Abstract
Aspergillus fumigatus is an opportunistic fungal pathogen responsible for invasive aspergillosis in immunocompromised individuals. The inefficiency of antifungal agents and high mortality rate resulting from invasive aspergillosis remain major clinical concerns. Recently, we reported on a new family of ultrashort cationic lipopeptides active in vitro against fungi. Mode of action studies supported a membranolytic or a detergent-like effect. Here, we screened several lipopeptides in vitro for their anti-A. fumigatus activity. To investigate the therapeutic properties of the selected peptides in vivo, we challenged immunosuppressed C57BL/6 wild-type mice intranasally with DsRed-labeled A. fumigatus conidia and subsequently treated the animals locally with the lipopeptides. Confocal microscopic analysis revealed the degradation of DsRed-labeled hyphal forms and residual conidia in the lungs of the mice. The most efficient peptide was tested further using a survival assay and was found to significantly prolong the life of the treated animals, whereas no mice survived with the current standard antifungal treatment with amphotericin B. Moreover, as opposed to the drug-treated lungs, the peptide-treated lungs did not display any toxicity of the peptide. Our results highlight the potential of this family of lipopeptides for the treatment of pulmonary invasive aspergillosis.
Aspergillus fumigatus is a respiratory pathogen usually acquired through the inhalation of conidia reaching the small airways and the alveolar spaces. Both mucociliary clearance and phagocytic defense prevent disease development in immunocompetent individuals (24). Macrophages (MΦ) ingest and kill resting conidia mainly through nonoxidative mechanisms, while neutrophils use oxygen-dependent mechanisms to attack hyphae germinating from conidia that escape MΦ surveillance (1, 8, 14). Pulmonary dendritic cells (DC) were reported to play a role in conidium/hypha internalization, transportation to the draining lymph nodes, and induction of local and peripheral Th cell reactivity to the fungus (3). The absence of these protective mechanisms in immunocompromised individuals allows conidia to germinate and invade the lung tissue (7). Invasive aspergillosis (IA) has become an increasing cause of morbidity and mortality in patients with AIDS or those undergoing allogeneic bone marrow transplantation, as well as intensive chemotherapy (1, 18, 19, 29, 31). Although newer drugs with activity against IA have reached the commercial market (4, 10), amphotericin B deoxycholate (D-AMB) is still used extensively for severe cases of IA (26). However, the reported toxicity of D-AMB remains a major concern (33). To reduce toxicity and increase dose delivery, AMB lipid formulations have been developed recently, such as liposomal AMB (L-AMB [AmBisome]) and AMB lipid complex (Abelcet) (2, 15, 30, 32). Corresponding preclinical studies revealed an increased antifungal activity. However, this activity correlated with increased drug levels in the tissues of mouse models of invasive fungal infection (6), which were toxic at doses within a limited range (5). Both drugs injected intravenously (12 mg/kg of body weight) were effective for prolonging survival, and the reduced nephrotoxicity observed with L-AMB allowed the therapeutic index to be increased (20). Using lipid formulations of AMB thus improved IA treatment. Nevertheless, the poor aqueous solubility and the toxicity toward host tissue remain unsolved issues. In addition, inhalation of aerosolized AMB is not well tolerated and is inefficient in preventing invasive pulmonary aspergillosis (IPA) in immunocompromised patients (9, 25). In this study, we selected an immunosuppressed murine model of pulmonary aspergillosis with which to investigate the effects of a new family of ultrashort antifungal lipopeptides on therapeutic efficacy. This family of de novo designed synthetic lipopeptides is composed of only four L and D amino acids linked to fatty acids that have a potent, broad spectrum of in vitro antimicrobial activity against human-pathogenic yeast, fungi, and bacteria. The sequence of the peptidic moiety and the length of the fatty acyl group determine the specificities of the lipopeptides against bacteria, fungi, and mammalian cells. Studies of their plausible modes of action support a membranolytic or detergent-like effect, probably via the carpet mechanism, that should make it difficult for the microorganisms to develop resistance (16). We found that one of the ultrashort lipopeptides exerts specific antifungal activity toward pathogenic A. fumigatus fungi in vivo and increases the life rate and survival of IPA model mice with low toxicity effects and no damage to the treated lung tissues.
MATERIALS AND METHODS
Mice.
This study involved the use of 8-week-old wild-type (WT) C57BL/6 and C57BL/6 CX3CR1GFP mice harboring a targeted replacement of the Cx3cr1 gene with a green fluorescent protein (GFP) reporter (12). All mice were maintained under specific-pathogen-free conditions and handled under protocols approved by the Weizmann Institute animal care committee according to international guidelines.
