Abstract
Intracellular concentrations of isoniazid and rifabutin resulting from administration of inhalable microparticles of these drugs to phorbol-differentiated THP-1 cells and the pharmacokinetics and biodistribution of these drugs upon inhalation of microparticles or intravenous administration of free drugs to mice were investigated. In cultured cells, both microparticles and dissolved drugs established peak concentrations of isoniazid (∼1.4 and 1.1 μg/106 cells) and rifabutin (∼2 μg/ml and ∼1.4 μg/106 cells) within 10 min. Microparticles maintained the intracellular concentration of isoniazid for 24 h and rifabutin for 96 h, whereas dissolved drugs did not. The following pharmacokinetic parameters were calculated using WinNonlin from samples obtained after inhalation using an in-house apparatus (figures in parentheses refer to parameters obtained after intravenous administration of an equivalent amount, i.e., 100 μg of either drug, to parallel groups): isoniazid, serum half-life (t1/2) = 18.63 ± 5.89 h (3.91 ± 1.06 h), maximum concentration in serum (Cmax) = 2.37 ± 0.23 μg·ml−1 (3.24 ± 0.57 μg·ml−1), area under the concentration-time curve from 0 to 24 h (AUC0-24) = 55.34 ± 13.72 μg/ml−1 h−1 (16.64 ± 1.80 μg/ml−1 h−1), and clearance (CL) = 63.90 ± 13.32 ml·h−1 (4.43 ± 1.85 ml·h−1); rifabutin, t1/2 = 119.49 ± 29.62 h (20.18 ± 4.02 h), Cmax = 1.59 ± 0.01 μg·ml−1 (3.47 ± 0.33 μg·ml−1), AUC0-96 = 109.35 ± 14.78 μg/ml−1 h−1 (90.82 ± 7.46 μg/ml−1 h−1), and CL = 11.68 ± 7.00 ml·h−1 (1.03 ± 0.11 ml·h−1). Drug targeting to the lungs in general and alveolar macrophages in particular was observed. It was concluded that inhaled microparticles can reduce dose frequency and improve the pharmacologic index of the drug combination.
Several species of the genus Mycobacterium have evolved to survive in lung macrophages of the mammalian host, leading to granulomatous or latent tuberculosis (TB) (27). Chemotherapeutic regimens for TB require prolonged administration of multiple drugs through the oral route, but their ability to provide a lasting cure has not yet been established (7). We (18, 21, 29, 30, 38; H. Sen, J. Suryakumar, R. Sinha, R. Sharma, and P. Muttil, 3 September 2003, PCT patent application PCT/IB2003/004694) and others (19, 23-26, 39, 40) have investigated several aspects of respirable or inhalable microparticles for treatment of pulmonary TB, first proposed by Hickey et al. (22, 33, 34). Particles developed by our group contain equal amounts of isoniazid and rifabutin and are meant for “adjunct therapy” of pulmonary TB through uptake by alveolar macrophages harboring Mycobacterium tuberculosis and/or related species. It is also speculated that, following uptake of microparticles, uninfected macrophages could traffic to lung areas where infected cells congregate. It has been demonstrated in animal experiments that inhaled microparticles target the drug payload to alveolar macrophages (21, 30) and enhance the efficacy of the incorporated agents (33, 34; Sen et al., PCT patent application PCT/IB2003/004694).
The biopharmaceutics of both isoniazid (15, 35, 36) and rifabutin (3, 4, 31, 32), both in humans and mice, are well known, and good correlations between the two species in terms of both kinetic data and therapeutic outcome have been observed (1).
This report addresses the single-dose pharmacokinetics of isoniazid and rifabutin in polylactide microparticles administered by dry-powder inhalation to mice. Khuller et al. have extensively investigated the steady-state pharmacokinetics of various anti-TB drugs in different formulations administered to animals by nebulization (23-26, 40). Their results generally show a lag time of 3 to 6 h between administration and appearance of drugs in the blood, first-order kinetics of absorption and elimination from the blood, and sustained blood and tissue concentrations over several days. These results arise from the administration of nebulized formulations, often containing particles or vesicles in the nanometer size range. The present investigations were undertaken to establish whether a dry-powder inhalation dose of micrometer-sized particles would behave similarly. Second, single-dose pharmacokinetics following pulmonary delivery were investigated in order to suggest a dosing interval. Since the delivery system is intended for targeting lung macrophages, blood levels were of secondary interest. The principal objectives of this study were to establish the time course of intracellular concentrations in lung macrophages and concentrations in lung lumen and homogenates of remaining lung tissue (the target sites) and the livers and kidneys (primary sites of toxic manifestations).
It was also of interest to examine whether the unusually high efficacy of microparticles reported by us (Sen et al., PCT patent application PCT/IB2003/004694) may also be due to a putative “pharmacokinetic synergy” between the two agents incorporated in them. Sustained rifabutin concentrations following depletion of isoniazid have the potential to reinforce early bactericidal activity (EBA) of the therapeutic regimen and minimize emergence of drug resistance (5, 9-12, 37). The present investigation also explores whether the desired pattern of drug concentrations is observed in vivo, as suggested by drug release studies reported earlier (21).
