Abstract
This study was aimed at identifying sex pheromones of the rat (Rattus norvegicus). We characterized the volatiles and semivolatiles of rat preputial gland and voided urine by using gas chromatography–mass spectrometry (GC–MS) and quantified them by their GC areas (abundances) and percentage of GC areas (relative abundances). Although all the compounds other than 4-heptanone and phenol detected were shared by males and females, the quantities for some of these sex-common compounds exhibited sexual dimorphism and decreased with gonadectomy. Thus, these compounds might be sex pheromones. Among them, squalene from preputial glands and 2-heptanone and 4-ethyl phenol from urine were 3 major compounds. They were richer in males and could be suppressed by castration. Adding any of the 3 compounds (at a concentration higher than its physiological level in male urine) to castrated male urine (CMU) increased the attractiveness of CMU to sex-naive females. Adding the 3 together (at the levels in normal male urine) to CMU significantly increased the attractiveness of CMU to females. However, such combination did not fully restore females' preference for urine from intact males, suggesting that some other trace compounds such as 4-heptanone and phenol might also play some roles in sex attractiveness. Thus, squalene, 2-heptanone, and 4-ethyl phenol were indeed male pheromone molecules in rats. Our study also indicates that E,E-β-farnesene and E-α-farnesene, both richer in females than males, might be putative female pheromones.
Keywords: 4-ethyl phenol, 2-heptanone, pheromone, preputial gland, rat, squalene, urine
Introduction
A pheromone is a molecule or a blend of molecules secreted to the outside by animals and used for communication with other conspecific members, which react by changing their behavior or developmental process (Wyatt 2003). As in insects, mammalian pheromones can function using one compound alone, such as the urinary 2,5-dimethylpyrazine, which suppresses the onset of puberty in female mice (Mus musculus), or in conjunction with others, such as R,R-dehydro-exo-brevicomin and S-2-sec-butyl-dihydrothiazole, which jointly attract female mice and promote aggression in male mice (Jemiolo et al. 1985; Novotny et al. 1985; Wyatt 2003). However, identification of pheromone compounds is much less attempted in mammals compared with insects (Novotny, Jemiolo, et al. 1999; Wyatt 2003; Brennan and Zufall 2006).
The brown rat (Rattus norvegicus) is a worldwide pest, but its albino variation is one of the most used animal models in biological research, for which much attention has been paid to its chemical communication (Brown 1985). Bioassay has revealed that the voided urine and preputial gland secretion (PGS) of the rat can convey a wealth of information including that about species, sex, reproductive condition, and stress status (Brown 1985; Brouette-Lahlou et al. 1991; Novotny, Ma, et al. 1999; Zhang, Rao, et al. 2007). However, little is known about which compounds are involved in coding for the information (Novotny, Ma, et al. 1999; Wyatt 2003; Brennan and Zufall 2006; Zhang, Rao, et al. 2007; Liu et al. 2008).
Rodent preputial glands are highly modified, paired holocrine sebum glands, and lying anterior to male prepuce or female clitoris between the skin and body wall (Brown 1985). The glands are widespread in rodents (Muridae and Arvicolinae) and vital for pheromone production (Brown 1985). Their excretions are discharged into the voided urine to communicate information about species, sex, and dominance status to conspecifics (Brinck and Hoffmeyer 1984; Brown 1985; Welsh et al. 1988; Harvey et al. 1989; Novotny et al. 1990; Novotny, Ma, et al. 1999). Under androgenic control, rodent preputial glands are often larger in males but smaller or absent in females (Nickerson et al. 1976; Gawienowski 1977; Brown 1985; Zhang, Rao, et al. 2007; Zhang, Zhao, et al. 2007). However, in some rat species (e.g., Rattus rattus, Rattus losea, Rattus nitidus, Niviventer confucianus, Niviventer fulvescens, and Leopoldamys edwardsi), they are like sized between the sexes (Brown 1985; Mallick 1991; Zhang J-X, Xiao Z-S, Liu X-H, Cong L, unpublished observation). In wild brown rats and albino rats, absence or presence of such sexual dimorphism varies with populations and strains (Mallick 1991; Natynczuk et al. 1995).
Despite the fact that rats have been much less studied than mice for pheromones, many attempts have been made in past decades. For instance, binary choice tests have revealed that aliphatic acetates from the preputial glands of male rats are attractive to females, but sexual dimorphism in these compounds has not been demonstrated in chemical analysis (Gawienowski 1977; Stacewicz-Sapuntzakis and Gawienowski 1977), probably due to technological limitations in quantifying the compounds. More recently, dodecyl propionate from preputial glands in rat pups has been shown to serve as an attractant to their mothers (Brouette-lahlou et al. 1991, 1999). In adult rats, PGSs are sexually attractive and contain rich squalene and aliphatic acids but poor in esters (Burgess and Wilson 1963; Albro and Moore 1974; Natynczuk et al. 1995; Zhang J-X, Xiao Z-S, Liu X-H and Cong L. unpublished observation). Principle component analysis has revealed that the quantitative composition of rat PGS is sexually dimorphic (Natynczuk et al. 1995), but such difference is not related to aliphatic acids (Gawienowski et al. 1975). Squalene in the rat has received little attention, although it is used as a pheromone in such vastly different species as the tamarin, Saguinus fuscicollis (Epple et al. 1979), and the red-sided garter snake, Thamnophis sirtalis (Mason et al. 1989). Recently, it has been characterized as a putative male pheromone in the giant panda, Ailuropoda melanoleuca, as well (Zhang et al. 2008). The convergent presence of squalene in a variety of vertebrates as a pheromone compound suggests that it may have some properties, such as the ability of conveying airborne cues over a distance, suited for chemical communication. Hence, it is logical to investigate whether squalene is sexually different in quantity and has pheromonal activity in rats.
Rats are well known for communication by urine marking (Brown 1985; Mennella and Moltz 1989). One of the major volatiles of urine metabolites is 2-heptanone (Holland et al. 1983), which has been identified to be an alarm pheromone (Gutiérrez-García et al. 2007). However, urine-metabolized sex pheromones remain poorly understood. Studies in mice have demonstrated that the major volatiles or semivolatiles of PGS and bladder urine, which are either sex specific (e.g., S-2-sec-butyl-dihydrothiazole of urine) or richer in males (e.g., R,R-dehydro-exo-brevicomin of urine; E,E-β-farnesene, E-α-farnesene, hexadecanol, and hexadecyl acetate of PGS), are male pheromones used to attract females (Jemiolo et al. 1985, 1991a; Zhang, Rao, et al. 2007; Liu et al. 2008; Zhang J-X, Xiao Z-S, Liu X-H, Cong L, unpublished observation). Despite the presence of male-specific compounds, most rodent scent constituents are sex-common compounds, especially in PGS (Welsh et al. 1988; Harvey et al. 1989; Zhang, Rao, et al. 2007; Zhang, Sun, and Novotny 2007). For these sex-common compounds, elevations in males in absolute and/or relative concentration and dependency on androgen and testis are often associated with their pheromonal activity (Singer et al. 1997; Novotny, Jemiolo, et al. 1999; Zhang et al. 2005; Zhang, Rao, et al. 2007; Zhang, Sun, and Novotny 2007).