Fungal strains.
The A. fumigatus CBS 144.89 strain was a clinical isolate. These conidia were transformed by electroporation with the plasmids pAN7-1 and pPgpd-DsRed, harboring a hygB resistance gene and a DsRed gene, respectively (28). Resting DsRed conidia were harvested by washing a 4-day-old culture with phosphate-buffered saline (PBS) supplemented with 0.1% Tween 20 (PBST) (Sigma-Aldrich). The suspension was filtered through a 40-μm cell strainer (Falcon) to separate DsRed conidia from contaminating mycelium and stored at 4°C. The DsRed conidium count was determined with a hemacytometer, and the DsRed conidium suspension was adjusted to the desired concentration by dilution in PBS.
Pulmonary challenge.
Mice were immunosuppressed and challenged as previously described (22). Briefly, we injected intraperitoneally 25 mg of cortisone acetate (Sigma-Aldrich) diluted in PBS supplemented with 0.02% Tween 80 (Sigma-Aldrich) on days 0 and 3. Before mice were infected, they were anesthetized with isoflurane (Nicholas Piramal), and 107 DsRed conidia in 25 μl of PBST were inoculated intranasally on day 0, using a micropipettor. Control nonimmunosuppressed mice received the same conidial suspension. At day 4, an infection readout was carried out on fresh tissue sections isolated from inflated lungs with PBS containing agarose 1% supplemented, or not, with 0.1 μM 5- (and 6)-{[(4 chloromethyl)benzoyl]amino}tetramethylrhodamine (CMTMR; Molecular Probes), using a confocal Axioplan LSM 510 microscope (Zeiss).
Lipopeptide synthesis and purification.
We synthesized lipopeptides composed of four amino acids linked to aliphatic acids with different lengths (28). The sequence of the peptidic moiety was KXXK (X here represents Leu [L], Ala [A], or Lys [K]). All of the peptides were amidated at their C termini, and one of their amino acids was replaced with the d-enantiomer. Lipopeptides were synthesized by a 9-fluorenylmethoxy carbonyl (Fmoc) solid phase method on Rink methylbenzhydrylamine (MBHA) salt resin, by using a 433A automatic peptide synthesizer unit (Applied Biosystems). Purification was performed by reversed-phase high-performance liquid chromatography (purity, >98%), and the lipopeptide composition was confirmed by electrospray mass spectroscopy and amino acid analysis.
Antifungal activity.
The antifungal activity of lipopeptides was measured according to the conditions described in the National Committee for Clinical Laboratory Standards document M27-A (20a). The peptides were examined in sterile polystyrene 96-well plates (F96 microtest plates; Falcon) in a 200-μl volume of solution as follows: 100 μl of a suspension containing fungi at a concentration of 2 × 103 CFU/ml in culture medium (RPMI 1640 medium and 0.165 M morpholinepropanesulfonic acid [pH 7.4], with l-glutamine, without NaHCO3 medium) was added to 100 μl of PBS containing the peptide in serial twofold dilutions. The fungi were incubated for 24 h for DsRed A. fumigatus (CBS 144.89), using a Binder (Tuttingen) KB115 incubator. Growth inhibition was determined by measuring the absorbance at 620 nm in an El309 microplate autoreader (Biotek Instruments). Antifungal activities are expressed as the MICs, the concentration at which no growth was observed. Three independent serial dilution experiments were done, and the results were similar.
Intratracheal instillation.
PBS (25 μl) only or PBS containing either lipopeptides or AMB (purity 80%; Sigma-Aldrich) was applied at the indicated doses to the trachea of mice, as previously described, with modifications (11). Briefly, mice were anesthetized using isoflurane (Nicholas Piramal) and placed vertically, and their tongues were pulled out of the way. Using a long nasal tip, liquid was placed at the top of the trachea and actively aspirated by the mouse.
Efficacy experiments.
After they were infected, immunosuppressed mice were intratracheally treated every 24 h, from day 0 to day 3, with repeated doses of 4.5 mg/kg of lipopeptide. As controls, mice received no treatment or received AMB at 1 mg/kg (nontoxic dose, as shown in toxicological studies cited below) after infection or received DsRed conidia preincubated with lipopeptides at a dose of 4.5 mg/kg. At day 4, fresh lung tissue was isolated from inflated lungs with PBS containing agarose 1% and cut into sections that were observed using a confocal Axioplan LSM 510 microscope (Zeiss).
Survival analysis.
