Abstract
Reporter clones of Staphylococcus aureus with different SOS response- and DNA repair-associated promoter-lux gene fusion constructs were constructed to study the effects of sub-MICs of antibiotics on the transcription of the SOS and methyl mismatch repair (MMR) genes. Fluoroquinolones (FQs) upmodulated both the SOS and the MMR genes. The patterns of antibiotic-induced transcriptional modulation were altered in FQ-resistant mutants.
Subinhibitory concentrations (sub-MICs) of antibiotics are known to provoke extensive transcriptional changes in bacteria (28, 29). The expression of virulence functions such as toxins, adhesins, and biofilm formation in the human pathogen Staphylococcus aureus is affected by exposure to sub-MICs of antibiotics (2, 6, 7, 11, 12, 14, 19). Sub-MICs of certain antibiotics, in particular, compounds whose primary mode of action is DNA damage, are known to enhance mutation rates in bacteria (15). This is usually the result of transcriptional changes in the genes responsible for DNA repair and preservation of the integrity of the genome, such as the SOS and methyl mismatch repair (MMR) pathways (10, 24, 25). DNA polymerases of the SOS system lack intrinsic proofreading activity, which leads to mutations when DNA replication bypasses lesions or errors (24). The MMR system maintains the fidelity of DNA replication by postreplicative correction of base mismatches, small insertions, or deletions (8); a strong mutator phenotype is associated with genetic defects in the MMR system (21).
We have studied the effects of sub-MICs of antibiotics of different chemical classes and with different modes of action on the principal mediators of the SOS response in S. aureus, lexA and recA (16) (Fig. 1A). We also examined other known or presumptive SOS response genes, umuC, sosA (SAOUHSC_01334), dinB, and recF (9), and known or presumptive MMR genes, mutSL (25, 27), mutS2 (SAOUHSC_01099), mutS3 (SAOUHSC_02276), and mutT (SAOUHSC_00429) (see Table SA in the supplemental material). The respective promoter regions (234 to 661 bp) were amplified by PCR and inserted into pAmiLux (L. R. Mesak et al., unpublished data), a promoter cloning vector, at a BamHI site upstream of a modified Photobacterium luminescens luxABCDE (lux) operon encoding luciferase (luxAB) and fatty acid reductase (luxCDE) from pAL2 (4). The constructs were introduced into S. aureus RN4220 (18), and the effects of the antibiotics on transcription in cells grown in NYE medium (26) were monitored by obtaining luminescence measurements. A single colony of S. aureus from NYE agar was resuspended in 200 μl water, mixed with 0.7% agar (1:1,000), and poured as an overlay on NYE agar. Paper disks containing selected antibiotics were placed on the overlay, and the culture was incubated at 37°C. After 20 h, luminescence was detected with a luminograph LB980 photon camera (Berthold). Liquid assays were performed at room temperature in a clear-bottom 96-well plate by using starting cultures with an optical density at 595 nm of 0.150. Luminescence was recorded hourly for 20 h in a Wallac 1420 Victor multilabel counter (Perkin-Elmer). Each determination was replicated two to four times (for experiments in broth) or six times (for experiments on solid medium).
FIG. 1.
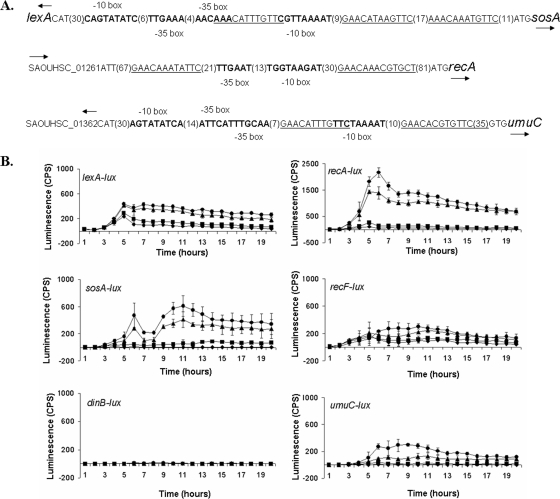
(A) Promoter regions of SOS response genes. The SOS boxes (underlined) are conserved sequences upstream of many genes in the SOS regulon that repress transcription when they are bound by the LexA protein (5, 9). The putative −10 and −35 boxes (boldface), the direction of transcription (arrow), and intervening nucleotides not shown (parenthetical numbers) are indicated. (B). Expression of SOS genes. The luminescence in the presence of 0 μg/ml (diamonds), 0.2 μg/ml (squares), 0.5 μg/ml (triangles), and 1 μg/ml (circles) CIP in broth was measured with a Wallac 1420 Victor multilabel counter (Perkin-Elmer).