A common approach to validate pheromonal activity is to add the synthetic analogs of urinary pheromone candidates to castrated urine and examine the recurrence of the activity of intact urine (Jemiolo et al. 1985, 1991a; Novotny et al. 1985; Lin et al. 2005). In this study, we adopted this general research strategy to screen for male pheromone compounds in albino rats. Specifically, we used gas chromatography–mass spectrometry (GC–MS) to look for male-specific or sex-dimorphic compounds and then attempted to verify their pheromonal activities by using behavioral 2-way choice tests. Because the voided urine of male rats is attractive to females and contains the compounds from both bladder urine metabolites and preputial glands, we hypothesized that 1) some of the compounds were either male specific or quantitatively richer in males than in females and 2) these compounds alone or in combination could enhance the attractiveness of castrated male urine (CMU) to females.
Materials and methods
Experimental animals
Ten male (weighing 386–478 g) and 10 female (weighing 245–293 g) adult Sprague–Dawley (SD) rats at the age of 5 months were used as urine and preputial gland donors. These subjects were born in our laboratory by a parental line purchased from Weitong-Lihua Animal Company Ltd, Beijing, China. Additional 10 females at the age of 10 weeks were purchased from the aforementioned company and used as odor recipients. Males were housed individually, whereas females were housed in groups of 3 or 4 in plastic cages with a dimension of 37 × 26 × 17 cm for each. The housing room was under a reversed 14:10 light:dark photoperiod (lights on at 1900 h) and at the temperature of 23 ± 2 °C. All rats used were sexually naive. Standard rat chew and tap water were provided ad libitum. For female subjects, we determined their estrous cycles by vaginal smears and used those with a regular estrous cycle of about 4 days. The procedure of animal handling complied with the Institutional Guidelines for Animal Use and Care at the Institute of Zoology, the Chinese Academy of Sciences.
Surgery
Five of the male odor donors underwent bilateral castration via a single midline incision. Five of the female odor donors underwent bilateral paralumbar incisions and ovariectomy. The incisions were closed with sterile sutures and treated with 75% ethanol and 5% tincture of iodine. All the operations were conducted following the sodium pentobarbital (40 mg/kg) anesthesia procedure. Four weeks later, the operated rats, together with intact ones, were used for urine collection before being sacrificed for surgical removal of the preputial glands, which were weighed (±0.1 mg) and then solvent extracted as described below. Relative weight of the glands was obtained in milligrams per 100 g body weight.
Scent collection and sample preparation
To collect urine, we placed each intact or castrated male donor in a clean plastic mouse cage (dimension: 31.8 × 20.2 × 31.5 cm) with a wire grid floor. Upon animal's urination, the urine was immediately absorbed and transferred to a vial by a disposable glass capillary (inter diameter [i.d.] 1.8 mm and 15 cm long) for behavioral and chemical assays. Paired preputial glands were dissected out immediately after sacrificing the rats by neck displacement. The collected urine and preputial glands were individually sealed in vials and kept at −20 °C until solvent extraction.
To characterize the volatiles in urine samples, we mixed 250 μl dichloromethane with 250 μl urine, let the mixture sit for 12 h at 0 °C, and then used the bottom phase (i.e., the layer with dichloromethane) for chemical analysis. To extract compounds in rat PGS, we weighed the whole gland tissue, added dichloromethane into the vial in the proportion of 1-mg tissue in 5-μl dichloromethane (purity >99.5%), and kept the solution at 0 °C for 12 h. Then, we removed the tissue and used the remaining solution for GC–MS analysis.
To prepare scented urine from castrated males (CMU) with the identified synthetic analogs (2-heptanone, 4-ethyl phenol, and squalene) for behavioral assays, we first diluted each of the CMU samples with dichloromethane to a manageable concentration. We then sampled each solution, transferred the sample into a clean vial, and allowed the vial uncovered for 5 min to vaporize the solvent before adding the CMU sample, which was mixed equally from 9 castrated donors into the vial, as desired scented CMU.
GC–MS assay
Analysis was performed on an Agilent Technologies Network 6890N GC System coupled with 5973 Mass Selective Detector (NIST 2002 Library). The GC was equipped with a HP5MS glass capillary column (30 m long, i.d. 0.25 mm × 0.25 μm film). The carrier gas was Helium (1.0 ml/min.). The injector temperature was set at 280 °C. The oven temperature was set initially at 50 °C, heated by 5 °C/min to 100 °C, then ramped by 10 °C/min until 280 °C, and held for 5 min. Electron impact ionization was at 70 eV. Transfer line temperature was set at 280 °C. Scanning mass ranged from 30 to 450 amu. One microliter sample was injected in a split mode (10:1) for PGS and 5 μl sample at a splitless mode for urine.
Tentative identifications were made by matching the mass spectra of GC peaks with those in the MS library (NIST 2002). Of the tentatively identified compounds, indole, E-ß-farnesene, tetradecanoic acid, hexadecanoic acid, octadecanoic acid, squalene, cholesterol, 2-heptanone, and 4-ethyl phenol were verified by matching the retention times and mass spectra of the authentic analogs (all purities ≥ 95% and purchased from J&K Chemical Ltd, Beijing, China). Z9-octadecenoic acid, E9-octadecenoic acid, and Z9, E9-octadecenoic acid were verified with corresponding methyl esters after methylation of the samples (Zhao et al. 2004). 4-Heptanone and E,E-α-farnesene were verified with their counterparts in mouse urine and preputial glands (Zhang, Rao, et al. 2007).
We quantified the abundance of a particular compound by its GC area. We then converted the peak area of a particular compound into the percentage of the summed peak areas from all targeted GC peaks of either urine or preputial glands as its relative abundance. The abundances and relative abundances of the scent constituents were used for quantitative comparisons between the treatment groups. If a given GC peak was too small to display the diagnostic MS ions of the corresponding compound, its GC area was taken as zero, which was also used for the GC peaks less than the detectable limit. We measured the genuine quantities of 2-heptanone, 4-ethyl phenol, and squalene in urine by comparing their GC areas in intact male urine samples with the established standard curve of GC area versus concentration.
Behavioral assay
We tested the preference of female rats for 2 aqueous scent samples from 0900 to 1100 h in the dark phase. Immediately prior to each trial, test rats in their home cages were transferred to a test room under dim light. For each test, we kept 1 subject as test rat in the home cage while temporarily removing its cage mates into a holding rat cage with exactly the same dimension. We injected 2 μl of each aqueous sample into a disposable glass capillary from one opening (i.d. 1.1–1.2 mm, outer diameter 1.3–1.4, 15 cm length). We then sealed the other opening of the capillary with odorless gum to suspend the sample aliquot inside the capillary, 1 cm away from the sample-containing end. As a result, the rats could sense only volatiles. The sample-containing end of the capillary was presented to test rats in their home cages, whereas the other end was held by us with disposable plastic gloves. Two capillaries scented by treatment and control aqueous samples, respectively, were lowered through the wire lid and kept approximately 3 cm apart. Thus, each test subject was simultaneously presented with a 2-way choice. We recorded its behavior for 3 min after it showed the initial sniffing response. The time that the test rats spent sniffing within the 1 cm apart from the tip and licking the end of the capillaries were recorded with 2 hand-held stopwatches.