In the first experiment, infected WT mice were intratracheally treated repeatedly every 24 h from day 0 to day 3 or once on day 1 with either lipopeptides at 4.5 mg/kg or with AMB at 1 mg/kg. Mice were followed for morbidity for 14 days postchallenge. In the second experiment, infected WT mice were intratracheally treated repeatedly on days 1, 4, 7, and 11 with lipopeptides at 4.5 mg/kg or with AMB at 1 mg/kg. Mice were followed for morbidity for 21 days postchallenge. As controls, infected immunocompetent and immunosuppressed mice were added to both experiments. Survival rates of the mice were analyzed for all of the treated groups (n = 8 mice per group). Two independent reproducible experiments were done. Lung tissues from each group were collected in the second experiment at the latest survival day along the challenge period. Tissues were fixed in 4% neutral buffered formalin, embedded in paraffin, and cut into 5-μm-thick sections. Sections were stained with periodic acid-Schiff (PAS) for fungal detection and examined microscopically (Olympus E800 microscope).
Evaluation of toxicity.
Noninfected, immunosuppressed mice (n = 3 per group) received intratracheal PBS or lipopeptides at 4.5 mg/kg or AMB at 1 to 4.5 mg/kg on days 1, 4, 7, and 11. Lung tissues from each group were collected at 24 h after the last drug treatment. Tissues were fixed in 4% neutral buffered formalin, embedded in paraffin, and cut into 5-μm-thick sections. Sections were stained with hematoxylin and eosin (H&E) and examined microscopically (Olympus E800) by a board-certified veterinary pathologist.
RESULTS
In vitro activity of lipopeptides.
In our previous study, we reported the MICs of the ultrashort lipopeptides and their lethal activity against representative pathogens including A. fumigatus (ATCC 26430) (16). Based on these results, we have selected five lipopeptides with efficient antifungal activity (amino acids in italics are the d-enantiomers): C16-KAAK, C16-KLLK, C14-KLLK, C16-KKKK and C12-KLLK. In addition, we included the lipopeptide C12-DL6K6 with a longer peptidic chain (12-amino-acid sequence, C12-LKKLLKKLLKKL) (17) and previously showed antifungal activity. These lipopeptides were assayed against the strain of A. fumigatus used in this study (CBS 144.89). The antifungal agent AMB served as a control. The data shown in Table 1 reveal MICs similar to those obtained previously against the ATCC fungal strain and confirmed the potent antifungal activity of these lipopeptides. C16-KAAK, C16-KLLK, and C14-KLLK have lower MICs than AMB, compared to the MICs of the three other lipopeptides. However, C16-KLLK and C14-KLLK were found to be highly hemolytic in our previous study. Furthermore, they were too hydrophobic for efficient in vivo treatment. Since C16-KAAK has potent antifungal activity along with low hemolytic activity, the in vivo study was conducted with this lipopeptide. In addition, we included C12-DL6K6, which revealed intermediate levels of activity.
TABLE 1.
Antimicrobial MICs of the ultrashort lipopeptides
In situ imaging of DsRed-labeled A. fumigatus in the lungs of untreated and treated mice.
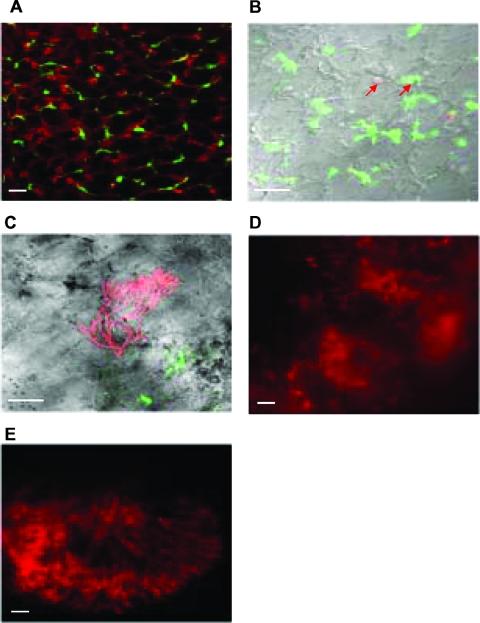
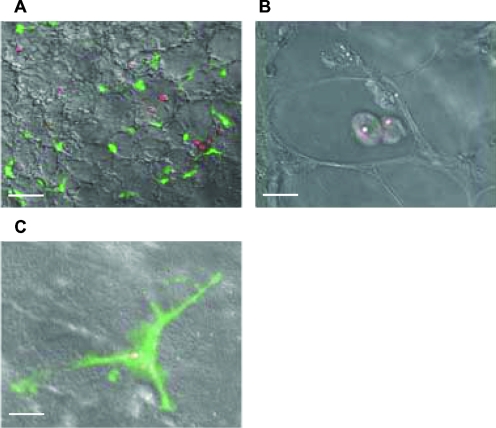
To first visualize, in our aspergillosis murine model, the uptake of DsRed-labeled A. fumigatus conidia by MΦ and DC in the lungs, we used transgenic Cx3cr1gfp/+ mice that expressed GFP in lung DC (12, 13). The lung architecture was revealed by confocal microscopic analysis after CMTMR was injected into inflated lungs (Fig. 1A). GFP-positive DC were located mainly within the lung tissue, whereas autofluorescent MΦ were observed mostly within alveolar spaces. Four days after immunocompetent Cx3cr1gfp/+ mice were intranasally infected, DsRed conidia were phagocytosed by both MΦ and DC in the