MATERIALS AND METHODS
Microparticles containing 1 part rifabutin {(9S, 12E, 14S, 15R, 16S, 17R, 18R, 19R, 20S, 21S, 22E, 24Z)-6-16,18,20-tetrahydroxy-1′-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethylspiro[9,4-epoxypentadeca[1,11,13]trienimino)- 2H-furo[2′,3′:7,8]naphth[1,2-d]imidazole-2,-4′-piperidine]-,10,26(3H,9H)-trione,16-acetate} (USP), 1 part isoniazid (4-pyridinecarboxylic acid hydrazide) (Indian Pharmacopoeia), and two parts poly(d,l-lactic acid) with an intrinsic viscosity of 0.8 cp, as well as free drugs and analytical standards, were donated by Lupin Laboratories Research Park, Pune, India. Microparticles were prepared by spray drying and conformed to specifications suitable for administration by dry-powder inhalation (21). Cell culture media and supplements were obtained from Sigma, and dichloromethane, n-pentane, acetonitrile, methanol, and other reagents and solvents used in the study were of high-performance liquid chromatography (HPLC) or analytical grade.
Drug uptake in cell culture.
The human monocyte cell line THP-1 (National Centre for Cell Science, Pune, India) was cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and 1% antimycotic-antibiotic mixture in 96-well culture plates at 37°C, 5% CO2, and 95% relative humidity. Prior to experiments, cells seeded at a density of 106 cells/well were induced to undergo terminal differentiation into macrophages by incubation in the presence of 20 nM/ml of phorbol myristate acetate for 14 h (13). At the end of this period, the cells were washed with incomplete medium (without fetal calf serum) three times. Fresh complete medium without antimycotic-antibiotic was added to the adherent cells, and incubation continued for another 10 h.
At 24 h after addition of phorbol ester, a solution of isoniazid in normal saline or of rifabutin dissolved in dimethyl sulfoxide-saline was added to achieve a final concentration of 3 μg/ml of either drug in wells assigned. A suspension of microparticles (12 μg/ml) containing an equivalent amount of the drugs was added to separate designated wells. Drugs or microparticles were incubated with the cells for the next 2 h, after which the wells were washed five times with incomplete medium and complete medium was replaced. Cells in duplicate wells were then recovered sequentially at various time points and lysed by six cycles of freezing at −20°C for 1 h followed by thawing at room temperature. Lysates were analyzed by HPLC as described below.
Animals, dosing, and sampling.
Animal experiments were conducted after receiving consent of and under the supervision of the Institutional Animal Ethics Committee of the Central Drug Research Institute. BALB/c mice of either sex (25 to 30 g) were bred, housed, and fed ethically in the Laboratory Animals Division of the institute. The dose level selected for the study was 100 μg of each drug (∼4 to 5 mg/kg of body weight), which is about one-half the recommended daily dose. This dose was selected because the highest efficacy was observed when inhalation doses were administered to experimental animals in combination with oral dosing at one-half the recommended daily dose (Sen et al., PCT patent application PCT/IB2003/004694). A total of 120 mice were used for the study, randomly divided into groups of 4. The following experimental groups were formed. Sixty-four mice were assigned to the isoniazid group. Of these, 32 received inhalation doses, while the remaining were administered intravenous injections of 100 μg isoniazid in saline (sterile filtered) through the caudal vein. Animals were administered microparticles by dry-powder inhalation using an in-house inhalation apparatus described elsewhere (21, 29, 30; Sen et al., PCT patent application PCT/IB2003/004694). Briefly, about 10 mg of microparticles was charged into the apparatus and fluidized by 30 actuations over 30 s. Mice were restrained with their nares inserted snugly into the fluidization chamber and inhaled the fluidized material under ambient pressure. Starting at 10 min after administration of inhalation doses, sequential sets of four mice each were sampled at 0.5, 1, 2, 4, 8, 12, and 24 h. Animals were administered ketamine-xylazine for deep anesthesia, and their thoracic cavities were opened. Cardiac puncture to exsanguinate the lungs and bronchoalveolar lavage (BAL) were carried out as described elsewhere (21, 29, 30). In brief, the trachea was cannulated and the lung lobes were repeatedly inflated and lavaged with chilled saline containing 0.5 mM EDTA. Lavage fluids were pooled, and macrophages were counted using a hemocytometer. Immediately after lavage, the lungs, livers, and kidneys were harvested, the fresh weights were recorded, and the tissue was placed in 1 ml of triple-distilled water.
Fifty-six BALB/c mice were assigned to the rifabutin group, subdivided into two groups of 28 animals each as described above. Intravenous administration of rifabutin required dissolution of accurately weighed rifabutin (∼2 mg) in 200 μl dimethyl sulfoxide and making up the volume with saline to get a solution of 1 mg/ml. As before, four animals were sacrificed at time points of 10 min and 6, 12, 24, 48, 72, and 96 h.
Sample preparation for bioanalysis.
