported as the absolute average frequency, averaged across all mice and trials. Error bars = SD.
Increases in sniff frequency habituated over subsequent presentations of the odorant (Figure 3B,C). In the example shown in Figure 3B from one mouse presented with the odorant hexanal, there were no clear changes in sniffing behavior evoked by the third or fourth odorant presentations. Similar response patterns were seen across all 6 mice, with a strong relationship between odorant presentation number and odorant-evoked sniffing frequency (repeated measures ANOVA; F(4,3) = 6.074, P = 0.0093). On average, novel odorants (i.e., “presentation 1,” n = 25) evoked a 75.0 ± 20.1% increase in sniffing frequency relative to the 2–3 s prior to odorant presentation (Figure 3C vs. the 4- to 5-s preodor). Three trials later, sniffing responses to the same odorant were only 18.3 ± 19.6% greater than preodor frequencies, not significantly different from baseline sniffing (n = 19 trials; Figure 3C). These results show that the presentation of novel odorants to mice is accompanied by spontaneous (viz., unlearned) increases in sniffing frequency which rapidly habituate over repeated presentations.
Two-choice odor discrimination–digging task
We next assessed sniffing behavior of mice in an operant task involving a 2-choice odor discrimination. We used the sand-digging paradigm, which is a commonly used paradigm for assessing both olfactory discrimination and generalization in mice and rats (Mihalick et al. 2000; Fortin et al. 2002; Wei et al. 2006; Tait et al. 2007). In this task, a CS+ (conditioned “rewarded”) odorant is placed on one cup of sand and a CS− (conditioned “unrewarded”) odorant on another cup and the animal is allowed to choose from the 2 cups (see Materials and methods and Figures 1B1,B2). Trials were initiated by the experimenter lifting a partition on one end of the testing chamber (trial start), freeing the animal from a holding location ∼20 cm from the cups. The mouse then was allowed to sample and dig in either cup, yet only a correct response (digging in the CS+ cup) was reinforced with a food reward (Figure 4A). An important aspect of this paradigm is that the reward was delivered manually to the mouse by the experimenter, ∼1–2 s after making the correct choice.
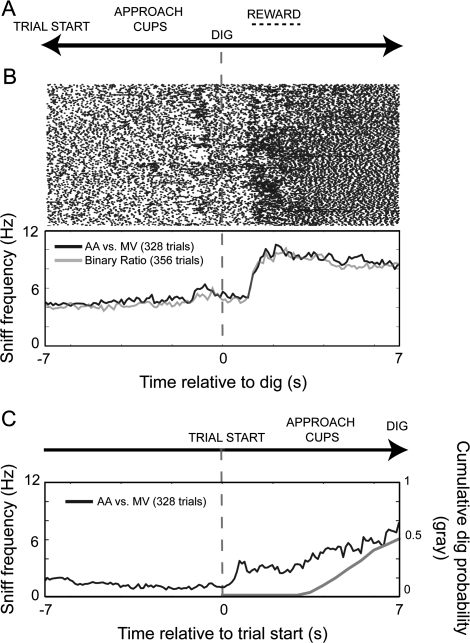
Figure 4.
Mouse sniffing behavior during a digging-based odor discrimination. (A) Diagram of the sand-digging odor-discrimination task structure. In this task, the animal approaches the stimulus cup at which time it can sample the odorant. The mouse is then free to dig in the cup to indicate discrimination (dig). If the animal dug in the correct cup, it was administered a small food reward (reward). (B) Raster and sniffing frequency plots showing sniffing data from 5 mice relative to the moment of dig, during discrimination of isoamyl acetate (AA) vs. methyl valerate (“MV;” black trace; n = 5 mice) and discriminating binary mixtures of AA:MV (gray trace, “binary ratio;” n = 4 mice). The raster shows the moment of sniff inhalation peaks, across 300 trials from the AA vs. MV odorant discrimination (includes data from all mice and all sessions, both CS+ and CS− trials). A small increase in sniffing frequency is noted in both conditions approximately 2 s prior to dig and an even greater increase in sniffing frequency following the moment of dig, related to the time of reward presentation. Time bins = 100 ms. (C) Sniff frequency plot showing same data as in (B) but aligned relative to trial start, during discrimination of isoamyl acetate (AA) vs. methyl valerate (“MV;” black trace; n = 5 mice). “Trial start” is initiated by the experimenter raising a partition, allowing the animal to approach the stimulus cups and eventually dig. During this approach period, a gradual rise in sniff frequency is observed, related to locomotion toward the stimulus cups. Gray plot indicates cumulative probability of digging as a function of time after trial start. Mice rarely dug in <4 s. The increase in mean sniff frequency after this time may reflect reward-driven responses as shown in (B). Time bins = 100 ms.