lungs, as shown by confocal microscopy (Fig. 1B). In immunosuppressed Cx3cr1gfp/+ mice, DsRed hyphal forms started to grow in the lungs (Fig. 1C and D) to further invade the tissue (Fig. 2E). When WT mice received four daily doses of C12-DL6K6, the hyphal forms observed on day 4 were partially degraded (Fig. 2B) compared to those observed with nontreated WT mice (Fig. 2A and C). Interestingly, the use of C16-KAAK, under the same conditions, provided more efficient degradation of hyphal forms (Fig. 2E) scattering red fluorescent spores than the use of AMB (Fig. 2D). Whereas large numbers of developed DsRed hyphal forms could be identified throughout the lungs in untreated control mice (Fig. 2F), only DsRed conidia phagocytosed mainly by macrophages could be identified in the C16-KAAK-treated WT or Cx3cr1gfp/+ mice (Fig. 2G and 3A, respectively). Closer examination of tissue at higher magnification revealed few residual fungi spores exclusively colocalized with MΦ and DC (Fig. 3B and C). Furthermore, this infection state was similar to the one obtained when DsRed conidia were incubated with C16-KAAK prior to infection (Fig. 2H). We therefore continued this study using only the C16-KAAK lipopeptide.
FIG. 1.
Ex vivo confocal imaging of cross sections of fresh lung tissue from CX3CR1GFP mice. (A) Lungs were injected with CMTMR to reveal tissue architecture. Mice were intranasally infected with 107 DsRed A. fumigatus conidia, and the course of infection was observed for immunocompetent mice at day 4 (B) and for immunosuppressed mice at day 4 (C and D) and at day 7 (E). (B) Examples of DsRed A. fumigatus phagocytosed by MΦ and DC are indicated by the red arrows. Hyphal forms are observed starting on day 4 in immunosuppressed mice (C, D, and E). Scale bars = 10 μm.
FIG. 2.
Confocal microscopic analysis of the lungs of untreated or treated immunocompromised WT mice. Four days after mice were intranasally infected with DsRed A. fumigatus conidia, fresh lung sections were isolated from untreated (A, C, and F), C12-DL6K6-treated (B), C16-KAAK-treated (E and G), or AMB-treated (D) mice or mice infected with preincubated DsRed conidia with C16-KAAK (H). Whereas hyphae were partially degraded by C12-DL6K6 (B), C16-KAAK efficiently damaged hyphal forms (E), and only residual conidia were observed (G and H). Scale bars = 10 μm (A, C, F, G, and H) and 5 μm (B, D, and E).
FIG. 3.
Confocal microscopic analysis of the lungs of C16-KAAK-treated immunocompromised CX3CR1GFP mice. Four days after mice were intranasally infected with DsRed A. fumigatus conidia, fresh lung sections were isolated. Residual conidia were detected within both MΦ (A and B) and DC (A and C). Scale bars = 10 μm (A) and 5 μm (B and C).
Animal survival.
Immunosuppressed WT mice were infected intranasally, and two groups received intratracheal administrations of either C16-KAAK, at a dose of 4.5 mg/kg, or AMB, at a nontoxic dose of 1 mg/kg, for the sequential 4 days' treatment experiment. The other two groups received intratracheal administrations of either C16-KAAK, at a single dose of 4.5 mg/kg, or AMB, at a single dose of 1 mg/kg. As controls, immunocompromised and immunocompetent WT mice were infected without delivering any treatment. As shown by Fig. 4A, the survival rate of the mice treated with C16-KAAK at both doses was markedly increased compared to that of the untreated mice. Furthermore, C16-KAAK was more efficient than AMB. Interestingly, the four sequential treatments with the lipopeptide initiated 1 day after infection improved survival at a rate similar to that of the single treatment on the day postinfection. Moreover, when the four sequential lipopeptide treatments were extended on days 1, 4, 7, and 11, we observed a 25% total recovery, whereas all AMB-treated mice died by day 12 (Fig. 4B).
FIG. 4.
