Abstract
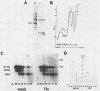
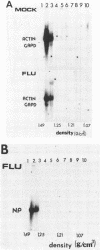
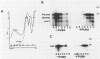
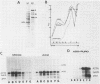
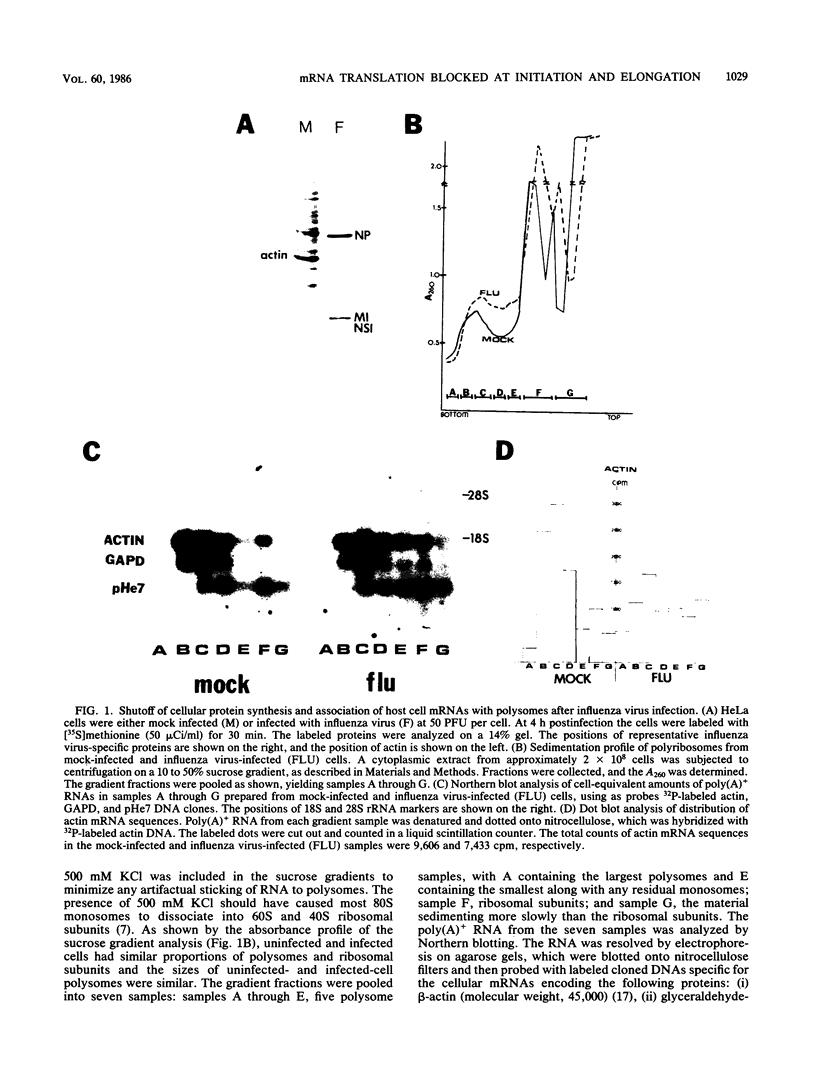
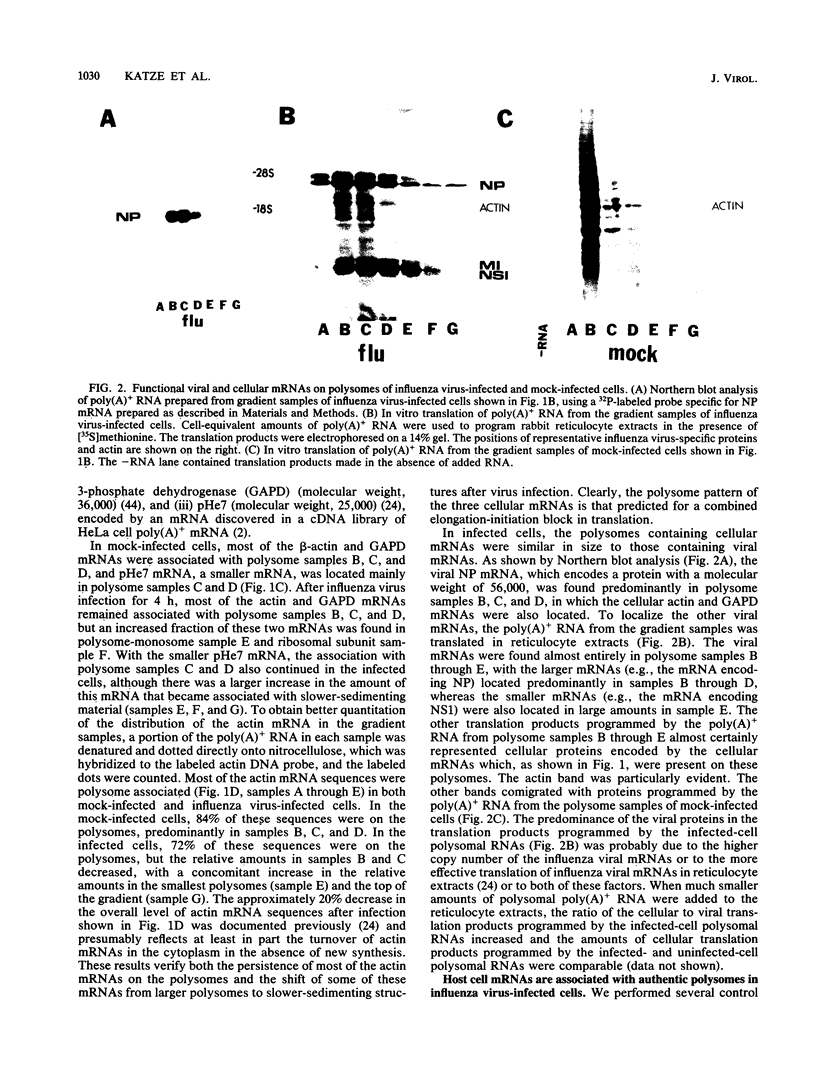
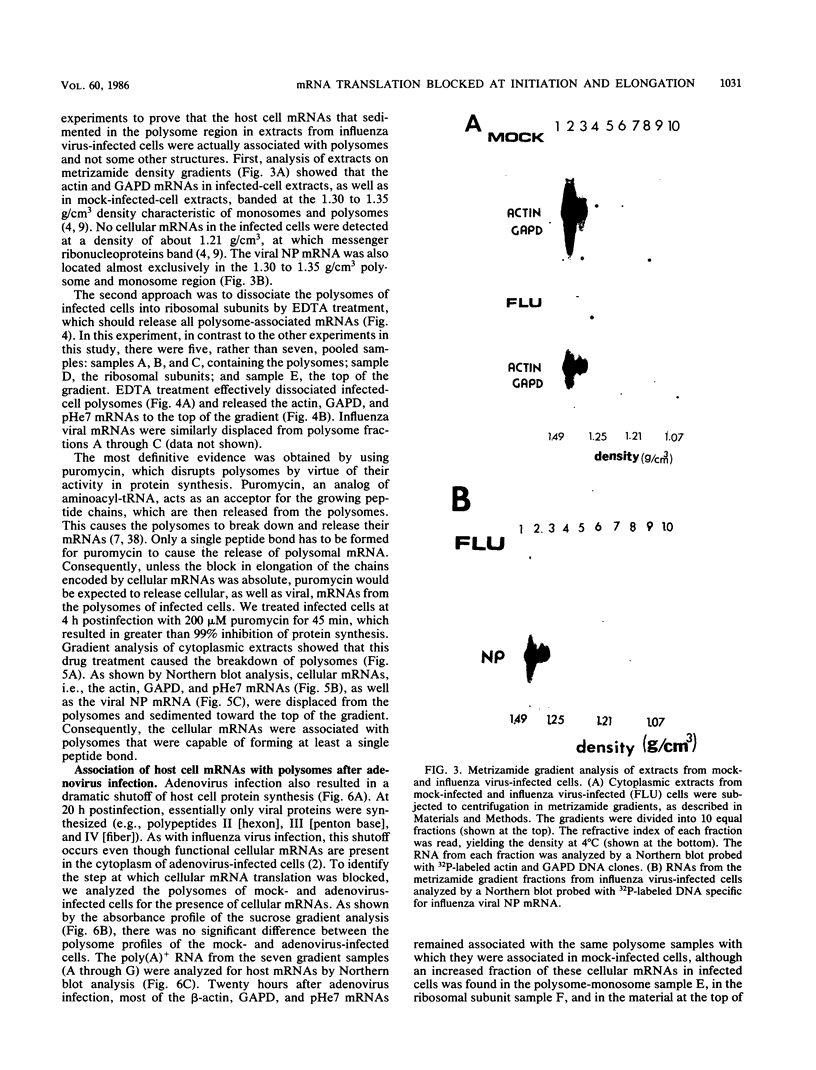
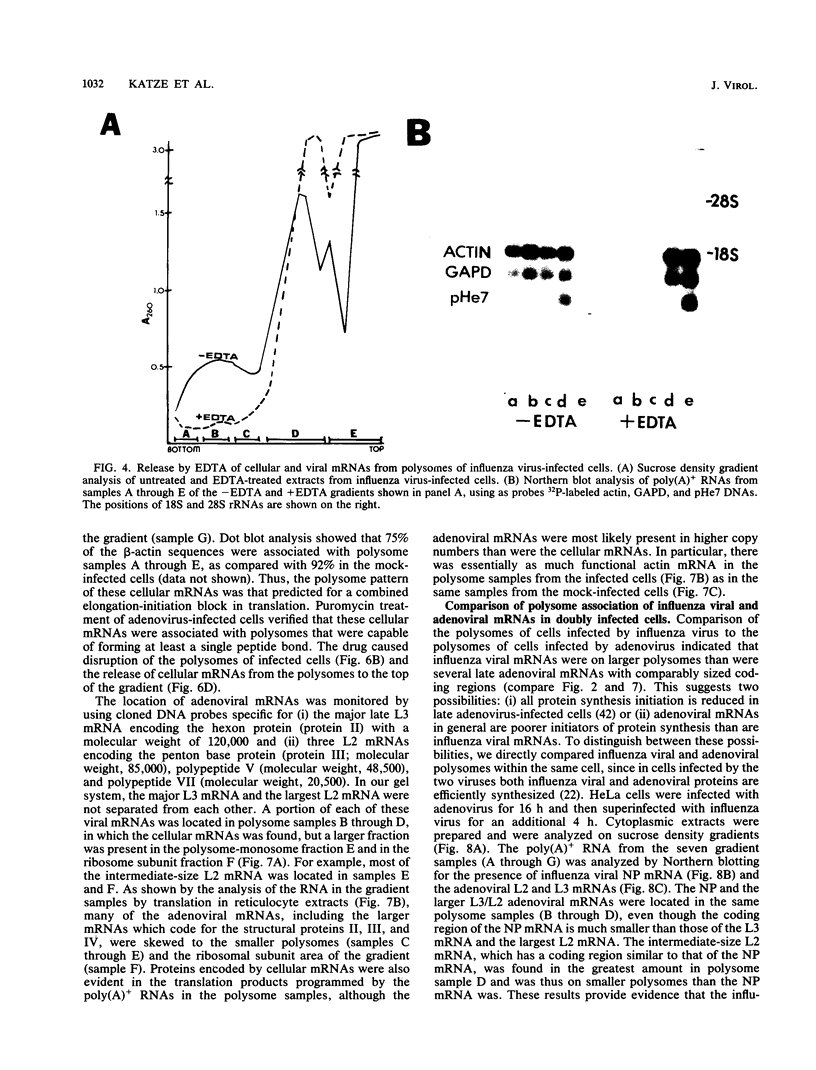
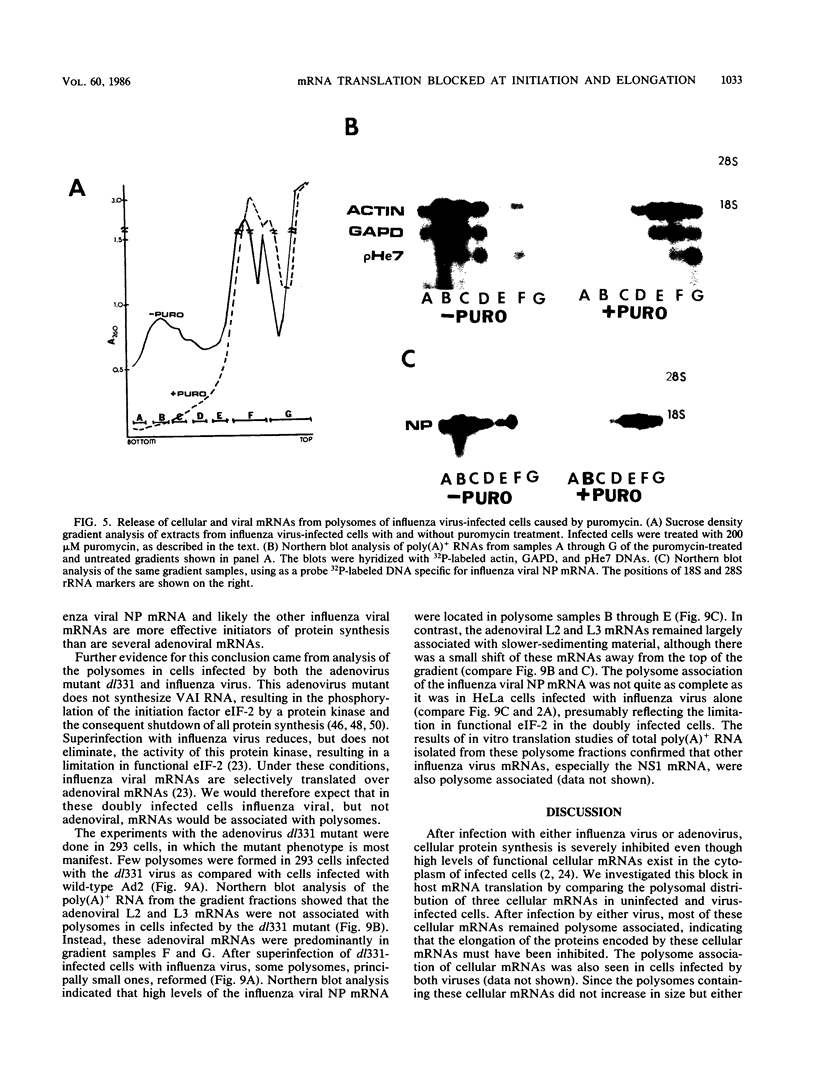
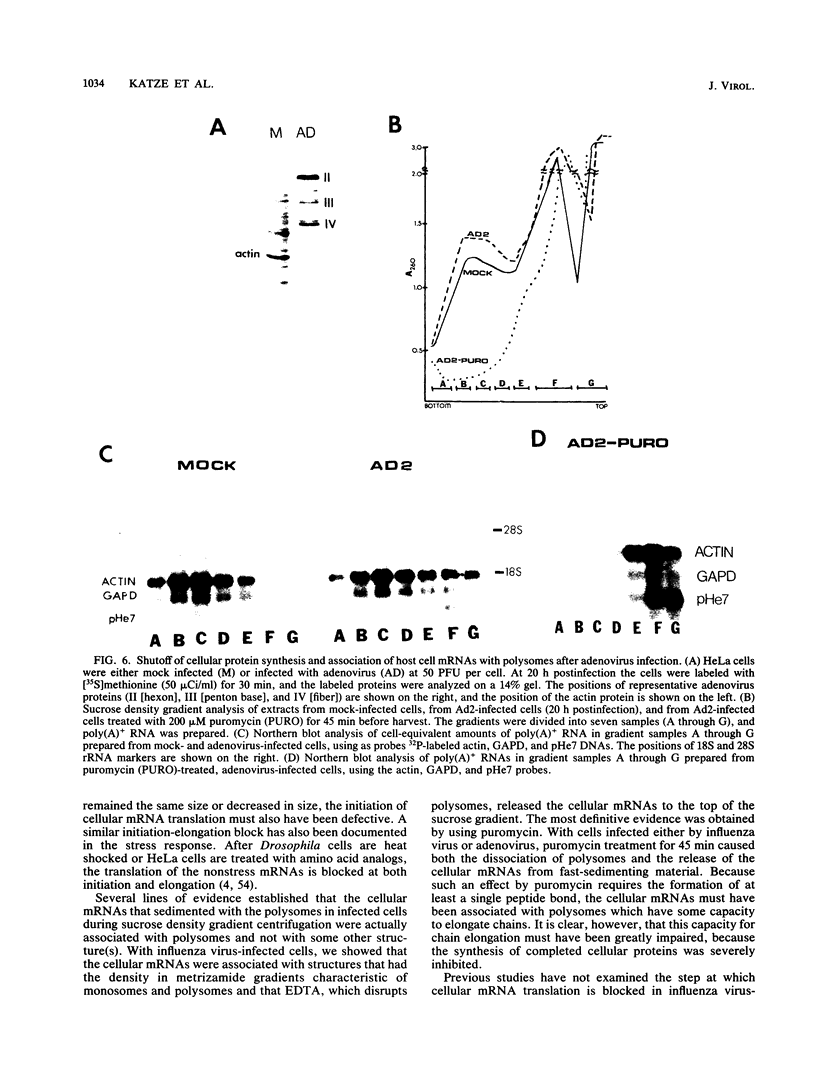
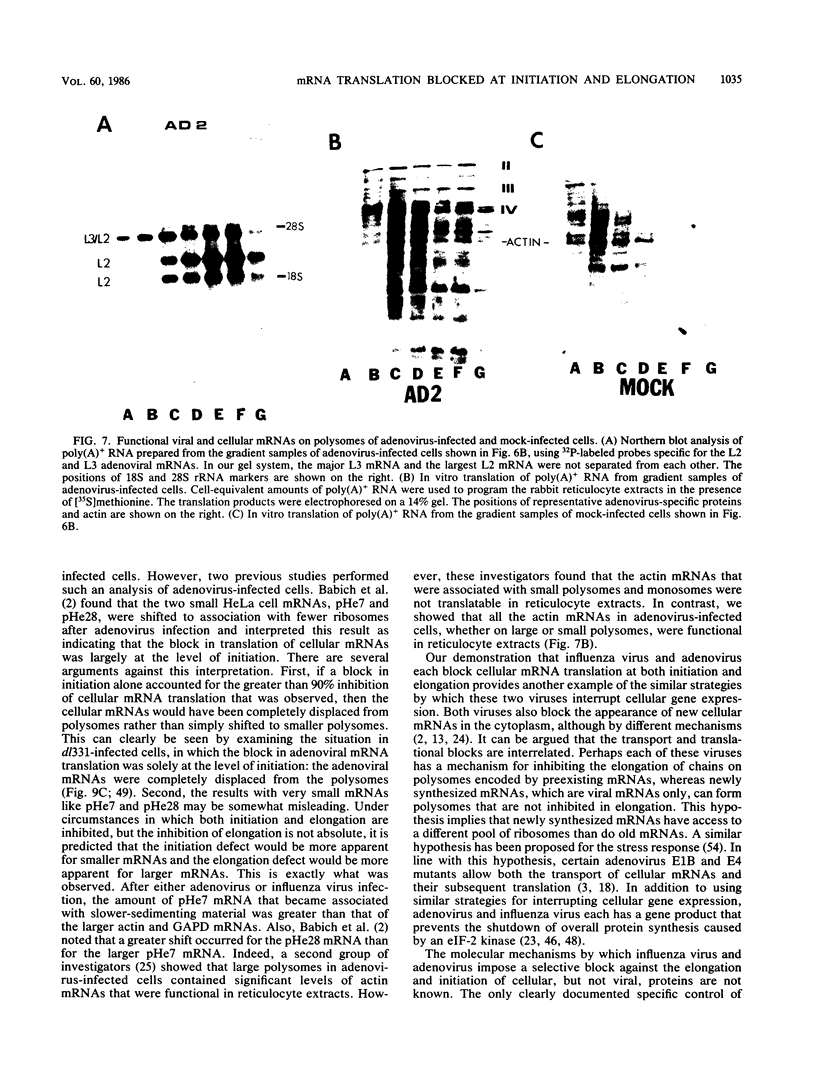
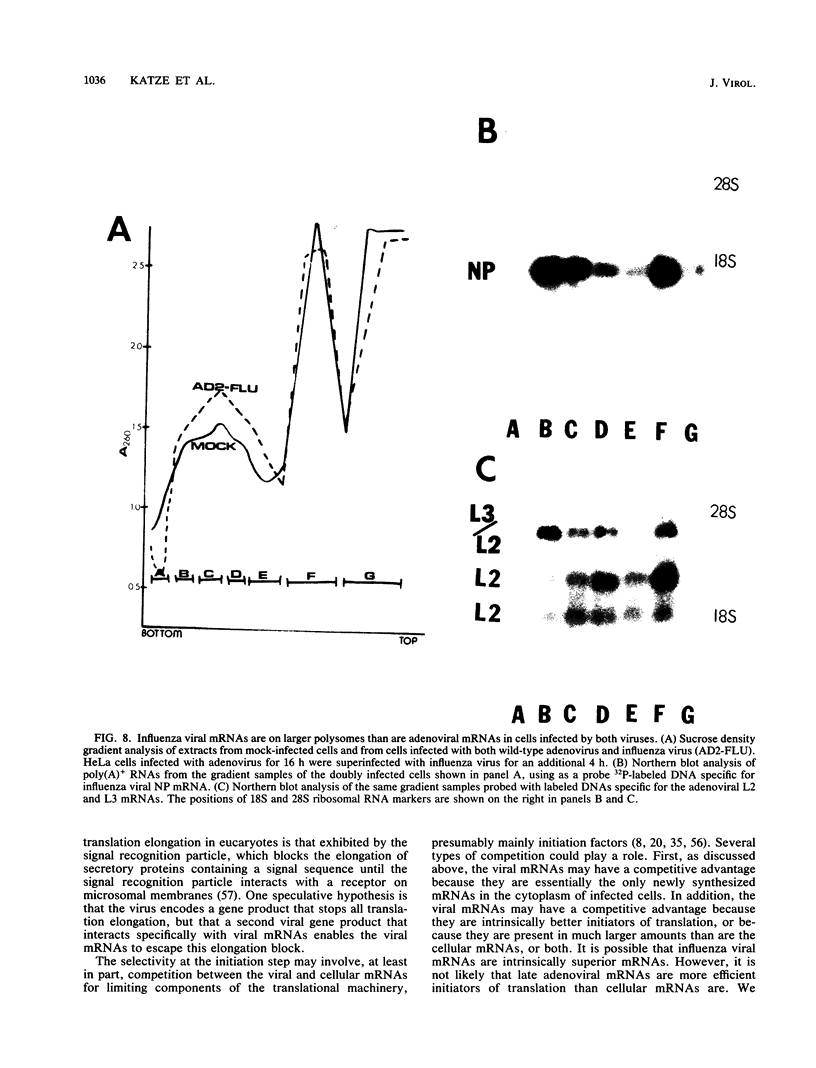
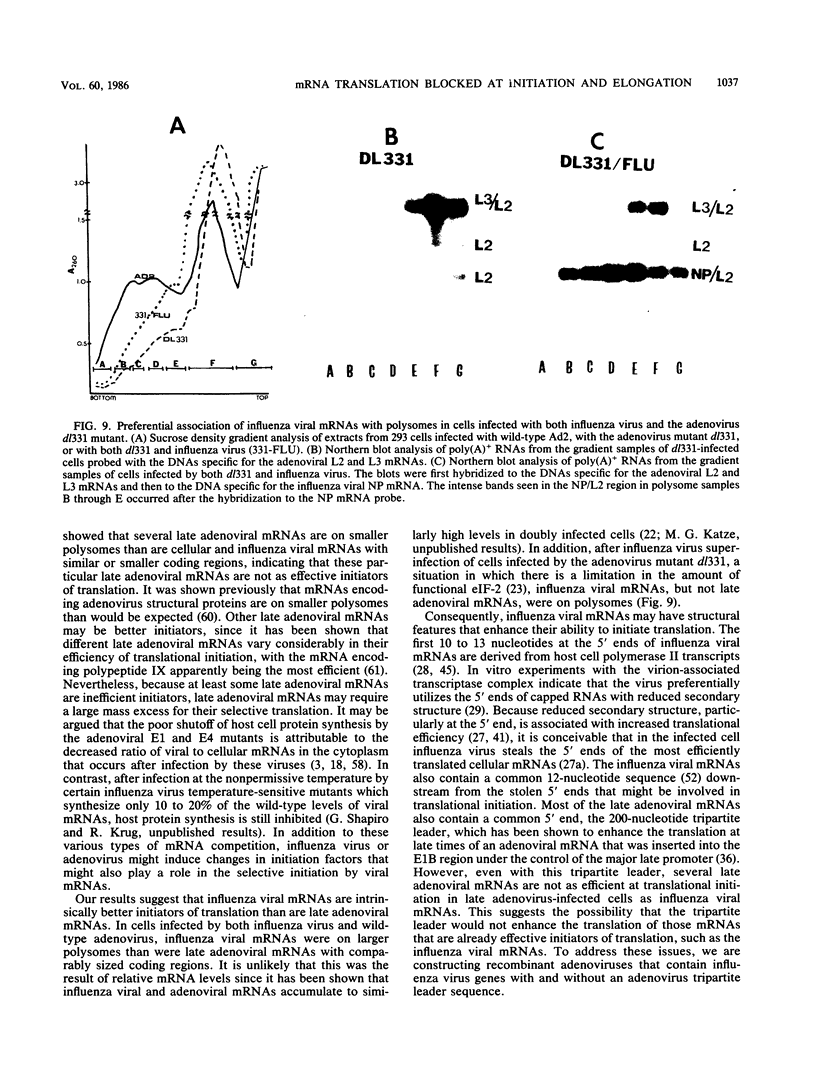
During influenza virus infection, protein synthesis is maintained at high levels and a dramatic switch from cellular to viral protein synthesis occurs despite the presence of high levels of functional cellular mRNAs in the cytoplasm of infected cells (M. G. Katze and R. M. Krug, Mol. Cell. Biol. 4:2198-2206, 1984). To determine the step at which the block in cellular mRNA translation occurs, we compared the polysome association of several representative cellular mRNAs (actin, glyceraldehyde-3-phosphate dehydrogenase, and pHe7 mRNAs) in infected and uninfected HeLa cells. We showed that most of these cellular mRNAs remained polysome associated after influenza viral infection, indicating that the elongation of the proteins encoded by these cellular mRNAs was severely inhibited. Because the