We monitored sniffing behavior from 5 mice performing this task. For all mice, the 2 conditioned odorants were isoamyl acetate and methyl valerate (see Materials and methods for details). A total of 328 trials (2–4 sessions/mouse) were recorded, wherein mice cumulatively performed with an average accuracy of 97.1 ± 4.6% correct responses. The median latency from trial start until the animal dug in a cup was 7.3 ± 3.9 s (average of correct choices only; inter-animal range of mean latencies: 6.6 ± 2.2 to 9.6 ± 4.6). Digging rarely occurred <4 s after trial start. Because of variability in digging times, we first analyzed sniffing behavior relative to the time the animal dug; odorant sampling and decision making presumably occurred in the seconds preceding this time point. Figure 4B shows sniff rasters and mean frequencies across all trials and animals relative to digging time. On average, mice showed relatively stable sniffing patterns during the 5 s prior to digging, with mean bin frequencies ranging from 4.3–6.4 Hz (average min–max bin values from all mice, 100-ms bins). Across all animals, we found a modest but insignificant increase (peak frequency = 6.4 ± 3.6 Hz) in sniffing frequency in the 1 s prior to digging in the stimulus cup (Figure 4B; F(1,8) = 4.248, P = 0.073; 2–3 s predig vs. 0–1 s predig). Interestingly, there was a striking 3-fold increase in sniff frequency beginning ∼1 s after digging (Figure 4B; F(1,8) = 31.237, P = 0.0005; 2–3 s predig vs. 2–3 s postdig). Sniff frequency reached a peak of 10.5 ± 4.9 Hz during this epoch. This increase coincided with the manual lowering of food reward to the mouse by the experimenter.
We also analyzed sniffing behavior relative to the time of trial start (Figure 4C). This analysis showed that mice—on average—increase sniff frequency from resting levels of ∼2 Hz to ∼4 Hz in the interval after trial start and preceding digging. This increase in sniff frequency could reflect active sampling of odorants as the mouse decided which cup to approach or it could simply reflect effects of locomotion or general arousal on sniff frequency. Overall, these data suggest that mice show increased sniff frequency while engaged in the task, no further increases at the time of digging, and large increases prior to receiving a reward.
We further investigated whether the perceptual difficulty of the odor discrimination has any effect on sniff frequency in this paradigm. A common method to increase difficulty during odor discriminations is to use a binary mixture of the CS+ and CS− odorants and ask the animal to respond to the mixture with the largest proportion of the CS+ (Uchida and Mainen 2003; Abraham et al. 2004). Therefore, we trained 4 of the mice which were previously performing in the pure odor task on this binary mixture discrimination, using a ratio of 68% CS+:32% CS− (see Materials and methods). Training on this task persisted until behavioral performance (i.e., % correct response) was similar to that during the pure odor condition (60–120 trials per mouse), allowing us to compare performance on a more difficult discrimination but during similar performance accuracy. Data were recorded from 356 trials (2–4 sessions/mouse), with a mean accuracy of 92.6 ± 0.1% correct responses. In this mixture task, as in the pure odor task, there was a very slight yet insignificant increase (peak frequency = 6.0 ± 3.9 Hz) in sniff frequency in the 1 s prior to digging in the stimulus cup (Figure 4B, gray trace; F(1,6) = 4.155, P = 0.087; 2–3 s predig vs. 0–1 s predig) and a large increase in frequency when reward was delivered (Figure 4B; F(1,6) = 7.288, P = 0.035; 2–3 s predig vs. 2–3 s postdig). Interestingly, there was no difference in sniffing behavior in the time prior to digging between the pure odor task and the binary mixture task (F(1,7) = 0.495, P = 0.504; 0–1 s predig). “Baseline” sniffing frequencies between the groups also did not differ (F(1,7) = 0.748, P = 0.416; 2–3 s predig). These results show that mice can perform even “difficult” odor discriminations without noticeably increasing sniff frequency.
Nose poke–based go/no-go odor-discrimination task
In a final set of experiments, we monitored sniffing behavior of mice during a go/no-go odor-discrimination task using a nose poke–based odor-sampling paradigm (see Materials and methods and Figure 1C). This paradigm has been frequently used to assess odor-discrimination abilities in mice and rats (Slotnick and Nigroshi 1974; Eichenbaum et al. 1991; Bodyak and Slotnick 1999; Kelliher et al. 2003; Uchida and Mainen 2003; Abraham et al. 2004; Kay et al. 2006; Rinberg et al. 2006b; Wesson et al. 2006; Beshel et al. 2007; Doucette et al. 2007). Mice were trained to nose poke into a sampling port and hold their snout in the port for 100 ms, after which either a CS+ or a CS− odorant was presented (Figure 5A). The mice could then move to a separate reward spout to receive a liquid food reward for CS+ odorants, and no reward was given for CS− odorants. We trained 3 mice to criterion performance (>80% correct responses) and then monitored sniffing behavior throughout the task for multiple sessions per mouse (n = 3,858 trials, mean 257.2 ± 84.4 trials/session, 4–7 sessions per mouse). Average discrimination performance was 90.5% correct accuracy (±4.8 SD; range of individual session performances: 80.5–98.1% correct). We also monitored the time spent sampling the odorant before head withdrawal form the port (Figure 5B). Mice spent an average of 747 ± 88 ms engaged in “odor sampling,” as defined by the latency from odorant onset (100 ms after initial nose poke) to withdrawal (average session range: 334–1124 ms).
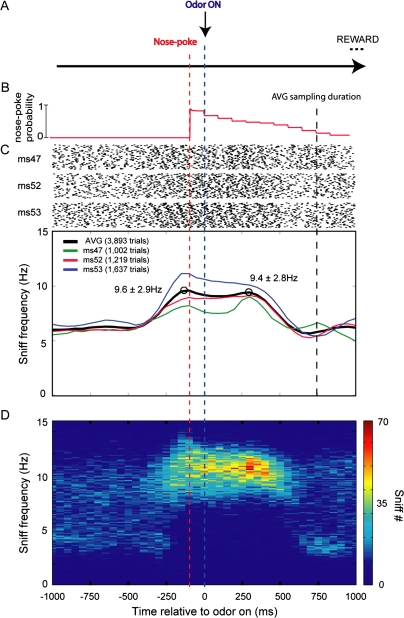
Figure 5.