When we completed a test, we put the tested rat to the holding cage and removed another rat from the holding cage to the home cage for the next test until all rats were tested. A total of 10 female rats were used for all trials, and a rat was used only once a day. Subjects whose investigating time was less than 1 s were excluded for the day (Zhang, Sun, and Novotny 2007).
Statistical analysis
To test the hypothesis of sexual dimorphism in the abundance and/or relative abundance of the targeted GC peaks in PGS and urine, we first examined the distribution of the raw data. Then, we used either parametric test (1-way analysis of variance with post hoc Bonferroni t-test), if the data did not violate the normal distribution prerequisite, or nonparametric tests (Kruskal–Wallis H with post hoc Mann–Whitney U test), if the data were not normal, for the relative abundances of the compounds. Likewise, we used paired t-test or Wilcoxon matched-pairs signed-rank test for behavioral data collected from 2-way choice tests. All statistical analyses were conducted using SPSS (Version 10.0) with the critical value of α = 0.05. We selectively applied these tests to the 3 pairs of treatment groups related to our hypotheses: males and females, intact males and castrated males, and intact females and ovariectomized females.
Results
Gonad and rat preputial gland size
The preputial glands did not exhibit sexual differences, but gonad-removal significantly suppressed the glands of male and female rats (Table 1).
Table 2.
Comparison of the relative abundances of the identified compounds of rat preputial glands between groups (mean ± standard deviation, N = 5 for each group)
| GC peak No. | Retention time (min) | Compound identification |
Experimental groups |
||||
| Compound names | Diagnostic ions [m/z(relative intensity)] | Males | Females | Castrated males | Ovariectomized females | ||
| 1 | 4.82 | Benzaldehyde | 106(100), 77(88), 105(86), 51(46) | 0.16 ± 0.11 | 0.21 ± 0.12c | 0.24 ± 0.25 | 0.09 ± 0.02c |
| 2 | 9.78 | Unkown | 59(100), 41(70), 56(66), 127(65), 43(53), 69(26), 84(18), 142(4) | 0.02 ± 0.003a,b | 0.21 ± 0.09a,c | 0.07 ± 0.02b | 0.11 ± 0.06c |
| 3 | 12.17 | 3,7-Dimethyl-2,6-octadienal | 41(100), 69(99), 84(34), 39(24), 94(15), 152(6) | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.05 ± 0.02 |
| 4 | 12.71 | Indole* | 117(100), 90(34), 89(26), 118(10), 63(8) | 0.08 ± 0.06 | 0.10 ± 0.08 | 0.10 ± 0.06 | 0.04 ± 0.01 |
| 5 | 15.17 | E-ß-farnesene* | 69(100), 41(86), 93(60), 81(24), 133(24), 120(22), 204(2) | 0.03 ± 0.01a,b | 0.13 ± 0.06a | 0.11 ± 0.04b | 0.12 ± 0.03 |
| 6 | 15.89 | E,E-α-farnesene* | 93(100), 41(74), 55(54), 69(50), 107(48), 119(46), 123(45), 91(38), 79(36), 204(1) | 0.08 ± 0.03a,b | 0.32 ± 0.14a | 0.28 ± 0.09b | 0.31 ± 0.09 |
| 7 | 16.71 | Dodecanoic acid | 60(100), 73(98), 43(78), 129(46), 157(28), 171(8), 200(6) | 0.47 ± 0.33a,b | 0.10 ± 0.09a | 0.04 ± 0.05b | 0.07 ± 0.03 |
| 8 | 18.98 | Tetradecanoic acid* | 73(100), 60(96), 43(86), 41(70), 57(76), 55(68), 129(66), 185(26), 228(12) | 0.82 ± 0.38a,b | 0.30 ± 0.10a | 0.28 ± 0.13b | 0.25 ± 0.10 |
| 9 | 19.49 | Hexadecanal | 57(100), 43(96), 68(46), 71(35), 82(86), 96(56), 110(18), 124(12), 138(6), 222(1) | 0.23 ± 0.17b | 0.47 ± 0.47 | 0.63 ± 0.37b | 0.12 ± 0.11 |
| 10 | 21.09 | Hexadecanoic acid* | 43(100), 73(94), 60(90), 129(52), 213(12), 256(14) | 7.61 ± 2.79 | 5.59 ± 1.72 | 8.27 ± 2.45 | 7.02 ± 2.07 |
| 11 | 22.69 | Z9,Z12-octadecenoic acid* | 67(100), 81(84), 55(82), 41(72), 95(64), 109(30), 60(10), 280(6) | 2.39 ± 0.96 | 2.30 ± 0.90 | 2.15 ± 0.79 | 2.47 ± 0.78 |
| 12 | 22.73 | Z9-octadecenoic acid* | 55(100), 69(80), 83(86), 97(82), 43(64), 60(16), 264(12), 282(1) | 1.87 ± 1.00 | 1.99 ± 0.85 | 2.20 ± 1.05 | 2.38 ± 1.23 |
| 13 | 22.76 | E9-octadecenoic acid* | 55(100), 41(66), 43(48), 69(56), 83(46), 97(42), 111(18), 60(12), 264(2), 282(1) | 1.42 ± 0.72a | 0.80 ± 0.05a | 1.35 ± 0.76 | 0.75 ± 0.22 |
| 14 | 22.95 | Octadecanoic acid* | 43(100), 57(80), 73(80), 60(74), 55(72), 129(56), 84(22), 185(20), 241(12) | 4.31 ± 1.58a | 2.72 ± 0.74a | 5.53 ± 2.41 | 2.97 ± 0.89 |
| 15 | 24.15 | (all-Z)5,8,11,14-eicosatetraenoic acid | 79(100), 41(86), 67(76), 91(70), 80(72), 55(70), 105(38), 119(30), 304(0.5) | 0.90 ± 0.43a | 0.44 ± 0.17a,c | 0.89 ± 0.66 | 0.84 ± 0.35c |
| 16 | 24.26 | (all-Z)8,11,14-eicosatrienoic acid | 67(100), 79(96), 41(92), 80(88), 55(86), 93(54), 94(42), 150(22), 60(14), 306(1) | 0.38 ± 0.22a | 0.13 ± 0.06a | 0.29 ± 0.19 | 0.17 ± 0.09 |
| 17 | 24.41 | Z11,Z14-eicosadienoic acid | 67(100), 55(88), 81(84), 41(74), 95(66), 109(32), 60(14), 123(12), 308(4) | 2.45 ± 0.64 | 2.06 ± 1.20 | 2.81 ± 1.64 | 1.38 ± 0.45 |
| 18 | 25.77 | A tricosadienoic acid | 79(100), 41(82), 67(82), 55(74), 80(80), 91(66), 93(56), 105(34), 119(26), 133(12), 150(12), 60(10), 340(0.5) | 0.83 ± 0.42 | 0.62 ± 0.33 | 1.66 ± 1.31 | 0.81 ± 0.41 |