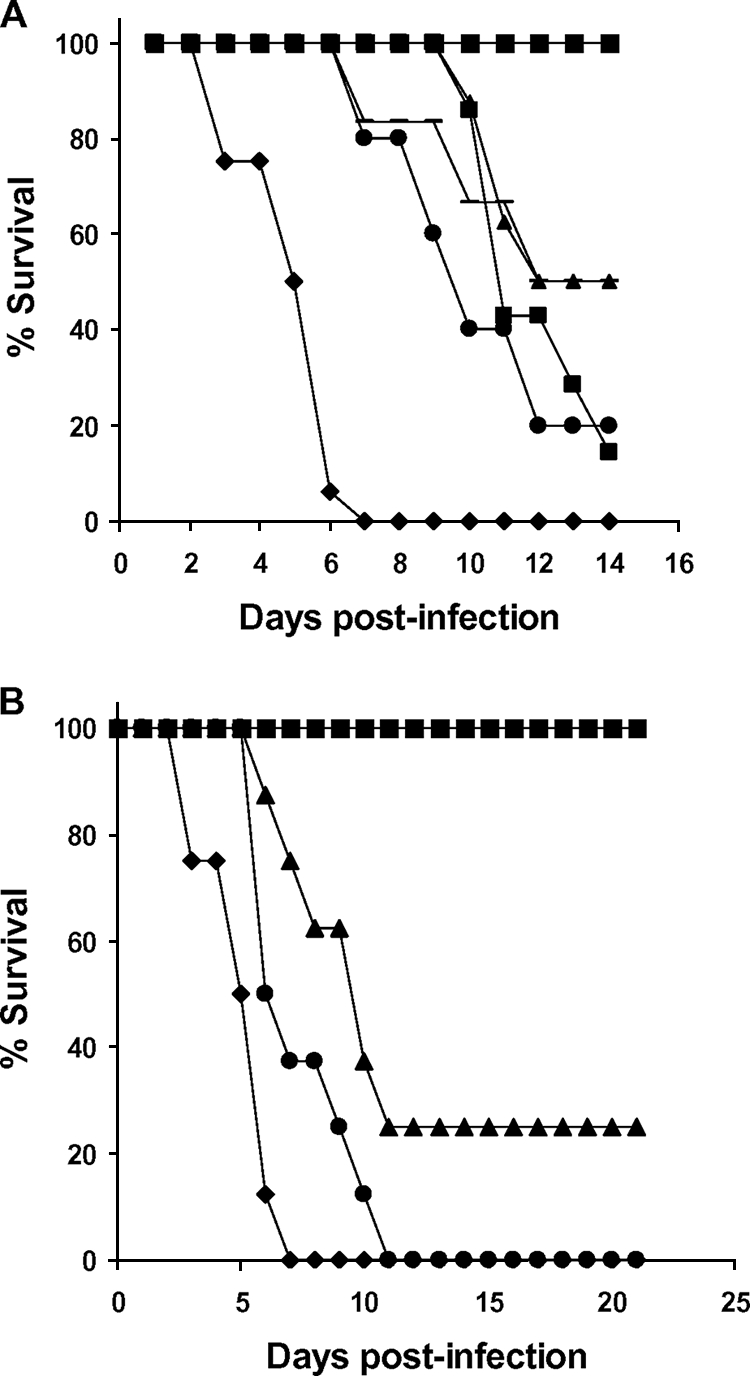
Survival of immunocompromised WT mice. In the first survival assay (A), mice were infected intranasally with DsRed A. fumigatus conidia and treated with vehicle control (♦) or with one dose of C16-KAAK at 4.5 mg/kg day after the infection (▴) or with four doses of C16-KAAK at 4.5 mg/kg every day from the day of the infection (▪) or with one dose of AMB at 1 mg/kg day after the infection (•), or with four doses of AMB at 1 mg/kg every day from the day of the infection (−). Survival was followed over a period of 14 days. In the second survival assay (B), treatments were given on days 1, 4, 7, and 11, and survival was followed over a period of 21 days: vehicle control (♦), C16-KAAK at 4.5 mg/kg (▴), AMB at 1 mg/kg (•). In both assays, infected immunocompetent mice served as a control (▪). Two independent reproducible experiments were done.
Histological examination.
