Abstract
Maillard reacted peptides (MRPs) were synthesized by conjugating a peptide fraction (1000–5000 Da) purified from soy protein hydrolyzate with galacturonic acid, glucosamine, xylose, fructose, or glucose. The effect of MRPs was investigated on human salt taste and on the chorda tympani (CT) taste nerve responses to NaCl in Sprague–Dawley rats, wild-type, and transient receptor potential vanilloid 1 (TRPV1) knockout mice. MRPs produced a biphasic effect on human salt taste perception and on the CT responses in rats and wild-type mice in the presence of NaCl + benzamil (Bz, a blocker of epithelial Na+ channels), enhancing the NaCl response at low concentrations and suppressing it at high concentrations. The effectiveness of MRPs as salt taste enhancers varied with the conjugated sugar moiety: galacturonic acid = glucosamine > xylose > fructose > glucose. The concentrations at which MRPs enhanced human salt taste were significantly lower than the concentrations of MRPs that produced increase in the NaCl CT response. Elevated temperature, resiniferatoxin, capsaicin, and ethanol produced additive effects on the NaCl CT responses in the presence of MRPs. Elevated temperature and ethanol also enhanced human salt taste perception. N-(3-methoxyphenyl)-4-chlorocinnamid (a blocker of TRPV1t) inhibited the Bz-insensitive NaCl CT responses in the absence and presence of MRPs. TRPV1 knockout mice demonstrated no Bz-insensitive NaCl CT response in the absence or presence of MRPs. The results suggest that MRPs modulate human salt taste and the NaCl + Bz CT responses by interacting with TRPV1t.
Keywords: benzamil, chorda tympani, ENaC, SB-366791, umami taste
Introduction
During the aging and/or cooking process, a reaction called the “Maillard reaction” occurs between reducing carbohydrate and amino acids (Maillard 1912; Hodge 1953). A Maillard reaction between D-glucose and D-alanine gives rise to N-(1-carboxyethyl) 6-hydroxymethyl-pyridinium-3-ol, a compound that has no taste by itself. However, it significantly enhanced sweet, salty, and umami taste in humans (Soldo et al. 2003). It was also reported that Maillard peptides (1000–5000 Da) generated during the aging of soy pesto and soy sauce affected not only basic taste qualities but also enhanced “kokumi,” a sensation of mouthfulness and continuity (Ogasawara et al. 2006a, 2006b, 2006c). In our preliminary studies, Maillard peptides, depending upon their concentration, enhanced or suppressed salt taste in human psychophysical evaluation (Rhyu et al. 2006; Katsumata et al. 2007). In this paper, we describe the purification of a 1000–5000 Da peptide fraction obtained from the enzymatic hydrolysis of soy protein that has no effect on human salt taste by itself. However, when the Maillard reaction is carried out between the peptide and different sugar moieties which is glucose (Glc), xylose (Xyl), fructose (Fru), N-glucosamine (GlcNH2), and galacturonic acid (GalA); the above Maillard reacted peptides (MRPs) produce a biphasic effect on human salt taste. We hypothesize that MRPs modulate salt taste by interacting with one or more salt taste receptors in taste receptor cells. To test if MRPs interact with the amiloride-sensitive and/or amiloride-insensitive salt taste receptors, we monitored chorda tympani (CT) taste nerve responses to NaCl in Sprague–Dawley rats and in transient receptor potential vanilloid 1 (TRPV1) knockout mice and their wild-type control mice.
In mammals, 2 salt taste receptors have been characterized so far: one that is Na+ specific and a second that does not discriminate among Na+, K+, and NH4+ (Stewart et al. 1997; Lindemann 2001). In the anterior tongue, the Na+-specific receptor in the fungiform taste receptor cells is the amiloride- and benzamil (Bz)-sensitive epithelial Na+ channel and is the predominant salt taste transducer in rats, many mice species and hamsters (DeSimone and Lyall 2006, 2008). The nonspecific salt taste receptor is amiloride and Bz insensitive and is the predominant transducer of salt taste in some mammalian species, including humans (Ossebaard and Smith 1995; Feldman et al. 2003; Dewis et al. 2006; Katsumata et al. 2007). It is a constitutively active, nonselective cation channel (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007) that demonstrates many similarities with the cloned pain receptor (TRPV1) (Caterina et al. 1997, 2000; Cortright et al. 2007). This candidate salt taste receptor is designated as TRPV1t (Simon and De Araujo 2005). However, additional amiloride-insensitive salt transduction mechanisms are present in taste receptor fields other than the anterior tongue (Lin et al. 1999; Ruiz et al. 2006; Treesukosol et al. 2007).
Both the amiloride-sensitive and amiloride-insensitive taste receptors are subject to acute modulation by effectors commonly present in food and beverages. Changes in pH (Lyall et al. 2002), osmolarity (Lyall et al. 1999), Ca2+ (DeSimone and Lyall 2008), and temperature (DeSimone and Lyall 2008) modulate the amiloride-sensitive CT responses to NaCl. On the other hand, vanilloids (resiniferatoxin [RTX] and capsaicin [CAP]), ethanol, nicotine, temperature, and external pH modulate the amiloride-insensitive NaCl CT response (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007). Thus, in principle, any one of the above effectors should have both salt taste enhancer and/or suppressor properties in both humans and in animal models. Because the amiloride-insensitive salt taste receptors are the predominant transducers of salt taste in humans (Ossebaard and Smith 1995; Feldman et al. 2003), it is more useful to consider TRPV1t modulators as perspective salt taste modifiers in humans. Consistent with this hypothesis, several studies suggest that TRPV1t modifiers, such as ethanol, elevated temperature (Hahn and Gunther 1932; Shizuyuki 1993; Martin and Pangborn 2006), N-geranyl cyclopropylcarboxamide (Dewis et al. 2006), naturally occurring Maillard peptides (Rhyu et al. 2006), and MRPs (Katsumata et al. 2007) modulate human salt taste. An ideal human salt taste enhancer or suppressor should be 1) tasteless, 2) odorless, 3) nonpungent, 4) stable at high temperatures, 5) effective at relatively low concentrations, 6) acutely effective in modulating the salt taste receptor, 7) completely reversible upon washout, and 8) additive with other TRPV1t modulators on the salt taste response.
The results presented in this paper show that MRPs at concentrations <0.01% enhanced and >0.01% suppressed human salt taste. MRPs also produced biphasic effects on the amiloride- and Bz-insensitive NaCl CT responses in control rats and wild-type TRPV1 mice. At concentrations <0.5%, MRPs enhanced and >0.5% inhibited the CT response. In both human salt taste evaluation and NaCl CT responses, the potency of MRPs conjugated with different sugar moieties was GalA ≥ GlcNH2 > Xyl > Fru > Glc. In TRPV1 knockout mice, that lack the Bz-insensitive component of the NaCl CT response, no effect of MRPs was observed on the CT response to NaCl + Bz. These studies suggest that a relationship exists between the MRPs-induced increase or decrease in human salt taste and TRPV1t. Some of the data have been published earlier as abstracts (Rhyu et al. 2006; Katsumata et al. 2007).
Materials and methods
All chemicals were of reagent grade or HPLC grade and were purchased from Sigma, St Louis, MO; Wako Pure Chemical Industries Ltd, Tokyo, Japan; or Nacalai Tesque Inc., Tokyo, Japan. Alcalase 2.4 L (Bacillus licheniformis) was purchased from Novozyme, Tokyo, Japan. Soybean protein, containing 90% protein, was purchased from Fuji Oil Co., Ltd, Tokyo, Japan.
Preparation of the 1000–5000 Da peptide fraction from soy protein
Fifteen grams of purified soy protein was dissolved in distilled water to obtain 15% (w/v) protein slurry and hydrolyzed at 50 °C at pH 8.0 for 24 h in the presence of 0.14 gm of the protease, alcalase. The hydrolyzate was incubated at 95 °C for 10 min to inactivate the protease and any resulting precipitate was removed by centrifugation at 5000 × g for 20 min at 4 °C. The supernatant was ultrafiltered through 1000 and 5000 Da cut-off membranes (Millipore Co., Milford, MA). The resulting 1000–5000 Da protein fraction was freeze dried and stored at −18 °C until used.
One-fourth of a gram of Xyl and 1 gm of the 1000- to 5000-Da protein fraction were dissolved in distilled water to obtain a 24% solution (w/v). Similarly, each of the sugar moieties GalA, GlcNH2, Glc, and Fru was used at a peptide to sugar moiety ratio of 4:1. This mixture was heated to 95 °C for 4.5 h and then fractionated with 1000 and 5000 Da membranes. The 1000–5000 Da fractions (MRPs) were freeze dried and stored at −18 °C until used (Ogasawara et al. 2006a).
CT taste nerve recordings
Animals were housed in the Virginia Commonwealth University animal facility in accordance with institutional guidelines. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Virginia Commonwealth University. Female Sprague–Dawley rats (150–200 gm) were anesthetized by intraperitoneal injection of pentobarbital (60 mg/kg), and supplemental pentobarbital (20 mg/kg) was administered as necessary to maintain surgical anesthesia. The animal's corneal reflex and toe-pinch reflex were used to monitor the depth of surgical anesthesia. Body temperatures were maintained at 37o C with a Deltaphase Isothermal PAD (Model 39 DP; Braintree Scientific Inc., Braintree, MA). The left CT nerve was exposed laterally as it exited the tympanic bulla and placed onto a 32-G platinum/iridium wire electrode. The CT responses were recorded under zero lingual current clamp and analyzed as described previously (Lyall et al. 2005a, 2005b, 2005c, 2007).
CT responses were also monitored in wild-type (C57BL/6J) and homozygous TRPV1 knockout mice (B6. 129S4-Trpv1tmijul; The Jackson Laboratory, Bar Harbor, ME). Mice (30–40 gm) were anesthetized by intraperitoneal injection of pentobarbital (30 mg/kg), and supplemental pentobarbital (10 mg/kg) was administered as necessary to maintain surgical anesthesia. The rest of the procedure was the same as described above for rats (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007). At the end of each experiment, animals were humanely killed by an intraperitoneal overdose of pentobarbital (c.a., 195 mg/kg body weight for rats and 150 mg/kg weight for mice).
