Abstract
This study extends earlier reports regarding the in vitro efficacies of the 1,4-di-N-oxide quinoxaline derivatives against Mycobacterium tuberculosis and has led to the discovery of a derivative with in vivo efficacy in the mouse model of tuberculosis. Quinoxaline-2-carboxylate 1,4-di-N-oxide derivatives were tested in vitro against a broad panel of single-drug-resistant M. tuberculosis strains. The susceptibilities of these strains to some compounds were comparable to those of strain H37Rv, as indicated by the ratios of MICs for resistant and nonresistant strains, supporting the premise that 1,4-di-N-oxide quinoxaline derivatives have a novel mode of action unrelated to those of the currently used antitubercular drugs. Specific derivatives were further evaluated in a series of in vivo assays, including evaluations of the maximum tolerated doses, the levels of oral bioavailability, and the efficacies in a low-dose aerosol model of tuberculosis in mice. One compound, ethyl 7-chloro-3-methylquinoxaline-2-carboxylate 1,4-dioxide, was found to be (i) active in reducing CFU counts in both the lungs and spleens of infected mice following oral administration, (ii) active against PA-824-resistant Mycobacterium bovis, indicating that the pathway of bioreduction/activation is different from that of PA-824 (a bioreduced nitroimidazole that is in clinical trials), and (iii) very active against nonreplicating bacteria adapted to low-oxygen conditions. These data indicate that 1,4-di-N-oxide quinoxalines hold promise for the treatment of tuberculosis.
The high number of multidrug-resistant Mycobacterium tuberculosis strains circulating worldwide has increased concern that tuberculosis (TB) may once again become an incurable disease and has emphasized the need for new drugs to treat this infection. Over the last few years, extensively drug-resistant TB, defined as TB caused by M. tuberculosis strains resistant to isoniazid (INH), rifampin, any fluoroquinolone, and one of the injectable drugs amikacin, kanamycin, and capreomycin, has become a major concern for TB treatment and control in the global setting (2, 7, 9, 11, 12, 16, 19, 28, 31). In addition, strains with resistance to all of the major clinically used antitubercular drugs are known (21), thus answering the question posed by Dye and Espinal, “Will tuberculosis become resistant to all antibiotics?” (8).
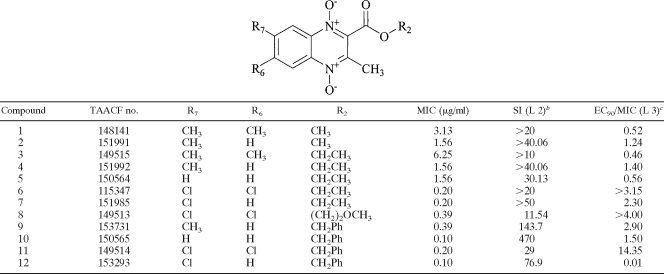
Our antitubercular research group has previously published several papers in which the synthesis and primary biological valuation of a large number of quinoxaline and quinoxaline 1,4-di-N-oxide derivatives were described (14, 22, 24-27, 32). In this context, different quinoxaline-2-carboxylate 1,4-dioxide derivatives were synthesized and evaluated as anti-M. tuberculosis agents (15). Some of them showed good in vitro parameters in a cytotoxicity assay and in an M. tuberculosis-infected macrophage model (Table 1). In this report, we detail the in vitro activities and in vivo efficacies of quinoxaline-2-carboxylate 1,4-di-N-oxide derivatives.
TABLE 1.
Structures of the quinoxaline derivatives and previously reported in vitro activities against M. tuberculosisa
Data are from reference 15.
The SI is the selectivity index calculated as the concentration inhibiting the growth of Vero cells in culture by 50% following 72 h of exposure (assessed using the CellTiter 96 nonradioactive cell proliferation assay reagent from Promega) divided by the MIC. L 2, assay level 2 according to the TAACF assay program.
EC90/MIC is a measure of the activity against intracellular M. tuberculosis taken up by mouse bone marrow macrophages, calculated as the 90% effective concentration (EC90), or the concentration required to inhibit the growth of intracellular M. tuberculosis by 90%, divided by the MIC. L 3, assay level 3 according to the TAACF assay program.
(Portions of these data were presented in abstract form at the Gordon Conference on Tuberculosis and Drug Development, Oxford, United Kingdom, August 2007, and the 38th Union World Conference on Lung Health of the International Union against Tuberculosis and Lung Disease, Cape Town, South Africa, November 2007.)
MATERIALS AND METHODS
Compound synthesis.
