Abstract
There were no significant differences in the pharmacokinetics of micafungin and expression of hepatic multidrug resistance-associated protein 2 (ABCC2/Mrp2) between analbuminemic and Sprague-Dawley rats. Micafungin bound strongly to high-density lipoprotein (HDL) and moderately to gamma globulin. These results suggest that HDL and gamma globulin contribute to the pharmacokinetics of micafungin.
Micafungin is widely used for the treatment and prevention of various fungal infections. This antibiotic appears to be predominantly eliminated by hepatic cytochrome P450 (CYP)-mediated metabolism and partly excreted in unchanged form in the feces via hepatobiliary excretion, whereas urinary excretion is a minor excretion route (6, 12, 20). Moreover, it has also been reported that micafungin has no effect on the metabolism of various substrates for CYP isoforms such as CYP3A4, CYP1A2, and CYP2C9 (4, 5, 14, 16). We have recently demonstrated that micafungin is excreted into bile mainly by multidrug resistance-associated protein 2 (ABCC2/Mrp2) and partly by ABCB1/P-glycoprotein, and its plasma protein binding level is very high (>99%) in Sprague-Dawley (SD) rats (1). The effect of plasma protein binding on the pharmacokinetics of micafungin, however, is not clear yet.
Nagase analbuminemic rats, which have been established from SD rats, are characterized by a considerably low plasma albumin concentration and hyperlipidemia (13). Many studies regarding the pharmacokinetic characteristics of various drugs in Nagase analbuminemic rats have been published (2, 3, 7-9, 18).
The present study aims to clarify the role of plasma proteins such as albumin, high-density lipoprotein (HDL), and gamma globulin in the pharmacokinetics of micafungin in analbuminemic rats.
Standard solutions of micafungin and FR195743 (an internal standard) were kindly supplied by Astellas Pharma Inc. (Tokyo, Japan). Micafungin for injection was purchased commercially. Human serum albumin (HSA) was obtained from Sigma Chemicals (St. Louis, MO). Human HDL and gamma globulin were purchased from Wako Chemicals (Tokyo, Japan). C219 mouse monoclonal antibody to P-glycoprotein (Dako, Glostrup, Denmark), human monoclonal antibody against Mrp2 (Alexis Biochemicals, San Diego, CA), mouse monoclonal antibody to β-actin (Sigma), and horseradish peroxidase-conjugated anti-mouse immunoglobulin G (GE Healthcare UK Ltd., Buckinghamshire, United Kingdom) were used for Western blotting. All other reagents were obtained commercially and were of the highest purity available. Micafungin, HSA, gamma globulin, and HDL were dissolved in saline.
Male SD rats (8 weeks old; body weight, 270 to 295 g) and male Nagase analbuminemic rats (body weight, 225 to 250 g) of the same age as the SD rats were obtained from Japan SLC Inc. (Hamamatsu, Japan). The rats were housed under controlled environmental conditions (temperature of 23 ± 1°C and humidity of 55% ± 5%) with a commercial diet and water freely available. All animal experiments were carried out in accordance with the guidelines of Aichi Medical University for the care and use of laboratory animals.
One day before examination, rats put under anesthesia by intraperitoneal injection of sodium pentobarbital (25 mg/kg of body weight) were cannulated with polyethylene tubes in the right jugular vein for drug administration and blood sampling. The rats received a single dose of micafungin (1 mg/kg) after awakening. The dose of micafungin was chosen according to previous studies (1, 17, 19). Blood samples (<0.2 ml) were collected at designated time intervals (5, 10, 20, 30, and 45 min and 1, 2, 4, 6, 8, and 12 h after injection of micafungin). Plasma samples obtained were stored at −70°C until analysis.
Protein binding experiments with micafungin were performed according to our previous studies (1). An aliquot (0.4 ml) of each sample (fresh plasma obtained from analbuminemic and SD rats and saline solution containing 0.28% HSA, 1% gamma globulin, and 0.05% HDL solution) spiked with the desired concentrations of micafungin was dialyzed against an equal volume of saline for 24 h at 37°C. These concentrations were chosen on the basis of the biochemical tests. The concentrations of albumin and total protein in plasma were determined with the bromcresol green method and the Bradford assay, respectively.