Mouse sniffing behavior during a nose poke odor discrimination. (A) Diagram showing the nose poke task structure. In this go/no-go design, animals were conditioned to nose poke into an odor-sampling port (“nose poke,” red dashed line), hold their snout in the port for 100 ms, upon which time either a CS+ or CS− odor was presented (rewarded or nonrewarded odor, respectively; “odor on,” blue dashed line). The animal was then conditioned to sample the odor while in the port (sample) and could lick a reward spout in exchange for a reward during CS+ trials. The odorant duration was controlled based upon the length of nose poke (up to 2 s). (B) Trace displaying the duration of nose poke as defined by the latency from nose poke till nose withdrawal from the sampling port across all trials. The average odorant sampling duration of all animals defined as the moment of odorant onset till nose withdrawal is displayed (“AVG sampling duration,” black dashed line). (C) Raster and sniff frequency plot of sniffing recorded from 3 mice while performing the nose poke odorant discrimination, relative to the moment of odorant onset. Rasters show 100 trials from each mouse (ms 47, 52, and 53) with points indicating moments of sniff inhalation peaks. (C, lower) Sniffing frequency plots of individual animals (colored traces) and average from all animals (black trace) throughout task performance. Time bins = 50 ms. There was a heightened increase in sniff frequency upon nose poke into the odor port (prior to odor onset), which was maintained during the “odor-sampling” period. Interestingly, animals decreased their sniffing frequency before their withdrawal from the odor port. These dynamics are also shown in a 2-dimensional histogram (D), with sniff count represented in each bin in pseudocolor. Note the reduction in sniff frequency variability during nose poke and odorant sampling across all trials. Time bin = 50 ms, frequency bin = 0.1 Hz. Data within all figures is from both CS+ and CS− trials.
Mice displayed strong modulations in sniff frequency during this task. Prior to nose poke, mice sniffed within a range of 6.0 ± 0.1 to 6.3 ± 0.1 Hz (min–max bin values from −1.0 to −0.5 s pre nose poke; average across all animals; Figure 5C). At approximately 250 ms prior to nose poke, sniff frequency increased, peaking at the time of the poke (peak frequency = 9.6 ± 2.9 Hz; Figure 5C, black trace). Although all 3 mice showed qualitatively similar behavior, there were substantial inter-animal differences in the magnitude of sniff frequency increase at nose poke. For example, whereas one mouse increased sniff frequency to 8.1 ± 0.5 Hz at nose poke (Figure 5C, green trace), another mouse in the same cohort sniffed at 10.8 ± 0.3 Hz (Figure 5C, blue trace). The frequencies from these 2 mice at the moment of nose poke significantly differed across all behavioral sessions (unpaired t(8) = −4.166, P = 0.0031; −150 to −100 ms preodor).
Mice generally maintained their elevated sniff frequency into the time of odorant presentation. Whereas the average frequency at nose poke was 9.4 ± 1.5 Hz (average frequency of all sessions, n = 3 mice, −150 to −100 ms prepoke), the average during odor presentation was 9.4 ± 2.8 Hz (average frequency of all sessions, n = 3 mice, 250–300 ms during odor, Figure 5C). Sniffing just prior to nose poke and during odorant sampling was also less variable across trials (Figure 5D). Interestingly, in all 3 mice, sniff frequency began returning to baseline levels “before” withdrawal from the odor-sampling port (Figure 5C). At the time of mean odor-sampling duration (750 ms), the mean sniff frequency was 5.8 ± 3.3 Hz (average frequency 750–800 ms during odor on). This frequency is significantly less than that displayed during poke (1 group t(5) = 3.922, P = 0.017; −150 to −100 ms pre odor) and during odor sampling (1 group t(5) = 8.657, P < 0.001; 250 to 300 ms during odor). A prior study using a similar go/no-go discrimination paradigm in mice reported a difference in odor-sampling time for CS+ and CS− trials (Slotnick 2007). Although we did not counterbalance our CS+ and CS− odors to allow an accurate assessment of the effects of stimulus valence on sniffing frequency independent of the stimulus per se, we did not find substantial differences in sniffing frequency related to valence (data not shown). Whereas the mean sniffing frequencies of mice during nose poke and odor sampling for all CS- trials was 9.7 ± 2.7 and 9.6 ± 2.6 Hz, the same values during CS+ trials were 9.6 ± 2.9 and 9.6 ± 2.3 Hz (poke and odor; poke = −150 to −100 ms, odor = 250–300 ms relative to odor on). Overall, these results show that in mice, performance in a go/no-go nose poke odor–discrimination task involves large increases in sniffing frequency that occur just prior to odor sampling and end prior to withdrawal from the sampling port.
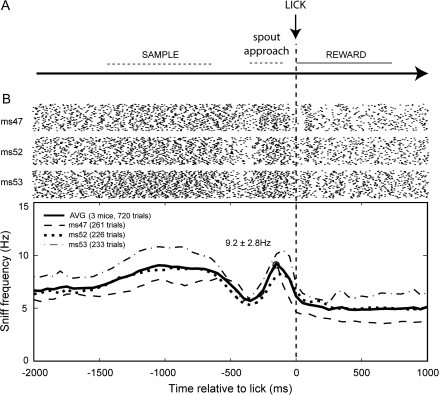
For CS+ trials, mice moved to the reward spout after port withdrawal and licked for a liquid food reward—typically within 500 ms after odor sampling (Figure 6A). Similar to previous reports in rats (Kepecs et al. 2007), we observed a substantial increase in sniffing frequency just before the moment of licking for the reward in CS+ trials (Figure 6B). This behavior was robust and conserved between animals. In particular, mice on average showed a rapid increase from 5.9 ± 2.8 Hz (average frequency, n = 3 mice, −450 to −400 pre lick) to 9.2 ± 2.8 Hz immediately prior to licking (average freq, n = 3 mice, −250 to −200 pre lick), which quickly returned to resting levels during reward drinking. These results confirm previous findings in rats (Kepecs et al. 2007), and our findings in mice seeking a reward in the sand-digging task (Figure 4), and suggest a strong relationship between reward-seeking and sniffing behavior.