The composition of rinse and NaCl stimulating solutions is shown in Table 1. The anterior lingual surface was stimulated with the rinse solution and salt solutions with or without MRPs and the nonreacted peptide (0–1%). The salt solutions containing MRPs had a pH of 6.4. In some experiments, Bz or N-(3-methoxyphenyl)-4-chlorocinnamide (SB-366791; SB) was added to the rinse and/or salt solutions to block Na+ entry into taste receptor cells via the apical epithelial Na+ channels and/or TRPV1t (Gunthorpe et al. 2004; Lyall et al. 2004, 2005b, 2005c, 2007). We also monitored the CT response to N + Bz containing a fixed concentration of Gal-MRP as a function of variation in pH between 4.1 and 9.1 (Table 1). In each case, the rinse solution was adjusted to the same pH as the stimulating salt solution. This was done to eliminate the contribution of changes in pH to the NaCl CT response (Lyall et al. 2002). CT responses were also recorded in the presence of TRPV1 modulators: RTX, CAP, ethanol, or elevated temperature. In some experiments, we tested the effect of MRPs on the CT response to monosodium glutamate (MSG) and MSG + inosine 5′-monophosphate (IMP) (Table 1). IMP is a specific modulator of umami taste (Yamaguchi 1991). CT responses to MSG were monitored in the presence of Bz and SB (Table 1). This was done to eliminate the contribution of Na+ flux via epithelial Na+ channels and TRPV1t to the CT response to glutamate (Lyall et al. 2004). Typically, stimulus solutions remained on the tongue for 1 min. Control stimuli consisting of 0.3 M NH4Cl and 0.3 M NaCl applied at the beginning and at the end of experiment were used to assess preparation stability. The following stimulus series were used in the CT experiments:
Table 1.
Stimuli used for CT experiments
| Rinse | M | Salt stimuli | M |
| R | 0.01 KCl | N | 0.01 KCl + 0.1 NaCl |
| R | 0.01 KCl | N + Bz | 0.01 KCl + 0.1 NaCl + 5 × 10−6 Bz |
| R | 0.01 KCl | N + Bz + MRPs | 0.01 KCl + 0.1 NaCl + 5 × 10−6 Bz + MRPs (0–1%) |
| R | 0.01 KCl | N + Bz + MRPs + TRPV1t modulatorsa | 0.01 KCl + 0.1 NaCl + 5 × 10−6 Bz + MRPs (0–1%) + TRPV1t modulatorsa |
| R | 0.01 KCl | MSG + Bz + SB | 0.01 KCl + 0.1 MSG + 5 × 10−6 Bz + 1 × 10−6 Bz SB |
| R | 0.01 KCl | MSG + Bz + SB + IMP | 0.01 KCl + 0.1 MSG + 5 × 10−6 Bz + 1 × 10−6 Bz SB + 1 × 10−3 IMP |
| (R)pHb | 0.01 KCl + 0.01 HEPES/Tris | (N + Bz + MRPs)pHb | 0.01 KCl + 0.01 HEPES/Tris + 0.1 NaCl + 5 × 10−6 Bz + MRPs (0–1%) |
| R | 0.01 KCl | Control solution 1 | 0.3 NH4Cl |
| R | 0.01 KCl | Control solution 2 | 0.3 NaCl |
MRPs: GalA-MRP; GlcNH2-MRP; Xyl-MRP; Fru-MRP; Glc-MRP. The concentration of MRPs is in percent. In some experiments, we also tested the effect of the unreacted soy protein fraction.
TRPV1 modulators: RTX (0.25 × 10−6 M); CAP (20 × 10−6 M); ethanol (20% or 40%); SB (N-(3-methoxyphenyl)-4-chlorocinnamide; SB-366791), a specific blocker of TRPV1t. In some experiments, SB was used at 5 × 10−6 M; Bz, a specific blocker of the epithelial Na+ channel.
HEPES (4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid) or Tris (tris(hydroxymethyl)aminomethane) were used to buffer the pH of (R)pH and (N + Bz + MRPs)pH between pH 4.1 and 9.1.
Series 1
The R → (N + Bz + MRP) → R step was repeated for each concentration of MRP between 0.1% and 1%. At the end of the MRP concentration series, the control stimuli were again applied (R → 0.3 M NH4Cl → R → 0.3 M NaCl → R). In some experiments, additional steps in the stimulus series protocol involved: (N + Bz), (N + Bz + RTX), (N + Bz + CAP), (N + Bz + MRPs), (N + Bz + MRPs + RTX), (N + Bz + MRP + CAP), and (N + Bz + ethanol).
Series 2
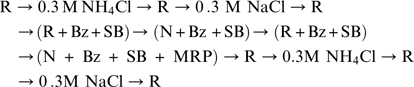 |
Series 3
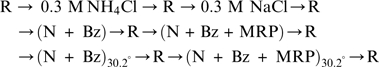 |
The R → (N + Bz) → R → (N + Bz + MRP) → R step was repeated for (N + Bz) and (N + Bz + MRP) maintained at 38.6 °C and 41.9 °C. At the end of the temperature series, the control stimuli were again applied (R → 0.3 M NH4Cl → R → 0.3 M NaCl → R). In these experiments, the lingual surface was superfused with salt solutions maintained at 30.2 °C, 38.6 °C, and 41.9 °C while rinse (R) was maintained at room temperature (23 °C).
In CT experiments, both transient (phasic) and tonic (steady state) parts of the NaCl CT responses were quantified. We also quantified the transient (phasic) response to the application of rinse (R) to a tongue already superfused with R. To quantify the phasic part of the CT response, the height of the stimulus-induced maximum CT response relative to baseline response was divided by the mean steady state (tonic) response to 0.3 M NH4Cl. To quantify the tonic part of a response, the area under the response versus time curve was taken over the final 30 s of the response. To normalize this result, this area was divided by the area under the 0.3 M NH4Cl response curve over the final 30s of the tonic response period. The normalized data were reported as mean ± standard error of the mean of the number of animals. Student's t-test was employed to analyze the differences between sets of data. Because we are comparing the normalized CT responses before and after TRPV1t modulators in the same CT preparation, paired t-test was used to evaluate statistical significance.
For clarity, the points on the graphs of the mean normalized phasic and tonic responses versus the logarithm of the GalA-MRP concentration were connected respectively by smooth curves. The curves were generated using a fitting function that models the characteristic biphasic property of the peptide agonists of TRPV1. The biphasic property has been observed with every agonist of TRPV1t thus far examined (Lyall et al. 2004, 2005b, 2005c, 2007). The fitting function used was
| (1) |
where
| (2) |
and
| (3) |
Here, R is the response, x is the logarithm of the GalA-MRP concentration expressed in percent and a, b, d, m, n, and r are parameters chosen by least squares criteria.
Human salt sensory evaluation
A total of 8–14 healthy volunteers (24–44 years old) participated in salt sensory evaluation experiments. All volunteers were employees of the Kyowa Hakko Food Specialties Co., Ltd, Ibaraki, Japan. The volunteers had training in taste sensory evaluation and extensive experience with psychophysical studies. Freshly distilled water was used to prepare the test solutions for taste evaluation. One-ounce (29.6 ml) samples were presented in opaque disposable plastic cups. All tests were conducted in individual sensory test booths.
Two independent methods were used to assess the effect of MRPs on human salt taste perception. In the first case, we used the constant stimuli method (Masuyama and Miura 1963; Johansson and Drake 1973; Kobayashi et al. 1974) to assess the effect of MRPs on human salt taste perception. Two experimental series, below called enhancing and suppressing, were performed with the test MRP concentration of 0.0025%, 0.005%, 0.01%, 0.025%, and 0.1% in 0.08 M NaCl. In the first case, 5 equidistant concentrations ranging from 0.078–0.086 M NaCl were used; and in the second case, the 5 equidistant NaCl concentrations ranging from 0.074–0.082 M were used (Table 2). In each session, the subjects were presented with 5 pairs of samples containing test MRP and pure NaCl solution. The subject's task was to taste each pair and to indicate which of the 2 samples was saltier. Each pair consisted of one sample of MRP + NaCl solution and one sample of solution that had higher or lower concentration of NaCl.
Table 2.
Stimuli used for human sensory evaluation
| Wash | Control | Salt stimuli |
| H2O | 0.074 M NaCl, 0.076 M NaCl, 0.078 M NaCl, 0.08 M NaCl, 0.082 M NaCl, 0.084 M NaCl, and 0.086 M NaCl | 0.08 M NaCl + MRPs (0.0025–0.1%) |
| H2O | 0.078 M NaCl, 0.080 M NaCl, 0.082 M NaCl, 0.084 M NaCl, and 0.086 M NaCl | 0.08 M NaCl + 4% ethanol + MRPs (0.02%) |
| H2O | 0.3% Vegetable soup + 0.1 M NaCl | 0.3% Vegetable soup + 0.1 M NaCl + MRPs (0.00001–0.02%) |
| H2O | 0.15 M NaCl + 50 × 10−6 M amiloride | 0.15 M NaCl + 50 × 10−6 M amiloride + GalA-MRP (0.002–0.1%) |
Vegetable soup = 41.52% sugar, 37.3% MSG, 13.84% onion powder, 4.21% WMP (IMP and guanosine 5’-monophosphate), 2.41% Na citrate, 0.6% garlic powder, and 0.12% celery powder. MRPs: GalA-MRP; GlcNH2-MRP; Xyl-MRP; Fru-MRP. The concentration of MRPs is in percent.