Lysates of cultured cells, blood serum, and tissue homogenates were extracted with different solvent systems. Isoniazid was extracted using the procedure of Hutchings et al. (16) with minor modifications (8). Fifty and 200 μl of a solution of 20% (wt/vol) NaCl was added to 200 μl of cell lysate or 500 μl of serum or tissue homogenate, respectively. Cell lysates were extracted with 1-ml portions of chloroform-butanol (70:30, vol/vol) and serum or tissue homogenates with 3-ml portions by vortexing for 1 min followed by centrifugation at 4,000 × g for 10 min. The supernatant was decanted, the process was repeated three times, and supernatants were pooled. Isoniazid was extracted from the organic phase with 0.5 ml of 30 mM phosphoric acid by vortex mixing for 1 min. The aqueous layer was separated by centrifugation at 4,000 × g for 10 min, and 200 μl was aspirated from the top. This was neutralized with 4 μl of 4 M KOH just before injection onto the HPLC column.
Rifabutin was extracted from 200 μl of cell lysate or 500 μl of serum and tissue homogenates. Butylated hydroxytoluene dissolved in acetonitrile was first added to the samples to achieve a final concentration of 0.1%, wt/vol, in order to minimize drug degradation (6). A solvent system comprising equal parts of dichloromethane and n-pentane was added to the samples (0.5 ml and 1 ml for the cell lysate and serum and tissue homogenates, respectively). The samples were vortex mixed for 1 min and centrifuged at 2,604 × g for 10 min. The organic layer was transferred to a glass test tube. This procedure was repeated three times, and the organic phase was pooled and vacuum dried (Heto-Holten, Denmark). Just prior to injection, the sample was reconstituted in 500 μl of methanol and filtered through a 0.22-μm filter.
HPLC.
A Shimadzu Class VP HPLC system with a Luna C18 column (5 μm, 4.6 by 250 mm; Phenomenex) was used for the analyses. Isocratic elution was carried out with different solvent systems for the two drugs. Isoniazid was eluted with 3% acetonitrile in triethylamine acetate buffer, pH 6.0, at 1 ml/min, with the detector set at 262 nm. The drug eluted at ∼6 min. Acetonitrile and 0.05 M potassium dihydrogen phosphate, pH 4.10 (55:45, vol/vol), were used to elute rifabutin at 1 ml/min, with the detection wavelength set at 275 nm. Rifabutin eluted at ∼5.5 min. Standard curves were generated by spiking known amounts of drugs for analysis of tissue, serum, and cell samples. Both methods were validated in accordance with International Conference on Harmonization guidelines, and satisfactory validation parameters were obtained (P. Muttil et al., unpublished data). Briefly, the validation parameters were as follows: linearity, 0.1 to 8 μg/ml (rifabutin) and 0.2 to 7 μg/ml (isoniazid); limits of detection and quantification, 10 and 20 ng/ml, respectively (rifabutin), and 60 and 200 ng/ml, respectively (isoniazid); inter- and intraday variation (nine quality control samples each), ±20% at 0.5 ng/ml rifabutin and 15 ng/ml isoniazid; and ±15% at 2 or 4 μg/ml of both drugs.
Data analysis.
Compartmental and noncompartment models were fitted to data obtained by HPLC analysis using WinNonlin, version 5.1. Acceptance criteria for goodness of fit of various modeling approaches, over and above minimization of Akaike's and Schwartz Bayesian criteria (AIC and SBC, respectively), were a correlation coefficient (r2) of >0.9 and coefficients of variance (CV) of <25% for each individual pharmacokinetic parameter. Arithmetic means and standard deviations are plotted in concentration-time curves.
Compartment modeling was first attempted for concentration-time data obtained in various experiments. If the acceptance criteria (r2 > 0.9 and CV < 25% for each calculated parameter) were satisfied, the model was adjudged to hold true. Otherwise, noncompartment modeling was employed, and the SBC/AIC criteria were compared with compartment modeling. Further minimization of the AIC and SBC criteria was accepted as evidence of better conformity of the model to the data.
RESULTS
Intracellular drug concentrations in phorbol myristate acetate-differentiated THP-1 cells.
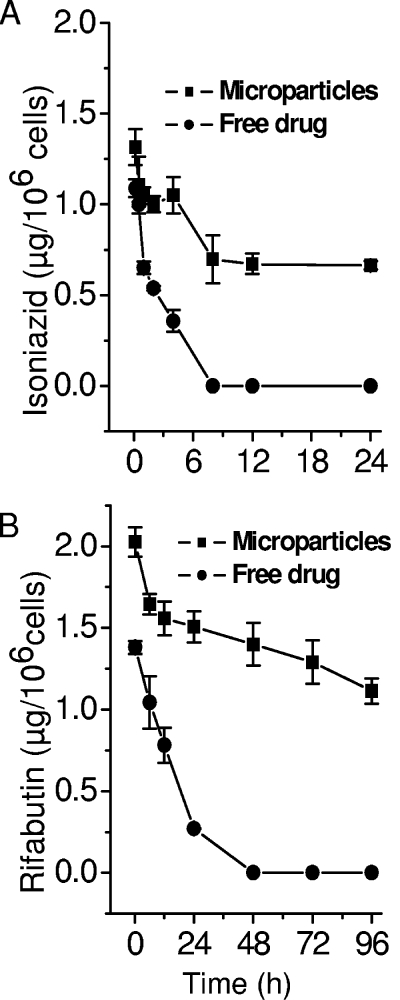
Phorbol-differentiated THP-1 cells were exposed to equivalent concentrations of free drugs or microparticles to ascertain the time course of intracellular drug concentrations resulting from treatment with microparticles and/or drugs in solution. It was observed that both drugs were rapidly taken up by the cells, but the kinetics of drug delivery to the intracellular compartment could not be elucidated with acceptable accuracy. As shown in Fig. 1, in the case of free drugs, peak intracellular drug concentrations of 1.09 and 1.38 μg/106 cells for isoniazid and rifabutin, respectively, were attained in 10 min. With microparticles, the corresponding figures were 1.31 and 2.03 μg/106 cells, respectively. Fitting a one-compartment model with no time lag and first-order elimination yielded elimination constants of 0.034 ± 0.01 h−1 in the case of isoniazid and 0.005 ± 0.001 h−1 in the case of rifabutin incorporated in microparticles. The corresponding values for isoniazid and rifabutin in solution were 0.369 ± 0.06 and 0.060 ± 0.006 h−1, respectively. These results indicated that microparticles deliver effective bactericidal concentrations more rapidly and maintain them for a longer time within the intracellular compartment than free drugs exposed to macrophages. Further, isoniazid concentrations fell within 48 h, while rifabutin was maintained for up to 96 h after a single exposure to microparticles, conforming to the expected performance required for pharmacokinetic synergy between the two agents. These observations also guided our design of sampling schedules in animal studies reported below.