Figure 6.
Mouse sniffing prior to reward administration during the nose poke task. Task and data herein are the same as in Figure 5, yet in this figure data are aligned relative to the moment of licking from the reward spout. (A) Diagram showing the nose poke task structure, relative to the time of lick (lick). Following sampling of the CS+ odorant (sample), animals were conditioned to approach the reward spout (spout approach) upon which time they were free to lick in exchange for a reward (reward). (B) Raster and sniff frequency plot of sniffing recorded from 3 mice while performing the nose poke odorant discrimination, relative to the moment of lick. Data shown are only from CS+ trials. Rasters show 100 trials from each mouse (ms 47, 52, and 53) with points indicating moments of sniff inhalation peaks. Sniffing frequency plots of individual animals (dashed traces) and averaged from all animals (solid trace) throughout task performance. There was a strong increase in sniffing frequency across animals prior to licking for the reward, which was similar to the frequency during odorant sampling. Time bins = 50 ms.
Nonolfactory nose poke–based operant task
Finally, given the popularity of nose poke–based tasks in olfactory studies and in prior characterizations of sniffing behavior (Macrides et al. 1982; Youngentob et al. 1987; Uchida and Mainen 2003; Kepecs et al. 2007), we wished to test the degree to which nonolfactory aspects of this task shape sniffing behavior. Therefore, we monitored sniffing in a separate cohort of 3 mice, naive to olfactory conditioning that were trained to simply nose poke in exchange for a reward. Mice were trained to nose poke for the same duration as in the odor-discrimination task (100 ms) and then were free to withdraw from the port and lick for a reward (Figure 7A). There were no CS- trials nor were any olfactory stimuli presented during training or data collection. Sniffing was recorded from 459 nose poke trials (1 session per mouse, 136 ± 16 trials/session) for which the mice licked to 92% of all trials. Interestingly, mean “sampling time” in the port was nearly as long as for the odor-discrimination task: 716 ± 164 ms (Figure 7B).
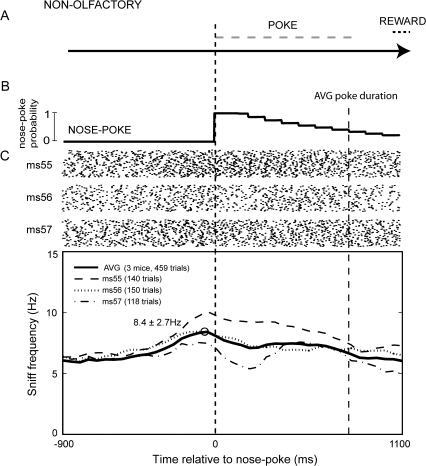
Figure 7.
Mouse sniffing behavior during a nonolfactory nose poke–based task. (A) Diagram showing the nose poke task structure. In this task, odor-naive mice (n = 3) were taught to nose poke (poke) and hold their snout in the port for 100 ms in exchange for a reward (reward). Importantly, these mice were neither trained on nor exposed to odors during testing. Testing took place in the same chamber as in Figure 1C. (B) Trace displaying the duration of nose poke as defined by the latency from nose entry till nose withdrawal from the sampling port across all trials. (C) Raster and sniff frequency plot of sniffing recorded from mice while performing the nonolfactory nose poke task relative to the moment of nose poke. Rasters show 100 trials from each mouse (55, 56, and 57 ms) with points indicating moments of sniff inhalation peaks. (C, lower) Sniffing frequency plot relative to nose poke, averaged from all animals throughout task performance. Average poke duration (AVG poke duration), defined as the latency between nose poke and withdrawal from odor port, is indicated. The act of nose poke itself was accompanied with increases in sniffing frequency, similar to that observed during the odor-discrimination nose poke task. Time bins = 50 ms.
As in the odor-discrimination task, mice showed increases in sniff frequency as they approached the port; sniff frequency peaked at or just prior to the time of nose poke. Average peak frequency within the 50 ms before nose poke was 8.4 ± 2.7 Hz (n = 3 mice; Figure 7C). This frequency was significantly greater than baseline frequencies (Figure 7; 1 group t(5) = 12.258, P < 0.0001; −500 to −450 ms prepoke [baseline] vs. −100 to −50 ms prepoke [poke]) and was statistically similar to the sniff frequency during nose poke of the cohort of animals in the odor-discrimination nose poke task (2 group t(4) = 1.015, P = 0.367). Interestingly, we observed a similar increase in sniffing frequency prior to licking for a reward in this task as in the odor-discrimination nose poke task (data not shown). These results suggest that much of the sniffing frequency changes observed during nose poke–based odor-guided tasks are related to the act of the poke itself rather than to odor sampling and discrimination.