Concentrations of micafungin in each sample were determined by high-performance liquid chromatography according to the method described previously (10) with minor modifications (1).
The expression of hepatic Mrp2 and P-glycoprotein in analbuminemic and SD rats was measured according to our previous studies (1). The relative levels of Mrp2, P-glycoprotein, and β-actin in each gel were measured with the NIH Image program (Bethesda, MD).
The plasma concentration-time data for micafungin were analyzed for each rat by the noncompartmental method. The area under the concentration-versus-time curve (AUC) and the area under the first-moment curve (AUMC) were calculated by the trapezoidal rule with extrapolation to infinity. The systemic clearance (CLsys) was calculated as dose/AUC. The mean residence time (MRT) was calculated as MRT = AUMC/AUC. The steady-state volume of distribution (Vss) was calculated as Vss = CLsys × MRT.
Results are expressed as means ± standard errors. Statistical comparisons were assessed by one-way analysis of variance. Statistical differences between analbuminemic and SD rats were assessed by Student's t test, and P values of less than 0.05 were taken as significant.
Total cholesterol, low-density lipoprotein, and HDL were significantly higher in analbuminemic rats than in SD rats (Table 1) . The concentration of albumin in analbuminemic rats was 1/10 of that in SD rats, although no significant difference in the total protein concentrations was observed between analbuminemic and SD rats.
TABLE 1.
Biochemical data for SD rats and Nagase analbuminemic ratsa
| Animal | Concn (mean ± SD [n = 4])
|
||||
|---|---|---|---|---|---|
| TP (g/liter) | Alb (g/liter) | TC (nmol/liter) | LDL (nmol/liter) | HDL (nmol/liter) | |
| SDR | 56.2 ± 3.3 | 31 ± 2.6 | 12.9 ± 1.7 | 0.12 ± 0.05 | 0.59 ± 0.13 |
| NAR | 53.9 ± 0.8 | 2.8 ± 0.2b | 34.1 ± 1.0b | 0.39 ± 0.08b | 1.43 ± 0.02b |
Abbreviations: SDR, SD rats; NAR, Nagase analbuminemic rats; TP, total protein; Alb, albumin; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Value is significantly different from that for SDR (P < 0.05).
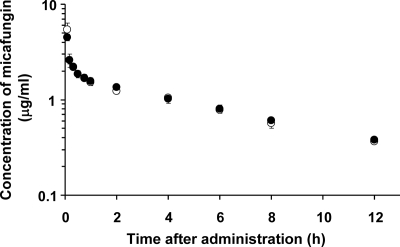
Figure 1 shows the mean plasma concentration-time curves for micafungin after a single intravenous injection in analbuminemic and SD rats. The CLsys and Vss of micafungin in analbuminemic rats were 1.20 ± 0.07 ml/min/kg and 0.52 ± 0.02 liter/kg, respectively, values which were not significantly different from those of SD rats (1.15 ± 0.05 ml/min/kg and 0.54 ± 0.08 liter/kg, respectively). These results indicate that micafungin exhibits the same pharmacokinetic behavior in analbuminemic and SD rats. The levels of plasma protein binding of micafungin in analbuminemic and SD rats were 99.8% ± 0.05% and 99.7% ± 0.06%, respectively, suggesting the possible involvement of other plasma proteins.
FIG. 1.
Mean semilogarithmic plots of plasma concentration-time profiles of micafungin after a single intravenous injection at a dose of 1 mg/kg to analbuminemic (○) and SD (•) rats. Each point represents the mean ± standard error of five experiments. When the standard error is small, it is included in the symbol. No significant differences in plasma concentration data for micafungin at all sampling points were observed between analbuminemic and SD rats.
The protein binding potencies of micafungin for HSA, gamma globulin, and HDL were 99.7% ± 0.06%, 35.1% ± 12.7%, and 97.4% ± 0.25%, respectively, suggesting that micafungin binds to other plasma proteins, gamma globulin, and HDL, besides albumin. It is reported elsewhere that globulins play an important role in the protein binding of azosemide and bumetanide in rats (7, 8). Considering that the concentration of HDL in plasma of analbuminemic rats is twofold higher than that in SD rats, it is reasonable that the plasma protein binding of micafungin in analbuminemic rats is fairly high.