The quinoxaline derivatives used in this study were prepared by procedures described in the literature (15, 26) by the reaction of the appropriate benzofuroxan with the corresponding β-ketoester. The structural properties of the compounds were confirmed by nuclear magnetic resonance, mass spectrometry, and infrared analyses, and the purity was established by elemental analyses. The compounds described in the present work have demonstrated activity in previous assays (15): a MIC and cytotoxicity assay and an assay of activity against M. tuberculosis growing in macrophages. A list of the compounds and their structures and biological activities is presented in Table 1.
Determination of minimum bactericidal concentrations (MBCs) and MICs for single-drug-resistant strains of M. tuberculosis.
Compounds were dissolved in dimethyl sulfoxide and diluted in test media to the desired concentrations, with the final dimethyl sulfoxide concentration kept below 0.625%. MICs for a minimum of three strains of drug-resistant M. tuberculosis (each strain resistant to a single TB drug) were determined by the microplate Alamar Blue assay (MABA) (10).
The MBCs for the M. tuberculosis H37Rv strain and the other strains tested were then determined by subculturing the strains onto drug-free solid medium and enumerating CFU after exposure for 5 days at 37°C in Middlebrook 7H9 medium supplemented with drug concentrations equivalent to and higher than the previously determined MICs for the respective strains. The MBC was the lowest concentration of the drug that killed more than 99.9% of the bacterial population present when the drug was added.
Potential for cross-resistance with PA-824.
PA-824 is a nitroimidazole agent in clinical trials for treating TB. Mycobacterium bovis strains resistant to PA-824, created using Tn5367 mutagenesis (4), were obtained from Lacy Daniels (Texas A&M College of Pharmacy, Kingsville, TX). MICs were determined by broth microdilution using the MABA. PA-824 was obtained from the Global Alliance for Tuberculosis Drug Development (New York, NY).
Maximum tolerated dose (MTD) and oral bioavailability.
All experiments using mice were approved by the Animal Care and Use Committee at Colorado State University (approval no. 06-221A-02; expiration date, 17 October 2008) and under Animal Welfare Assurance number A3572-01. Single doses of drugs at 100, 300, or 1,000 mg/kg of body weight were administered (by gavage) to three C57BL/6 female mice per dose. Mice were observed postadministration at 4 and 6 h and then twice daily for the duration of the study (1 week). The oral bioavailability was determined by bioassays as described previously (13).
Rapid in vivo screen.
Eight- to 10-week-old female specific-pathogen-free C57BL/6-Ifngtm1ts mice (gamma interferon gene knockout [GKO] mice) were purchased from Jackson Laboratories, Bar Harbor, ME (6). Mice were infected via low-dose aerosol exposure to M. tuberculosis Erdman by using a Middlebrook aerosol generation device (Glas-Col Inc., Terre Haute, IN), and the rapid screening model was carried out as described previously (18). One day postinfection, three mice were sacrificed to verify the uptake of 50 to 100 CFU of bacteria per mouse. Negative control mice remained untreated. An INH-treated control group, receiving the drug via oral gavage at 25 mg/kg/day, was included in each study. Each treatment group consisted of five mice. Treatment was started 18 days postinfection and continued for nine consecutive days. Five infected mice were killed at the start of treatment as pretreatment controls. Drugs were administered daily by oral gavage using 0.5% methyl cellulose (200-μl volumes).
Dose-response analysis in the rapid in vivo screen.
The in vivo activity of compound 8 (Tuberculosis Antimicrobial Acquisition and Coordinating Facility [TAACF] no. 151985) was examined in the rapid screening model to repeat the efficacy testing and to estimate the minimal effective dose. Doses of 25, 100, and 300 mg/kg were tested using the same methods as those in the initial in vivo test.
Statistical analysis.
The viable counts were converted to logarithms, which were then evaluated by a one-way analysis of variance followed by a multiple-comparison analysis of variance using a one-way Tukey test (in the SigmaStat software program). Differences were considered significant at the 95% level of confidence.
Activity against nonreplicating persistent M. tuberculosis.
The activity of compound 8 against nonreplicating M. tuberculosis was determined by measuring the activity against M. tuberculosis bacteria adapted to a low-oxygen environment under anaerobic conditions (3). This low-oxygen-recovery assay (LORA) quantifies antibacterial activity by measuring the ability of an M. tuberculosis recombinant strain, containing a plasmid with an acetamidase promoter driving a bacterial luciferase gene, to produce a luminescent signal when placed back into an environment with ambient oxygen.
RESULTS AND DISCUSSION
The most potent compounds from our previous studies were subjected to the following set of tests: determination of (i) the MICs for different single-drug-resistant strains of M. tuberculosis, (ii) the MBCs, (iii) the activities against nonreplicating bacteria, (iv) the MTDs, (v) the levels of oral bioavailability, and (vi) the in vivo efficacies.