The overall level of lux expression in the absence of antibiotics was low for all the constructs tested, whether expression was tested on agar or in broth, although the basal levels of expression of lexA, recA, and recF were higher than those of the other SOS genes (Fig. 1B). When antibiotic disks were placed on lawns seeded with reporter strains, promoter activation, as indicated by luminescence at the border between the inhibition zone around the disks (concentrations greater than the MIC) and the growing cells beyond (concentrations less than the MICs), was observed after 20 h at 37°C. Expression of the lexA, recA, sosA, recF, and umuC genes was upmodulated by all nine fluoroquinolones (FQs) tested to levels higher than those in the presence of mitomycin C, a DNA-damaging agent known to activate lexA-regulated genes (3) (Fig. 2A). Although the function of sosA, a gene that appears to be unique to Staphylococcus spp., remains to be identified, the upmodulation of sosA transcription by FQs was similar to that of the SOS genes, suggesting that the SOS boxes associated with lexA may influence sosA (Fig. 1A). In recent studies, sosA has been shown to be regulated by lexA (3, 9). SOS boxes were not found in the dinB promoter region, and the expression of dinB was not affected by FQs. MMR gene transcription was upmodulated to a lesser extent than SOS gene transcription by FQs after 20 h of incubation at 37°C (Fig. 2A).
FIG. 2.
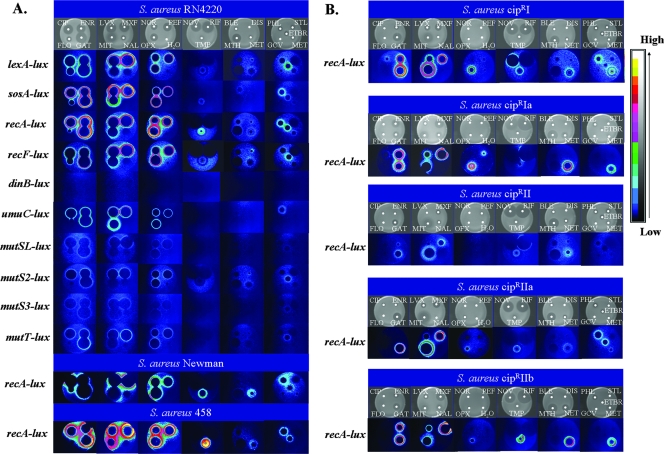
Effects of antibiotics on SOS and MMR gene expression in the indicated wild-type S. aureus strains (RN4220, Newman, and 458) (A) and Cipr mutants (B). The first row in each set shows the inhibition zones in representative disk diffusion assays after 20 h; the other rows show the effects of the antibiotics on the indicated reporter strains, as seen with a luminograph LB980 photon camera (Berthold) and as converted to the color scale on the right. FQs and mitomycin C did not affect the promoterless-lux constructs. The antibiotic disks are as follows: CIP, CIP at 5 μg; ENR; enrofloxacin at 5 μg; FLO, florofloxacin at 5 μg; GAT, gatifloxacin at 5 μg; LVX, levofloxacin at 5 μg; MXF, moxifloxacin at 5 μg; MIT, mitomycin C at 5 μg; NAL, nalidixic acid at 30 μg; NOR, norfloxacin at 5 μg; PEF, pefloxacin at 5 μg; OFX, ofloxacin at 5 μg; H2O, water; NOV, novobiocin at 5 μg; RIF, rifampin at 5 μg; TMP, trimethoprim at 5 μg; BLE, bleomycin A at 10 μg; DIS, distamycin at 10 μg; MTH, mithramycin A at 10 μg; NET, netropsin at 10 μg; PHL, phleomycin at 10 μg; STL, streptolydigin at 10 μg; GCV, ganciclovir at 10 μg; MET, methotrexate at 50 μg; ETBR, ethidium bromide at 10 μg.
The results from the studies with solid medium were confirmed by monitoring the luminescence during the growth of the reporter strains in liquid culture with ciprofloxacin (CIP); recA and umuC expression was strongly activated by 1 μg/ml CIP (Fig. 1B). The level of induction of the lexA, recF, and MMR genes was lower than that of the recA, umuC, and sosA genes (Fig. 1B). The SOS response was dependent on the antibiotic concentration, but mutSL expression was not. Interestingly, the behavior of mutS2 and mutT, proposed here to be MMR genes on the basis of their deduced similarity to proteins of the MutS and MutT families, respectively, was similar to that of mutSL. Further studies are necessary to determine if they have a role in the MMR pathway or other pathways.