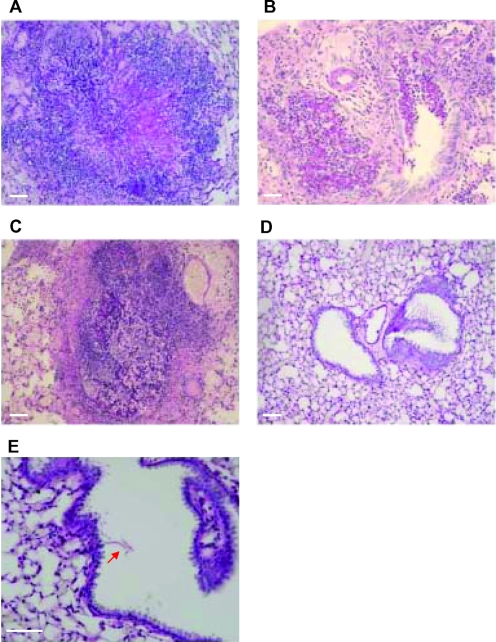
Lung tissues from each group of the prolonged survival assay were removed on the last day for histological examination by PAS. In immunosuppressed mice, untreated (Fig. 5A and B) or treated with AMB (Fig. 5C), large numbers of isolated foci of hyphae and tissue-invasive hyphae could be identified throughout the lungs, with prominent tissue distraction. Interestingly, the lungs of the recovered mice sacrificed on day 21 were almost devoid of fungal contamination. Only residual amounts of damaged A. fumigatus hyphae were identified (Fig. 5D and E).
FIG. 5.
Fungus detection on lung sections of infected immunocompromised WT mice. Lungs of mice from each group of the second survival assay were isolated at the last survival day. Lung paraffin sections were stained with PAS. Focal lesions of IA were observed in both untreated (A and B) and AMB-treated (C) mice. Lung tissue of C16-KAAK-treated mice, at 21 days after infection, was cleared of fungal forms (D), and only a few damaged hyphae were found as indicated by the red arrow (E). Scale bars = 10 μm.
Toxicological studies.
To determine the relative toxicity of the C16-KAAK lipopeptide, we compared its effects at a dose of 4.5 mg/kg on the murine lung tissue to that of AMB at doses ranging from 1 mg/kg to 4.5 mg/kg. Noninfected immunosuppressed WT mice received four sequential, extended, intratracheal treatments. Lung tissues were isolated for histological examination by H&E staining. In control mice injected with PBS only, mild multifocal interstitial plasmacytic infiltration was observed (Fig. 6A). This type of infiltration was the only effect discernible with C16-KAAK at the indicated dose and for AMB at 1 mg/kg (Fig. 6C and B, respectively). However, higher concentrations of AMB resulted in toxic effects such as macrophage infiltration in the airways (Fig. 6D), vasculitis (Fig. 6E), edema (Fig. 6F), airway infiltration of neutrophils, blood vessel formation (Fig. 6G), and hemorrhages (Fig. 6H).
FIG. 6.
Toxicity in lungs of treated immunocompromised WT mice. H&E staining of a lung paraffin section after intratracheal injection of PBS (A), C16-KAAK at 4.5 mg/kg (C), or AMB at 1 mg/kg (B), 2 mg/kg (D), 3 mg/kg (E and F), and 4.5 mg/kg (G and H). Toxic effects were not detected in lungs injected with PBS or C16-KAAK but were detected in lungs injected only with doses of AMB ranging from 2 to 4.5 mg/kg. Scale bars = 10 μm.
DISCUSSION
Numerous strategies to treat IPA infections have been reported, all based on different formulations and applications of AMB and designed to overcome the high toxicity of this antibiotic. Here, we provide direct evidence that ultrashort lipopeptides can specifically damage fungal structures in vivo. Moreover, the C16-KAAK lipopeptide was more efficient against IPA than AMB, at nontoxic doses. Indeed, intratracheal treatment with this lipopeptide provided prolonged survival and a 25% rate of total recovery among infected immunosuppressed mice, with fungal clearance revealed by histology. In addition, no toxic effects associated with the treatment were noticed. The use of AMB at a nontoxic dose of 1 mg/kg was not sufficient to clear fungal structures in infected immunosuppressed mice and consequently resulted in the deaths of 100% of the animals. Higher doses of AMB were not tested in our study due to its toxic effects observed for murine lungs by histology. However, we observed that higher doses of the lipopeptide, up to 10 mg/kg, were tolerated by mice, providing a window for increasing antifungal activity. Moreover, no acute systemic toxicity was observed after an intravenous injection of 10 mg/kg C16-KAAK. As observed for lung sections of C16-KAAK-treated mice, phagocytosed conidia within MΦ and DC on day 4 could be the origin of further invasive aspergillosis once the treatment is aborted. Therefore, increasing the lipopeptide dose could allow a better targeting of the conidia within mononuclear phagocytes in immunosuppressed mice, in which the clearance mechanism of MΦ is impaired. Moreover, we are currently testing this lipopeptide by using other fungi to determine the cross-range of killing activity. As reported in our previous study (16), the attachment of an aliphatic chain to otherwise very short, inert cationic peptides provides lower MICs than many native antimicrobial peptides and specificity toward different cell types. Importantly, most of these ultrashort lipopeptides show low hemolytic levels and several members offered both antibacterial and antifungal activity, as opposed to most known antimicrobial peptides or natural lipopeptides. Furthermore, the incorporation of d- amino acids could give these lipopeptides several advantages compared with their parental lipopeptides which contain all l- amino acids, such as controlled enzymatic degradation. Also, the in vivo prophylactic potential of this lipopeptide remains to be determined. In fact, the few trials that investigated prophylaxis against IA infections prospectively failed to demonstrate a benefit of any strategies, mainly due to toxicity issues (21, 23, 27). In addition, the goal of developing different formulations of AMB is to improve antifungal activity by increasing the antibiotic dose, which potentially raises the issue of resistance that may not be encountered using ultrashort lipopeptides that are naturally degraded in the body.