Table 2 shows the MICs obtained for single-drug-resistant strains of M. tuberculosis, including those resistant to INH, rifampin, thiacetazone, ethambutol (EMB), ciprofloxacin (CIP), kanamycin (KAM), ethionamide, and p-aminosalicylic acid. In general, all the compounds showed good MICs for resistant strains; MICs ranged between 0.1 and 6.25 μg/ml, with the exception of that of 3,7-dimethylquinoxaline-2-carboxylate 1,4-di-N-oxide (compound 2), a drug which showed poor activity against EMB-resistant and KAM-resistant strains, with MICs of 25 μg/ml. The susceptibilities of resistant strains can be considered comparable to those of H37Rv, as indicated by the ratios of MICs for resistant and nonresistant M. tuberculosis strains (calculations not shown). This finding suggests that there is minimal if any cross-resistance with the current anti-TB drugs, thereby supporting the notion of a novel mechanism of action. These results are promising for the development of new effective compounds against the growing number of drug-resistant strains. The quinoxaline-2-carboxylate derivative with a benzyl group in the carboxylate (compound 10) was the most active compound, with MICs of 0.4 μg/ml or less for the resistant strains.
TABLE 2.
MICs for strains of single-drug-resistant M. tuberculosis
| Compound | MIC (μg/ml)a for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| H37Rv | INH-R | RIF-R | TAC-R | EMB-R | CIP-R | KAM-R | ETA-R | PAS-R | |
| 1 | 3.13 | 3.13 | 3.13 | ≤1.56 | ≤1.56 | 3.13 | 3.13 | 3.13 | 3.13 |
| 2 | 3.13 | 6.25 | 3.13 | 1.56 | 25 | 6.25 | 25 | 3.13 | 3.13 |
| 3 | ≤3.13 | 6.25 | ≤3.13 | ≤3.13 | ≤3.13 | 12.5 | ≤3.13 | 6.25 | NDb |
| 4 | 3.13 | ND | 3.13 | 0.39 | ND | 6.25 | ND | 6.25 | 3.13 |
| 5 | 1.56 | 1.56 | 1.56 | ND | 1.56 | 3.13 | 1.56 | ND | ND |
| 6 | 0.2 | 0.39 | 0.2 | ND | 0.2 | 0.2 | ≤0.1 | ND | ND |
| 7 | 0.20 | 0.39 | 0.39 | ND | 0.39 | 0.78 | 0.39 | ND | ND |
| 8 | ≤0.2 | ≤0.2 | ≤0.2 | 0.39 | ND | 0.39 | ND | 0.78 | 0.78 |
| 9 | 0.39 | ND | 0.39 | 0.39 | ND | 0.39 | ND | 1.56 | 0.78 |
| 10 | 0.2 | 0.2 | 0.2 | 0.2 | ND | 0.1 | ND | 0.4 | 0.2 |
| 11 | 0.39 | 1.56 | 0.39 | 0.78 | 0.78 | 0.39 | 0.39 | 0.78 | ND |
INH-R, INH-resistant strain; RIF-R, rifampin-resistant strain; TAC-R, thiacetazone-resistant strain; EMB-R, EMB-resistant strain; CIP-R, ciprofloxacin-resistant strain; KAM-R, KAM-resistant strain; ETA-R, ethionamide-resistant strain; PAS-R, p-aminosalicylic acid-resistant strain.
ND, not determined.
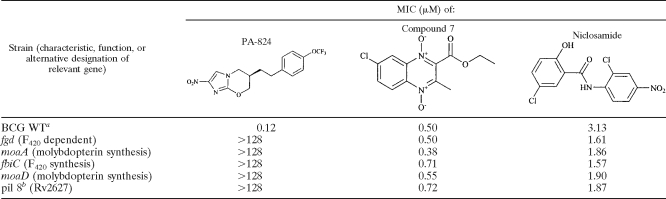
Compound 7 is likely activated via bioreduction in bacteria, similar to the reduction observed for other N-oxides (29). Since PA-824, a nitroimidazole in clinical trials for treating TB, is bioreduced into an active intermediate (4, 5, 20), we tested the activity of compound 7 against an isogenic set of M. bovis strains with defined resistance to PA-824. PA-824 is bioreduced into an active form by a pathway involving the deazaflavin F420 cofactor-dependent glucose dehydrogenase (Fdg1) and other cellular factors, including molybdopterin, a cofactor for many oxidoreductase enzymes. Thus, the loss of function of Fdg1 or the loss of the ability to synthesize the F420 cofactor leads to resistance to PA-824 (Table 3). Compound 7 was active against all PA-824-resistant M. bovis strains tested, thus showing the lack of cross-resistance and supporting the evidence for a different pathway of drug activation. Niclosamide (an anthelmintic agent that shows poor absorption from the host intestine) was included as another control compound that is structurally different from PA-824 and activated by a different pathway (1).