Discussion
Despite their prominent use as models for olfactory sensation, little is known about the sniffing behavior of mice during odor-guided behaviors. Here, we investigated sniffing behavior in mice during task performance in 3 commonly used olfactory behavior paradigms as well as in 1 nonolfactory paradigm and during free exploration of a novel environment. To our knowledge, this study provides the first descriptive data on sniffing behavior in mice, and so should provide a useful starting point for further studies on the role of sniffing in shaping odor information processing as well as the further development of tools using sniffing behavior as a measure of olfactory performance (Youngentob et al. 1987; Youngentob 2005; Wesson et al. 2008).
Sniffing as a function of behavioral context
In general, we found that sniffing behavior in the mouse is complex and highly dynamic as is the case for sniffing in other rodents (Welker 1964; Youngentob et al. 1987; Kepecs et al. 2007). Sniff parameters that were modulated included frequency, amplitude, inhalation/exhalation waveform, and regularity across multiple sniffs. A previous study (Youngentob 2005) has shown that these and additional parameters can be combined into a univariate measure of sniffing behavior (the “sniffing index”) that can reliably detect inter-animal differences in the response to odorant stimulation. Thus, mice clearly alter many aspects of their sniffing behavior in response to olfactory stimuli. To provide a quantitative and more concrete description of sniffing in mice, we chose to focus our analysis on sniff frequency. Sniff frequency has been widely used as a descriptor of sniffing behavior in other animals (Welker 1964; Youngentob et al. 1987; Thesen et al. 1993; Steen et al. 1996; Kepecs et al. 2007), and sniff timing (rather than waveform) is the major parameter considered in current models of how sniffing shapes olfactory processing (Macrides 1975; Kepecs et al. 2006; Wachowiak and Shipley 2006; Verhagen et al. 2007; Bathellier et al. 2008).
During free exploration of a novel environment, sniffing behavior was unstructured, with rapid transitions from low-frequency but irregular sniffing to bouts of high-frequency sniffing lasting usually less than 2 s and reaching sustained rates of ∼10 Hz. Such transitions to high-frequency (5–10 Hz) sniffing have been previously reported during exploratory behavior in rats (Welker 1964; Verhagen et al. 2007; Wesson et al. 2008) and hamsters (Macrides 1975). One interesting difference between this study and previous ones is that here high-frequency sniffing was observed in response to nonolfactory cues—for example, unexpected auditory stimuli. This finding is in agreement with previous reports in rats (Harrison 1979). Transitions to high-frequency sniffing also occurred in the absence of external stimuli but when the animal began locomotor behavior. Thus, many factors other than odorant stimulation strongly modulate sniffing behavior in mice.
Partly because of this complexity, we further characterized sniffing during performance in several common odor-guided tasks. The tasks were chosen to represent major classes of behavioral assays of olfactory performance: a spontaneous discrimination task, an operant 2-choice task, and an operant go/no-go task. Interestingly, sniffing behavior was different in each type of task. In general, however, sniffing was more stereotyped and less dynamic than in freely exploring mice.
The odor habituation/dishabituation paradigm is routinely used to assay an animal's ability to spontaneously detect and discriminate odors (Sundberg et al. 1982; Baum and Keverne 2002; Cleland et al. 2002; McNamara et al. 2008). In this task, an increase in investigative behavior to an odorant (typically defined as approach to the odor source or visually monitored sniffing) is used as an indication that the animal discriminated the odorant from a prior one. Our measurements show that increased sniff frequency is a reliable indicator of dishabituation to a novel odorant. On average, mice increased sniff frequency by approximately 4 Hz above baseline levels for novel presentations. Equally reliable was the decrease in sniff frequency for subsequent odorant presentations. Thus, measuring sniffing during odor habituation/dishabituation may yield more robust (i.e., less noisy) behavioral readouts than more common measures that rely on visual judgments from the experimenter. More precise quantitative measurements may be particularly important when using this paradigm to make graded measurements of perceptual odor similarity (Cleland et al. 2002).
Sniffing behavior in the 2 operant paradigms was more stereotyped, possibly because each task involved a well-defined sequence of motor actions repeated by the animal in each trial. In the 2-choice sand-digging task, the animal moves toward 1 of the 2 stimulus cups, samples the odorant, and makes a decision to dig or to investigate the other cup, after which it receives a food reward from the experimenter. Although the exact time of odor sampling and decision making was unclear, it was striking that mice showed no significant increases in sniff frequency just prior to digging, nor did they do so at the time of digging. This result held true even for more difficult odor discriminations. Thus in this paradigm, sniffing behavior was not informative as to either the timing or the nature of the odor-discrimination event. One explanation for this result is that discrimination occurs rapidly—for example, in a single sniff of odorant, as has been previously reported for rats (Uchida and Mainen 2003; Rajan et al. 2006; Wesson et al. 2008)—and thus does not require a sustained increase in sniff frequency. Instead, there was a consistent and dramatic increase in sniff frequency as the animal received the reward. The relationship between reward anticipation and sniffing behavior has been previously reported in rats (Clarke and Trowill 1971; Ikemoto and Panksepp 1994; Kepecs et al. 2007) and appears even in nonolfactory paradigms (Clarke and Trowill 1971; Ikemoto and Panksepp 1994).
