Abstract
Recent studies of nonneutropenic patients with candidemia or candidiasis suggest that fluconazole pharmacodynamic parameters correlate with clinical outcomes; however, additional data of correlation to mortality in patients with candidemia would be valuable. We assessed the impact of MICs for Candida, fluconazole pharmacodynamics, and patient characteristics on all-cause mortality with use of a prospective cohort of 96 hospitalized patients with candidemia. Among 84 patients for whom Candida isolates were available for testing, the most frequent Candida species isolated were Candida albicans (44%), followed by Candida parapsilosis (20.2%), and Candida glabrata (20.2%). Fluconazole resistance (MIC of ≥64 μg/ml) was present in 7 (8.3%) to 10 (11.9%) of 84 isolates, depending on the MIC endpoint determination method (50% or 80% inhibition read at 24 or 48 h). Overall mortality occurred in 27 (28.1%) of 96 patients, and nonsurvivors were more likely to have fluconazole-resistant isolates (25% versus 6.7%; P = 0.02). Multivariable analysis demonstrated an association between fluconazole resistance and mortality, but it did not reach statistical significance (odds ratio, 5.3; 95% confidence interval, 0.8 to 33.4; P = 0.08). By pharmacodynamic analysis, a fluconazole area under the concentration-time curve/MIC of <11.5 or MIC of ≥64 was associated with increased patient mortality (P ≤ 0.09). These data support previous findings of an antifungal exposure-response relationship to mortality in patients with candidemia. In addition, similar MICs were obtained using a 24- or 48-h MIC endpoint determination, thus providing the opportunity to assess earlier the impact of isolate susceptibility on therapy.
Fluconazole has been a recommended treatment option for patients with invasive candidiasis and candidemia because of its proven safety and efficacy (20, 23). However, because of an increase in non-albicans Candida species and associated fluconazole resistance in certain Candida species, specifically Candida glabrata and Candida krusei, the treatment of candidemia has become more challenging.
As there has been increased awareness of Candida infections and their impact on morbidity and mortality in the past decade (23), there has also been the development of a reliable, standardized, methodology to test antifungal susceptibility of Candida isolates to fluconazole (15). For any particular susceptibility test, the MIC will give information under fixed, laboratory conditions; but other important factors, especially drug dosage, distribution, and elimination, are valuable in addition to MICs for correlating treatment to outcomes. The field of study that considers the relationship among MIC, antimicrobial pharmacokinetics, and treatment outcome is pharmacodynamics.
Multiple nonclinical experiments have demonstrated that the ratio of the 24-h free-drug area under the plasma concentration-time curve (AUC) to the MIC (AUC/MIC) is the pharmacodynamic index predictive of triazole efficacy (3, 13). Moreover, the 24-h AUC/MIC target of >25 has been associated with efficacy in animal models of Candida albicans infection (2, 3, 12, 13). Analysis of clinical outcomes in patients with mucosal candidiasis suggests that a fluconazole AUC/MIC target near 25 is similarly predictive of a favorable outcome in patients (4, 5, 10, 11, 15, 22). Importantly, these pharmacodynamic data are supportive of the CLSI fluconazole susceptibility breakpoints and the creation of the susceptible dose-dependent category. More recent studies of nonneutropenic patients with candidemia suggest that this pharmacodynamic target also correlates with mortality and therapeutic success for systemic infection (6, 7, 16). A concern that remains is the paucity of data on the fluconazole MIC and its correlation to mortality among patients with candidemia. Although there are adequate data on the relation of the fluconazole MIC to clinical response, additional data of a correlation to mortality would be valuable (16, 19, 21, 24, 25). Herein, with the use of a prospective, observational study of patients with candidemia, we assess the impact of the MIC for Candida, fluconazole pharmacodynamics, and other patient characteristics on all-cause mortality. In addition, using CLSI methodology, this study attempts to compare the performance of the recently approved 24-h MIC reading endpoint with that of the previously approved 48-h endpoint for prediction of patient mortality.
(This work was presented in part at the 44th Annual Meeting of the Infectious Diseases Society of America, 12 to 15 October 2006, Toronto, Canada.)
MATERIALS AND METHODS
Clinical data.
Patients were identified from a prospective, observational study of 119 consecutive adult patients with candidemia that was conducted at University Hospital in Birmingham, AL, from 15 July 2002 to 15 July 2003 (18). For the current study, patients were included if they received initial monotherapy with fluconazole for 3 days or more and had mortality data available (96 patients). Data collected included demographics, Candida species, fluconazole MICs, fluconazole dose, and outcome (all-cause mortality at 6 weeks after first positive culture for Candida). Other data, including information on factors that previous studies suggested potentially impact mortality, were collected on underlying diseases, Acute Physiology and Chronic Health Evaluation (APACHE II) score at the time of drawing blood for culture, time to administration of fluconazole, location in the intensive care unit, Candida colonization, and previous surgery or antifungal use (8, 9). Fluconazole doses ranged from 200 to 800 mg daily and were determined by the primary healthcare providers or consulting physicians and pharmacists. Neutropenia was defined as ≤500 neutrophils per mm3. Time to fluconazole administration was defined as the number of days from the first blood sample positive for yeast to the administration of fluconazole.