With the exceptions of trimethoprim, phleomycin, and ethidium bromide, none of the other compounds tested (Table 1), regardless of their modes of action, upmodulated the S. aureus RN4220 SOS and MMR promoter-lux fusions (Fig. 2A). Trimethoprim had a strong upmodulating effect on recA (Fig. 2A); this compound inhibits nucleoside biosynthesis and results in the accumulation of damaged DNA (1, 17). Phleomycin is a member of the glycopeptide antibiotic family, generates free radicals, and produces DNA breaks. It is considerably more cytotoxic than bleomycin (22). Ethidium bromide is a well-known intercalating agent. Sub-MICs of the β-lactams, e.g., penicillin G, had no effect on the transcription of the SOS genes. This is surprising, because β-lactams have been reported to induce lexA transcription and recA-dependent prophage induction in S. aureus lysogens (20). The reason for the difference is not clear.
TABLE 1.
S. aureus strains used in this studya
| Strain | Relevant characteristic(s) | CIP MICb (μg/ml) | Reference or source |
|---|---|---|---|
| RN4220 | Restriction-deficient derivative of 8325-4 rK− mK+ | 0.65-1.3 | 18 |
| Newman | Clinical isolate | 0.3-0.65 | 13 |
| 458 | Clinical isolate | 0.3-0.65 | L. Friedman |
| CiprI | grlA (GrlA [S80F]) | 42 | This study |
| CiprIa | Insertion in norA promoterc | 21 | This study |
| CiprII | grlA (GrlA [S80Y]), gyrA (GyrA [E88K]) | 42 | This study |
| CiprIIa | grlA (GrlA [S80F]), gyrA (GyrA [E88Q]) | 10.5 | This study |
| CiprIIb | grlA (GrlA [S80Y]), insertion in norA promoterd | 42 | This study |
The promoter-lux fusion constructs were transformed into S. aureus strains and then tested with FQs, other DNA-damaging agents (bleomycin A, phleomycin, distamycin, netropsin, nalidixic acid, mithramycin A, ethidium bromide), and a variety of antibiotics with other modes of action: cell membrane-damaging agents (daptomycin, polymyxin B), cell wall biosynthesis inhibitors (bacitracin, fosfomycin, imipenem, penicillin G, vancomycin), protein synthesis inhibitors (clindamycin, erythromycin, gentamicin, kanamycin, neomycin, pristinamycins I and II, spectinomycin, streptomycin, tetracycline, tobramycin), the nucleoside analog ganciclavir, a DNA replication inhibitor (novobiocin), the metabolic inhibitor trimethoprim, RNA polymerase inhibitors (rifampin, streptolydigin), and a nucleotide biosynthesis inhibitor (methotrexate). Antibiotic disks were obtained from Becton Dickinson or Difco or were made by using the laboratory collection.
The MICs of CIP were determined in NYE broth: an aliquot of a culture grown overnight from a single colony at 37°C was diluted in water (1:100), and 10 μl was inoculated into 1.5 ml NYE broth in tubes containing CIP representing twofold serial dilutions starting from 170 μg/ml.
A direct tandem duplication (ATATGTAGCAATGTTGTAATACAAT) of native sequences was observed at position 379 within the promoter region of norA (23), adjacent to the −10 box.
A direct tandem duplication (TGTTGTAATACAAT) of native sequences was observed at position 379 within the promoter region of norA (23), adjacent to the −10 box.
The effects of sub-MICs of antibiotics on recA expression in S. aureus Newman and 458, both of which are clinical strains, was largely similar to the effect in RN4220 (Fig. 2A); however, the level of induction of recA expression by netropsin in S. aureus Newman was increased compared to that in RN4220 and 458 (Fig. 2A).