FIG. 1.
Intracellular concentrations of isoniazid (A) and rifabutin (B) in phorbol-differentiated THP-1 cells treated with dissolved (free) drugs or microparticles containing equivalent amounts of the two drugs.
Intracellular concentrations in BAL fluid and macrophages.
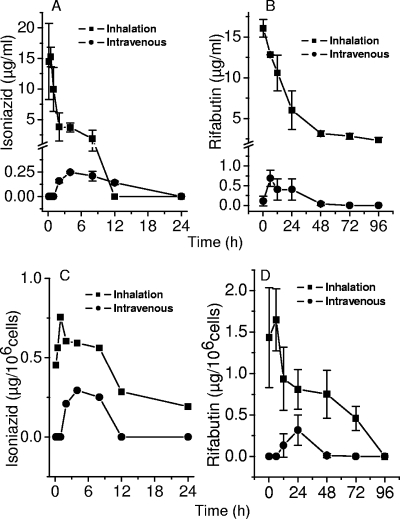
BAL was carried out at different time points after administration of inhalation doses or intravenous injections to mice in order to examine the time course of lung lumen concentrations. Alveolar macrophages recovered by BAL were assayed to establish intracellular concentrations. As shown in Fig. 2, intravenous administration resulted in delayed attainment of drug concentrations in the lung lumen. In contrast, inhalation resulted in immediate peak concentrations, since the microparticles were deposited in the lung lumen itself. Further, drug concentrations achieved in the lung lumen after intravenous administration were 2 orders of magnitude lower than contemporaneous concentrations resulting from microparticle inhalation. Pharmacokinetic model fitting was not applied to these data, since the time course of concentrations is demonstrably attributable to deposition of drug-containing microparticles in the lung lumen in the case of inhalation.
FIG. 2.
(A and C) Concentrations of isoniazid and rifabutin in BAL fluid, indicating drug concentrations in the lung lumen following inhalation or intravenous administration of 100 μg of each drug. (C and D) Intracellular concentrations of isoniazid and rifabutin in cells recovered at the same time points.
Intracellular levels of both drugs rose much slower than was observed in vitro. Isoniazid rose immediately and peaked at 6 h postinhalation at 0.56 μg/106 cells, while a peak of 0.29 μg/106 cells was observed 4 h after intravenous administration. Maximal concentrations of rifabutin (1.65 and 0.32 μg/106 cells) were observed at 6 and 24 h after inhalation and intravenous administration, respectively.
Serum pharmacokinetics.
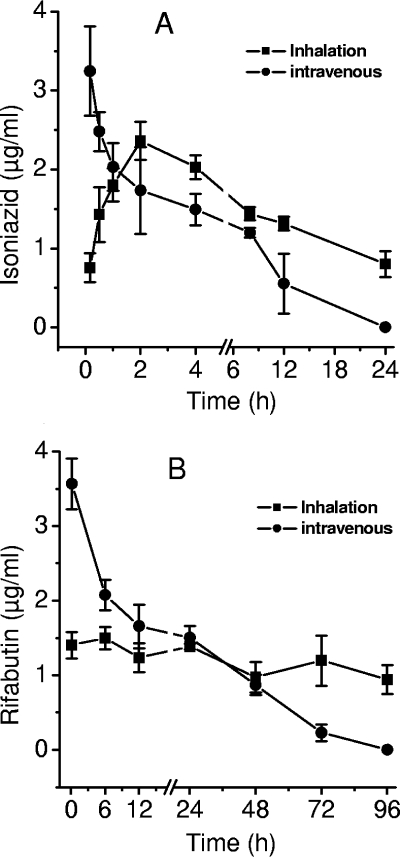
The serum concentration versus time profiles of the two drugs are shown in Fig. 3. Inhalation did not lead immediately to high concentrations of either drug, despite pulmonary delivery. About 2 h elapsed before peak serum concentrations were established.
FIG. 3.