Susceptibility testing.
Susceptibility to fluconazole was evaluated by a broth microdilution method per CLSI document M27-A2 (15). MIC endpoints were determined after incubation at both 24 and 48 h at 35°C. MICs of fluconazole were measured visually at two endpoints and were based on the concentration that produced a 50% or 80% (MIC2450, MIC2480, MIC4850, and MIC4880, respectively) inhibition in growth compared to that of the drug-free control. Candida parapsilosis (ATCC 22019) and C. krusei (ATCC 6258) organisms were included with each testing for quality control. All isolates were run in duplicate. Susceptibility to fluconazole was defined as an MIC of ≤8μg/ml; susceptible-dose dependent (S-DD) was defined as an MIC of 16 to 32 μg/ml; and resistance to fluconazole was defined as an MIC of ≥64 μg/ml.
Analyses of factors associated with patient outcome.
Frequencies of categorical variables and means, medians, and standard deviations of continuous variables were calculated. Univariate analyses were performed using chi-square or Fisher's exact methods for categorical variables and a student's t test or the Wilcoxon rank sum test for continuous variables. To assess the association of factors to mortality, univariate and multivariable logistic regression analysis was used. All variables significant at a P value of <0.20 in univariate analyses were included as possible predictor variables in the models in addition to time to fluconazole administration. APACHE II score (1-point intervals) and time to fluconazole administration (days) were entered into the final models as continuous variables. Using classification and regression tree (CART) analysis as implemented in the SYSTAT program (version 11.0; SYSTAT Software, Inc., Richmond, CA), categorical breakpoint values that identified the greatest difference in probability for mortality for each continuous independent variable were assessed, and any such categorical variables were evaluated as part of the logistic regression analysis. However, the CART breakpoint value for the APACHE II score was not placed in the final model as a categorical variable due to the small number of patients and model instability. One final logistic regression model, using stepwise regression, was run with an MIC endpoint variable that showed the strongest association with mortality. Model goodness-of-fit was determined with use of the Hosmer-Lemeshow statistic, and the final model fit the data well. The ability of the different MIC endpoints to agree in detection of fluconazole resistance among the isolates was measured using the kappa statistic. Statistical tests were two tailed and were performed using a 0.05 significance level. Statistical analyses, excluding CART analyses, were conducted using SAS (version 9.1; SAS Institute, Inc., Cary, NC).
Pharmacodynamic modeling and outcome.
We examined the relationship among fluconazole exposure, MIC for the organism, and mortality in patients with candidemia using univariate logistic regression and nonlinear regression (using a Hill-type model). We considered both the 24-h and 48-h MIC endpoints. Fluconazole exposure was expressed as the ratio of the 24-h free-drug AUC/MIC. Free-drug AUC values were derived for each patient using the daily dose received, a point estimate for fluconazole clearance (0.96 ml/min), and protein binding (12%) (16). MICs and AUC/MICs were evaluated both as continuous and categorical variables. CART analysis was used to identify categorical breakpoint values that identified the greatest difference in probability for patient survival for each of these measures. All pharmcodynamic analyses, including evaluations using CART, were conducted using SYSTAT (version 11.0; SYSTAT Software, Inc., Richmond, CA).
RESULTS
Clinical characteristics.
Ninety-six patients were identified who received initial fluconazole monotherapy for ≥3 days and for whom mortality data were available. The mean age of patients was 51.9 years; 52% were male, and 58% were Caucasian. The overall mean APACHE II score was 16, and it was greater in nonsurvivors than survivors (22 versus 13.7; P < 0.001). Common characteristics among the study patients are listed in Table 1 and included use of central venous catheters (89.6%), location in an intensive care unit (43.8%), malignancy (22.9%), diabetes mellitus (33.3%), previous surgery (30.2%), total parenteral nutrition (30.2%), neutropenia (11.5%), transplantation (12.5%), and previous antifungal use (23.9%). Antifungal therapy with amphotericin B formulations, caspofungin, or voriconazole after initial fluconazole dosing was given in 12 (12.5%) of 96 patients; however, no significant difference in frequency of receipt of other antifungals was present in patients infected with fluconazole-susceptible or -resistant isolates or among those who lived or died. Patients who had received antifungal therapy within 2 weeks prior to the first positive blood culture for Candida were more likely to have been infected with a fluconazole-resistant isolate (25% versus 7.5%; P = 0.053).
TABLE 1.