The data were analyzed using Probit analysis (methods of maximum likelihood) (Finney 1980) to obtain an estimate of the median effective dose that produced a change in salt taste intensity. The number of times (expressed as percent) an individual identified the saltier sample correctly or incorrectly were designated as PC and PI, respectively. The values of PC and PI were plotted as a function of the standard NaCl solution concentrations given above. The x-intercept of the fitted lines was calculated for all PC (P′C) and PI (P′I) values. The median effective dose was calculated using the fitting parameters P′C, P′I and values of parameters Y, w, and y from Probits analysis (Harris and Lieberman 1983). Y and y were designated as Probits and working probits from standard tables (Finney 1980) by using parameters P′C and P′I.
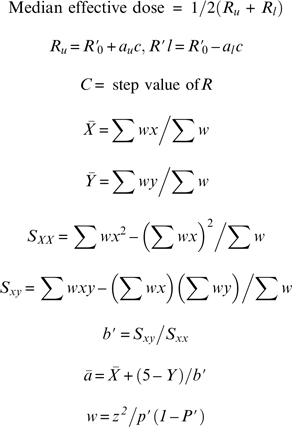 |
where, P′ = P′C or P′I, and z = (2Π)−1/2 exp(−(y − 5)2/2).
The additive effects between GalA-MRP and ethanol were also evaluated by the above method. This experiment was performed with 0.01% GalA-MRP and 4% ethanol in 0.08 M NaCl solution. The additive effects between GalA-MRP and elevated temperature were also evaluated by the above method. In this experiment, the temperature of pure NaCl standard solution and the test solution containing 0.01% GalA-MRP were maintained at 30 °C or 45 °C during salt taste evaluation. Equations (1–3) were also used to fit human sensory data.
The effect of MRPs on the salt taste was also assessed using a quantitative descriptive analysis method and a 7-point intensity scale (1 = none and 7 = very strong) (Ogasawara et al. 2006a) in a 0.3% model vegetable soup (Table 2). The soup without MRPs was presented as a control and given an intensity of 4 on a 7-point intensity scale. This experiment was performed with the test MRPs concentrations of 0.00001%, 0.00002%, 0.00005%, 0.0001%, 0.0002%, 0.001%, 0.005%, and 0.01% in 0.3% vegetable soup. The final salinity of the test solution was adjusted to 0.1 M NaCl. Volunteers tasted the soup without and with MRPs and indicated how strong the salt taste perception was of the soup containing MRPs relative to the soup without MRPs. In some experiments, the quantitative descriptive analysis method was used to evaluate the effect of GalA-MRP on human salt perception in 0.15 M NaCl solution containing 50 × 10−6 M amiloride, a blocker of epithelial Na+ channels. Two-sided paired t-test was used to evaluate statistical significance. All statistical significant values were Bonferroni adjusted.
Results
Effect of GalA-MRP on the Bz-insensitive NaCl CT response in rats
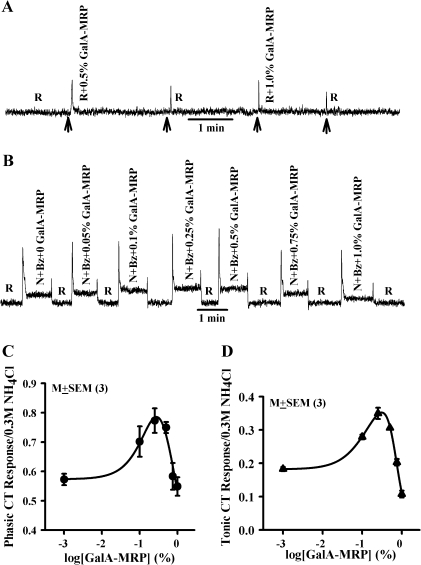
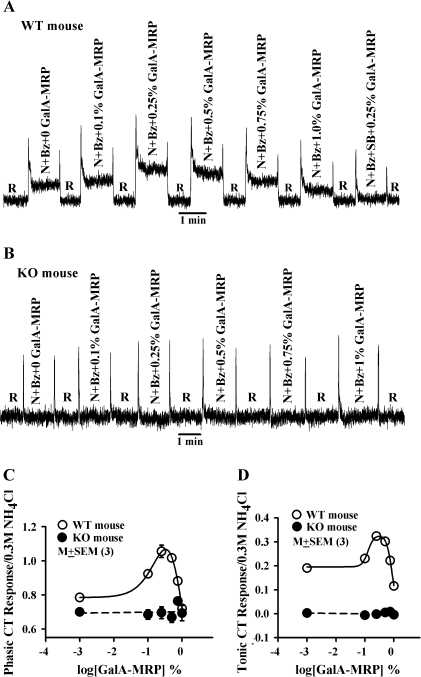
GalA-MRP dissolved in the rinse solution (R; Table 1) elicited only transient CT responses that were concentration independent and were indistinguishable from the mechanical rinse artifact (Figure 1A). This suggests that within the range of concentrations (0.1–1%) used in this study, GalA-MRP by itself does not elicit a CT response. Adding increasing concentrations of GalA-MRP to stimulating solutions containing N + Bz (Table 1) initially produced an increase in both phasic and tonic NaCl CT responses between 0.1% and 0.25% (Figure 1B). Above 0.5% GalA-MRP, the magnitudes of the phasic and tonic CT responses were less than their respective maximum values. At 1% GalA-MRP, the tonic CT response decreased below the value of N + Bz alone (Figure 1B). The normalized mean results from 3 such experiments are summarized in Figure 1C,D. GalA-MRP produced a biphasic dose-response relationship for both phasic (Figure 1C) and tonic (Figure 1D) NaCl CT responses. The maximum increase in the mean normalized phasic and tonic CT responses occurred at approximately the same GalA-MRP concentration (0.31% and 0.27% GalA-MRP, respectively) as estimated from the fitted curves. At a concentration of 0.27%, GalA-MRP enhanced the normalized mean Bz-insensitive NaCl tonic CT response by 101% relative to N + Bz. At 0.75% GalA-MRP, the tonic CT response was not statistically different from its value in N + Bz alone (P > 0.05). At 1%, the tonic CT response was inhibited by 54.8% relative to N + Bz (Figure 1D). The magnitude of the phasic CT response at 0.75% and 1% GalA-MRP was not different from its value in N + Bz alone (Figure 1C). These results demonstrate that both the phasic and tonic components of the Bz-insensitive NaCl CT response produce similar GalA-MRP concentration-response relations.
Figure 1.
Effect of GalA-MRP on the Bz-insensitive NaCl CT response. (A) Shows a representative trace of a CT response obtained while the rat tongue was stimulated with the rinse (R) alone and then with R + GalA-MRP at 0.5% and 1.0% maintained at 23 °C (Table 1). The arrows show the time period when the tongue was superfused with R or R + GalA-MRP. (B) Shows a representative trace of a CT response obtained while the rat tongue was first stimulated with R and then with N + Bz solutions containing GalA-MRP (0–1%) maintained at 23 °C (Table 1) using Stimulus Series 1 protocol (see Material and methods). The mean ± standard error of the mean values of the normalized phasic (C) and tonic (D) CT responses from 3 animals are plotted as a function of log[GalA-MRP] concentration. At 0.1%, 0.25%, 0.5%, 0.75%, and 1% GalA-MRP, the paired P values for the phasic response were 0.047, 0.005, 0.001, 0.825, and 0.52, respectively, and for the tonic response were 0.0001, 0.0001, 0.0001, 0.13, and 0.0011, respectively, with reference to 0 GalA-MRP.
Effect of SB on the NaCl CT response in the absence and presence of GalA-MRP
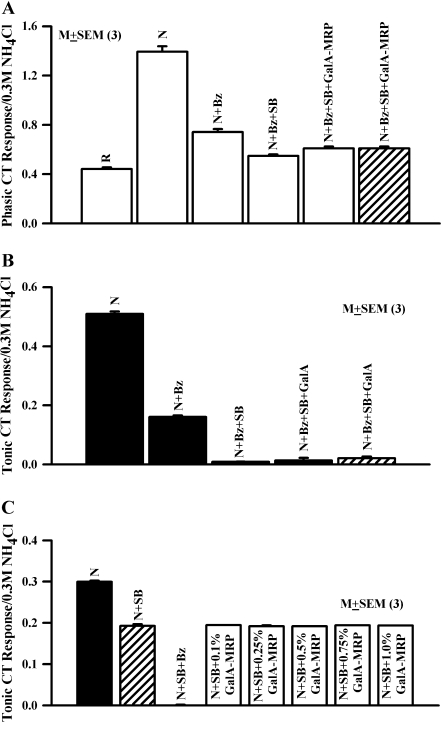
Rat NaCl CT responses were monitored under control condition and in the presence of Bz alone, SB alone, or Bz + SB relative to the rinse (R; Table 1). Bz decreased the mean normalized phasic NaCl CT response (Figure 2A) by 46.8% (N + Bz). In the presence of Bz + SB, the mean normalized phasic CT response (N + Bz + SB) was further decreased to 60.4% of control, a value that was slightly bigger than the rinse artifact (R; P < 0.002). Bz decreased the mean normalized tonic NaCl CT response by 68.6% (N + Bz); and in the presence of Bz + SB, it further decreased to 98.0% of control (N + Bz + SB), a value that was not statistically different from baseline (Figure 2B; P > 0.05 with respect to zero). These results show that in the presence of Bz, SB blocks both phasic and tonic NaCl CT responses.
Figure 2.
Effect of GalA-MRP on the NaCl CT responses in the presence of SB. CT responses were monitored while the rat tongue was stimulated with R, N, N + Bz, N + Bz + SB and N + Bz + SB + 0.25% GalA-MRP (Table 1) using Stimulus Series 2 protocol (see Materials and methods). The mean ± standard error of the mean (SEM) values of the normalized phasic (A) and tonic (B) NaCl CT responses from 3 animals are shown. The hatched bars show the CT response when Bz + SB were present in both rinse and the salt stimulus. In (A), the P values for phasic response for N + Bz, N + Bz + SB, N + Bz + SB + GalA-MRP with respect to R were 0.0004, 0.0042, and 0.0011, respectively. The P value for N + Bz + SB + GalA-MRP with respect to N + Bz + SB was 0.035. In (B), the values of the tonic response for N + Bz + SB, N + Bz + SB + GalA-MRP were not different from zero (P > 0.05). (C) Shows the mean ± SEM values of the normalized tonic NaCl CT response in 3 animals as a function of GalA-MRP concentration in the presence of N + SB (Table 1).