Serum pharmacokinetics of isoniazid (A) and rifabutin (B) in mice (n = 4/time point) after administration of intravenous injection of 100 μg of each drug or inhalation of microparticles containing equivalent amounts.
A one-compartment model could be fitted to the isoniazid concentrations following inhalation of microparticles, but rifabutin kinetics were not amenable to compartment analysis. Noncompartmental analysis of inhalation data typically resulted in minimization of the SBC and AIC and yielded regression coefficients >0.92. This was true for data collected after intravenous administration as well.
The model selected for intravenous administration was a single-intravenous-bolus model, while an extravascular-bolus model was used to fit data emerging from inhalation experiments. In general, inhalation resulted in sustained drug concentrations in the serum compared to intravenous injection. The most pronounced effects observed were the enhancement of serum half-life (t1/2), clearance (CL), and the volume of distribution in the terminal portion of the curve (Vz). Maximal plasma concentrations (Cmax) resulting from intravenous administration were greater than those observed after inhalation. Pharmacokinetic parameters calculated by noncompartmental fit to the concentration-time data are reported in Table 1.
TABLE 1.
Selected pharmacokinetic parameters of isoniazid and rifabutin delivered via the intravenous and inhalation routes in the serum of micea
| Drug and route | Cmax (μg·ml−1) | AUCOBSb (μg·ml−1·h−1) | t1/2 (h) | Vz (ml) | CL (ml·h−1) |
|---|---|---|---|---|---|
| Isoniazid | |||||
| Intravenous | 3.24 ± 0.57 | 16.64 ± 1.80 | 3.91 ± 1.06 | 41.34 ± 14.92 | 4.43 ± 1.85 |
| Inhalation | 2.37 ± 0.23 | 55.34 ± 13.72 | 18.62 ± 5.89 | 1,667.78 ± 48.09 | 63.90 ± 13.32 |
| Rifabutin | |||||
| Intravenous | 3.47 ± 0.33 | 90.82 ± 7.46 | 20.18 ± 4.02 | 29.52 ± 4.29 | 1.02 ± 0.11 |
| Inhalation | 1.59 ± 0.01 | 109.35 ± 14.78 | 119.49 ± 29.62 | 2,308.84 ± 137.78 | 11.68 ± 7.00 |
Values are means ± standard deviations.
AUCOBS, observed area under the concentration-time curve.
Tissue distribution and pharmacokinetics.
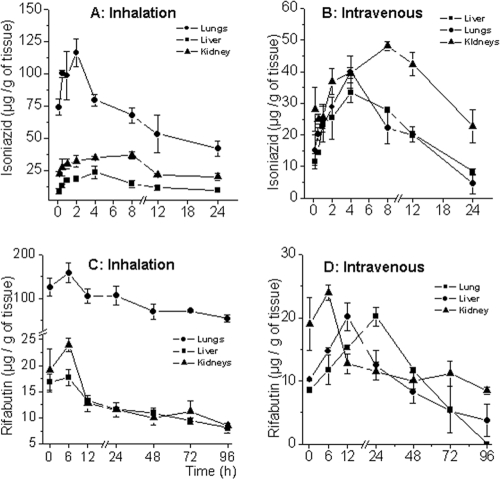
Tissue pharmacokinetics and a partial biodistribution of the two drugs were established over the time course studied by determining drug concentrations in the lungs (target tissue depleted of alveolar macrophages), livers (as an indicator of metabolism and hepatotoxic potential), and kidneys (as indicators of elimination and nephrotoxic potential) at each sampling point. The results are shown in Fig. 4 in terms of amounts of drug per unit weight of tissue. As expected, both drugs were found to accumulate in the liver following intravenous injection, with amounts found in the kidneys marginally exceeding the levels attained in the lungs (Fig. 4B and D). Inhalation of microparticles resulted in targeting both drugs to the lungs, with the effect being more pronounced in the case of rifabutin (C) than isoniazid (A).
FIG. 4.
Tissue pharmacokinetics of isoniazid in lungs, livers, and kidneys following inhalation of microparticles (A) and intravenous injection of free drugs (B), compared to those of rifabutin (C and D). The dose level was 100 μg of each drug. The mean fresh weight of the lungs was 208.10 ± 8.39 mg, that of the livers was 513.24 ± 5.10 mg, and that of the kidneys was 223.33 ± 9.72 mg.
DISCUSSION
The experiments reported here were primarily focused on providing information on the time course of sustained drug concentration at the target sites, i.e., lung macrophages, the lung lumen, and gross lung tissue. Drug concentrations at nontarget sites, including the blood, livers, and kidneys, reflected “leakage” of the payload to nontarget sites. Inhalation delivery of microparticles was expected to generate a pharmacokinetic ensemble, in which compartment-wise distribution of the drug payload was affected by drug release from microparticles, partitioning, and diffusion of the released drugs from the lung lumen to the bloodstream and subsequent tissue accumulation. Difficulties encountered during attempts to undertake compartment modeling on inhalation data supported this conception, such that noncompartment models fitted the data better. Therefore, noncompartment modeling was used to evaluate even those data where compartment modeling was successful, such as the case of intravenous administration of free drugs.