Characteristics of 96 patients with candidemia
| Parameter | Value for the parameter
|
P valueb | ||
|---|---|---|---|---|
| Total group (n = 96) | Survivors (n = 69)a | Nonsurvivors (n = 27)a | ||
| Mean age ± SD (yr) | 51.9 ± 15.8 | 49.7 ± 14.8 | 57.7 ± 17.1 | 0.039 |
| Age of ≥63 yr (n [%])c | 25 (26) | 12 (17.4) | 13 (48.1) | 0.002 |
| No. (%) of males sex | 50 (52) | 38 (55.1) | 12 (44.4) | 0.35 |
| No. (%) of Caucasians | 56 (58.3) | 40 (58.0) | 16 (59.3) | 0.90 |
| Mean APACHE II score (SD) | 47 (8) | 13.7 (5.8) | 22 (9.7) | 0.0002 |
| APACHE II score of >23 (n [%])c | 10 (10.4) | 2 (2.9) | 8 (29.6) | 0.0001 |
| Patient medical profile (n [%]) | ||||
| Diabetes mellitus | 32 (33.3) | 20 (29.0) | 12 (44.4) | 0.15 |
| Renal insufficiencyd | 22 (22.9) | 14 (20.3) | 8 (29.6) | 0.33 |
| Neutropeniae | 11 (11.5) | 10 (14.5) | 1 (3.7) | 0.14 |
| Transplantation | 12 (12.5) | 7 (10.4) | 5 (18.5) | 0.27 |
| Malignancy | 22 (22.9) | 19 (27.5) | 3 (11.1) | 0.09 |
| ICU locationf | 42 (43.8) | 24 (34.8) | 18 (66.7) | 0.005 |
| Previous surgeryg | 29 (30.2) | 19 (27.5) | 10 (37.0) | 0.36 |
| Intubation | 33 (34.4) | 18 (26.0) | 15 (55.6) | 0.006 |
| Central venous catheter | 86 (89.6) | 61 (88.4) | 25 (92.3) | 0.54 |
| Total parenteral nutrition | 29 (30.2) | 20 (29.0) | 9 (33.3) | 0.68 |
| Candida colonization | 42 (43.8) | 24 (34.8) | 18 (66.7) | 0.005 |
| Previous antifungal treatmenth | 23 (23.9) | 16 (23.2) | 7 (25.9) | 0.78 |
| Fluconazole resistance (no. of resistant isolates/no. in group [%])i | ||||
| MIC2450 | 7/84 (8.3) | 3/60 (5.0) | 4/24 (16.7) | 0.08 |
| MIC2480 | 8/84 (9.5) | 4/60 (6.7) | 4/24 (16.7) | 0.16 |
| MIC4850 | 10/84 (11.9) | 4/60 (6.7) | 6/24 (25.0) | 0.02 |
| MIC4880 | 10/84 (11.9) | 4/60 (6.7) | 6/24 (25.0) | 0.02 |
| Time to fluconazole treatment (mean no. of days [SD])j | 1.59 (1.2) | 1.5 (1.3) | 1.8 (1.1) | 0.3 |
| Mortality (no. of nonsurvivors/total no. in group [%])k | 27/96 (28.1) | |||
Mortality data were available for 96 patients.
P values were computed with chi-square or Fisher's exact testing for categorical variables and a student's t test for the variable age.
Based on CART analysis breakpoint value (boldface values).
Defined as serum creatinine of >2.0 mg/dl.
Defined as absolute neutrophil count of <500 at time of culture.
Location at time blood culture was drawn. ICU, intensive care unit.
Surgery within previous 1 month prior to culture.
Receipt of a systemic antifungal agent within 2 weeks prior to first positive Candida blood culture.
Isolates from only 84 of the 96 patients were available for susceptibility testing. Fluconazole was defined as a MIC of ≥64 μg/ml. MICs were read visually after 24 h or 48 h of incubation and determined as 20% (MIC2480, MIC4880) or 50% inhibition (MIC2450, MIC4850) compared with growth control.
Defined as time from culture to start of fluconazole (mean days). Comparison is by a Wilcoxon rank sum test.
Mortality was defined as all-cause death at 6 weeks after first positive Candida blood culture.
All-cause mortality at 6 weeks after the first positive Candida blood culture occurred in 27 (28.1%) of 96 patients. Mortality was higher in patients infected with C. albicans than non-albicans species (35.7% versus 22.2%; P = 0.14). Overall, mortality was highest with C. krusei (40%), followed by C. albicans (35.7%), C. parapsilosis (26.4%), Candida tropicalis (20%), and C. glabrata (15.8%). On univariate analysis, nonsurvivors were more likely to be located in the intensive care unit (66.7% versus 34.8%; P = 0.005), intubated at the time of positive Candida culture (55.6% versus 26%; P = 0.006), and colonized with Candida species (66.7% versus 34.8%, P = 0.005); they were more likely than survivors to have APACHE scores of >23 (29.6% versus 2.9%, P = 0.0001) (Table 1).
Candida species susceptibilities.