In the presence of SB (1 × 10−6 M), GalA-MRP (0.25%) produced no significant increase in the normalized mean tonic CT response (N + Bz + SB + GalA-MRP) above baseline (Figure 2B). GalA-MRP elicited no tonic CT response above baseline whether Bz + SB were added in the salt stimulus alone (Figure 2B; open bar) or when Bz + SB were present in both rinse (R + Bz + SB) and the salt stimulus (Figure 2B; hatched bar). In the presence of SB, the mean normalized phasic CT response in the presence of 0.25% GalA-MRP (N + Bz + SB + GalA-MRP) was slightly bigger than its value in (N + Bz) (P < 0.05). There was no difference in the magnitude of the phasic response in the presence of GalA-MRP whether Bz + SB were added to the salt stimulus alone or to both R and the salt stimulus (Figure 2A; hatched bar). The results show that SB inhibits the effect of GalA-MRP on both phasic and tonic Bz-insensitive NaCl CT responses. These results further suggest that SB inhibits the constitutively active TRPV1t in fungiform taste receptor cells and its subsequent activation by GalA-MRP.
To test if GalA-MRP specifically affects TRPV1t, we also monitored the effect of GalA-MRP on the Bz-sensitive NaCl CT responses in the presence SB alone. In the presence of SB, the NaCl CT response is derived only from the Na+ influx into taste receptor cells through apical epithelial Na+ channels. In the presence of 5 × 10−6 M SB, GalA-MRP (0.1–1%) had no effect on the tonic (Figure 2C) and phasic (data not shown) NaCl CT response relative to N + SB alone. These results suggest that GalA-MRP does not modulate the activity of epithelial Na+ channels in the apical membrane of fungiform taste receptor cells.
External pH, temperature, RTX, CAP, and ethanol modulate the effects of GalA-MRP on the NaCl CT response
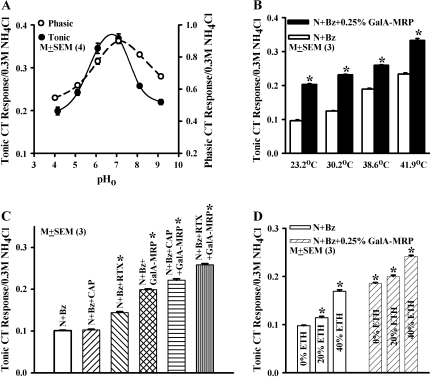
At a fixed GalA-MRP concentration (0.25%), both phasic (Figure 3A; open circles) and tonic (Figure 3A; filled circles), Bz-insensitive NaCl CT responses demonstrated a biphasic relationship with changes in external pH between 4.1 and 9.1 (Supplement Figure 1A). Similarly, nicotine, a TRPV1t agonist also enhanced the Bz-insensitive NaCl CT responses at all pHs between 4 and 9 relative to N + Bz alone (Lyall et al. 2007). The maximum increase in the phasic CT response was observed around pH 7, whereas the tonic CT response showed maximum activity between pH 6 and 7. In 4 animals, the mean normalized tonic CT responses in the presence of GalA-MRP at pH 4.1 and pH 9.1 were significantly greater than the N + Bz tonic CT response (P < 0.05 and P < 0.002, respectively). These results suggest that GalA-MRP enhances the N + Bz CT response at all pHs between 4.1 and 9.1. In our previous studies, all TRPV1t modulators tested (RTX, CAP, ethanol, nicotine, and cetylpyridinium chloride) produced a maximum increase in the Bz-insensitive tonic NaCl CT responses between pH 6 and pH 7 (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007). Accordingly, in this study, all salt solutions containing MRPs used in CT experiments and in human psychophysical studies had a pH of 6.4.
Figure 3.
Modulatory effect of external pH (pH0), elevated temperature, capsaicin (CAP), resiniferatoxin (RTX), and ethanol (ETH) on the Bz-insensitive NaCl CT response. (A) Rat tongue was first stimulated with R and then with N + Bz containing 0.25% GalA-MRP maintained at 23 °C. Both R and N + Bz + GalA-MRP solutions were adjusted to pHs between 4.1 and 9.1 (Table 1). The mean ± standard error of the mean (SEM) values of the normalized phasic (open circle) tonic (filled circles) CT response from 3 animals are plotted as a function of pH0. (B) Rat tongue was first stimulated with R and then with N + Bz solutions containing 0.25% GalA-MRP maintained at 23 °C, 30.2 °C, 38.6 °C, and 41.9 °C (Table 1) using Stimulus Series 3 protocol (see Materials and methods). The normalized mean ± SEM values of the tonic CT response from 3 animals are plotted as a function of temperature. *P < 0.0001. (C) Rat tongue was first stimulated with R and then with N + Bz, N + Bz + CAP (20 × 10−6 M), N + Bz + RTX (0.25 × 10−6 M), N + Bz + 0.25% GalA-MRP, N + Bz + CAP + GalA-MRP, and N + Bz + RTX + GalA-MRP maintained at 23 °C (Table 1). The mean ± SEM values of the mean normalized tonic CT response from 3 animals are plotted in the presence of above TRPV1t modulators. *P < 0.0001. (D) Rat tongue was first stimulated with R and then with N + Bz, N + Bz + 20% ETH, N + Bz + 40% ETH, N + Bz + 0.25% GalA-MRP, N + Bz + 20% ETH + GalA-MRP, and N + Bz + 40% ETH + GalA-MRP maintained at 23 °C (Table 1). The mean SEM values of the normalized tonic CT response from 3 animals are plotted in the absence and presence of above TRPV1t modulators. *P < 0.0001.
Increasing the temperature from 23.2 °C to 30.2 °C, 38.6 °C, and 41.9 °C (Table 1) increased the magnitude of the phasic and tonic Bz-insensitive NaCl CT responses (N + Bz) relative to 23.2 °C (Figure 3B and Supplement Figure 1B). At all temperatures studied, GalA-MRP produced a significantly greater increase (P < 0.0001) in the tonic CT response (N + Bz + GalA-MRP) relative to (N + Bz) (Figure 3B). These results suggest that elevated temperature and GalA-MRP produce additive effects on the Bz-insensitive NaCl CT response. Because our results demonstrate a strong relation between phasic and tonic NaCl CT responses under a variety of conditions, in the rest of the paper results only from the tonic Bz-insensitive NaCl CT response will be reported.
The Bz-insensitive NaCl CT response was enhanced in the presence of RTX (0.25 × 10−6 M) (Figure 3C). In mixtures containing RTX + GalA-MRP (0.25%), the tonic CT response was enhanced relative to NaCl solutions containing RTX alone or GalA-MRP alone (Figure 3C). Similarly, in the presence of a subthreshold concentration of CAP (20 × 10−6 M), which by itself did not give a significant increase in the tonic CT response relative to N + Bz, GalA-MRP also produced a significantly greater response relative to its value in the presence of CAP alone and GalA-MRP alone (Figure 3C). It is important to note that the effect of CAP + GalA-MRP and RTX + GalA-MRP was superadditive, that is, the combined response was greater than the sum of the individual CT responses to CAP, RTX, and GalA-MRP.
Ethanol, a modulator of TRPV1t (Lyall et al. 2005a, 2005b), at 20% and 40% enhanced the N + Bz tonic CT response in a dose-dependent manner (Figure 3D). In mixtures containing ethanol and GalA-MRP (0.25%), the CT responses were enhanced relative to their respective values in ethanol alone (Figure 3D). In the mixture, tonic CT responses to ethanol and GalA-MRP were additive.
Effect of MRPs conjugated with different sugar moieties on the Bz-insensitive NaCl CT response
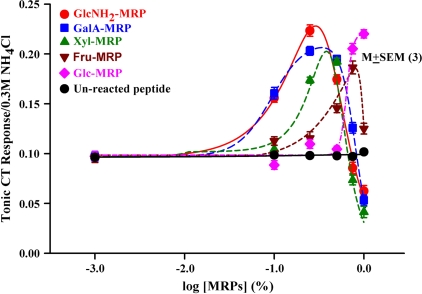
To test if the effect of MRPs on the Bz-insensitive NaCl CT response is dependent upon the conjugated sugar moiety, the 1000–5000 Da peptide was conjugated with GalA, GlcNH2, Glc, Fru, or Xyl. We obtained the dose-response relation between MRP concentration and the magnitude of the Bz-insensitive NaCl CT response in rats for each of the MRPs. As shown in Figure 4 and Table 3, both the maximum increase in the Bz-insensitive NaCl tonic CT response and the MRP concentration at which the maximum increase in the tonic CT response is observed varied with the sugar moiety. The effectiveness of various MRPs conjugated with different sugar moieties to modulate the Bz-insensitive NaCl tonic CT response was GlcNH2 ≥ GalA > Xyl > Fru > Glc (Table 3). The most effective modulators of the Bz-insensitive NaCl response were GalA-MRP and GlcNH2-MRP. In contrast to MRPs, the unreacted peptide had no effect on the tonic (Figure 5; filled circle) and phasic (data not shown) CT response in the presence of N + Bz.
Figure 4.
Effect of MRPs conjugated with different sugar moieties on the Bz-insensitive NaCl CT response. Rat CT responses were measured while the tongue was stimulated with R and then with N + Bz solutions containing 0–1% MRPs conjugated with glucosamine (GlcNH2), galacturonic acid (GalA), xylose (Xyl), fructose (Fru), glucose (Glc), and the unreacted peptide maintained at 23 °C. The normalized mean ± standard error of the mean values of the tonic CT response from 3 animals are plotted as a function of log[MRP] concentration. The normalized mean values of maximum CT response and the MRP concentration that produces a maximum response are summarized in Table 3.
Table 3.