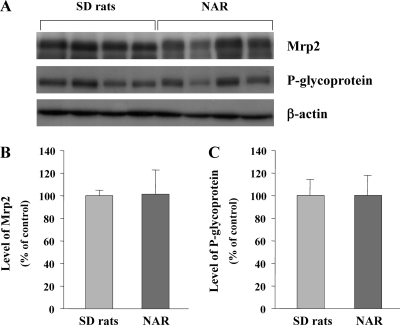
No significant differences in the expression of hepatic Mrp2 and P-glycoprotein were observed between analbuminemic and SD rats, suggesting that analbuminemic rats possess the same transport systems as do SD rats (Fig. 2).
FIG. 2.
Expression of hepatic Mrp2 and P-glycoprotein in Nagase analbuminemic rats (NAR) and SD rats. (A) Western blot analysis of Mrp2, P-glycoprotein, and β-actin in SD rats and NAR. (B) Protein levels of Mrp2 in SD rats and NAR. (C) Protein levels of P-glycoprotein in SD rats and NAR. The expression levels of Mrp2 and P-glycoprotein were evaluated by referring to that of β-actin, and each bar shows the intensity ratio to the value for SD rats (control). Each bar represents the mean ± standard error of four experiments. No significant differences in the expression of Mrp2 and P-glycoprotein were observed between NAR and SD rats.
It is reported elsewhere that the expression of hepatic CYP1A2 increases in analbuminemic rats whereas the expression of CYP2E1 and CYP3A2 is unchanged (8). Considering that micafungin is not metabolized by such CYP isozymes (4, 5, 11, 14-16), it is likely that the metabolism of micafungin is not altered in analbuminemic rats. Analbuminemic rats have been reported to possess normal liver function with no remarkable pathological changes (13). These findings suggest that the pharmacokinetics of micafungin in analbuminemic rats is almost the same as that in SD rats.
In conclusion, the present study is the first to report that the pharmacokinetics of micafungin is not altered in analbuminemic rats because micafungin binds to other plasma proteins, gamma globulin and HDL, besides albumin.
Acknowledgments
We are extremely grateful to Astellas Pharma Inc. (Tokyo, Japan) for the generous contribution of the drug.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Scientific Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Abe, F., J. Ueyama, M. A. Kimata, M. Kato, T. Hayashi, M. Nadai, H. Saito, N. Takeyama, H. Noguchi, and T. Hasegawa. Involvement of multidrug resistance-associated protein 2 (ABCC2/Mrp2) in biliary excretion of micafungin in rats. Life Sci., in press. [DOI] [PubMed]
- 2.Bae, S. K., H. E. Kang, M. K. Kang, J. W. Kim, T. Kim, and M. G. Lee. 2006. Pharmacokinetics of oltipraz in mutant Nagase analbuminemic rats. J. Pharm. Sci. 95:998-1005. [DOI] [PubMed] [Google Scholar]
- 3.Choi, Y. H., S. K. Bae, S. O. Kim, and M. G. Lee. 2007. Pharmacokinetics of 5-fluorouracil in mutant Nagase analbuminemic rats: faster metabolism of 5-fluorouracil via CYP1A. Biopharm. Drug Dispos. 28:87-95. [DOI] [PubMed] [Google Scholar]
- 4.Hebert, M. F., D. K. Blough, R. W. Townsend, M. Allison, D. Buell, J. Keims, and I. Bekersky. 2005. Concomitant tacrolimus and micafungin pharmacokinetics in healthy volunteers. J. Clin. Pharmacol. 45:1018-1024. [DOI] [PubMed] [Google Scholar]
- 5.Hebert, M. F., R. W. Townsend, S. Austin, G. Balan, D. K. Blough, D. Buell, J. Keims, and I. Bekersky. 2005. Concomitant cyclosporine and micafungin pharmacokinetics in healthy volunteers. J. Clin. Pharmacol. 45:954-960. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko, H., Y. Yamato, Y. Teramura, T. Fujiwara, A. Suzuki, A. Kawarura, M. Terakawa, and A. Kagayama. 2002. Metabolites of micafungin in rats and dogs. Jpn. J. Chemother. 50(Suppl. 1):88-93. [Google Scholar]