Of the 96 study patients, 84 (87.5%) had isolates available for susceptibility testing. Among these 84 patients, the most frequent cause of candidemia was C. albicans (44%), followed by C. parapsilosis (20.2%), C. glabrata (20.2%), C. tropicalis (11.9%), and C. krusei (2.3%). Fluconazole resistance (MIC of ≥64 μg/ml) was present in 7 (8.3%, MIC at 24 h) to 10 (11.9%; MIC at 48 h) of 84 isolates, depending on the MIC reading endpoint (Table 1). Fluconazole susceptibility (MIC of ≤ 8μg/ml) was present in 69 (82.1%, MIC at 24 h) to 74 (88%, MIC at 48 h) of 84 isolates, whereas 2 (2.4%, MIC at 24 h) to 5 (6.0%, MIC at 48 h) of 84 isolates were determined to be S-DD (MIC of 16 to 32 μg/ml). Overall, the four different MIC determination methods were consistent with each other in identifying fluconazole resistance (kappa coefficients ranging from 0.80 to 0.88). Resistance was most common among C. krusei (2/2, 100%) and C. glabrata (4/17, 23.5%; MIC50 of 8 μg/ml; MIC90 of 64 μg/ml) isolates. Nonsurvivors were more likely than survivors to have fluconazole-resistant isolates (at all MIC endpoints) (Tables 1 and 2), but this was significant only at the 48-h endpoints (25% versus 6.7%; P = 0.02). This difference at the time points was explained by the death of two patients whose isolates were determined to be susceptible at 24 h but resistant at 48 h. Overall mortality was 25.4% (18/71), 20% (1/5), and 60% (6/10) for patients with fluconazole-susceptible, S-DD, and resistant isolates, respectively (MIC4850) (Table 2).
TABLE 2.
Relationship of MIC to mortality in patients with candidemia
| MIC (μg/ml) by susceptibility levela | Mortality at the indicated MIC (no. of nonsurvivors/total no. in group [%])b
|
|||
|---|---|---|---|---|
| MIC2450 | MIC2480 | MIC4850 | MIC4880 | |
| Susceptible | ||||
| 0.125 | 5/20 (25) | 2/5 (40) | 3/9 (33.3) | 1/3 (33.3) |
| 0.25 | 4/21 (19) | 3/20 (15) | 3/18 (16.7) | 2/12 (16.7) |
| 0.5 | 6/15 (40) | 5/21 (23.8) | 5/22 (22.7) | 5/26 (31) |
| 1 | 0/1 (0) | 5/11 (45.5) | 1/4 (25) | 2/9 (22.2) |
| 2 | 2/8 (25) | 1/1 (100) | 0/2 (0) | 2/2 (50) |
| 4 | 3/8 (37.5) | 1/12 (8.3) | 3/11 (27.3) | 1/9 (11.1) |
| 8 | 0/1 (0) | 3/4 (75) | 3/5 (60) | 4/6 (66.7) |
| Total | 20/74 (27) | 20/74 (27) | 18/71 (25) | 17/69 (24.6) |
| S-DD | ||||
| 16 | 0 | 0 | 0/1 (0) | 1/3 (33.3) |
| 32 | 0/3 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) |
| Total | 0/3 (0) | 0/2 (0) | 0/3 (0) | |
| Resistant (64) | 4/7 (57.1) | 4/8 (50) | 6/10 (60) | 6/10 (60) |
Susceptible, MIC of ≤8 μg/ml; S-DD, MIC of 16 to 32 μg/ml; resistant, MIC of ≥64 μg/ml.
MICs were read visually after 24 h or 48 h of incubation and determined at 50% or 80% inhibition (MIC2450, MIC2480, MIC4850, and MIC4880, respectively) compared with growth control. Eighty-four patient isolates were included.
Independent factors related to mortality.
A multivariable logistic regression model was constructed that included age, gender, race, cancer, neutropenia, intensive care unit location, intubation, APACHE II score, Candida colonization, time to fluconazole administration, and fluconazole resistance (MIC4850). For the variable age, ≥63 years was used based on a breakpoint determined by CART analysis. Age of ≥63 years (odds ratio [OR], 8.9; 95% confidence interval [CI], 2.0 to 38.9; P = 0.004) and APACHE II (OR, 1.2; 95% CI, 1.03 to 1.3; P = 0.01) were independently associated with mortality (Table 3). Having a fluconazole-resistant isolate was associated with mortality (ORs, 1.8 to 5.0), with 48-h MIC readings showing the strongest association (OR, 5.3; 95% CI, 0.8 to 33.4; P = 0.08). However, these associations did not reach statistical significance in the final model.
TABLE 3.