Maximum CT response to MRPs
| MRPs | CT Response |
| 0.25% GalA-MRP | 0.203 |
| 0.25% GlcNH2-MRP | 0.223 |
| 0.50% Xyl-MRP | 0.192 |
| 0.75% Fru-MRP | 0.186 |
| 1.0% Glc-MRP | 0.220 |
CT response = maximum value of the Bz-insensitive NaCl tonic CT response/0.3 M NH4Cl. MRP concentration that produces maximum CT response was calculated from Figure 4.
Figure 5.
Effect of GalA-MRP on wild-type and TRPV1 knockout mice. Mouse tongues were first stimulated with R and then with N + Bz solutions containing increasing concentrations of GalA-MRP maintained at 23 °C (Table 1) using Stimulus Series 1 protocol (see Materials and methods). Representative traces of a Bz-insensitive NaCl CT response are shown for a wild-type (WT) mouse (A) and a TRPV1 knockout (KO) mouse (B) as a function of GalA-MRP concentration. The relation between normalized mean ± standard error of the mean phasic (C) and tonic (D) Bz-insensitive NaCl CT response and log[GalA-MRP] concentration in 3 WT (open circle) and 3 KO (filled circles) are also shown. In WT mice, at 0.1%, 0.25%, 0.5%, and 0.75%, GalA-MRP the paired P values for the phasic response were 0.008, 0.003, 0.0013, and 0.016, respectively and for the tonic response were 0.0006, 0.0006, 0.0001, and 0.012, respectively, with reference to 0 GalA-MRP. In KO mice, the phasic and tonic CT responses were not significantly different from their respective values in the absence of GalA-MRP.
CT responses in the wild-type and TRPV1 knockout mice
In order to confirm that GalA-MRP produces its effect by interacting with TRPV1t, NaCl CT recordings were made in both wild-type and TRPV1 knockout mice. Similar to the case in rats (Figure 1), in wild-type mice, the phasic and tonic Bz-insensitive NaCl CT responses demonstrated a biphasic response to increasing GalA-MRP concentrations (Figure 5A). The maximum increase in the phasic and tonic CT response was observed around 0.25% GalA-MRP. SB (1 × 10−6 M) inhibited the CT response to 0.25% GalA-MRP to near baseline. However, in the TRPV1 knockout mice, which lack the Bz-insensitive component of the NaCl CT response, GalA-MRP between 0.1% and 1% did not increase the tonic CT response to N + Bz above baseline (Figure 5B). Similarly, the phasic CT response was not affected with increasing GalA-MRP concentrations relative to N + Bz. The mean phasic and tonic CT responses in 3 wild-type and 3 knockout mice are summarized in Figure 5C,D, respectively. The results suggest that in mice in which TRPV1 is silenced, GalA-MRP does not modulate CT responses in the presence of N + Bz. The results in TRPV1 knockout mice are equivalent to the results obtained in rats and wild-type mice in the presence of Bz + SB (Figure 2A,B).
Effect of MRPs on the CT response to MSG
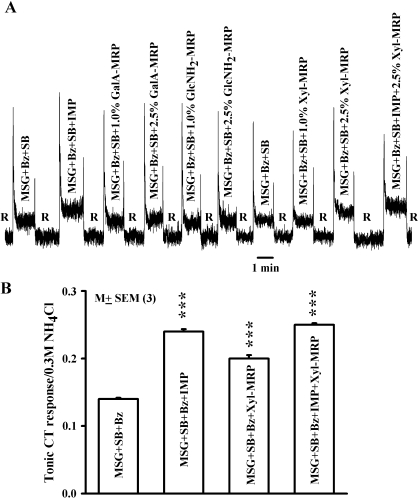
Xyl-MRP has been reported to modulate not only human salt taste but also to affect umami taste (Ogasawara et al. 2006a). Accordingly, we tested the effect of MRPs on the rat CT response to MSG and MSG + IMP (Table 1). Bz and SB were added to eliminate the contribution of Na+ to the CT response (Figure 2). Thus, in the presence of Bz + SB the CT response to MSG is due to glutamate only. Consistent with previous reports (Li et al. 2002; Damak et al. 2003), IMP enhanced the CT response relative to MSG alone (Figure 6A). In 3 animals, IMP (1 × 10−3 M) or Xyl-MRP (2.5%) significantly increased the mean normalized tonic CT response to MSG + SB + Bz (Figure 6B; P < 0.001). The effect of Xyl-MRP on the CT response to MSG + Bz + SB was not observed at concentrations ≤1%, that is, at concentrations at which Xyl-MRP modulated the Bz-insensitive NaCl CT responses (Figure 6A). However, in the presence of IMP + Xyl-MRP the magnitude of the mean normalized CT response to MSG + SB + Bz was less than additive relative to the CT response to IMP alone or Xyl-MRP alone (Figure 6B). At 1% and 2.5%, both GalA-MRP and GlcNH2-MRP did not modulate the CT response to MSG + Bz + SB.
Figure 6.
Effect of MRPs on the CT response to MSG, IMP, and MSG + IMP. (A) Rat tongue was first stimulated with R and then with MSG solutions containing Bz + SB (MSG + Bz + SB), IMP (MSG + Bz + SB + IMP), GalA-MRP (MSG + Bz + SB + GalA-MRP), GlcNH2 (MSG + Bz + SB + GlcNH2-MRP), Xyl-MRP (MSG + Bz + SB + Xyl-MRP), and MSG + Bz + SB + Xyl-MRP + IMP) maintained at 23 °C (Table 1). (B) Shows the normalized mean ± standard error of the mean tonic CT response from 3 animals for MSG + SB + Bz, MSG + SB + Bz + IMP, MSG + SB + Bz + Xyl-MRP, and MSG + SB + Bz + IMP + Xyl-MRP. ***P < 0.001 relative to MSG + SB + Bz.
Effect of MRPs on human salt taste
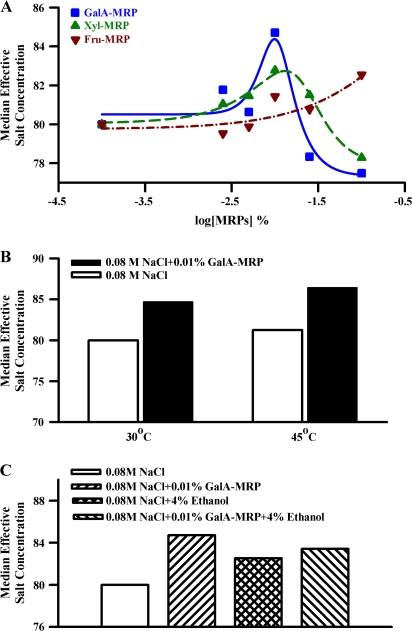
We used an estimation of the median effective dose to assess the effect of MRPs on the salt intensity of 0.08 M NaCl in human psychophysical tests (Figure 7A). Between 0.0025% and 0.01%, both GalA-MRP and Xyl-MRP (Figure 7A) increased, and above 0.01% suppressed the salt taste intensity. In the presence of 0.01% GalA-MRP and Xyl-MRP, the median effective salt concentration was maximally enhanced by 5.9% and 3.5%, respectively. In contrast, Fru-MRP (Figure 7A; inverted filled triangle) showed a maximum salt-enhancing effect (3.2%) at 0.1%. The data demonstrate that in the presence of 0.01% and 0.1% GalA–MRP, the salt intensity rating of 0.08 M NaCl solution was equivalent to that of 0.0847 M NaCl and 0.0775 M NaCl, respectively. Xyl-MRP showed a similar biphasic effect on NaCl intensity but was slightly less effective than GalA-MRP. In contrast, the NaCl intensity response curve was shifted to the right in the presence of 0.08 M NaCl + Fru-MRP. In this case, the maximum increase in the NaCl intensity was observed at 0.1% Fru-MRP. These results are consistent with the biphasic effect of MRPs observed on the CT response to NaCl + Bz (Figure 4). However, the increase in the human sensory response of MRPs was observed at significantly lower concentrations of MRPs relative to the concentrations of MRPs that produce an increase in the Bz-insensitive NaCl CT response in both rat and mouse models. This may be due to the fact that the human amiloride- and Bz-insensitive salt taste receptor is more sensitive to MRPs than in the rat.
Figure 7.
Effect of MRPs on human salt taste perception using constant stimuli method. (A) The effect of MRPs was assessed on salt taste using the constant stimuli method and the median effective salt concentration scale in 0.08 M NaCl. This experiment was performed with the MRPs concentrations at 0.0025%, 0.005%, 0.01%, 0.025%, and 0.1% in 0.08 M NaCl. (B) The effect of 0.01% GalA-MRP was assessed on salt taste using the constant stimuli method and the median effective salt concentration scale in 0.08 M NaCl (pH 6.4) maintained at 30 °C and 45 °C. (C) The effect of 0.01% GalA-MRP was assessed on salt taste using the constant stimuli method and the median effective salt concentration scale in 0.08 M NaCl (pH 6.4) in the absence and presence of 4% ethanol (ETH). In each case, 5 equidistant concentrations of NaCl ranging from 0.078 to 0.086 M (for the enhancing effect) and 0.074 to 0.082 M (for suppressing effect) were used (Table 2). All samples were evaluated by 10–14 trained panelists.
Consistent with the CT studies, in humans, the salt taste intensity increased with increasing the temperature of the test solution from 30 °C to 45 °C (Figure 7B). In addition, the increase in the salt intensity in the presence of GalA-MRP was also enhanced at 45 °C relative to 30 °C. Thus, the changes in human salt taste intensity mimic the effect of temperature on the Bz-insensitive NaCl CT response.
In humans, in mixtures containing 0.08 M NaCl + 4% ethanol, the salt intensity was greater relative to NaCl alone (Figure 7C). This suggests that ethanol, a modulator of TRPV1t, also enhances salt taste in humans. In mixtures containing 0.01% GalA-MRP + 4% ethanol, the perceived salt intensity was greater than that of 4% ethanol alone. However, the perceived salt taste intensity was less than that of GalA-MRP alone.