| 19 | 26.03 | A pentanoic acid ester | 103(100), 55(34), 81(26), 57(24), 67(20), 69(20), 96(18), 95(16), 97(10), 41(18), 43(16), 85(4) | 0.14 ± 0.05 | 0.11 ± 0.02 | 0.23 ± 0.11 | 0.10 ± 0.03 |
| 20 | 26.18 | A pentanoic acid ester | 103(100), 57(26), 43(12), 41(8), 55(44), 81(2), 83(2), 85(1) | 0.13 ± 0.05b | 0.14 ± 0.09 | 0.29 ± 0.14b | 0.13 ± 0.04 |
| 21 | 28.17 | Squalene* | 69(100), 81(56), 41(26), 95(16), 93(14), 55(8), 107(8), 121(12), 123(10), 136(10), 137(10), 109(9) | 46.67 ± 7.22b | 50.49 ± 6.00 | 35.24 ± 10.77b | 50.13 ± 5.71 |
| 22 | 28.79 | A terpenoid polyene | 69(100), 93(44), 41(42), 135(40), 81(32), 55(20), 43(18), 107(18), 148(14), 147(12), 175(4), 203(2) | 3.15 ± 0.50 | 3.37 ± 1.18 | 3.07 ± 0.59 | 2.35 ± 0.55 |
| 23 | 29.04 | A terpenoid polyene | 69(100), 81(100), 43(68), 41(50), 71(49), 95(34), 123(30), 109(26), 93(25), 107(22), 121(20), 135(20), 136(16), 203(2) | 0.35 ± 0.19b | 1.97 ± 2.75 | 0.65 ± 0.26b | 0.27 ± 0.29 |
| 24 | 29.17 | A terpenoid polyene | 69(100), 151(66), 109(56), 41(50), 123(34), 81(28), 93(12), 95(12), 203(2) | 1.51 ± 0.31b | 1.11 ± 0.38 | 1.10 ± 0.29b | 0.90 ± 0.28 |
| 25 | 29.44 | A terpenoid polyene | 69(100), 151(82), 41(32), 123(20), 81(10), 109(4), 93(2), 95(2) | 2.26 ± 0.41b | 2.35 ± 0.27 | 1.31 ± 0.82b | 1.91 ± 0.79 |
| 26 | 29.55 | A terpenoid polyene | 69(100), 95(38), 41(36), 81(28), 59(20), 153(14), 109(12), 107(10), 123(10), 121(8) | 5.10 ± 0.68a,b | 4.30 ± 0.33a | 2.44 ± 1.27b | 3.38 ± 1.18 |
| 27 | 29.76 | A terpenoid polyene | 69(100), 41(36), 81(26), 203(24), 119(20), 123(16), 93(12), 95(8) | 4.89 ± 0.68 | 5.31 ± 1.51 | 5.40 ± 0.94 | 4.51 ± 1.04 |
| 28 | 30.54 | A terpenoid polyene | 43(100), 69(78), 71(78), 121(66), 41(54), 95(52), 81(50), 59(52), 125(48), 139(16), 149(10), 167(4) | 1.29 ± 0.18 | 1.64 ± 0.54c | 0.91 ± 0.36 | 0.96 ± 0.31c |
| 29 | 30.90 | Cholesterol* | 43(100), 55(78), 107(72), 57(70), 105(68), 145(66), 95(64), 386(52), 91(50), 275(38), 213(26), 301(32), 369(20) | 10.34 ± 1.26b | 10.28 ± 2.36c | 22.02 ± 4.86b | 15.07 ± 2.32c |
The means in a row marked by same superscript letters show significant differences (P < 0.05, using independent t-test or Mann–Whitney U test). The compounds marked by superscript asterisk (*) were verified with authentic standards; other components were identified by comparison with spectra listed in the NIST (Agilent Technologies 2002) mass spectral library and analogous data.
GC–MS analysis of rat PGS
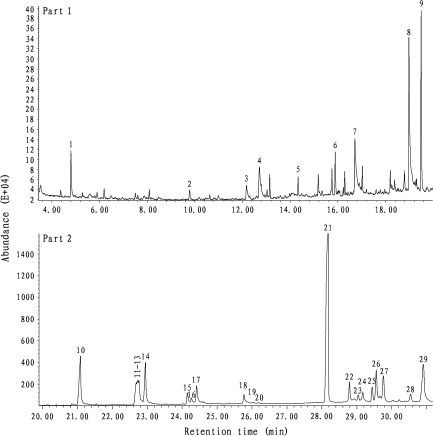
From rat preputial gland, we found 29 compounds including aldehydes, aliphatic acids, indole, esters, cholesterol, and terpenes (Figure 1 and Table 2). Squalene was the most abundant, accounting for about 50% of all the compounds. Each of GC peaks 22–28 had a similar spectrum with squalene (Table 2), showing 6 isoprene units (molecule weight = 68 for each) with the largest peak at m/z 69 and smaller peaks at or near m/z 137 (2 isoprene units) and m/z 203 (3 isoprene units). Thus, these compounds might be high–molecular weight terpenoid polyenes. Compound 28 also had m/z 43 and m/z 71, suggesting a butyrate ester contamination. Compound 24 had small peaks at m/z 355 rather than m/z 341, m/z 109 other than m/z 95, and m/z 151 other than m/z 137, suggesting an extra methyl branch off the chain.
Figure 1.
Representative GC profile of dichloromethane extract of male rat preputial gland (top: 3.8–20 min; bottom: 20–31 min.). GC conditions were described in Materials and methods of the text. The numbered GC peaks correspond to the compounds in Table 2.
Ten of the compounds exhibited sexual difference in relative abundance. Among them, compounds 2, 5, and 6 were richer in females than in males, and compounds 7, 8, 13, 14, 15, and 16 were richer in males than in females (Table 2). Castration significantly suppressed compounds 7, 8, 21, 24, 25, and 26 while promoted compounds 2, 5, 6, 9, 20, 23, and 29. Ovariectomy significantly reduced compounds 1, 2, and 28 but promoted compounds 17 and 29 (Table 2). In addition, both weights and relative weights of rat preputial glands did not exhibit significant differences between the sexes, although they were reduced by gonadectomy (Table 1).
Table 1.
Comparison of weight and relative weight of preputial gland (PG) between groups (mean ± standard deviation, N = 5 for each group)
| Items | Experimental groups |
|||
| Males | Females | Castrated males | Ovariectomized females | |
| Body weight (BW) (g) | 462.6 ± 45.68a | 258.1 ± 22.57a | 435.1 ± 44.74 | 297.0 ± 43.42 |
| PG weight (mg) | 103.5 ± 30.65a | 81.56 ± 13.28c | 69.88 ± 12.77a | 60.26 ± 13.93c |
| Relative PG weight (mg/100 g BW) | 22.36 ± 6.093a | 32.08 ± 7.776c | 16.15 ± 3.080a | 20.53 ± 5.367c |
The means in a row marked by same superscript letters show significant differences (P < 0.05, using independent t-test or Mann–Whitney U test).