Analysis of factors related to mortality
| Variable | Univariate analysisa
|
Multivariate analysisa
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age of >63b | 4.4 | 1.7-11.7 | 0.003 | 8.9 | 2.0-38.9 | 0.004 |
| Male sex | 0.7 | 0.3-1.6 | 0.35 | |||
| White race | 1.1 | 0.4-2.6 | 0.90 | |||
| APACHE IIc | 1.2 | 1.08-1.3 | 0.0002 | 1.2 | 1.03-1.3 | 0.01 |
| Malignancy | 0.3 | 0.09-1.2 | 0.10 | |||
| Neutropeniad | 0.2 | 0.03-1.9 | 0.17 | |||
| Intubated | 3.5 | 1.4-9.0 | 0.008 | |||
| Candida colonization | 3.8 | 1.5-9.6 | 0.006 | |||
| ICU locatione | 3.8 | 1.5-9.6 | 0.006 | |||
| Time to fluconazolef | 1.2 | 0.8-1.8 | 0.31 | |||
| Fluconazole resistant (MIC4850)g | 4.7 | 1.2-18.4 | 0.03 | 5.3 | 0.8-33.4 | 0.08 |
For multivariate logistic regression analysis, variables with P of <0.20 on univariate analysis were included in a stepwise regression model in addition to variables for age, race, sex, and time to fluconazole administration. When a continuous variable was significantly related to mortality as such or as a categorical variable, the most significant of these variables was evaluated by logistic regression analysis. One MIC (MIC4850) was chosen for the final model based on strength of association with mortality. The best model fit was with the values of the MIC4850 or MIC4880, which showed identical results. Results are listed for significant variables (P < 0.05) and the MIC variable. P values obtained are two tailed.
The value 63 years was based on breakpoint from CART analysis.
At time of culture as acontinuous variable (1-point increments).
Defined as absolute neutrophil count of <500 at time of culture.
Location at time blood culture was drawn. ICU, intensive care unit.
Defined as time from culture to start of fluconazole (days).
Fluconazole resistance was defined as a MIC of ≥64 μg/ml.
Pharmacodynamic analyses.
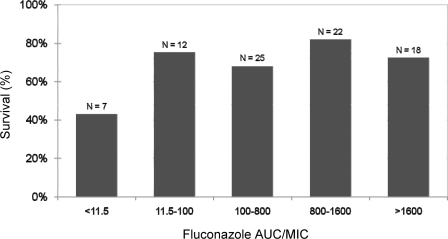
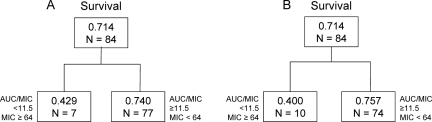
Among 84 patients with isolates available for susceptibility testing, 60 (71%) survived. The majority of patients (n = 81) received daily fluconazole doses of 400 mg. The remaining three patients received 200 mg every 24 h. The MIC2450 and MIC4850 values ranged from 0.125 to 64 μg/ml. The MIC50 and MIC90 values of 0.5 and 32 μg/ml at 24-h and 0.5 and 64 μg/ml at 48-h endpoints were very similar (Spearman's rank correlation coefficient, 0.91); MIC2450 values were within 1 dilution of MIC4850 values for 90% of paired observations. The median (range) for AUC/MICs, based on either MIC2450 or MIC4850 values, was 733 (5.72 to 2,933). With the exception of seven patients for whom the AUC/MICs (based on MIC2450 values) were 5.73 and among whom the survival rate of 42.9%, the percentage of patients surviving among groups ranked by increasing AUC/MICs of ≥11.5 was reasonably similar (68.0 to 81.8%) (Fig. 1). As shown in Fig. 2, CART analysis revealed breakpoints for survival for both the AUC/MIC and MIC of 11.5 and 64 μg/ml, respectively. For MIC2450 values, 74.0% (57/77) of patients survived when the AUC/MIC or MIC values were at or above these thresholds. Below these thresholds, 42.9% (3/7) of patients survived. Similar results were evident for MIC4850 values; 75.7% (56/74) of patients survived at or above each of these thresholds while only 40% (4/10) of patients survived below these thresholds. Note that in units of dose/MIC, an AUC/MIC ratio of 11.5 translated to a dose/MIC ratio of 12.5.
FIG. 1.
Relationship between the fluconazole 24-h AUC/MIC and survival in patients with candidemia (n = 84).
FIG. 2.
Results of CART analysis for AUC/MIC and MIC values associated with survival based on MICs assessed at 24 (A) and 48 (B) h. Within each box, the proportion of patients surviving and the sample size (N) are shown.
Univariate logistic regression revealed significant associations for each of these categorical variables. As shown in Table 3, for patients infected with isolates demonstrating fluconazole resistance, which is defined by the same MIC as identified by the CART analysis (64 μg/ml), the OR was 4.7 (95% CI, 1.2 to 18.4; P = 0.03) for categorical MIC and AUC/MIC variables based on the MIC at 48 h.