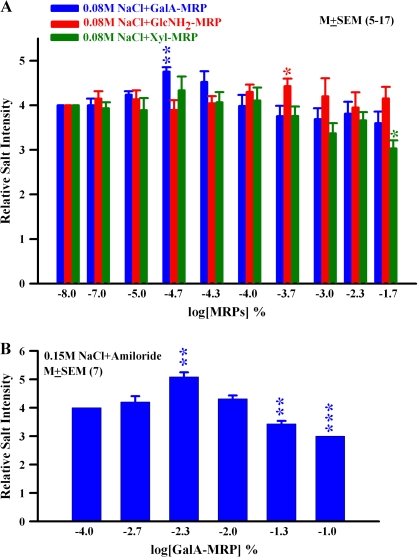
Human sensory evaluation was also performed using a model soup containing MRPs conjugated with GalA, GlcNH2, and Xyl using a quantitative descriptive analysis and a 7-point intensity scale. Between 0.00001% and 0.00005% GalA-MRP (Figure 8A; blue bar) increased and above 0.0001% suppressed the salt taste. At 0.00005%, the relative salt intensity was significantly (P < 0.001) enhanced relative to control solution by 19%. At 0.0002%, GlcNH2-MRP (Figure 8A; red bar) significantly (P < 0.05) enhanced the relative salt intensity relative to control solution by 10%. In contrast, Xyl-MRP did not show an increase in the relative salt intensity; however, at 0.02% (Figure 8A; green bar) it showed a significant (P < 0.05) salt-masking activity relative to control solution by 24%.
Figure 8.
Effect of MRPs on human salt taste perception using quantitative descriptive analysis. (A) Effect of MRPs was assessed on salt taste using a quantitative descriptive analysis method and a 7-point intensity scale in a 0.3% model vegetable soup (Table 2). The soup without MRPs was presented as a control and given an intensity of 4 on a 7-point intensity scale. MRPs were added to the soup at 0.00001%, 0.00002%, 0.00005%, 0.0001%, 0.0002%, 0.001%, 0.005%, and 0.02%. The final salinity of the test solution was adjusted to 0.1 M NaCl. All samples were evaluated by 5–17 trained panelists. Values are presented as mean ± standard error of the mean (SEM) of relative salt intensity. *P < 0.025 and **P < 0.005 (Bonferroni adjusted). (B) Effect of GalA-MRP on salt taste using a quantitative descriptive analysis method and a 7-point intensity scale in a 0.15 M NaCl solution containing 50 × 10−6 M amiloride. Values are presented as mean ± SEM of relative salt intensity from 7 trained panelists. **P < 0.005; ***P < 0.001 (Bonferroni adjusted).
In order to confirm that GalA-MRP also modulates the amiloride-insensitive salt taste in humans, additional experiments were conducted using 0.15 M NaCl solutions containing amiloride. As shown in Figure 8B, GalA-MRP produced a biphasic effect on salt taste in the presence of amiloride. However, in the presence of NaCl + amiloride, GalA-MRP produced a maximum increase in the perceived salt taste intensity at 0.005%.
Discussion
The results presented in this paper suggest that a relationship exists between the modulation of human salt taste by MRPs and their effects on the Bz-insensitive NaCl CT responses in rat and mouse models. This relationship is derived from mixture interactions between NaCl and MRPs, vanilloids, temperature, pH, amiloride, Bz, and SB observed in CT taste nerve recordings in rats and mice and in human sensory evaluation of salt taste. Below, we will discuss the proposed underlying physiological basis of this relationship.
MRPs as salt taste modifiers
In human sensory evaluation, MRPs were reported to have no taste of their own. This is consistent with the observations that in the absence of mineral salts, MRPs failed to elicit a CT response above baseline (Figure 1A), and thus, by themselves, MRPs are not gustatory stimuli. MRPs are nonpungent. In contrast to MRPs, some of the modulators of TRPV1t are not only gustatory stimuli by themselves but they also produce additional pharmacological and psychoactive effects. This seriously limits their use as possible salt taste modifiers. At low concentrations, humans report the taste of alcohol as sweet and at high concentrations it tastes bitter. Humans report the taste of nicotine as bitter. Both alcohol and nicotine also produce pharmacological and psychoactive effects. Moreover, alcohol and nicotine are only effective as TRPV1t modifiers at relatively high concentrations. Although the vanilloids (RTX and CAP) are the most potent agonists of TRPV1t (Lyall et al. 2004, 2005c), they are extremely pungent and thus have a strong trigeminal component. Thus, MRPs offer a distinct advantage over most of the above TRPV1t agonists as possible salt taste modifiers. In humans, MRPs modulate salt at relatively low concentrations (Figures 7 and 8) without affecting other taste qualities and are not associated with additional complications involving trigeminal, pharmacological, and psychoactive effects.
Biphasic effects of MRPs on human salt taste and the Bz-insensitive NaCl CT responses in rodents
All modulators of TRPV1t tested so far (RTX, CAP, ethanol, nicotine, temperature, cetylpyridinium chloride, N-geranyl cyclopropylcarboximide, and naturally occurring Maillard peptides) produced biphasic effects on the Bz-insensitive NaCl CT responses in both rats and mice (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007; Dewis et al. 2006; Rhyu et al. 2006). Consistent with these studies, all MRPs conjugated with different sugar moieties also produced biphasic effects on the Bz-insensitive NaCl CT responses (Figures 1,4 and 5). The dose-response profiles of MRPs were strongly dependent upon the conjugated sugar moiety. Among the sugars tested, MRPs conjugated with GlcNH2 and GalA were most effective and gave a maximum increase in the Bz-insensitive NaCl CT response by almost 100% relative to N + Bz at 0.25% (Table 3). The results suggest that the binding of MRPs to the activating site on TRPV1t is determined by the conjugated sugar moiety.
In comparison to RTX (0.0000063%) and CAP (0.0012%) (Lyall et al. 2004, 2005c), GalA-MRP and GluNH2-MRP produced maximum increase in the CT response at 0.25%. However, the increase in the maximum CT response was equivalent to that seen with RTX and CAP. At the highest concentration of GlcNH2-MRP and GalA-MRP (1%), the CT response was inhibited by 35% and 45%, respectively. In contrast, RTX (0.00063%) and CAP (0.006%) inhibited the CT response by 100% to the baseline, respectively. Thus, in comparison to RTX and CAP, MRPs are far less potent modulators of TRPV1t. In contrast, MRPs conjugated with Xyl, Fru, and Glc are about 2, 3, and 4 fold less potent than GlcNH2-MRP and GalA-MRP, respectively.
The Bz-insensitive NaCl CT response is observed in the absence of TRPV1t agonists and when stimulating solutions are presented at room temperature (Figures 1–5). This suggests that TRPV1t is constitutively active at room temperature and its activity is further enhanced in the presence of TRPV1t modulators. This is a major difference between TRPV1t and the pain receptor TRPV1. The native TRPV1 channel in dorsal root ganglia or the cloned TRPV1 expressed in human embryonic kidney (HEK-293) cell line is not constitutively active (Caterina et al. 1997, 2000). TRPV1 is opened in the presence of low pH, vanilloids, or elevated temperature. However, recent reports suggest that TRPV1 is also tonically activated in vivo abdominal viscera and is involved in body temperature regulation (Gavva et al. 2007; Steiner et al. 2007). In mesenteric resistance arteries and the renal cortex and medulla, tonically active TRPV1 is involved in blood pressure regulation (Wang Y and Wang DH 2006; Wang et al. 2007). Recently, additional roles of TRPV1 receptors or its variants have been proposed in the off taste of artificial sweeteners and metallic taste of Cu2+, Zn2+, and Fe2+ (Riera et al. 2007) and in the sensitization of changes in osmolarity to CAP in trigeminal sensory neurons (Liu et al. 2007).
It is important to note that in human psychophysical studies (Figures 7 and 8), MRPs conjugated with GalA, GlcNH2, Xyl, or Fru produced a biphasic effect on salt taste. Consistent with the CT data, the potency of the MRPs was related to the conjugated sugar moiety and the order of potency was the same as seen in the CT experiments (GalA = GlcNH2 > Xyl > Fru > Glc). A significant difference between the CT data and the human psychophysical data was in the concentration range at which MRPs modulate the human salt responses. The GalA-MRP and GlcNH2-MRP produced their maximum increase in salt perception using quantitative descriptive analysis at 0.0005% and 0.002%, respectively. In comparison, using the magnitude scale GalA-MRP produced its maximum increase in salt perception at 0.01%. These differences in the concentration of MRPs between the 2 methods may arise because of the use of 0.1 M NaCl dissolved in the model soup in the quantitative descriptive analysis and the use of 0.08 M NaCl solution in the analysis using the magnitude scale method. These results suggest that the presence of additional TRPV1t modulators and/or endogenous naturally occurring Maillard peptides in the model soup shift the dose-response curve to the left on the MRP concentration axis. GalA-MRP also produced a biphasic effect on human salt taste in the presence of amiloride (Figure 8B) providing additional support for the hypothesis that GalA-MRP also modulates an amiloride-insensitive salt taste receptor in humans.
In human sensory studies, the increase in salt taste intensity occurred over a very narrow concentration range of MRPs and become antagonists at higher concentrations. This may have been a limiting factor in the discovery of effective human salt taste enhancers. Depending upon where an individual may fall on the MRP dose–response curve, a particular concentration of MRP may act as an agonist, produce no change or act as an antagonist of the salt response, thus producing inconsistent effects on salt perception.