The results obtained serve to explain in part the unexpectedly high efficacy of inhalable microparticles reported in the literature (33, 34; Sen et al., PCT patent application PCT/IB2003/004694). Earlier reports have examined whether macrophage activation evoked by phagocytosed microparticles, including the generation of bactericidal free radicals, secretion of proinflammatory cytokines (29), and induction of apoptotic rather than necrotic cell death (38), could contribute to bactericidal efficacy. The role of pharmacokinetics in enhancing efficacy has not been considered in the investigations cited above.
Pharmacokinetics may be expected to influence efficacy in two ways. First, maintenance of bactericidal concentrations at the target site can enhance bactericidal efficacy. Second, in a clinical scenario wherein the infection comprises strains with various sensitivities, the EBA of isoniazid can work in synergy with maintained rifabutin concentrations (9, 11, 12). The formulation of the drug delivery system provides grounds to expect that isoniazid concentrations following inhalation would be initially high and deplete rapidly. Rifabutin concentrations, on the other hand, would be slow to peak as well as to fall. These two drugs differ significantly in solubility—whereas isoniazid is readily water-soluble, rifabutin is not. This difference leads to strong divergence in in vitro drug release profiles, with isoniazid being released much more rapidly than rifabutin (21). This observation lends useful support to the expected therapeutic action and pharmacological indices (2) of the two drugs: isoniazid has the highest EBA among anti-TB drugs (9), but this activity ceases rapidly, primarily through emergence of resistance (11, 12). Maintaining bactericidal concentrations of rifabutin in alveolar macrophages, caseous lesions, and general lung tissue as well as blood is desirable in the case of this drug (3, 4, 31, 32). Since the inherent physicochemical properties and biopharmaceutics of these two agents lead to just such a situation upon dosing, the principal tasks of the drug delivery system reduce to (i) ensuring a more favorable biodistribution, such that the drugs are targeted to the site of infection and the target organs of toxicity are protected, and (ii) sustaining drug delivery such that the relative pharmacokinetics of the two agents are not modified except for enhancing duration of action. The results indicate that the delivery system fulfils both the above functions.
In vitro studies conducted on cultured cells indicated that the inhalable microparticles used here are amenable to phagocytosis by macrophages and yield sustained intracellular drug concentrations (Fig. 1). As expected, isoniazid fell to low levels in 24 h and was undetectable after 48 h. Rifabutin levels could be observed for up to 96 h. In the same experimental system, intracellular concentrations of both drugs administered in solution decayed within one-half the time observed in the case of microparticles. These observations suggested that similar time frames should be tracked in vivo, wherein recovery of lung and airway macrophages by BAL was envisaged. Thus, cell culture can serve as a useful tool to suggest residence times and sampling intervals to determine intracellular concentrations in vivo.
An unusual experiment design was adopted for the studies conducted in vivo, since alveolar macrophages were required to be isolated at every time point. There is no way whereby these cells may be recovered by survival surgery, specially from rodents, and therefore it was decided to sacrifice four mice for every time point reported. Mice were selected as the animal model since efficacy studies had been conducted with this species and strain (Sen et al., PCT patent application PCT/IB2003/004694). Some concerns accompanied the adoption of this experimental design. Foremost was the issue of interindividual variability. Reduction of variability was sought by using inbred mice, and the results appear to have justified the choice. In most cases, the CV in drug concentrations determined in four samples representing a single time point were within 17%. A few sample groups did display larger variation, specially at the initial time points after dosing (Fig. 2D, 3A, and 4B). Standard deviations from the arithmetic means are therefore plotted to enable realistic appreciation of the data sets.
The approach described above was used to assay drug concentrations in the lung lumen and also within lung and airway macrophages at various time points after administration of intravenous injections or inhalation doses. A dose level of 100 μg/animal (rather than mg/kg) was used for convenience since the number of animals was large and surgical procedures were required after every dose. BAL fluid was used as an indicator of concentrations in the lung lumen and mucosa. Since the delivery system is intended for pulmonary delivery, it was not surprising to see 15- to 20-fold-higher drug concentrations in the lung lumen (BAL fluid) at every time point. The study also showed that blood-borne drugs do not readily diffuse out to the mucosal surface of the lungs and are thus not recovered in significant quantities by BAL. The only unexpected result in Fig. 2 was the depletion of intracellular rifabutin concentrations to undetectable levels in 96 h, while there was evidently a sufficient supply of the drug in the lung lumen or mucosal surface (Fig. 2B and D). It is speculated that this result might have arisen because lung macrophages that had taken up microparticles over the previous 72 h had begun to migrate away from the mucosal surface and fresh infiltration of macrophages had not been expedited during this time.
Serum pharmacokinetics of isoniazid and rifabutin after intravenous administration, as depicted in Fig. 3, and pharmacokinetic parameters listed in Table 1 compare well with the literature (1, 3, 4, 14, 15, 17, 31, 32, 35, 36). For instance, isoniazid displayed a t1/2 of 3.91 h in the present experiments. In humans, this varies between 3.59, 1.61, and 1.02 h in individuals with NAT-2 genotypes corresponding to slow, intermediate, and rapid acylators, respectively (28). The t1/2 of rifabutin is reported variously in the region of 16 to 96 h in humans after oral administration (3, 4, 31). In the present report, a value of 20.18 h after intravenous administration was calculated. Such concordance also indicates the credibility of data obtained from mice administered inhalation doses. Across-the-board enhancement of the area under the concentration-time curve, t1/2, Vz, and CL, with small differences in Cmax, confirms that sustained drug release is provided by the delivery system.