DISCUSSION
Fluconazole remains an effective drug for the treatment of invasive candidiasis and candidemia, but its use has been complicated by emerging non-albicans Candida species and associated azole resistance (17, 23). With the advent of standardized methodology to test antifungal susceptibility of Candida isolates to fluconazole, a variety of in vitro, animal model, and clinical studies have demonstrated the impact of the MIC on treatment outcome (1, 3, 7, 11, 14, 21, 24). More recently, studies of the impact of the MIC have also begun to consider the influence of exposure to drugs on patient outcome. Antimicrobial pharmacodynamics involves the evaluation of relationships among the MIC, drug exposure, and outcome. In vitro and animal model studies with antifungals from the triazole class have consistently shown that the total drug exposure or AUC/MIC is the pharmacodynamic index that is associated with treatment effect (1). Subsequent investigations have demonstrated that the amount of triazole or the pharmacodynamic target needed for efficacy is a 24-h free-drug AUC/MIC near 25 (1, 3, 12). This value can be thought of as the in vivo MIC and is essentially the same as averaging the MIC over a 24-h period.
Recently, in human studies, there have been important reports of a correlation of fluconazole pharmacodynamic exposures to therapeutic success (7, 11, 16, 21, 24). In a retrospective analysis of the MIC data set used for the development of susceptibility breakpoints, it became clear that a fluconazole dose/MIC ratio near 25 (or a value near an AUC/MIC of 25) was associated with clinical success (21). The majority of this data set included patients with mucosal candidiasis; however, for the 108 patients that had candidemia, the relationship was similar. More recently, small data sets have provided additional clinical information allowing analysis of fluconazole pharmacodynamics with respect to invasive candidiasis and candidemia (7, 11, 16). Clancy and colleagues reported the relationship between fluconazole dose, MIC, and outcome in 32 patients with candidemia, and overall, patients infected with isolates for which the MICs were elevated fared worse than those infected with susceptible organisms (7). All 6 patients infected with fluconazole-resistant (MIC of ≥64 μg/ml at 48 h) isolates had therapeutic failure, while among 21 patients infected with fluconazole-susceptible (MIC of ≤8 μg/ml) isolates, 14 (67%) had therapeutic success. The clinical data set also include the fluconazole dose level, and the same correlation was found for the dose/MIC ratio and therapeutic response. Therapeutic response was significantly greater for a 48-h fluconazole dose/MIC ratio of >50 than for a ratio of ≤50 (74% versus 8%, P = 0.0003) (7).
In a contemporary cohort, Lee et al. examined data from 32 human immunodeficiency virus-negative patients with systemic Candida infection treated with fluconazole (11). This patient group included 22 bloodstream infections and 10 other patients with peritonitis, pyelonephritis, or pulmonary infection. Of 32 patients, 8 (25%) were infected with organisms for which the MIC was ≥32 μg/liter. With the fluconazole dosing (400 mg/day) used in these patients, the 24-h AUC/MIC was below 20 for all of these patients, and treatment failed in 75%. In the remaining cases, the 24-h AUC/MIC was above 20, and patients received successful treatment in most cases (79%).
Pai and colleagues evaluated the fluconazole pharmacodynamic relationship in 77 patients with candidemia but focused on the endpoint of mortality at hospital discharge instead of therapeutic success (16). Only 2 (2.5%) of 77 isolates were fluconazole resistant, but an increase in mortality was found in patients for whom the 24-h AUC/MIC ratios were lower (0 to 15), when controlling for time to initiation of fluconazole therapy (P = 0.09). When the analysis of mortality was stratified by the 24-h fluconazole dose/MIC ratio, mortality declined significantly with increased ratios (P = 0.03); however, a significant correlation between mortality and the 48-h fluconazole dose/MIC ratio assessed as a continuous variable was not found. CART analysis demonstrated that a fluconazole dose/24-h MIC ratio of 12 was significantly associated with mortality (P = 0.007), which is consistent with the dose/MIC threshold of 11.5 based on the analyses described herein.
Most recently, Rodriquez-Tudela and colleagues evaluated the correlation of outcomes between fluconazole MIC and the dose/MIC ratio for patients with mucosal candidiasis (110 episodes) and candidemia (126 episodes) using the EUCAST standard (24). The outcome of interest was cure or resolution of infection. Overall, for those infected with strains for which the MIC was 4 mg/liter, the response was 66%, whereas the cure rate was only 12% for those infected with isolates for which the MICs were ≥8 mg/liter. Moreover, the cure rate was increased in patients for whom the dose/MIC ratio was ≥100 compared to those for whom the ratio was less (93.9% versus 14.6%). CART analysis indicated that a breakpoint of 35.5 best separated groups into those cured or not (24). This value is higher than the breakpoint value among our patients and may be related to different study populations (ours with candidemia only), the different outcome endpoint, or differences in susceptibility methodologies.
These studies, although small and with few fluconazole-resistant isolates, described therapeutic failure and increased mortality in patients infected with fluconazole-resistant isolates compared to those infected with fluconazole-susceptible isolates. Moreover, consideration of the pharmacokinetics and pharmacodynamics of fluconazole provided the tools to demonstrate the relevance of the MIC even though the data set contained very few resistant isolates. The current study provides critical corroborative data demonstrating a correlation between AUC/MIC and MIC and all-cause mortality at 6 weeks.