The nose poke–based task is a third commonly used behavioral paradigm in olfaction (Slotnick and Nigroshi 1974; Eichenbaum et al. 1991; Bodyak and Slotnick 1999; Kelliher et al. 2003; Uchida and Mainen 2003; Abraham et al. 2004; Kay et al. 2006; Rinberg et al. 2006b; Wesson et al. 2006; Beshel et al. 2007; Doucette et al. 2007) and has the advantage that the time of odorant sampling can be precisely defined. Several studies have measured rat sniffing behavior in this paradigm, and all have reported sniff frequencies of 4–12 Hz at the time of nose poke and the beginning of odor sampling (Macrides et al. 1982; Youngentob et al. 1987; Kepecs et al. 2007). Our results show that mice engage in a similar sniffing behavior. We also found that periods of high-frequency sniffing were very short in this paradigm, returning to baseline levels after ∼500 ms of sampling and before the animal withdrew its snout from the sampling port. This result appears consistent with a previous study in the rat (Macrides et al. 1982) and raises the possibility that measures of “odor sampling” based purely on time spent in an odorant port (Abraham et al. 2004; Rinberg et al. 2006b; Slotnick 2007) may not accurately reflect the time the animal was actively sampling the odorant. Finally, we observed a brief yet robust increase in sniff frequency at the time mice were approaching the reward spout. This result agrees with a previous report in rats (Kepecs et al. 2007) and also with our current work from that of the sand-digging task.
By measuring sniffing behavior in mice performing a nonolfactory variant of the nose poke task, we also found that mice showed increases in sniff frequency simply when performing the nose poke itself, even when completely naive to olfactory conditioning. Increased sniff frequency during nose poke highlights sniffing as part of a stereotyped behavioral repertoire exhibited during exploration or spatial navigation. For example, although not monitored here, whisking likely occurs as the animal approaches and inserts its nose into the sampling port as whisking and sniffing are tightly coupled sensorimotor systems that are often coactivated and possibly are synchronized (Welker 1964; Komisaruk 1970).
This result underscores the importance of considering sniffing as part of a general sensorimotor program that is shaped by many different factors, both external and internal to the animal. The fact that sniffing behavior in mice appears more dependent on the behavioral paradigm than the sensory demands on the olfactory system is consistent with this idea. Thus, although sniffing behavior is a useful behavioral readout for evaluating olfactory performance/perception in some paradigms (i.e., habituation/dishabituation), it is less useful in other paradigms (i.e., 2-odor sand digging). In all cases, potential confounds from nonolfactory aspects of the task should be carefully considered. One parameter that we did not explore in this study is how the hedonic value of an olfactory stimulus influences sniffing behavior. For example, it would be interesting to test whether socially relevant or complex food odors increase sniff frequency in the operant paradigms—possibly as a function of their effect on motivational state (Wesson et al. 2006; Sorwell et al. 2008). Another interpretation of our results is that sniffing frequency increases specifically during active learning about an odorant (i.e., during exposure to a novel odorant in the dishabituation task) but not during discrimination of learned odorants. This possibility could be explored by monitoring sniffing behavior during the acquisition phases of the operant tasks. Finally, given that we observed similar sniff frequencies during different phases of a mouse's behavior (e.g., sampling a habituated odor and during quiescent sniffing in free exploration), it seems problematic to classify sniffing behavior into discrete “modes” based solely on frequency, as has been done previously in rats (Kepecs et al. 2007).
Sniffing behavior and olfactory coding
The importance of sniffing in shaping how the nervous system represents and processes olfactory information has been recognized since the earliest recordings of odorant-evoked neural activity (Adrian 1950, 1953). Since that time, numerous hypotheses on the role of sniffing in odor coding have been articulated. These include shaping spatiotemporal patterns of receptor neuron activation via chromatographic effects at the level of the olfactory epithelium (Mozell 1973; Youngentob et al. 1987; Schoenfeld and Cleland 2005; Scott 2006), providing a means for regulating the strength of receptor activation as a function of odorant concentration (Youngentob et al. 1987; Sobel et al. 2000; Johnson et al. 2003), controlling the level of adaptation of receptor neurons to background odorants (Schmitt and Ache 1979; Dethier 1987; Verhagen et al. 2007), and temporally organizing sensory input and central processing to enable timing-based coding strategies to encode odor information (Macrides 1975; Chaput 1986; Kepecs et al. 2006; Spors et al. 2006; Schaefer and Margrie 2007; Wesson et al. 2008). All these hypotheses critically depend on the particular parameters of sniffing. The fact that these parameters are highly dynamic in the behaving animal suggests that the nature of odor coding and information processing itself may change as a function of the animal's sniffing strategy. Olfactory processing strategies might also differ between animals that show differences in sniffing behavior.
For example, in rats, a change in sniff frequency from resting respiration at 1–2 Hz to high-frequency sniffing above 4 Hz leads to attenuation of odorant-evoked receptor neuron inputs to the OB and a loss of temporal patterning of these inputs relative to the sniff cycle (Verhagen et al. 2007); the presumed function of this attenuation is to adaptively filter out background odorants during exploratory behavior. In mice, however, we found that resting sniff frequencies occur at 3–5 Hz and exploratory sniffing occurs at 8–10 Hz. Thus, responses to background odorants may be attenuated even during quiescent behavior in mice. Alternatively, the frequency at which adaptive filtering occurs may be shifted to higher frequencies in mice. This possibility needs to be tested with combined measurements of sniffing behavior and odorant-evoked neural activity in the awake mouse.