MRPs produced only acute effects on TRPV1t that were completely reversible (Figure 1B). Stimulating the tongue repeatedly with GalA-MRP concentrations <0.25% gave reproducible changes in the CT responses, suggesting that TRPV1t channel does not desensitize with repeated stimulations with MRPs. In our previous studies, no channel desensitization was observed by repeated application of RTX (<1 × 10−6 M), CAP (<40 × 10−6 M), ethanol (<40%), nicotine (<10 × 10−3 M), and elevated temperature (<42 °C) (Lyall et al. 2005a, 2005b, 2005c, 2007). In contrast, prolonged or repeated activation of TRPV1 induces desensitization and insensitivity of the receptor to subsequent stimuli (Caterina et al. 1997, 2000). However, at high concentrations, the above modulators decrease the magnitude of the Bz-insensitive NaCl CT response from its maximum value (Lyall et al. 2005a, 2005b, 2005c, 2007). Our preliminary studies suggest that desensitization of the channel in the presence of TRPV1t modulators is related to changes in intracellular Ca2+ ([Ca2+]i) of taste receptor cells (Heck et al. 2005). An increase in taste receptor cell [Ca2+]i reduced the response magnitude in the agonist dose-response curve at all agonist concentrations, and a decrease in [Ca2+]i increased the response magnitude in the agonist dose-response curve at all agonist concentrations and significantly inhibited the decline from maximal response at the higher agonist concentrations seen under control conditions (Heck et al. 2005). It is likely that Na+ entry through TRPV1t depolarizes taste receptor cells resulting in an increase in [Ca2+]i via the voltage-gated Ca2+ channels in taste receptor cells or the release of Ca2+ from intracellular stores (Lyall et al. 2007). This suggests that preventing the desensitization of TRPV1t at higher MRP concentrations would be beneficial in extending the concentration range over which MRPs can act as salt taste enhancers. In addition, the maximum activation of the TRPV1t will be maintained over a wider range of MRP concentrations. This means that MRPs can act as salt taste enhancers in a much larger human population displaying different affinities of MRPs for TRPV1t.
Interaction of MRPs and other TRPV1t modulators on the salt responses
In the absence of a TRPV1t agonist, the Bz-insensitive NaCl CT response is not affected by changes in external pH (Lyall et al. 2002). Also, in the absence of a TRPV1t agonist, the temperature threshold of the Bz-insensitive NaCl CT response is independent of changes in external pH (Lyall et al. 2004, 2005c). This indicates that the constitutive TRPV1t activity is not modulated by external pH. This is a significant difference between the cloned pain receptor, TRPV1, and the amiloride- and Bz-insensitive salt taste receptor, TRPV1t. TRPV1 is not constitutively active. A decrease in external pH is one of several stimuli that can open the channel (Davis et al. 2002; Gunthorpe et al. 2002; Geppetti and Trevisani 2004). However, the Bz-insensitive NaCl CT responses in the presence of TRPV1t agonists are strongly dependent upon external pH. In the presence of TRPV1t agonists, a change in external pH from 6 to either 4.7 or 9.7 increased the temperature threshold of the Bz-insensitive NaCl CT responses (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007). In our studies, the CT response to N + Bz + GalA-MRP gave the maximal response between pH 6 and 7 (Figure 3A). At constant external pH, changes in intracellular pH do not affect salt responses in the presence of RTX (Lyall et al. 2004, 2005c). This suggests that H+ binding to an external site on the TRPV1t channel protein modulates the affinity of TRPV1t agonists, including that of MRPs, to their respective intracellular binding sites on the channel protein.
An important property of TRPV1t is that it can integrate the effect of multiple stimuli to produce additive effects on the Bz-insensitive NaCl CT responses (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007). Consistent with this, in the presence of GalA-MRP, the effect of temperature and the peptide were additive (Figure 3B). GalA-MRP enhanced the Bz-insensitive NaCl CT response without a shift in the temperature threshold of the CT response. Consistent with this, ethanol and nicotine also increased the Bz-insensitive NaCl CT response without a shift in the temperature threshold of the CT response (Lyall et al. 2005a, 2005b, 2007). In contrast to ethanol, nicotine, and GalA-MRP, RTX shifted the temperature threshold of the Bz-insensitive NaCl CT response to lower temperatures (Lyall et al. 2004, 2005c). This suggests that the mechanism by which vanilloids (RTX and CAP) activate the channel is different from that of ethanol, nicotine, and MRPs.
The results shown in Figure 7B suggest that elevated temperature and GalA-MRP produce additive effects on human salt taste. In rats, increasing the temperature from 30 °C to 43 °C inhibited the Bz-sensitive NaCl CT responses from its maximum value to baseline in a dose-dependent manner (DeSimone and Lyall 2008). This suggests that at 43 °C the contribution of epithelial Na+ channel to salt taste is negligible. Thus, at 45 °C both the GalA-MRP–induced increase in the Bz-insensitive NaCl CT response and the GalA-MRP–induced increase in the salt taste perception in humans are most likely due to the amiloride- and Bz-insensitive salt taste receptor and not due to the amiloride-sensitive epithelial Na+ channels. This is further supported by the observations that GalA-MRP produced increase in human sensory salt taste evaluation in the presence of amiloride (Figure 8B).
The increase in the Bz-insensitive NaCl CT response by GalA-MRP and ethanol were additive (Figure 3D), suggesting that ethanol and GalA-MRP bind to different intracellular-binding sites on the TRPV1t receptor. In human psychophysical studies, in mixtures containing ethanol and GalA-MRP, the salt intensity was slightly higher than ethanol alone but was less than that of GalA-MRP (Figure 7C). This suggests that ethanol produces mixture interactions with GalA-MRP resulting in a decrease in the salt perception. This further suggests that the relationship between human salt perception and ethanol is shifted to the left on the MRP concentration axis relative to that of rat (Lyall et al. 2005c).
In contrast to ethanol, in mixtures containing a subthreshold concentration of RTX or CAP plus GalA-MRP, GalA-MRP enhanced the Bz-insensitive NaCl CT response. In each case, the GalA-MRP + RTX or GalA–MRP + CAP induced increase in the CT responses was greater than the increase in the CT response in the presence of RTX alone, CAP alone, or GalA-MRP alone. These results suggest that the binding of RTX or CAP to an intracellular-binding site on the TRPV1t alters the conformation of the channel and sensitizes the channel to further stimulation by GalA-MRP. These results are in agreement with previous studies (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007) in which a subthreshold concentration of RTX shifted the temperature threshold of the Bz-insensitive NaCl CT response to the left on the agonist concentration axis in the presence of ethanol or nicotine. These results support the notion that at subthreshold concentrations, vanilloids (RTX and CAP) produce allosteric effects on the Bz-insensitive NaCl CT response in the presence of ethanol, nicotine, and GalA-MRP.
MRPs produce their effects by interacting with TRPV1t
The specificity of MRPs as modulators of TRPV1t is supported by the observations that SB inhibits the effects of MRPs on the Bz-insensitive NaCl CT response (Figures 2 and 5). The constitutive TRPV1t activity is inhibited to baseline in the presence of SB and no further increase in the Bz-insensitive NaCl CT response above baseline is observed when stimulated with RTX, CAP, ethanol, nicotine, and elevated temperature (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007) or GalA-MRP (Figures 2 and 5). At an external pH of 6.4, RTX, CAP, ethanol, nicotine (Lyall et al. 2004, 2005a, 2005b, 2005c, 2007), and GalA-MRP (Figure 2) have no effect on the Bz-sensitive NaCl CT response. In addition, SB does not block ENaC (Figure 2C). The additional proof that GalA-MRP interacts with TRPV1t is provided by the observation that TRPV1 knockout mice, that lack the Bz-insensitive component of the NaCl CT response, also do not respond to Gal-MRP (Figure 5B–D), ethanol, RTX, nicotine, and elevated temperatures (Lyall et al. 2005a, 2005c, 2007). Taken together, the data support the conclusion that MRPs produce their effect on salt responses via the amiloride- and Bz-insensitive TRPV1t cation channels in fungiform taste receptor cells. However, the exact mechanism of how MRPs modulate the TRPV1t cation channel in taste receptor cells remains to be established.
MRPs as umami taste modifiers
Similar to other MRPs, Xyl-MRP also produced effects on salt responses between 0.1% and 1%. However, at 2.5%, it demonstrated a significant increase in the CT response to MSG. Similarly, another TRPV1t modulator, N-geranyl cyclopropylcarboximide, at higher concentrations enhanced the CT response to MSG and increased umami taste in humans (Dewis et al. 2006). It is important to point out that in our studies, CT responses to MSG were monitored in the presence of Bz + SB. This eliminates the contribution of Na+ to the MSG CT response and elicits a response only to glutamate. In the past, studies have only used amiloride to block epithelial Na+ channels while measuring the CT or glossopharyngeal taste nerve responses to MSG (Damak et al. 2003; Zhao et al. 2003). Our studies further suggest that another ideal way to measure CT responses to MSG is to use TRPV1 knockout mice and to record CT nerve responses in the presence of amiloride or Bz. Our results suggest that at high concentrations, Xyl-MRP and N-geranyl cyclopropylcarboximide interact with umami taste receptor (T1R1 + T1R3) and modulate umami taste in humans. In contrast to the wild-type mice, in T1R3 knockout mice, IMP did not potentiate the CT response to MSG (Damak et al. 2003). This suggests that IMP targets T1R3 to potentiate the CT response to MSG. It is likely that Xyl-MRP and IMP produce their effects on the CT response to MSG by acting on the T1R3 part of the umami taste receptor (T1R1 + T1R3). The observation that in the presence of IMP + Xyl-MRP the CT response to MSG is less than additive relative to IMP alone or Xyl-MRP alone (Figure 7), suggests that they compete for the same binding site on T1R3 (Damak et al. 2003). Xyl-MRP is a salt taste modifier at low concentrations and an umami taste modifier at high concentrations in both humans and animal models. This suggests that it acts on similar umami (Li et al. 2002; Damak et al. 2003) and amiloride-insensitive salt taste receptors in humans, rats, and mice.