polysomes containing these cellular mRNAs did not increase in size but either remained the same size or decreased in size, the initiation step in cellular protein synthesis must also have been defective. Several control experiments established that the cellular mRNAs sedimenting in the polysome region of sucrose gradients were in fact associated with polyribosomes. Most definitively, puromycin treatment of infected cells caused the dissociation of polysomes and the release of cellular, as well as viral, mRNAs from the polysomes, indicating that the cellular mRNAs were associated with polysomes that were capable of forming at least a single peptide bond. A similar analysis was performed with HeLa cells infected by adenovirus, which also dramatically shuts down cellular protein synthesis. Again, it was found that most of the cellular mRNAs, which were translatable in reticulocyte extracts, remained associated with polysomes and that there was a combined initiation-elongation block to cellular protein synthesis. In cells infected by both adenovirus and influenza virus, influenza viral mRNAs were on larger polysomes than were several late adenoviral mRNAs with comparably sized coding regions. In addition, after influenza virus superinfection of cells infected by the adenovirus mutant dl331, a situation in which there is a limitation in the amount of functional initiation factor eIF-2 (M. G. Katze, B. M. Detjen, B. Safer, and R. M. Krug, Mol. Cell. Biol. 6:1741-1750, 1986), influenza viral mRNAs, but not late adenoviral mRNAs, were on polysomes. These results indicate that influenza viral mRNAs are better initiators of translation than are late adenoviral mRNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich A., Feldman L. T., Nevins J. R., Darnell J. E., Jr, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol Cell Biol. 1983 Jul;3(7):1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S., Darnell J. E., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985 Oct;5(10):2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Inhibition of host protein synthesis in type 5 adenovirus-infected cells. J Virol. 1967 Oct;1(5):843–850. doi: 10.1128/jvi.1.5.843-850.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendler T., Godefroy-Colburn T., Yu S., Thach R. E. The role of mRNA competition in regulating translation. III. Comparison of in vitro and in vivo results. J Biol Chem. 1981 Nov 25;256(22):11755–11761. [PubMed] [Google Scholar]
- Buckingham M. E., Gros F. The use of metrizamide to separate cytoplasmic ribonucleoprotein particles in muscle cell cultures: a method for the isolation of messenger RNA, independent of its poly A content. FEBS Lett. 1975 May 15;53(3):355–359. doi: 10.1016/0014-5793(75)80054-8. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Etchison D., Milburn S. C., Edery I., Sonenberg N., Hershey J. W. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982 Dec 25;257(24):14806–14810. [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J Virol. 1975 Dec;16(6):1464–1475. doi: 10.1128/jvi.16.6.1464-1475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Beltz G. A., Linzer D. I. Synthesis and processing of simian virus 40-specific RNA in adenovirus-infected, simian virus 40-transformed human cells. J Mol Biol. 1983 Jun 25;167(2):335–359. doi: 10.1016/s0022-2836(83)80339-8. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Adamson S. D., Herbert E. Effects of cycloheximide on polyribosome function in reticulocytes. J Mol Biol. 1967 Jul 14;27(1):57–72. doi: 10.1016/0022-2836(67)90351-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert D. N., Cutt J. R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985 Oct;56(1):250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Translational initiation factor and ribosome association with the cytoskeletal framework fraction from HeLa cells. Cell. 1984 May;37(1):85–93. doi: 10.1016/0092-8674(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Jen G., Birge C. H., Thach R. E. Comparison of initiation rates of encephalomyocarditis virus and host protein synthesis in infected cells. J Virol. 1978 Sep;27(3):640–647. doi: 10.1128/jvi.27.3.640-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., Chen Y. T., Krug R. M. Nuclear-cytoplasmic transport and VAI RNA-independent translation of influenza viral messenger RNAs in late adenovirus-infected cells. Cell. 1984 Jun;37(2):483–490. doi: 10.1016/0092-8674(84)90378-7. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Detjen B. M., Safer B., Krug R. M. Translational control by influenza virus: suppression of the kinase that phosphorylates the alpha subunit of initiation factor eIF-2 and selective translation of influenza viral mRNAs. Mol Cell Biol. 1986 May;6(5):1741–1750. doi: 10.1128/mcb.6.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., Krug R. M. Metabolism and expression of RNA polymerase II transcripts in influenza virus-infected cells. Mol Cell Biol. 1984 Oct;4(10):2198–2206. doi: 10.1128/mcb.4.10.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili K., Weinmann R. Shut-off of actin biosynthesis in adenovirus serotype-2-infected cells. J Mol Biol. 1984 Jun 5;175(4):453–468. doi: 10.1016/0022-2836(84)90179-7. [DOI] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986 Apr 25;45(2):195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1986 May;83(9):2850–2854. doi: 10.1073/pnas.83.