Likewise, numerous studies have provided evidence that the timing of neural activity relative to the sniff cycle may play a role in encoding odor information (Chaput 1986; Spors et al. 2006; Schaefer and Margrie 2007). These temporal dynamics have often been analyzed in anesthetized animals showing regular low-frequency breathing, allowing neural dynamics to be characterized in terms of phase of the respiratory cycle (Chaput 1986; Rennaker et al. 2007). However, we found that instantaneous sniff frequency varied dramatically and was rarely maintained at a fixed frequency for more than a few cycles. Thus, in the awake, actively sniffing animal, respiratory phase is not clearly defined. Instead, neural dynamics of odorant-evoked activity may be best described in terms of latencies relative to the preceding inhalation (Margrie and Schaefer 2003; Spors et al. 2006). How robust these latency differences are across changes in sniffing behavior is another important question.
Several approaches are available for monitoring odorant-evoked neural activity with high temporal and spatial resolution in vivo, including multielectrode recording (Kay and Laurent 1999; Rinberg et al. 2006a; Rennaker et al. 2007) and optical imaging with synthetic and genetically encoded probes (Bozza et al. 2004; Wachowiak et al. 2004; Verhagen et al. 2007; Wesson et al. 2008). Given the integral link between sniffing and olfactory sensation, this description of mouse sniffing behavior should be useful in bringing these approaches to bear on the question of how odor information is represented and processed in the awake-behaving animal.
Funding
US National Institutes of Health (DC06441) and Boston University.
Supplementary Material
Acknowledgments
We thank Arbora Resulaj, Dima Rinberg, Michael Baum, and John McGann for comments on the manuscript. D.W.W. and M.W. conceived of the project and designed experiments. D.W.W., T.N.D., and M.O.J. trained mice, collected data, and analyzed data. D.W.W. and M.W. wrote the manuscript.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44(5):865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol. 1950;2:377–388. doi: 10.1016/0013-4694(50)90075-7. [DOI] [PubMed] [Google Scholar]
- Adrian ED. Sensory messages and sensation. The response of the olfactory organ to different smells. Acta Physiol Scand. 1953;29:5–14. doi: 10.1111/j.1748-1716.1953.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57(4):586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41(2):213–219. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci. 2007;27(31):8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 1999;24(6):637–645. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42(1):9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Catania KC, Hare JF, Campbell KL. Water shrews detect movement, shape, and smell to find prey underwater. Proc Natl Acad Sci USA. 2008;105(2):571–576. doi: 10.1073/pnas.0709534104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput MA. Respiratory-phase-related coding of olfactory information in the olfactory bulb of awake freely-breathing rabbits. Physiol Behav. 1986;36:319–324. doi: 10.1016/0031-9384(86)90023-5. [DOI] [PubMed] [Google Scholar]
- Clarke S, Trowill JA. Sniffing and motivated behavior in the rat. Physiol Behav. 1971;6(1):49–52. doi: 10.1016/0031-9384(71)90013-8. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116(2):222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Dethier VG. Sniff, flick, and pulse: an appreciation of interruption. Proc Am Philos Soc. 1987;131(2):159–176. [Google Scholar]
- Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn Mem. 2007;14(8):539–547. doi: 10.1101/lm.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum HB, Otto TA, Wible CG, Piper JM. Building a model of the hippocampus in olfaction and memory. In: Davis JL, Eichenbaum HB, editors. Olfaction: a model system for computational neuroscience. Cambridge (MA): MIT Press; 1991. pp. 167–210. [Google Scholar]
- Fortin NJ, Aqster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JM. The control of responding by sounds: unusual effect of reinforcement. J Exp Anal Behav. 1979;32(2):167–181. doi: 10.1901/jeab.1979.32-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The relationship between self-stimulation and sniffing in rats: does a common brain system mediate these behaviors? Behav Brain Res. 1994;61(2):143–162. doi: 10.1016/0166-4328(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Mainland JD, Sobel N. Rapid olfactory processing implicates subcortical control of an olfactomotor system. J Neurophysiol. 2003;90(2):1084–1094. doi: 10.1152/jn.00115.2003. [DOI] [PubMed] [Google Scholar]
- Kay LM, Krysiak M, Barlas L, Edgerton GB. Grading odor similarities in a go/no-go task. Physiol Behav. 2006;88(4–5):339–346. doi: 10.1016/j.physbeh.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2(11):1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Kelliher KR, Ziesmann J, Munger SD, Reed RR, Zufall F. Importance of the CNGA4 channel gene for odor discrimination and adaptation in behaving mice. Proc Natl Acad Sci USA. 2003;100(7):4299–4304. doi: 10.1073/pnas.0736071100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses. 2006;31(2):167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol. 2007;98(1):205–213. doi: 10.1152/jn.00071.2007. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR. Synchrony between limbic system theta activity and rhythmical behavior in rats. J Comp Physiol Psychol. 1970;70(3):482–492. doi: 10.1037/h0028709. [DOI] [PubMed] [Google Scholar]
- Laing DG. Characterization of human behaviour during odour perception. Perception. 1982;11:221–230. doi: 10.1068/p110221. [DOI] [PubMed] [Google Scholar]
- Macrides F. Temporal relationships between hippocampal slow waves and exploratory sniffing in hamsters. Behav Biol. 1975;14:295–308. doi: 10.1016/s0091-6773(75)90419-8. [DOI] [PubMed] [Google Scholar]
- Macrides F, Chorover SL. Olfactory bulb units: activity correlated with inhalation cycles and odor quality. Science. 1972;175:84–87. doi: 10.1126/science.175.4017.84. [DOI] [PubMed] [Google Scholar]