The content of squalene quantified by its GC area was significantly higher in males [(8.8 ± 5.4) × 108] than in females [(5.1 ± 2.87) × 108] (t8 = 3.115, P = 0.014). So was hexadecanoic acid (male = [15 ± 9.7] × 107; female = [5.3 ± 1.87] × 107; Z = 2.193, n = 10, P = 0.028). The contents of squalene and hexadecanoic acid were unaffected by ovariectomy in females (intact vs. ovariectomy—squalene: [5.1 ± 2.87] × 108 vs. [3.9 ± 1.67] × 108, t8 = 1.568, P = 0.155; hexadecanoic acid: [5.3 ± 1.87] × 107 vs. [4.9 ± 1.06] × 107, t8 = 0.262, P = 0.800). The content of cholesterol was significantly higher in males [(1.73 ± 0.47) × 108] than in females [(0.89 ± 0.09) × 108] (t8 = 3.595, P = 0.007) but was unaffected by gonadectomy (castrated male = [1.71 ± 0.90] × 108, t8 = 0.075, P = 0.942; ovariectomized female = [1.0 ± 0.25] × 108, t8 = 1.150, P = 0.283).
GC–MS analysis of rat urine
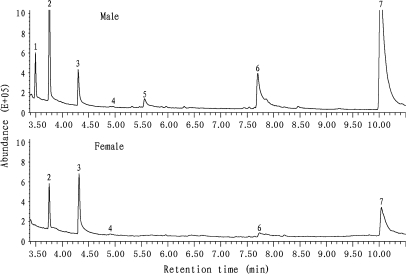
From rat urine, we characterized 7 compounds including 2 ketones, 1 aldehyde, 1 sulfone, and 3 phenols (Figure 2 and Table 3). In males, compound 7 (4-ethyl phenol) constituted more than 60% of all volatiles and compound 2 (2-heptanone) accounted for approximately 20%. Both were quantified by the percentage of GC peaks. In terms of relative abundance, compounds 1, 2, and 5 were higher and compound 3 was lower in males than in females; compounds 1 and 2 were suppressed by castration, whereas compound 5 was promoted by ovariectomy (Table 3). The abundances, which were also quantified by GC peaks, of compounds 1, 2, 5, 6, and 7 were higher in males than in females. Compounds 1, 2, 5, and 7 were suppressed by castration and compound 5 was promoted by ovariectomy (Table 4). The 2-heptanone, 4-ethyl phenol, and squalene levels in male rat urine were roughly estimated to be 10, 40, and 1–0.5 ppm, respectively.
Figure 2.
Representative GC profile of dichloromethane extract of male (top panel) and female (bottom panel) rat urine. GC conditions were described in Materials and methods of the text. The numbered GC peaks correspond to the compounds in Table 3.
Table 3.
Comparison of the relative abundances of the identified compounds of rat urine between groups (mean ± standard deviation, N = 5 for each group)
| GC peak No. | GC retention time | Compound identification |
Experimental groups |
||||
| Compound names | Diagnostic ions [m/z(relative intensity)] | Males | Females | Castrated males | Ovariectomized females | ||
| 1 | 3.48 | 4-Heptanone | 43(100), 71(66), 114(16), 58(6) | 3.04 ± 1.74a,b | 0 ± 0a | 0.16 ± 0.31b | 0 ± 0 |
| 2 | 3.77 | 2-Heptanone* | 43(100), 58(66), 71(12), 114(4) | 19.27 ± 9.30a,b | 6.60 ± 6.44a | 5.59 ± 7.52b | 6.16 ± 4.30 |
| 3 | 4.33 | Dimethyl sulfone | 79(100), 94(64) | 4.19 ± 3.57a | 38.90 ± 26.56a | 16.85 ± 21.42 | 32.28 ± 22.06 |
| 4 | 4.89 | Benzaldehyde | 106(100), 105(99), 77(96), 51(48) | 0.32 ± 0.40 | 0.63 ± 0.87 | 0.61 ± 0.97 | 0.64 ± 0.51 |
| 5 | 5.51 | Phenol | 94(100), 66(24), 65(22), 39(19) | 1.70 ± 1.43a | 0 ± 0a,c | 0.50 ± 0.34 | 0.29 ± 0.30c |
| 6 | 7.74 | 4-Methyl phenol | 107(100), 108(88), 77(20), 79(13) | 9.48 ± 6.80 | 3.53 ± 2.75 | 11.13 ± 9.79 | 3.11 ± 3.51 |
| 7 | 10.04 | 4-Ethyl phenol* | 107(100), 122(36), 77(12), 108(6) | 61.99 ± 10.20 | 50.34 ± 28.60 | 65.16 ± 23.39 | 57.52 ± 23.10 |
The means in a row marked by same superscript letters show significant differences (P < 0.05, using independent t-test or Mann–Whitney U test). The compounds marked by superscript asterisk (*) were verified with authentic standards; other components were identified by comparison with spectra listed in the NIST (Agilent Technologies 2002) mass spectral library and analogous data.
Table 4.
Comparison of the abundances of the identified compounds of the rat urine between groups (mean ± standard deviation, N = 5 for each group)
| GC peak No. | Retention time (min) | Identified compound names | Males | Females | Castrated males | Ovariectomized females |
| 1 | 3.48 | 4-Heptanone | 73.86 ± 61.99a,b | 0 ± 0a | 0.72 ± 1.44b | 0 ± 0 |
| 2 | 3.77 | 2-Heptanone* | 515.2 ± 604.0a,b | 21.02 ± 28.58a | 12.43 ± 9.69b | 26.59 ± 19.64 |
| 3 | 4.33 | Dimethyl sulfone | 67.38 ± 44.20 | 92.49 ± 48.62 | 62.28 ± 36.91 | 118.3 ± 68.30 |
| 4 | 4.89 | Benzaldehyde | 3.79 ± 3.53 | 1.46 ± 1.19 | 1.56 ± 0.93 | 2.58 ± 2.21 |
| 5 | 5.51 | Phenol | 35.55 ± 31.15a,b | 0 ± 0a,c | 5.34 ± 4.90b | 0.94 ± 0.68c |
| 6 | 7.74 | 4-Methyl phenol | 338.1 ± 447.7a | 12.87 ± 10.88a | 101.6 ± 93.8 | 15.94 ± 20.56 |
| 7 | 10.04 | 4-Ethyl phenol* | 1360.0 ± 1075.5a,b | 221.0 ± 257.7a | 635.2 ± 619.8b | 272.4 ± 164.9 |
The means in a row marked by same superscript letters show significant differences (P < 0.05, using independent t-test or Mann–Whitney U test). The compounds marked by superscript asterisk (*) were verified with authentic standards; other components were identified by comparison with spectra listed in the NIST (Agilent Technologies 2002) mass spectral library and analogous data.