Conclusion.
Cell culture experiments on intracellular drug concentration provide valuable information regarding in vivo intracellular pharmacokinetics. Inhalable microparticles appear to increase the time frame of action of the drugs incorporated in them by sustained release and drug targeting, as reflected in the pharmacokinetic parameters. The pharmacokinetic profiles of the two agents consequent to oral dosing, as in conventional drug delivery, are not modified apart from increasing durations of action.
Acknowledgments
Financial support from CSIR through grant 5/258/6/2001-NMITLI and senior research fellowships to R.K.V. and A.B.Y. is acknowledged. J.K. received a senior research fellowship from ICMR.
This is CDRI communication no. 7454.
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 2.Barger, A., C. Fuhst, and B. Wiedemann. 2003. Pharmacological indices in antibiotic therapy. J. Antimicrob. Chemother. 52:893-898. [DOI] [PubMed] [Google Scholar]
- 3.Blaschke, T. F., and M. H. Skinner. 1996. The clinical pharmacokinetics of rifabutin. Clin. Infect. Dis. 22(Suppl. 1):S15-S21. [PubMed] [Google Scholar]
- 4.Brogden, R. N., and A. Fitton. 1994. Rifabutin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 47:983-1009. [DOI] [PubMed] [Google Scholar]
- 5.Burman, W., D. Benator, A. Vernon, A. Khan, B. Jones, C. Silva, C. Lahart, S. Weis, B. King, B. Mangura, M. Weiner, and W. El-Sadr. 2006. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am. J. Respir. Crit. Care Med. 173:350-356. [DOI] [PubMed] [Google Scholar]
- 6.Calleja, I., M. J. Blanco-Príeto, N. Ruz, M. J. Renedo, and M. C. Dios-Viéitez. 2004. High-performance liquid-chromatographic determination of rifampicin in plasma and tissues. J. Chromatogr. 1031:289-294. [DOI] [PubMed] [Google Scholar]
- 7.Cox, H. S., M. Morrow, and P. W. Deutschmann. 2008. Long term efficacy of DOTS regimens for tuberculosis: systematic review. BMJ 336:484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delahunty, T., B. Lee, and J. E. Conte. 1998. Sensitive liquid chromatographic technique to measure isoniazid in alveolar cells, bronchoalveolar lavage and plasma in HIV-infected patients. J. Chromatogr. B 705:323-329. [DOI] [PubMed] [Google Scholar]
- 9.Donald, P. R. 2006. The early bactericidal activity of anti-tuberculosis agents. Int. J. Tuberc. Lung Dis. 10:591. [PubMed] [Google Scholar]
- 10.Gumbo, T., A. Louie, M. R. Deziel, W. Liu, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumbo, T., A. Louie, W. Liu, P. G. Ambrose, S. M. Bhavnani, D. Brown, and G. L. Drusano. 2007. Isoniazid's bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J. Infect. Dis. 195:194-201. [DOI] [PubMed] [Google Scholar]
- 12.Gumbo, T., A. Louie, W. Liu, D. Brown, P. G. Ambrose, S. M. Bhavnani, and G. L. Drusano. 2007. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob. Agents Chemother. 51:2329-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hestvik, A. L., Z. Hmama, and Y. Av-Gay. 2005. Mycobacterial manipulation of the host cell. FEMS Microbiol. Rev. 29:1041-1050. [DOI] [PubMed] [Google Scholar]
- 14.Hirata, T., H. Saito, H. Tomioka, K. Sato, J. Jidoi, K. Hosoe, and T. Hidaka. 1995. In vitro and in vivo activities of the benzoxazinorifamycin KRM-1648 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 39:2295-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holdiness, M. R. 1984. Clinical pharmacokinetics of the antituberculosis drugs. Clin. Pharmacokinet. 9:511-544. [DOI] [PubMed] [Google Scholar]
- 16.Hutchings, A., R. D. Monie, B. Spragg, and P. A. Routledge. 1983. High-performance liquid chromatographic analysis of isoniazid and acetylisoniazid in biological fluids. J. Chromatogr. 277:385-390. [DOI] [PubMed] [Google Scholar]
- 17.Jayaram, R., R. K. Shandil, S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharath, E. Kantharaj, and V. Balasubramanian. 2004. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 48:2951-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur, J., P. Muttil, R. K. Verma, K. Kumar, A. B. Yadav, R. Sharma, and A. Misra. 2008. A hand-held apparatus for “nose-only” exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages. Eur. J. Pharm. Sci. 34:56-65. [DOI] [PubMed] [Google Scholar]