- Macrides F, Eichenbaum HB, Forbes WB. Temporal relationship between sniffing and the limbic theta rhythm during odor discrimination reversal learning. J Neurosci. 1982;2:1705–1711. doi: 10.1523/JNEUROSCI.02-12-01705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainland J, Sobel N. The sniff is part of the olfactory percept. Chem Senses. 2006;31(2):181–196. doi: 10.1093/chemse/bjj012. [DOI] [PubMed] [Google Scholar]
- Margrie TW, Schaefer AT. Theta oscillation coupled spike latencies yield computational vigour in a mammalian sensory system. J Physiol. 2003;546(Pt 2):363–374. doi: 10.1113/jphysiol.2002.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, Linster C, Wilson DA, Cleland TA. Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn Mem. 2008;15(3):117–125. doi: 10.1101/lm.785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalick SA, Langlois JC, Krienke JD, Dube WV. An olfactory discrimination procedure for mice. J Exp Anal Behav. 2000;73(3):305–318. doi: 10.1901/jeab.2000.73-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozell MM. Evidence for sorption as a mechanism of the olfactory analysis of vapours. Nature. 1964;203:1181–1182. doi: 10.1038/2031181a0. [DOI] [PubMed] [Google Scholar]
- Mozell MM. Evidence for a chromatographic model of olfaction. J Gen Physiol. 1970;56(1):46–63. doi: 10.1085/jgp.56.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozell MM, Jagodowicz M. Chromatographic separation of odorants by the nose: retention times measured across in vivo olfactory mucosa. Science. 1973;181(106):1247–1249. doi: 10.1126/science.181.4106.1247. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24(42):9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JC, Craven B, Khan RM, Chang S, Kang I, Judkewitz B, Volpe J, Settles G, Sobel N. Mechanisms of scent-tracking in humans. Nat Neurosci. 2007;10(1):27–29. doi: 10.1038/nn1819. [DOI] [PubMed] [Google Scholar]
- Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311(5761):666–670. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- Rennaker RL, Chen CF, Ruyle AM, Sloan AM, Wilson DA. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J. Neurosci. 2007;27(7):1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006a;26(34):8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006b;51(3):351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Margrie TW. Spatiotemporal representations in the olfactory system. Trends Neurosci. 2007;30:92–100. doi: 10.1016/j.tins.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Schmitt BC, Ache BW. Olfaction: responses of a decapod crustacean are enhanced by flicking. Science. 1979;205:204–206. doi: 10.1126/science.205.4402.204. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Cleland TA. The anatomical logic of smell. Trends Neurosci. 2005;28(11):620–627. doi: 10.1016/j.tins.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Scott JW. Sniffing and spatiotemporal coding in olfaction. Chem Senses. 2006;31(2):119–130. doi: 10.1093/chemse/bjj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Acevedo HP, Sherrill L. Effects of concentration and sniff flow rate on the rat electroolfactogram. Chem Senses. 2006;31(6):581–593. doi: 10.1093/chemse/bjj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM. Odor-sampling time of mice under different conditions. Chem Senses. 2007;32(5):445–454. doi: 10.1093/chemse/bjm013. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Nigroshi BJ. Olfactory stimulus control evaluated in a small animal olfactometer. Percept Mot Skills. 1974;39:583–597. doi: 10.2466/pms.1974.39.1.583. [DOI] [PubMed] [Google Scholar]
- Sobel N, Khan RM, Hartley CA, Sullivan EV, Gabrieli JD. Sniffing longer rather than stronger to maintain olfactory detection threshold. Chem Senses. 2000;25(1):1–8. doi: 10.1093/chemse/25.1.1. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JD, Sullivan EV. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998;18(21):8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell KG, Wesson DW, Baum MJ, Forthcoming Sexually dimorphic enhancement by estradiol of male urinary odor detection thresholds in mice. Behav Neurosci. 2008 doi: 10.1037/0735-7044.122.4.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 2006;26(4):1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen JB, Mohus I, Kvesetberg TK, Walloe L. Olfaction in bird dogs during hunting. Acta Physiol Scand. 1996;157:115–119. doi: 10.1046/j.1365-201X.1996.479227000.x. [DOI] [PubMed] [Google Scholar]
- Sundberg H, Doving K, Novikov S, Ursin H. A method for studying responses and habituation to odors in rats. Behav Neural Biol. 1982;34(1):113–119. doi: 10.1016/s0163-1047(82)91501-1. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25(12):3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Thesen A, Steen JB, Doving KB. Behaviour of dogs during olfactory tracking. J Exp Biol. 1993;180:247–251. doi: 10.1242/jeb.180.1.247. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6(11):1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10(5):631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Denk W, Friedrich RW. Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging. Proc Natl Acad Sci USA. 2004;101(24):9097–9102. doi: 10.1073/pnas.0400438101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol. 2006;17(4):411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Linster C, Cleland TA. Dopamine D(2) receptor activation modulates perceived odor intensity. Behav Neurosci. 2006;120(2):393–400. doi: 10.1037/0735-7044.120.2.393. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing in the albino rat. Behavior. 1964;22:223–244. [Google Scholar]
- Wesson DW, Carey RC, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. PLoS Biol. 2008;6(4):e82. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Keller M, Douhard Q, Baum MJ, Bakker J. Enhanced urinary odor discrimination in female aromatase knockout (ArKO) mice. Horm Behav. 2006;49(5):580–586. doi: 10.1016/j.yhbeh.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL. A method for the rapid automated assessment of olfactory function. Chem Senses. 2005;30(3):219–229. doi: 10.1093/chemse/bji017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor discrimination tasks. Physiol Behav. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.