Therefore, our results suggest that the use of the C16-KAAK lipopeptide could offer a more efficient treatment of IPA and opportunities toward other antifungal applications.
Acknowledgments
We thank Ori Brenner for expertise in pathology, Dorit Natan for technical assistance, and Vladimir Kiss for help with confocal microscopy.
This study was supported by the Association of the Swiss Friends of the Weizmann Institute (to S.J.) and by a grant from the Conseil Pasteur-Weizmann (to Y.S.). Y.S. holds the Harold S. and Harriet B. Brady Professorial Chair in Cancer Research.
Footnotes
Published ahead of print on 7 July 2008.
REFERENCES
- 1.Baddley, J. W., T. P. Stroud, D. Salzman, and P. G. Pappas. 2001. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 32:1319-1324. [DOI] [PubMed] [Google Scholar]
- 2.Bowden, R., P. Chandrasekar, M. H. White, X. Li, L. Pietrelli, M. Gurwith, J. A. van Burik, M. Laverdiere, S. Safrin, and J. R. Wingard. 2002. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 35:359-366. [DOI] [PubMed] [Google Scholar]
- 3.Bozza, S., R. Gaziano, A. Spreca, A. Bacci, C. Montagnoli, P. di Francesco, and L. Romani. 2002. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 168:1362-1371. [DOI] [PubMed] [Google Scholar]
- 4.Caillot, D., H. Bassaris, A. McGeer, C. Arthur, H. G. Prentice, W. Seifert, and K. De Beule. 2001. Intravenous itraconazole followed by oral itraconazole in the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancies, chronic granulomatous disease, or AIDS. Clin. Infect. Dis. 33:e83-e90. [DOI] [PubMed] [Google Scholar]
- 5.Clark, J. M., R. R. Whitney, S. J. Olsen, R. J. George, M. R. Swerdel, L. Kunselman, and D. P. Bonner. 1991. Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob. Agents Chemother. 35:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemons, K. V., and D. A. Stevens. 2004. Comparative efficacies of four amphotericin B formulations—fungizone, amphotec (Amphocil), AmBisome, and Abelcet—against systemic murine aspergillosis. Antimicrob. Agents Chemother. 48:1047-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 8.Dubourdeau, M., R. Athman, V. Balloy, M. Huerre, M. Chignard, D. J. Philpott, J. P. Latge, and O. Ibrahim-Granet. 2006. Aspergillus fumigatus induces innate immune responses in alveolar macrophages through the MAPK pathway independently of TLR2 and TLR4. J. Immunol. 177:3994-4001. [DOI] [PubMed] [Google Scholar]
- 9.Erjavec, Z., G. M. Woolthuis, H. G. de Vries-Hospers, W. J. Sluiter, S. M. Daenen, B. de Pauw, and M. R. Halie. 1997. Tolerance and efficacy of Amphotericin B inhalations for prevention of invasive pulmonary aspergillosis in haematological patients. Eur. J. Clin. Microbiol. Infect. Dis. 16:364-368. [DOI] [PubMed] [Google Scholar]
- 10.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 11.Ho, W., and A. Furst. 1973. Intratracheal instillation method for mouse lungs. Oncology 27:385-393. [DOI] [PubMed] [Google Scholar]
- 12.Jung, S., J. Aliberti, P. Graemmel, M. J. Sunshine, G. W. Kreutzberg, A. Sher, and D. R. Littman. 2000. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20:4106-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landsman, L., C. Varol, and S. Jung. 2007. Distinct differentiation potential of blood monocyte subsets in the lung. J. Immunol. 178:2000-2007. [DOI] [PubMed] [Google Scholar]
- 14.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leenders, A. C., S. Daenen, R. L. Jansen, W. C. Hop, B. Lowenberg, P. W. Wijermans, J. Cornelissen, R. Herbrecht, H. van der Lelie, H. C. Hoogsteden, H. A. Verbrugh, and S. de Marie. 1998. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br. J. Haematol 103:205-212. [DOI] [PubMed] [Google Scholar]
- 16.Makovitzki, A., D. Avrahami, and Y. Shai. 2006. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. USA 103:15997-16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makovitzki, A., and Y. Shai. 2005. pH-dependent antifungal lipopeptides and their plausible mode of action. Biochemistry 44:9775-9784. [DOI] [PubMed] [Google Scholar]