- 19.Makino, K., T. Nakajima, M. Shikamura, F. Ito, S. Ando, C. Kochi, H. Inagawa, G. Soma, and H. Terada. 2004. Efficient intracellular delivery of rifampicin to alveolar macrophages using rifampicin-loaded PLGA microspheres: effects of molecular weight and composition of PLGA on release of rifampicin. Colloids Surf. B 36:35-42. [DOI] [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Muttil, P., J. Kaur, K. Kumar, A. B. Yadav, R. Sharma, and A. Misra. 2007. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur. J. Pharm. Sci. 32:140-150. [DOI] [PubMed] [Google Scholar]
- 22.O'Hara, P., and A. J. Hickey. 2000. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: manufacture and characterization. Pharm. Res. 17:955-961. [DOI] [PubMed] [Google Scholar]
- 23.Pandey, R., and G. K. Khuller. 2005. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis (Edinburgh) 85:227-234. [DOI] [PubMed] [Google Scholar]
- 24.Pandey, R., A. Sharma, A. Zahoor, S. Sharma, G. K. Khuller, and B. Prasad. 2003. Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J. Antimicrob. Chemother. 52:981-986. [DOI] [PubMed] [Google Scholar]
- 25.Pandey, R., S. Sharma, and G. K. Khuller. 2004. Lung specific stealth liposomes as antitubercular drug carriers in guinea pigs. Indian J. Exp. Biol. 42:562-566. [PubMed] [Google Scholar]
- 26.Pandey, R., S. Sharma, and G. K. Khuller. 2004. Nebulization of liposome encapsulated antitubercular drugs in guinea pigs. Int. J. Antimicrob. Agents 24:93-94. [DOI] [PubMed] [Google Scholar]
- 27.Russell, D. G. 2007. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 5:39-47. [DOI] [PubMed] [Google Scholar]
- 28.Schaaf, H. S., D. P. Parkin, H. I. Seifart, C. J. Werely, P. B. Hesseling, P. D. van Helden, J. S. Maritz, and P. R. Donald. 2005. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Arch. Dis. Child. 90:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma, R., P. Muttil, A. B. Yadav, S. K. Rath, V. K. Bajpai, U. Mani, and A. Misra. 2007. Uptake of inhalable microparticles affects defence responses of macrophages infected with Mycobacterium tuberculosis H37Ra. J. Antimicrob. Chemother. 59:499-506. [DOI] [PubMed] [Google Scholar]
- 30.Sharma, R., D. Saxena, A. K. Dwivedi, and A. Misra. 2001. Inhalable microparticles containing drug combinations to target alveolar macrophages for treatment of pulmonary tuberculosis. Pharm. Res. 18:1405-1410. [DOI] [PubMed] [Google Scholar]
- 31.Skinner, M. H., and T. F. Blaschke. 1995. Clinical pharmacokinetics of rifabutin. Clin. Pharmacokinet. 28:115-125. [DOI] [PubMed] [Google Scholar]
- 32.Skinner, M. H., M. Hsieh, J. Torseth, D. Pauloin, G. Bhatia, S. Harkonen, T. C. Merigan, and T. F. Blaschke. 1989. Pharmacokinetics of rifabutin. Antimicrob. Agents Chemother. 33:1237-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suarez, S., P. O'Hara, M. Kazantseva, C. E. Newcomer, R. Hopfer, D. N. McMurray, and A. J. Hickey. 2001. Airways delivery of rifampicin microparticles for the treatment of tuberculosis. J. Antimicrob. Chemother. 48:431-434. [DOI] [PubMed] [Google Scholar]
- 34.Suarez, S., P. O'Hara, M. Kazantseva, C. E. Newcomer, R. Hopfer, D. N. McMurray, and A. J. Hickey. 2001. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: screening in an infectious disease model. Pharm. Res. 18:1315-1319. [DOI] [PubMed] [Google Scholar]
- 35.Tannen, R. H., and W. W. Weber. 1979. Rodent models of the human isoniazid-acetylator polymorphism. Drug Metab. Dispos. 7:274-279. [PubMed] [Google Scholar]
- 36.Weber, W. W., and D. W. Hein. 1979. Clinical pharmacokinetics of isoniazid. Clin. Pharmacokinet. 4:401-422. [DOI] [PubMed] [Google Scholar]
- 37.Weiner, M., D. Benator, W. Burman, C. A. Peloquin, A. Khan, A. Vernon, B. Jones, C. Silva-Trigo, Z. Zhao, and T. Hodge. 2005. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin. Infect. Dis. 40:1481-1491. [DOI] [PubMed] [Google Scholar]
- 38.Yadav, A. B., and A. Misra. 2007. Enhancement of apoptosis of THP-1 cells infected with Mycobacterium tuberculosis by inhalable microparticles and relevance to bactericidal activity. Antimicrob. Agents Chemother. 51:3740-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida, A., M. Matumoto, H. Hshizume, Y. Oba, T. Tomishige, H. Inagawa, C. Kohchi, M. Hino, F. Ito, K. Tomoda, T. Nakajima, K. Makino, H. Terada, H. Hori, and G. Soma. 2006. Selective delivery of rifampicin incorporated into poly(DL-lactic-co-glycolic) acid microspheres after phagocytotic uptake by alveolar macrophages, and the killing effect against intracellular Mycobacterium bovis Calmette-Guerin. Microbes Infect. 8:2484-2491. [DOI] [PubMed] [Google Scholar]
- 40.Zahoor, A., S. Sharma, and G. K. Khuller. 2005. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 26:298-303. [DOI] [PubMed] [Google Scholar]