Attractiveness of 2-heptanone, 4-ethyl phenol, and squalene to females
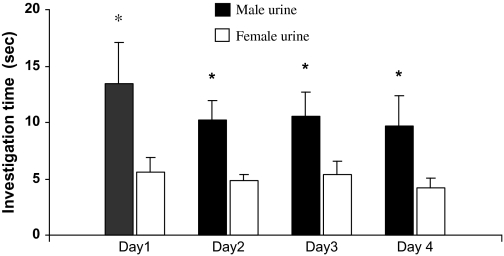
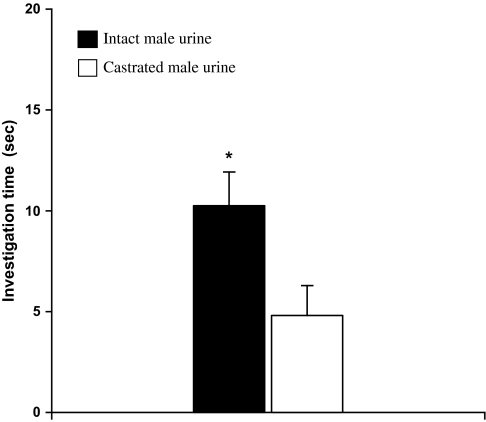
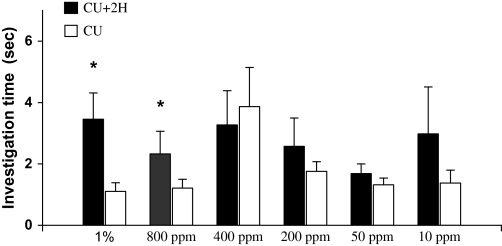
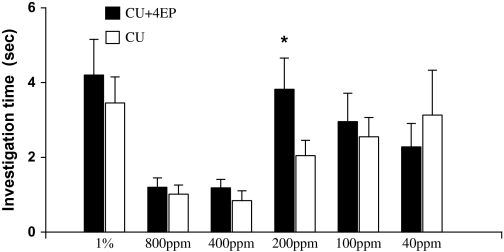
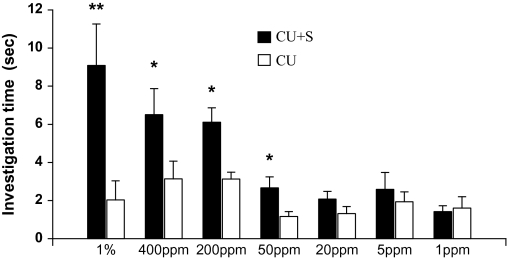
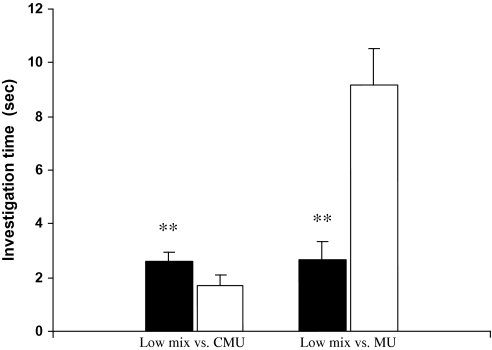
Binary choice test revealed that female rats, which exhibited an estrous cycle of about 4 days without synchrony, showed a significant preference for male urine over female urine during 4 consecutive days (Day 1: t9 = 2.791, P = 0.021; Day 2: t9 = 3.151, P = 0.012; Day 3: t9 = 2.283, P = 0.048; Day 4: t9 = 2.657, P = 0.026) (Figure 3). These responsive females, regardless of their stages of estrous cycles, were used to test for their preferences for castrated male urine (CMU) added with putative pheromone analogs over blank CMU (see below). As a result, females showed a significant preference for intact male urine over CMU (t9 = 3.157, P = 0.012) (Figure 4). Replenishing CMU with 2-heptanone at 800 ppm (Z = 2.395, n = 10, P = 0.017) or 1% 2-heptanone (t9 = 2.359, P = 0.040) significantly increased the attractiveness of CMU to females, but the preference was not shown when 10–400 ppm of either of the compounds was added (Figure 5). Likewise, females showed a higher level of preference for CMU added with 4-ethyl phenol at 200 ppm (t9 = 2.537, P = 0.032) but did not show a significant preference between blank CMU and CMU added with 40, 100, 400, 800 ppm, or 1% of the compound (Figure 6). Female also showed a heightened level of preference to CMU added with squalene at 50 ppm (Z = 2.293, n = 10, P = 0.022), 200 ppm (t8 = 2.073, P = 0.038), 400 ppm (t9 = 3.228, degrees of freedom = 9, P = 0.010), or 1% (Z = 2.803, n = 10, P = 0.005), compared with the control (blank CMU). However, females showed no preference between blank CMU and CMU added with squalene at 1–20 ppm (Figure 7). Furthermore, adding a mixture of squalene (1 ppm), 2-heptanone (10 ppm), and 4-ethyl phenol (40 ppm) at the levels approximate to those present in male urine enhanced the preferential response of females (t9 = 3.303, P = 0.009) (Figure 8). However, such a scented CMU was still less attractive to females than was intact male urine (t9 = 5.918, P = 0.000) (Figure 8).
Figure 3.
Duration of investigation (mean ± standard error, s) of female rats on male and female urine during a 3-min choice test in 4 consecutive days (*, P < 0.05, paired t-test).
Figure 4.
Duration of investigation (mean ± standard error, s) of female rats on intact and CMU during a 3-min choice test (*, P < 0.05, paired t-test).
Figure 5.
Duration of investigation (mean ± standard error, s) of female rats on castrate urine (CU) versus CU added with 2-heptanone (2H) during a 3-min choice test (*, P < 0.05, paired t-test or Wilcoxon matched-pairs signed-rank test).
Figure 6.
Duration of investigation (mean ± standard error, s) of female rats on castrate urine (CU) versus CU added with 4-ethyl phenol (4EP) during a 3-min choice test (*, P < 0.05, paired t-test or Wilcoxon matched-pairs signed-rank test).
Figure 7.
Duration of investigation (mean ± standard error, s) of female rats on castrate urine (CU) versus CU added with squalene (S) during a 3-min choice test (*, P < 0.05, paired t-test or Wilcoxon matched-pairs signed-rank test).
Figure 8.
Duration of investigation (mean ± standard error, s) of female rats on castrate urine (CMU) added with a mixture of 2-heptanone, 4-ethyl phenol, and squalene versus CMU or intact male urine (MU) during a 3-min choice test (*, P < 0.05, paired t-test).
Discussion
The house mouse, which is among the best studied species for mammalian pheromones, shows sexual dimorphism in the size of the preputial glands and specific compounds of major urinary volatiles in the male (Brown 1985; Novotny, Ma, et al. 1999). In contrast, our data from the rat show that there was little sexual difference in the size of the preputial glands and all urinary volatiles other than 4-heptanone and phenol, both having only a trace amount, were shared by males and females. Furthermore, the rat, as several other rodent species such as the house mouse and the Brandt's vole, had no sex-specific compounds from preputial glands (Zhang, Rao, et al. 2007; Zhang, Zhao, et al. 2007). However, the relative abundances of some aliphatic acids and a terpenoid polyene of minor amount from preputial glands and urine-derived heptanone were significantly higher in males. Numerous studies have demonstrated that relative abundance is a useful quantitative measure, indicative of putative pheromones (Singer et al. 1997; Wyatt 2003; Zhang, Rao, et al. 2007; Zhang, Zhao, et al. 2007). Hence, a compound showing either unique to or richer in males can be regarded as a putative male pheromone molecule. On the other hand, the absolute abundance of a scent constituent, though more difficult to quantify, is still a valuable GC attribute used to screen for pheromone candidates in mammals (Novotny, Ma, et al. 1999; Zhang et al. 2005). Our data indicate that preputial gland–derived squalene and urine-metabolized 4-methyl phenol and 4-ethyl phenol had apparently higher abundances in males than in females. The 3 male-biased compounds can also be viewed as putative male pheromone molecules, awaiting behavioral verification in the future.