We also investigated the effects of FQs on the expression of recA in Cipr mutants of RN4220 (Table 1). Spontaneous CIP-resistant mutants of S. aureus were selected by plating 2.5 × 109 cells on NYE medium containing 2 μg/ml of CIP. Five colonies that appeared after 3 days were picked and purified on medium containing 2, 4, or 8 μg/ml CIP to identify those mutants that were more resistant to CIP. One derivative of each mutant (mutants CiprI, CiprIa, CiprII, CiprIIa, and CiprIIb; Table 1) was selected from medium containing 8 μg/ml CIP and characterized by sequencing of the norA promoter and the gyrA, gyrB, grlA, and grlB genes (23). The strains were cross tolerant to most FQs, including extended-spectrum FQs (moxifloxacin and gatifloxacin), with mutant CiprII being generally more resistant than the other mutants. The mutants with an altered GrlA (S80F), mutants CiprI and CiprIIa, were more resistant to nalidixic acid. The plasmid carrying the recA-lux construct was then introduced into the different Cipr strains and the strains were tested for their responses to antibiotics (Fig. 2B). CIP did not stimulate recA transcription in the mutants; but enrofloxacin, levofloxacin, and ofloxacin upmodulated recA expression in mutants CiprI, CiprIa, and CiprIIb. Gatifloxacin and moxifloxacin, which both have GrlA and GyrA as targets (23), induced recA expression in all mutants. Unexpectedly, certain inhibitors that had no effect on the transcription of the SOS and MMR genes in RN4220 influenced recA transcription in the Cipr strains (Fig. 2B). Notably, novobiocin induced recA expression in CiprI, while rifampin induced recA expression in CiprII. Phleomycin and ethidium bromide did not induce recA expression in CiprII or the norA promoter mutants (mutants CiprIa and CiprIIb). In these two mutants, recA expression was also strongly induced by the DNA-binding agent netropsin. The atypical responses of spontaneous Cipr mutants indicate that mutations to resistance may lead to a variety of phenotypes affecting bacterial responses to completely different classes of antibiotics. However, the latter possibility could be due to the presence of additional unidentified mutations. There is no simple explanation for this phenomenon, but the fact that resistance to one class of antibiotic may influence bacterial responses to other inhibitors could be of therapeutic importance.
Using a modified lux operon expression system, we have demonstrated that a number of antibiotics at their sub-MICs have specific transcriptional effects on the SOS and MMR systems of S. aureus. One consequence of increased expression might be that the therapeutic use of one antibiotic could increase the numbers of mutations to resistance to other classes of antibiotics. The effects of sub-MICs of antibiotics on the mutation rate in wild-type and resistant mutants of S. aureus are under investigation. Given the specificities of the responses, the promoter-lux constructs generated in this study have utility in screening for different antibiotic classes or for use in promoter-lux reporter panels for screening of antibiotics for their modes of action.
Supplementary Material
Acknowledgments
We thank V. Salisbury for pAL2 and L. Friedman for S. aureus 458. We are grateful to A. L. Cheung (Dartmouth Medical School), D. E. Heinrichs (University of Western Ontario, London, Ontario, Canada), E. A. Meighen (McGill University, Montreal, Quebec, Canada), and Grace Yim (University of British Columbia) for helpful discussions.
This research was supported by the Canadian Institute of Health Research.
Footnotes
Published ahead of print on 30 June 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Abou-Eisha, A. 2006. Evaluation of cytogenetic and DNA damage induced by the antibacterial drug, trimethoprim. Toxicol. In Vitro 20:601-607. [DOI] [PubMed] [Google Scholar]
- 2.Adhikari, R. P., and R. P. Novick. 2005. Subinhibitory cerulenin inhibits staphylococcal exoprotein production by blocking transcription rather than by blocking secretion. Microbiology 151:3059-3069. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, K. L., C. Roberts, T. Disz, V. Vonstein, K. Hwang, R. Overbeek, P. D. Olson, S. J. Projan, and P. M. Dunman. 2006. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J. Bacteriol. 188:6739-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beard, S. J., V. Salisbury, R. J. Lewis, J. A. Sharpe, and A. P. MacGowan. 2002. Expression of lux genes in a clinical isolate of Streptococcus pneumoniae: using bioluminescence to monitor gemifloxacin activity. Antimicrob. Agents Chemother. 46:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisognano, C., W. L. Kelley, T. Estoppey, P. Francois, J. Schrenzel, D. Li, D. P. Lew, D. C. Hooper, A. L. Cheung, and P. Vaudaux. 2004. A RecA-LexA-dependent pathway mediates ciprofloxacin-induced fibronectin binding in Staphylococcus aureus. J. Biol. Chem. 279:9064-9071. [DOI] [PubMed] [Google Scholar]