- 7.Kim, E. J., and M. G. Lee. 2003. Pharmacokinetics and pharmacodynamics of intravenous trasemide in mutant Nagase analbuminemic rats. Biopharm. Drug Dispos. 24:27-35. [DOI] [PubMed] [Google Scholar]
- 8.Kim, E. J., A. K. Lee, S. H. Kim, S. G. Kim, and M. G. Lee. 2003. Pharmacokinetics and pharmacodynamics of intravenous azosemide in mutant Nagase analbuminemic rats. Drug Metab. Dispos. 31:194-201. [DOI] [PubMed] [Google Scholar]
- 9.Kim, E. J., O. K. Suh, and M. G. Lee. 2003. Pharmacokinetics of intravenous theophylline in mutant Nagase analbuminemic rats. Life Sci. 31:1231-1245. [DOI] [PubMed] [Google Scholar]
- 10.Konishi, H., M. Sudo, M. Sumi, H. Morii, T. Minouchi, T. Aimoto, and A. Yamaji. 2005. Pharmacokinetic behavior of micafungin in rats with carbon tetrachloride-induced acute hepatic failure. Biol. Pharm. Bull. 28:556-559. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki, N., K. Matsumoto, K. Ohno, T. Shimamura, H. Furukawa, S. Todo, and S. Kishino. 2006. Effects of hepatic CYP3A4 activity on disposition of micafungin in liver transplant recipients with markedly small-for-size grafts. Transplant. Proc. 38:3649-3650. [DOI] [PubMed] [Google Scholar]
- 12.Mukai, T., T. Ohkuma, K. Nakahara, M. Terakawa, M. Shimizu, T. Uematsu, and J. Azuma. 2001. Pharmacokinetics of FK463, a novel echinocandin analogue, in elderly and non-elderly subjects, abstr. A-30. Program Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 13.Nagase, S., K. Shimamune, and S. Shumiya. 1979. Albumin-deficient rat mutant. Science 205:590-591. [DOI] [PubMed] [Google Scholar]
- 14.Niwa, T., T. Shiraga, and A. Takagi. 2005. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol. Pharm. Bull. 28:1805-1808. [DOI] [PubMed] [Google Scholar]
- 15.Niwa, T., S. Inoue-Yamamoto, T. Shiraga, and A. Takagi. 2005. Effect of antifungal drugs on cytochrome P450 (CYP) 1A2, CYP2D6, and CYP2E1 activities in human liver microsomes. Biol. Pharm. Bull. 28:1813-1816. [DOI] [PubMed] [Google Scholar]
- 16.Sakaeda, T., K. Iwaki, M. Kakumoto, M. Nishikawa, T. Niwa, J. S. Jin, T. Nakamura, K. Nishiguchi, N. Okamura, and K. Okumura. 2005. Effect of micafungin on cytochrome P450 3A4 and multidrug resistance protein 1 activities, and its comparison with azole antifungal drugs. J. Pharm. Pharmacol. 57:759-764. [DOI] [PubMed] [Google Scholar]
- 17.Seibel, N. L., C. Schwartz, A. Arrieta, P. Flynn, A. Shad, E. Albano, J. Keirns, W. M. Lau, D. P. Facklam, D. N. Buell, and T. J. Walsh. 2005. Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob. Agents Chemother. 49:3317-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takada, K., T. Kawamura, M. Inai, S. Masuda, T. Oka, Y. Yoshikawa, N. Shibata, H. Yoshikawa, O. Ike, H. Wada, and S. Hitomi. 1999. Pharmacokinetics of cisplatin in analbuminemic rats. Biopharm. Drug Dispos. 20:421-428. [DOI] [PubMed] [Google Scholar]
- 19.Yamato, Y., H. Kaneko, T. Hashimoto, M. Katashima, K. Ishibashi, A. Kawarura, M. Terakawa, and A. Kagayama. 2002. Pharmacokinetics of the antifungal drug micafungin in mice, rats and dogs, and its in vitro protein binding and distribution to blood cells. Jpn. J. Chemother. 50(Suppl. 1):74-79. [Google Scholar]
- 20.Yamato, Y., H. Kaneko, S. Yamasaki, T. Fujiwara, M. Katashima, A. Kawarura, M. Terakawa, and A. Kagayama. 2002. Distribution and excretion after intravenous dosing of [14C] micafungin to rats. Jpn. J. Chemother. 50(Suppl. 1):80-87. [Google Scholar]