Our study also shows that the 3 major putative male pheromone molecules, 2-heptanone, 4-ethyl phenol, and squalene, could be suppressed by castration. Previous behavioral bioassays have revealed that rat preputial gland contains androgen-dependent male pheromones (Gawienowski et al. 1976; Gawienowski 1977) and that 2-heptanone in male rat urine is an alarm pheromone that can induce fear and anxiety in male rats (Gutiérrez-García et al. 2007). Likewise, male mice avoid and fear E,E-β-farnesene and E-α-farnesene, 2 typical male pheromones (Jemiolo et al. 1991b). It is reasonable to question whether these urinary compounds, especially 2-heptanone, are also male pheromones. Indeed, our behavioral tests validated their pheromonal activities by testing their chemosensory attractiveness to female rats. Although hexadecanoic acid and cholesterol were also richer in males than in females, the former does not attract females (Gawienowski et al. 1975) and the latter has never been reported as an animal pheromone compound (Wyatt 2003). Additionally, our results about the testis and ovary dependency of rat preputial gland were consistent with previous work in a sense that both androgen and estrogen can hypertrophy preputial glands in the rat (Marois M and Marois G 1974; Nickerson et al. 1976; Lucas et al. 1982).
It has been exemplified in house mice that some male pheromone molecules such as E,E-β-farnesene and E-α-farnesene need higher concentrations than their natural levels in male urine to attract sexually inexperienced females (Jemiolo et al. 1991a). This seems to explain why in our study a high concentration of 2-heptanone, 4-ethyl phenol, or squalene was needed to attract sexually naive female rats. Although replenishing the 3 compounds together to CMU at their physiological levels could significantly increase sex attractiveness, it could still not completely restore female rats’ preference for intact male urine. This suggests that other minor compounds such as 4-heptanone, phenol, and 4-methyl phenol might also play some small roles in eliciting female response. Interestingly, in disagreement with some previous work (Orasulak and Gawienowski 1972; Lucas et al. 1982; Kashiwayanagi 2005), our behavioral data show that sexually naive female rats preferred male urine over female urine.
It has been evidenced that the body odor of female rats contains estrogen-dependent female pheromones to attract males (Pietras 1981; Lucas et al. 1982). In our study, we found that E,E-β-farnesene and E-α-farnesene from rat preputial gland were richer in females than in males, suggesting that they could be female pheromone candidates. Intriguingly, in house mice, these 2 terpenes are richer in males than in females and can be suppressed by castration, typical of male pheromones (Harvey et al. 1989; Novotny, Ma, et al. 1999; Zhang, Rao, et al. 2007). Urine-metabolized dimethyl sulfone was richer in females than in males and might be another female pheromone candidate in the rat. Squalene had a lower content in preputial gland in female rats than in male rats, and its amount was unaffected by ovariectomy, suggesting a low possibility of being a female pheromone. It seems that ovariectomy imparted a much smaller effect on both preputial gland and urine constituents in female rats as compared with castration on the corresponding compounds in male rats.
Funding
Chinese NSF (No. 30670268 to J.X.Z and L.S.); International Partnership Project of CAS (CXTD2005-4); Ministry of Science and Technology, China (2005BA529A05).
Supplementary Material
Acknowledgments
We are grateful to Dr D. Wiesler for his elucidation in MS spectra and to Prof. C. Zhao for his provision of the synthetic analogs. We thank D. Wang, X. Qin, and R. Wang for their assistance.
References
- Albro PW, Moore B. Identification of the metabolites of simple phthalate diesters in rat urine. J Chromatogr. 1974;94:209–218. doi: 10.1016/s0021-9673(01)92368-4. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- Brinck C, Hoffmeyer I. Marking urine and preputial gland secretion of male bank voles (Clethrionomys glareolus): chemical analyses and behavioral tests. J Chem Ecol. 1984;10:1295–1308. doi: 10.1007/BF00988112. [DOI] [PubMed] [Google Scholar]
- Brouette-Lahlou I, Amouroux R, Chastrette F, Cosnier J, Stoffelsma J, Vernet-maury E. Dodecyl propionate, attractant from rat pup preputial gland: characterization and identification. J Chem Ecol. 1991;17:1343–1354. doi: 10.1007/BF00983767. [DOI] [PubMed] [Google Scholar]
- Brouette-Lahlou I, Godinot F, Vernet-Maury E. The mother rat's vomeronasal organ is involved in detection of dodecyl propionate, the pup's preputial gland pheromone. Physiol Behav. 1999;66:427–436. doi: 10.1016/s0031-9384(98)00334-5. [DOI] [PubMed] [Google Scholar]
- Brown RE. The rodents. II. Suborder Myomorpha. In: Brown RE, Macdonald DW, editors. Social odours in mammals. Oxford: Clarendon Press; 1985. pp. 345–417. [Google Scholar]
- Burgess TL, Wilson JD. Studies on hormonal regulation of squalene synthesis in preputial gland and skin of the rat. Proc Soc Exp Biol Med. 1963;113:747–750. doi: 10.3181/00379727-113-28479. [DOI] [PubMed] [Google Scholar]
- Epple G, Golob NF, Smith ABI. Odor communication in the trmarin Saguinus fuscicollis (Callitrichidae): behavioral and chemical studies. In: Ritter FJ, editor. Chemical ecology: odour communication in animals. Amsterdam (the Netherlands): Elsevier; 1979. pp. 117–130. [Google Scholar]
- Gawienowski AM. Chemical attractants of the rat preputial gland. In: Muller-Schwarze D, Mozell MM, editors. Chemical signals in vertebrates. Vol. 1. New York: Plenum; 1977. pp. 45–59. [Google Scholar]
- Gawienowski AM, Denicola DB, Stacewicz-Sapuntzakis M. Androgen dependent of a pheromone in rat urine. Horm Behav. 1976;7:401–405. doi: 10.1016/0018-506x(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Gawienowski AM, Orsulak PJ, Stacewicz-Sapuntzakis M, Joseph BM. Presence of sex pheromone in preputial glands of male rats. J Endocrinol. 1975;67:283–288. doi: 10.1677/joe.0.0670283. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-García AG, Contreras CM, Mendoza-López MR, García-Barradas O, Cruz-Sánchez JS. Urine from stressed rats increases immobility in receptor rats forced to swim: role of 2-heptanone. Physiol Behav. 2007;91:166–172. doi: 10.1016/j.physbeh.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Harvey S, Jemiolo B, Novotny M. Pattern of volatile compounds in dominant and subordinate male mouse urine. J Chem Ecol. 1989;15:2061–2071. doi: 10.1007/BF01207438. [DOI] [PubMed] [Google Scholar]