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., LaFiandra A. J., Morgan M. A., Shatkin A. J. Priming and inhibitory activities of RNAs for the influenza viral transcriptase do not require base pairing with the virion template RNA. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5874–5878. doi: 10.1073/pnas.77.10.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Ranki M. Inhibition of cell functions by RNA-virus infections. Annu Rev Microbiol. 1984;38:91–109. doi: 10.1146/annurev.mi.38.100184.000515. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Froshauer S. Rates of initiation of protein synthesis by two purified species of vesicular stomatitis virus messenger RNA. J Biol Chem. 1977 Dec 25;252(24):8804–8811. [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Translational control of protein synthesis after infection by vesicular stomatitis virus. J Virol. 1980 Dec;36(3):719–733. doi: 10.1128/jvi.36.3.719-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Aldrich C., Summers J., Taylor J. M. Asymmetric replication of duck hepatitis B virus DNA in liver cells: Free minus-strand DNA. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3997–4001. doi: 10.1073/pnas.79.13.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATHANS D. PUROMYCIN INHIBITION OF PROTEIN SYNTHESIS: INCORPORATION OF PUROMYCIN INTO PEPTIDE CHAINS. Proc Natl Acad Sci U S A. 1964 Apr;51:585–592. doi: 10.1073/pnas.51.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley R. P., Mariano T. M., Siekierka J., Mathews M. B. A mechanism for the control of protein synthesis by adenovirus VA RNAI. Cell. 1986 Feb 14;44(3):391–400. doi: 10.1016/0092-8674(86)90460-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Perlman S., Hirsch M., Penman S. Utilization of messenger in adenovirus-2-infected cells at normal and elevated temperatures. Nat New Biol. 1972 Aug 2;238(83):143–144. doi: 10.1038/newbio238143a0. [DOI] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel P. A., Merrick W. C., Siekierka J., Mathews M. B. Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature. 1985 Jan 17;313(5999):196–200. doi: 10.1038/313196a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Weinberger C., Shenk T. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell. 1984 May;37(1):291–298. doi: 10.1016/0092-8674(84)90325-8. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Mariano T. M., Reichel P. A., Mathews M. B. Translational control by adenovirus: lack of virus-associated RNAI during adenovirus infection results in phosphorylation of initiation factor eIF-2 and inhibition of protein synthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1959–1963. doi: 10.1073/pnas.82.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. Polypeptide synthesis in influenza virus-infected cells. Virology. 1972 Jul;49(1):23–36. doi: 10.1016/s0042-6822(72)80004-7. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. Alterations of transcription and translation in HeLa cells exposed to amino acid analogs. Mol Cell Biol. 1984 Jun;4(6):1063–1072. doi: 10.1128/mcb.4.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. E., Godefroy-Colburn T., Thach R. E. The role of mRNA competition in regulating translation. I. Demonstration of competition in vivo. J Biol Chem. 1981 Nov 25;256(22):11739–11746. [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Weinberg D. H., Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986 Mar;57(3):833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wilhelm J. M., Ginsberg H. S. Synthesis in vitro of type 5 adenovirus capsid proteins. J Virol. 1972 Jun;9(6):973–980. doi: 10.1128/jvi.9.6.973-980.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth D. J., Yu H. Y., Hsu M. T. Studies on the relationship between 5' leader sequences and initiation of translation of adenovirus 2 and simian virus 40 late mRNAs. Virology. 1980 Mar;101(2):363–375. doi: 10.1016/0042-6822(80)90450-x. [DOI] [PubMed] [Google Scholar]