Although these important pharmacodynamic relationships have recently been well defined, outcomes among patients with invasive candidiasis and candidemia are often dependent on many factors, including MICs, severity of illness, underlying conditions, and timing of antifungal therapy, among other things (4, 5, 9, 21). Our study aimed to address these other factors and MICs as potential predictors of mortality. Important factors related to increased mortality were identified, including older age and APACHE II score. CART analysis indicated that the age of 63 years or greater best separated the groups who died or did not. Regardless of method of MIC endpoint determination, infection with a fluconazole-resistant isolate was associated with increased mortality. The appropriateness of the susceptibility breakpoint for fluconazole resistance was further supported by the results of the CART analysis, which identified a threshold of 64 μg/ml to be significantly associated with survival. After multivariable analysis, the association with a 48-h MIC endpoint remained strong (OR, 5.4) but failed to be a significant independent predictor. We suspect that lack of significance was probably related to insufficient power. In the final model, the timing of fluconazole administration was not significantly related to mortality (OR, 1.5; 95% CI, 0.9 to 2.8; P = 0.13). However, the association with mortality was similar to that seen in the recent study by Garey and colleagues, where the adjusted OR was 1.5, and this proved to be a significant predictor of mortality. The lack of independence as a predictor of mortality in our cohort may be underestimated because of the small sample size.
This study was also able to evaluate the agreement of multiple endpoint determinations and their impact on mortality. The new CLSI reference method for antifungal susceptibility testing of yeasts will provide the opportunity to read MIC endpoints at 24 h instead of 48 h. In our analysis, overall agreement of the four endpoint determinations was good, with differences in only a few resistant isolates at 24 and 48 h. However, the strongest association of fluconazole resistance to mortality was found by using the MIC endpoint of 48 h, read either at 50% or 80% inhibition, compared to growth control. The small number of organisms for which the MICs and outcomes were different does not provide convincing evidence to suggest a clinically meaningful impact; nevertheless, future studies should continue to examine the clinical relevance of these endpoint determinations.
Our study has several limitations that should be considered. Although this represents a large cohort of patients with candidemia, only 10 isolates were fluconazole resistant, perhaps limiting the multivariable analysis. If this data set is considered in aggregate with other studies of invasive candidiasis examining the impact of MIC and dose on outcome, there are now data for more than 425 patients suggesting a similar relationship. There are possible limitations in our ability to accurately estimate fluconazole pharmacokinetics since factors such as patient weight were not available and thus may have impacted our values. The fluconazole AUC estimates were based upon a point estimate of clearance in patients with normal renal function. Given that some patients in this evaluation had reduced renal function (as is commonly the case in critically ill patients) and that the individual variability in fluconazole clearance could not be considered, it is likely that we underestimated the fluconazole exposure in some patients. The lack of ability to account for these factors may help explain why the pharmacodynamic target identified in this analysis is of a somewhat lower magnitude than targets previously identified based on preclinical and available clinical pharmacodynamic analyses (1, 3, 7, 12, 16). However, in nearly all clinical scenarios fluconazole kinetics are not measured, and dosing in adults is rarely, if ever, based upon weight. The strength of the associations despite this limitation suggests a “real-life” value to the consideration of fluconazole dose and the MIC for Candida in this disease state.
Although only 12 patients received other antifungals after initial fluconazole dosing and although these agents were balanced among patients who lived or died, the exact impact is unknown. Finally, the mortality endpoint at 6 weeks after the first positive culture for Candida is different from that of the study by Pai and colleagues, in which mortality at discharge was the endpoint. We are unable to determine if our findings would be similar at other mortality or efficacy endpoints. Despite the above-described limitations, we were encouraged by the general degree of concordance between the pharmacodynamic targets described herein and previous findings.
In summary, we have demonstrated the association between patient characteristics, MICs for Candida, fluconazole pharmacodynamics, and mortality among hospitalized patients with candidemia. These studies suggest that a clinician-controlled variable, fluconazole dose, may impact individual patient survival. Larger, prospective studies in patients with candidemia are needed to confirm the pharmacodynamic relationships observed herein. Careful attention to important host factors, fluconazole dose, and MICs may be helpful in managing and optimizing outcomes in such patients.
Acknowledgments
We thank Anita Smith for managing the isolate collection and performing susceptibility testing.
J.W.B. receives grant support from Merck, Inc., and Astellas Pharma, Inc.; provides consulting services for Pfizer, Inc., and Enzon; and serves on the speaker's bureau for Enzon, Merck, Inc., and Schering-Plough. D.R.A. receives research support from Astellas, Pfizer, Schering, and Enzon; serves on the speaker's bureau for Pfizer, Astellas, and Schering; and provides advisory services for Pfizer, Schering, Astellas, and Merck. M.P., S.B., and S.A.M. have no conflicts of interest.