- 18.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 19.Mylonakis, E., T. F. Barlam, T. Flanigan, and J. D. Rich. 1998. Pulmonary aspergillosis and invasive disease in AIDS: review of 342 cases. Chest 114:251-262. [DOI] [PubMed] [Google Scholar]
- 20.Olson, J. A., J. P. Adler-Moore, J. Schwartz, G. M. Jensen, and R. T. Proffitt. 2006. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob. Agents Chemother. 50:2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Pai, M. P., and A. L. Jones. 2004. Altered susceptibility of Candida glabrata bloodstream isolates to triazoles at clinically relevant pH values: comparison of the NCCLS M27-A2, Sensititre YeastOne, and Etest methods. Antimicrob. Agents Chemother. 48:4441-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perfect, J. R., M. E. Klotman, C. C. Gilbert, D. D. Crawford, G. L. Rosner, K. A. Wright, and W. P. Peters. 1992. Prophylactic intravenous amphotericin B in neutropenic autologous bone marrow transplant recipients. J. Infect. Dis. 165:891-897. [DOI] [PubMed] [Google Scholar]
- 22.Philippe, B., O. Ibrahim-Granet, M. C. Prevost, M. A. Gougerot-Pocidalo, M. Sanchez Perez, A. Van der Meeren, and J. P. Latge. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71:3034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieg, G., B. Spellberg, J. Schwartz, Y. Fu, J. E. Edwards, Jr., D. C. Sheppard, and A. S. Ibrahim. 2006. Antifungal prophylaxis is effective against murine invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:2895-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaffner, A., H. Douglas, and A. Braude. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Investig. 69:617-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz, S., G. Behre, V. Heinemann, H. Wandt, E. Schilling, M. Arning, A. Trittin, W. V. Kern, O. Boenisch, D. Bosse, K. Lenz, W. D. Ludwig, W. Hiddemann, W. Siegert, and J. Beyer. 1999. Aerosolized amphotericin B inhalations as prophylaxis of invasive aspergillus infections during prolonged neutropenia: results of a prospective randomized multicenter trial. Blood 93:3654-3661. [PubMed] [Google Scholar]
- 26.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey for the Infectious Diseases Society of America. 2000. Practice guidelines for diseases caused by Aspergillus. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 27.Tollemar, J., O. Ringden, S. Andersson, B. Sundberg, P. Ljungman, and G. Tyden. 1993. Randomized double-blind study of liposomal amphotericin B (Ambisome) prophylaxis of invasive fungal infections in bone marrow transplant recipients. Bone Marrow Transplant 12:577-582. [PubMed] [Google Scholar]
- 28.Vallon-Eberhard, A., L. Landsman, N. Yogev, B. Verrier, and S. Jung. 2006. Transepithelial pathogen uptake into the small intestinal lamina propria. J. Immunol. 176:2465-2469. [DOI] [PubMed] [Google Scholar]
- 29.Wald, A., W. Leisenring, J. A. van Burik, and R. A. Bowden. 1997. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J. Infect. Dis. 175:1459-1466. [DOI] [PubMed] [Google Scholar]
- 30.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh, T. J., J. W. Hiemenz, and E. Anaissie. 1996. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect. Dis. Clin. North Am. 10:365-400. [DOI] [PubMed] [Google Scholar]
- 32.Walsh, T. J., J. W. Hiemenz, N. L. Seibel, J. R. Perfect, G. Horwith, L. Lee, J. L. Silber, M. J. DiNubile, A. Reboli, E. Bow, J. Lister, and E. J. Anaissie. 1998. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 26:1383-1396. [DOI] [PubMed] [Google Scholar]
- 33.Wingard, J. R., P. Kubilis, L. Lee, G. Yee, M. White, L. Walshe, R. Bowden, E. Anaissie, J. Hiemenz, and J. Lister. 1999. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29:1402-1407. [DOI] [PubMed] [Google Scholar]