- Holland M, Rhodes G, Dalleave M, Wiesler D, Novotny M. Urinary profiles of volatile and acid metabolites in germfree and conventional rats. Life Sci. 1983;32:787–794. doi: 10.1016/0024-3205(83)90314-4. [DOI] [PubMed] [Google Scholar]
- Jemiolo B, Alberts J, Sochinski-Wiggins S, Harvey S, Novotny M. Behavioural and endocrine responses of female mice to synthetic analogues of volatile compounds in male urine. Anim Behav. 1985;33:1114–1118. [Google Scholar]
- Jemiolo B, Xie T-M, Novotny M. Socio-sexual olfactory preference in female mice: attractiveness of synthetic chemosignals. Physiol Behav. 1991a;50:1119–1122. doi: 10.1016/0031-9384(91)90570-e. [DOI] [PubMed] [Google Scholar]
- Jemiolo B, Xie T-M, Novotny M. Urine marking in male mice: responses to natural and synthetic chemosignals. Physiol Behav. 1991b;52:521–526. doi: 10.1016/0031-9384(92)90341-x. [DOI] [PubMed] [Google Scholar]
- Kashiwayanagi M. Augmentation of sensitivity to urinary pheromone and excreting of urinary pheromone by sexual experiences. Chem Senses. 2005;30(Suppl 1):i138–i139. doi: 10.1093/chemse/bjh152. [DOI] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Zhang JX, Zhang JH, Bao WD. Social dominance, sexual attractiveness and pheromonal communication in male mice, Mus musculus. Acta Zool Sin. 2008;54:399–406. [Google Scholar]
- Lucas PD, Donohoe SM, Thody AJ. The role of estrogen and progesterone in the control of preputial gland sex attractant odors in the female rat. Physiol Behav. 1982;28:601–607. doi: 10.1016/0031-9384(82)90037-3. [DOI] [PubMed] [Google Scholar]
- Mallick SA. Observations on the preputial glands of three species of Rattus. J Mammal. 1991;72:198–201. [Google Scholar]
- Marois M, Marois G. Action of estradiol and testosterone on the preputial glands of the rat fetus. J Gynecol Obstet Biol Reprod. 1974;3:971–980. [PubMed] [Google Scholar]
- Mason RT, Fales HM, Jones TH, Pannell LK, Chinn JW, Crews D. Sex pheromones in snakes. Science. 1989;245:290–293. doi: 10.1126/science.2749261. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Moltz H. Pheromonal emission by pregnant rats protects against infanticide by nulliparous conspecifics. Physiol Behav. 1989;46:591–595. doi: 10.1016/0031-9384(89)90337-5. [DOI] [PubMed] [Google Scholar]
- Natynczuk SE, Macdonald DW, Tattersall FH. Morphology and chemistry of brown rat, Rattus norvegicus, preputial and clitoral glands. J Chem Ecol. 1995;21:247–260. doi: 10.1007/BF02036655. [DOI] [PubMed] [Google Scholar]
- Nickerson PA, Freeman JJ, Brownie AC. Effect of testosterone propionate on the ultrastructure of the preputial gland in the rat. Acta Anat (Basel) 1976;94:481–489. doi: 10.1159/000144580. [DOI] [PubMed] [Google Scholar]
- Novotny M, Harvey S, Jemiolo B. Chemistry of male dominance in the house mouse (Mus domesticus) Experientia. 1990;46:109–113. doi: 10.1007/BF01955433. [DOI] [PubMed] [Google Scholar]
- Novotny M, Harvey S, Jemiolo B, Alberts J. Synthetic pheromones that promote inter-male aggression in mice. Proc Natl Acad Sci USA. 1985;82:2059–2061. doi: 10.1073/pnas.82.7.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny M, Jemiolo B, Wiesler D, Ma W, Harvey S, Xu F, Xie T-M, Carmack MA. Unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem Biol. 1999;6:377–383. doi: 10.1016/S1074-5521(99)80049-0. [DOI] [PubMed] [Google Scholar]
- Novotny M, Ma W, Zidek L, Daev E. Recent biochemical insights into puberty acceleration, estrus induction and puberty delay in the house mouse. In: Johnston RE, Müller-Schwarze D, Sorenson P, editors. Advances in chemical communication in vertebrates. New York: Plenum; 1999. pp. 99–116. [Google Scholar]
- Orasulak PJ, Gawienowski AM. Olfactory preferences for the rat preputial gland. Biol Reprod. 1972;6:219–223. doi: 10.1093/biolreprod/6.2.219. [DOI] [PubMed] [Google Scholar]
- Pietras RJ. Sex pheromone production by preputial gland: the regulatory role of estrogen. Chem Senses. 1981;6:391–408. [Google Scholar]
- Singer AG, Beauchamp GK, Yamazaki K. Volatile signals of the major histocompatibility complex in male mouse urine. Proc Natl Acad Sci USA. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacewicz-Sapuntzakis M, Gawienowski AM. Rat olfactory response to aliphatic acetates. J Chem Ecol. 1977;3:411–417. [Google Scholar]
- Welsh CJ, Moore RE, Bartelt RJ, Jackson LL. Species-typical esters from preputial glands of sympatric voles, Microtus montanus and M. Pennsylvanicus. J Chem Ecol. 1988;14:143–158. doi: 10.1007/BF01022538. [DOI] [PubMed] [Google Scholar]
- Wyatt TD. Pheromones and animal behaviour. Cambridge (UK): Cambridge University Press; 2003. [Google Scholar]
- Zhang JX, Liu DZ, Sun L, Wei RP, Zhang GQ, Wu HL, Zhang HM, Zhao CH. Potential chemosignals in the anogenital gland secretion of giant pandas, Ailuropoda melanoleuca, associated with sex and individual identity. J Chem Ecol. 2008;34:398–407. doi: 10.1007/s10886-008-9441-3. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Rao XP, Sun L, Zhao CH, Qin XW. Putative chemical signals about sex, individual and genetic background in the preputial gland and urine of the house mouse (Mus musculus) Chem Senses. 2007;32:293–303. doi: 10.1093/chemse/bjl058. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Soini HA, Bruce KE, Wiesler D, Woodley SK, Baum MJ, Novotny MV. Putative chemosignals of the ferret (Mustela furo) associated with individual and gender recognition. Chem Senses. 2005;30:727–737. doi: 10.1093/chemse/bji065. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Sun L, Novotny MV. Mice respond differently to urine and its major volatile constituents from male and female ferrets. J Chem Ecol. 2007;33:603–612. doi: 10.1007/s10886-006-9220-y. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Zhao CH, Rao XP, Wang DW, Liu XH, Qin XW, Zhang ZB. Gender and individual information coded by insect pheromone analogs in the preputial glands in male brandt's voles (Lasiopodomys brandtii) Acta Zool Sin. 2007;53:616–624. [Google Scholar]
- Zhao CH, Adlof RO, Löfstedt C. Sex pheromone biosynthesis in the pine caterpillar moth, Dendrolimus punctatus (Lepidoptera: Lasiocampidae): pathways leading to Z5-monoene and 5,7-conjugated diene components. Insect Biochem Mol Biol. 2004;34:261–271. doi: 10.1016/j.ibmb.2003.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.