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Andes, D. 2006. Pharmacokinetics and pharmacodynamics of antifungals. Infect. Dis. Clin. N. Am. 20:679-697. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., and M. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arikan, S., M. Akova, M. Hayran, O. Ozdemir, M. Erman, D. Gur, and S. Unal. 1998. Correlation of in vitro fluconazole susceptibility with clinical outcome for severely ill patients with oropharyngeal candidiasis. Clin. Infect. Dis. 26:903-908. [DOI] [PubMed] [Google Scholar]
- 5.Baddley, J. W., M. Patel, M. Jones, G. Cloud, A. C. Smith, and S. A. Moser. 2004. Utility of real-time antifungal susceptibility testing for fluconazole in the treatment of candidemia. Diagn. Microbiol. Infect. Dis. 50:119-124. [DOI] [PubMed] [Google Scholar]
- 6.Clancy, C. J., B. Staley, and M. H. Nguyen. 2006. In vitro susceptibility of breakthrough Candida bloodstream isolates correlates with daily and cumulative doses of fluconazole. Antimicrob. Agents Chemother. 50:3496-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy, C. J., V. L. Yu, A. J. Morris, D. R. Snydman, and M. H. Nguyen. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49:3171-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo, A. L., T. Guimaraes, L. R. Silva, L. P. de Almeida Monfardini, A. K. Cunha, P. Rady, T. Alves, and R. C. Rosas. 2007. Prospective observational study of candidemia in Sao Paulo, Brazil: incidence rate, epidemiology, and predictors of mortality. Infect. Control Hosp. Epidemiol. 28:570-576. [DOI] [PubMed] [Google Scholar]
- 9.Garey, K. W., M. Rege, M. P. Pai, D. E. Mingo, K. J. Suda, R. S. Turpin, and D. T. Bearden. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25-31. [DOI] [PubMed] [Google Scholar]
- 10.Kovacicova, G., Y. Krupova, M. Lovaszova, A. Roidova, J. Trupl, A. Liskova, J. Hanzen, P. Milosovic, M. Lamosova, L. Macekova, Z. Szovenyiova, A. Purgelova, T. Obertik, J. Bille, and V. Krcmery. 2000. Antifungal susceptibility of 262 bloodstream yeast isolates from a mixed cancer and non-cancer patient population: is there a correlation between in vitro resistance to fluconazole and the outcome of fungemia? J. Infect. Chemother. 6:216-221. [DOI] [PubMed] [Google Scholar]
- 11.Lee, S. C., C. P. Fung, J. S. Huang, C. J. Tsai, K. S. Chen, H. Y. Chen, N. Lee, L. C. See, and W. B. Shieh. 2000. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob. Agents Chemother. 44:2715-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie, A., G. L. Drusano, P. Banerjee, Q. F. Liu, W. Liu, P. Kaw, M. Shayegani, H. Taber, and M. H. Miller. 1998. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 42:1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louie, A., Q. F. Liu, G. L. Drusano, W. Liu, M. Mayers, E. Anaissie, and M. H. Miller. 1998. Pharmacokinetic studies of fluconazole in rabbits characterizing doses which achieve peak levels in serum and area under the concentration-time curve values which mimic those of high-dose fluconazole in humans. Antimicrob. Agents Chemother. 42:1512-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura, T., and H. Takahashi. 2006. Epidemiological study of Candida infections in blood: susceptibilities of Candida spp. to antifungal agents, and clinical features associated with the candidemia. J. Infect. Chemother. 12:132-138. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 16.Pai, M. P., R. S. Turpin, and K. W. Garey. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 51:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappas, P. G. 2006. Invasive candidiasis. Infect. Dis. Clin. N. Am. 20:485-506. [DOI] [PubMed] [Google Scholar]
- 18.Patel, M., D. F. Kunz, V. M. Trivedi, M. G. Jones, S. A. Moser, and J. W. Baddley. 2005. Initial management of candidemia at an academic medical center: evaluation of the IDSA guidelines. Diagn. Microbiol. Infect. Dis. 52:29-34. [DOI] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., D. J. Diekema, and D. J. Sheehan. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19:435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van der Horst, J. E. Edwards, R. G. Washburn, W. M. Scheld, A. W. Karchmer, A. P. Dine, et al. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]
- 21.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, A. L. Barry, et al. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 22.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, J. E. Edwards, et al. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Tudela, J. L., B. Almirante, D. Rodriguez-Pardo, F. Laguna, J. P. Donnelly, J. W. Mouton, A. Pahissa, and M. Cuenca-Estrella. 2007. Correlation of the MIC and dose/MIC ratio of fluconazole to the therapeutic response of patients with mucosal candidiasis and candidemia. Antimicrob. Agents Chemother. 51:3599-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takakura, S., N. Fujihara, T. Saito, T. Kudo, Y. Iinuma, and S. Ichiyama. 2004. Clinical factors associated with fluconazole resistance and short-term survival in patients with Candida bloodstream infection. Eur. J. Clin. Microbiol. Infect. Dis. 23:380-388. [DOI] [PubMed] [Google Scholar]