In summary, our studies show that in the presence of NaCl, MRPs increase the Bz-insensitive NaCl CT response. Below 0.25%, GlcNH2-MRP and GalA-MRP enhanced and above 0.25% inhibited CT responses to 0.1 M NaCl. GalA-MRP produced maximum increase in the CT response between pH 6 and 7. Stimulating the tongue with solutions containing RTX, CAP, ethanol, and elevated temperature increased the sensitivity of the CT response to GalA-MRP. Because the effects of GalA-MRP on NaCl are blocked by SB and because TRPV1 knockout mice are insensitive to GalA-MRP, ethanol, RTX, and temperature, we conclude that MRPs produce effects on salt taste by a direct action on the Bz-insensitive TRPV1t salt taste receptor. Thus, among the MRPs studied, GlcNH2-MRP and GalA-MRP satisfy most of the properties necessary for them to qualify as salt taste modifiers, namely that they are tasteless, odorless, nonpungent, stable at elevated temperatures, effective at relatively low concentrations, produce only acute effects on salt taste that are completely reversible, and produce additive effects with other TRPV1t modulators. At concentration ≥ 2.5%, Xyl-MRP increases CT responses to MSG and MSG + IMP and umami taste in humans.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
National Institutes of Health (DC-00122 to J.A.D., DC-005981 to V.L.); Campbell Soup Company (to J.A.D.); and Kyowa Hakko Food Specialties Co., Ltd, Ibaraki, Japan (to V.L.).
Supplementary Material
Acknowledgments
During his stay at Virginia Commonwealth University, Mr Tadayoshi Katsumata was fully supported by Kyowa Hakko Food Specialties Co., Ltd, Ibaraki, Japan. We thank Dr Gerard L. Heck for helping with CT studies.
References
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cortright DN, Krause JE, Broom DC. TRP channels and pain. Biochim Biophys Acta. 2007;1772:978–988. doi: 10.1016/j.bbadis.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301(5634):850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Davis JB, Smart D, Gunthorpe MJ. The vanilloid receptors and vanilloid receptor-like genes: a hot topic getting hotter. Cell Transm. 2002;18:3–9. [Google Scholar]
- DeSimone JA, Lyall V. Taste receptors in the gastrointestinal tract III. Salty and sour taste: sensing of sodium and protons by the tongue. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1005–G1010. doi: 10.1152/ajpgi.00235.2006. [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V. Amiloride-sensitive ion channels. In: Basbaum Allen I, Kaneko Akimichi, Shepherd Gordon M, Westheimer Gerald., editors. The senses: a comprehensive reference. Vol. 4: Olfaction & Taste, Stuart Firestein and Gary K. Beauchamp. San Diego (CA): Academic Press; 2008. pp. 281–288. [Google Scholar]
- Dewis M, DeSimone JA, Phan THT, Heck GL, Lyall V. Effect of N-geranyl cyclopropylcarboximide (NGCC) on TRPV1 variant salt taste receptor (TRPV1t) [abstract] Chem Senses. 2006;31:A105. [Google Scholar]
- Feldman GM, Mogyorósi A, Heck GL, DeSimone JA, Santos CR, Clary RA, Lyall V. Salt-evoked lingual surface potential in humans. J Neurophysiol. 2003;90:2060–2064. doi: 10.1152/jn.00158.2003. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Estimation of the median effective dose. 3rd ed. Cambridge: Cambridge University Press; 1980. Probit analysis; pp. 20–80. and p. 283–321. [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P, Trevisani M. Activation and sensitization of the vanilloid receptor: role in gastrointestinal inflammation and function. Br J Pharmacol. 2004;141:1313–1320. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Luis Harris S, Lappin SC, Egerton J, Smith GD, et al. Identification and characterization of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacol. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- Hahn H, Gunther H. Uber die Reize und die Reizbedungunden des Geschmackssinnes. Pflugers Arch Physiol. 1932;231:48–67. [Google Scholar]
- Harris R, Lieberman HR. Computation of psychophysical threshold using the probit technique. Behav Res. 1983;15:446–448. [Google Scholar]
- Heck GL, Phan THT, Lyall V, DeSimone JA. Changes in taste receptor cell calcium modulate the amiloride-insensitive non-specific salt taste receptor [abstract] Chem Senses. 2005;30:A22. [Google Scholar]
- Hodge JE. Dehydrated foods, chemistry of browning reaction in model system. J Agric Food Chem. 1953;1:928–943. [Google Scholar]
- Johansson B, Drake B. Difference taste thresholds for sodium chloride among young adults: an inter-laboratory study. J Food Sci. 1973;38:524–527. [Google Scholar]
- Katsumata T, Tokunaga C, Fuji N, Egi M, Phan THT, Heck GL, DeSimone JA, Lyall V. Evaluation of Maillard reacted peptides (MPs) as novel salt taste enhancers and their effect on TRPV1 variant salt taste receptor (TRPV1t) [abstract] Chem Senses. 2007;32:A1–A125. [Google Scholar]
- Kobayashi N, Kamimura M, Yamane T. On aspartame, a new sweetner. GAKUEN. 1974;418:7–17. [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol. 1999;405:406–420. doi: 10.1002/(sici)1096-9861(19990315)405:3<406::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lindemann B. Receptors and transduction in taste. Nature. 2001;413(6852):219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen L, Liedtke W, Simon SA. Changes in osmolality sensitize the response to capsaicin in trigeminal sensory neurons. J Neurophysiol. 2007;97:2001–2015. doi: 10.1152/jn.00887.2006. [DOI] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan T-HT, Russell OF, Malik SA, Heck GL, DeSimone JA. Modulation of rat chorda tympani NaCl responses and intracellular Na+ activity in polarized taste receptor cells by pH. J Gen Physiol. 2002;120:793–815. doi: 10.1085/jgp.20028656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, DeSimone JA, Feldman GM. Effects of osmolarity on taste receptor cell size and function. Am J Physiol. 1999;277:C800–C813. doi: 10.1152/ajpcell.1999.277.4.C800. [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan THT, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. I. Effect on TRC volume and Na+ flux. J Gen Physiol. 2005a;125:569–585. doi: 10.1085/jgp.200409213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan THT, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J Gen Physiol. 2005b;125:587–600. doi: 10.1085/jgp.200509264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan THT, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 2004;558:147–159. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan THT, DeSimone JA. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem Senses. 2005c;30(Suppl 1):i42–i43. doi: 10.1093/chemse/bjh104. [DOI] [PubMed] [Google Scholar]
- Lyall V, Phan THT, Mummalaneni S, Mansouri M, Heck GL, Kobal G, DeSimone JA. Effect of nicotine on chorda tympani responses to salty and sour stimuli. J Neurophysiol. 2007;98:1662–1674. doi: 10.1152/jn.00366.2007. [DOI] [PubMed] [Google Scholar]
- Maillard LC. Réaction de Maillard. Action des acides aminés sur les sucres: formation des mélanoïdines par voie méthodique. Compte-rendu de l'Académie des sciencestome. 1912;154:66–68. [Google Scholar]
- Martin S, Pangborn RM. Taste interaction of ethyl alcohol with sweet, salty, sour and bitter compounds. J Sci Food Agric. 2006;21:653–655. doi: 10.1002/jsfa.2740211213. [DOI] [PubMed] [Google Scholar]
- Masuyama G, Miura S. Hand book of sensory evaluation for industry 14-39. Tokyo (Japan): Union of Japanese Scientists and Engineers Press; 1963. [Google Scholar]
- Ogasawara M, Katsumata T, Egi M. Taste properties of Maillard-reaction products prepared from 1000 to 5000 Da peptide. Food Chem. 2006a;99:600–604. [Google Scholar]
- Ogasawara M, Katsumata T, Yamada Y, Tokunaga C, Egi M. Maillard reacted peptide, the taste enhancer that increase the intensity of mouthfulness and continuity in food. Jpn J Taste Smell Res. 2006c;13:281–282. [Google Scholar]
- Ogasawara M, Yamada Y, Egi M. Taste enhancer from the long-term ripening of miso (soybean paste) Food Chem. 2006b;99:736–741. [Google Scholar]
- Ossebaard CA, Smith DV. Effect of amiloride on the taste of NaCl, Na-gluconate and KCl in humans: implications for Na+ receptor mechanisms. Chem Senses. 1995;20:37–46. doi: 10.1093/chemse/20.1.37. [DOI] [PubMed] [Google Scholar]
- Rhyu M, Ogasawara M, Egi M, Phan THT, DeSimone JA, Heck GL, Lyall V. Effect of Maillard peptides (MPs) on TRPV1 variant salt taste receptor (TRPV1t) [abstract] Chem Senses. 2006;31:A105. [Google Scholar]
- Riera CE, Vogel H, Simon SA, le Coutre J. Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R626–R634. doi: 10.1152/ajpregu.00286.2007. [DOI] [PubMed] [Google Scholar]
- Ruiz C, Gutknecht S, Delay E, Kinnamon S. Detection of NaCl and KCl in TRPV1 knockout mice. Chem Senses. 2006;31:813–820. doi: 10.1093/chemse/bjl024. [DOI] [PubMed] [Google Scholar]
- Shizuyuki O. Salt and other sensing taste compounds. In: Harada Hiroshi., editor. Knowledge of reducing salt. Japan: Saiwai Book Co., Ltd; 1993. p. 72. [Google Scholar]
- Simon SA, De Araujo IE. Commentary: the salty and burning taste of capsaicin. J Gen Physiol. 2005;125:531–534. doi: 10.1085/jgp.200509329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldo T, Blank I, Hofmann T. (+)-(S)-Alapyridaine—A general taste enhancer? Chem Senses. 2003;28:371–379. doi: 10.1093/chemse/28.5.371. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RE, DeSimone JA, Hill DL. New perspectives in a gustatory physiology: transduction, development, and plasticity. Am J Physiol. 1997;272:C1–C26. doi: 10.1152/ajpcell.1997.272.1.C1. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1799–R1809. doi: 10.1152/ajpregu.00587.2006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kaminski NE, Wang DH. Endocannabinoid regulates blood pressure via activation of the transient receptor potential vanilloid type 1 in Wistar rats fed a high-salt diet. J Pharmacol Exp Ther. 2007;321:763–769. doi: 10.1124/jpet.106.112904. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47:609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. Basic properties of umami and effects on humans. Physiol Behav. 1991;49:833–841. doi: 10.1016/0031-9384(91)90192-q. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.