- 6.Braga, P. C., M. Dal Sasso, and S. Maci. 1997. Cefodizime: effects of sub-inhibitory concentrations on adhesiveness and bacterial morphology of Staphylococcus aureus and Escherichia coli: comparison with cefotaxime and ceftriaxone. J. Antimicrob. Chemother. 39:79-84. [DOI] [PubMed] [Google Scholar]
- 7.Braga, P. C., and G. Piatti. 1993. Favourable effects of sub-MIC rufloxacin concentrations in decreasing the pathogen-host cell adhesion. Pharmacol. Res. 28:11-19. [DOI] [PubMed] [Google Scholar]
- 8.Chopra, I., A. J. O'Neill, and K. Miller. 2003. The role of mutators in the emergence of antibiotic-resistant bacteria. Drug Resist. Updat. 6:137-145. [DOI] [PubMed] [Google Scholar]
- 9.Cirz, R. T., M. B. Jones, N. A. Gingles, T. D. Minogue, B. Jarrahi, S. N. Peterson, and F. E. Romesberg. 2007. Complete and SOS-mediated response of Staphylococcus aureus to the antibiotic ciprofloxacin. J. Bacteriol. 189:531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirz, R. T., and F. E. Romesberg. 2006. Induction and inhibition of ciprofloxacin resistance-conferring mutations in hypermutator bacteria. Antimicrob. Agents Chemother. 50:220-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dal Sasso, M., M. Culici, C. Bovio, and P. C. Braga. 2003. Gemifloxacin: effects of sub-inhibitory concentrations on various factors affecting bacterial virulence. Int. J. Antimicrob. Agents 21:325-333. [DOI] [PubMed] [Google Scholar]
- 12.Dancer, S. J. 2008. The effect of antibiotics on methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 61:246-253. [DOI] [PubMed] [Google Scholar]
- 13.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase; mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 14.Gemmell, C. G., and C. W. Ford. 2002. Virulence factor expression by gram-positive cocci exposed to subinhibitory concentrations of linezolid. J. Antimicrob. Chemother. 50:665-672. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie, S. H., S. Basu, A. L. Dickens, D. M. O'Sullivan, and T. D. McHugh. 2005. Effect of subinhibitory concentrations of ciprofloxacin on Mycobacterium fortuitum mutation rates. J. Antimicrob. Chemother. 56:344-348. [DOI] [PubMed] [Google Scholar]
- 16.Kelley, W. L. 2006. Lex marks the spot: the virulent side of SOS and a closer look at the LexA regulon. Mol. Microbiol. 62:1228-1238. [DOI] [PubMed] [Google Scholar]
- 17.Kimmitt, P. T., C. R. Harwood, and M. R. Barer. 2000. Toxin gene expression by Shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6:458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 19.Li, D., A. Renzoni, T. Estoppey, C. Bisognano, P. Francois, W. L. Kelley, D. P. Lew, J. Schrenzel, and P. Vaudaux. 2005. Induction of fibronectin adhesins in quinolone-resistant Staphylococcus aureus by subinhibitory levels of ciprofloxacin or by sigma B transcription factor activity is mediated by two separate pathways. Antimicrob. Agents Chemother. 49:916-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiques, E., C. Ubeda, S. Campoy, N. Salvador, I. Lasa, R. P. Novick, J. Barbe, and J. R. Penades. 2006. β-Lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus. J. Bacteriol. 188:2726-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marti, T. M., C. Kunz, and O. Fleck. 2002. DNA mismatch repair and mutation avoidance pathways. J. Cell. Physiol. 191:28-41. [DOI] [PubMed] [Google Scholar]
- 22.Moore, C. W. 1989. Cleavage of cellular and extracellular Saccharomyces cerevisiae DNA by bleomycin and phleomycin. Cancer Res. 49:6935-6940. [PubMed] [Google Scholar]
- 23.Noguchi, N., T. Okihara, Y. Namiki, Y. Kumaki, Y. Yamanaka, M. Koyama, K. Wakasugi, and M. Sasatsu. 2005. Susceptibility and resistance genes to fluoroquinolones in methicillin-resistant Staphylococcus aureus isolated in 2002. Int. J. Antimicrob. Agents 25:374-379. [DOI] [PubMed] [Google Scholar]
- 24.Nohmi, T. 2006. Environmental stress and lesion-bypass DNA polymerases. Annu. Rev. Microbiol. 60:231-253. [DOI] [PubMed] [Google Scholar]
- 25.Prunier, A. L., and R. Leclercq. 2005. Role of mutS and mutL genes in hypermutability and recombination in Staphylococcus aureus. J. Bacteriol. 187:3455-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schenk, S., and R. A. Laddaga. 1992. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol. Lett. 73:133-138. [DOI] [PubMed] [Google Scholar]
- 27.Trong, H. N., A. L. Prunier, and R. Leclercq. 2005. Hypermutable and fluoroquinolone-resistant clinical isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 49:2098-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsui, W. H., G. Yim, H. H. Wang, J. E. McClure, M. G. Surette, and J. Davies. 2004. Dual effects of MLS antibiotics: transcriptional modulation and interactions on the ribosome. Chem. Biol. 11:1307-1316. [DOI] [PubMed] [Google Scholar]
- 29.Yim, G., H. H. Wang, and J. Davies. 2006. The truth about antibiotics. Int. J. Med. Microbiol. 296:163-170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.