TABLE 3.
In vitro activities against susceptible and genetically defined PA-824-resistant mycobacteria
BCG WT, wild-type M. bovis strain BCG Montreal; MICs for this strain were measured by the MABA, as were those for all other M. bovis strains.
The precise function of pil 8 (Rv2627) is unknown.
Compound 7 was also tested under aerobic and anaerobic (LORA) conditions for activity against M. tuberculosis strain H37Rv containing the luciferase reporter. Compound 7 was active under aerobic growth conditions (MIC = 0.24 μg/ml) and under anaerobic conditions (LORA MIC = 0.42 μg/ml). The corresponding values for PA-824 and niclosamide were as follows: MICs under aerobic growth conditions, 0.10 and 0.28 μg/ml, respectively, and LORA MICs, 1.4 and 0.32 μg/ml, respectively. Activity against nonreplicating bacteria is another unique property of quinoxaline 1,4-di-N-oxides. Although the idea is speculative, activity targeting nonreplicating bacteria may translate into faster sterilization of infected tissues, and we are in the process of testing this possibility in animal models. The long continuation phase for the treatment of TB is believed to be due in part to the presence of nonreplicating organisms that persist even in the presence of antitubercular drugs. PA-824 is an experimental nitroimidazole that is in phase I clinical trials. PA-824 is bioreduced by M. tuberculosis into an active component. Niclosamide is another bioreduced drug that is active in the LORA. Compound 7 was slightly more active than PA-824 in the LORA on a molar basis (1.5 μM versus 4.85 μM).
The MBCs of select compounds for H37Rv, along with the MICs, were determined using the MABA (10). The MIC of compound 7 tested as 0.6 μg/ml in this assay, and the MBC was 2.5 μg/ml (MBC/MIC ratio = 4.2). The MIC of compound 6 was 0.2 μg/ml, with an MBC of 3.13 μg/ml (MBC/MIC ratio = 15.7), and the MIC of compound 10 was 0.39 μg/ml, with an MBC of >6.25 μg/ml (MBC/MIC ratio > 16.0). A compound is generally considered to be bactericidal if the ratio of the MBC to the MIC is ≤4 (30), so the ratios for these compounds against H37Rv indicated bacteriostatic rather than bactericidal activity by this definition. However, bactericidal activity in vivo is determined by peak levels in blood and tissues and exposure times following oral administration. The in vivo peak levels of compound 7 in serum reached 5 to 8 μg/ml 15 to 30 min following the administration of 100- to 200-mg/kg doses, as determined by bioassays, and bactericidal activity was observed in vivo (see below for more on in vivo efficacy).
Selected compounds 6 to 12 were evaluated in in vivo assays, including tests for efficacy in the mouse model of TB infection. The MTD of each compound was determined by using an escalating single dose of the drug given to mice by oral gavage. No acute adverse effects, reactions, or toxicity were observed at oral doses of up to 1,000 mg/kg (the highest dose tested). Subsequent in vivo evaluations of the efficacies of compounds 6 to 12 were done with doses of 300 mg/kg administered via oral gavage to infected GKO C57BL/6 mice (18). Six of the seven compounds were found to be inactive in that they did not effectively reduce the bacterial numbers in the lungs and spleens with respect to those in the untreated controls (Table 4). While we have not definitively determined the reason for the lack of in vivo efficacy of compounds 6 and 8 to 12, it appears that the lack of activity of compound 10 may be due to extensive serum binding, since the MIC is reduced 200-fold in the presence of serum. Likewise, the lack of efficacy of compound 6 appears to be due to a short in vivo half-life, based on the results of preliminary studies. The lack of activity was not due to the potency of M. tuberculosis strain Erdman, as all of the compounds were active against this strain (Table 4).
TABLE 4.
Evaluation of in vivo efficacies in the GKO mouse modela
| Treatment (dose [mg/kg] or time point) | TAACF no. (MIC)b | Organ | Log CFU/organ (SD) | Log CFU decrease vs control |
|---|---|---|---|---|
| None (day 15) | Lung | 6.81 (±0.09) | ||
| Spleen | 5.37 (±0.18) | |||
| None (day 24) | Lung | 7.57 (±0.11) | ||
| Spleen | 6.57 (±0.17) | |||
| INH (25) | Lung | 4.47 (±0.12) | 3.10 | |
| Spleen | 2.20 (±0.45) | 4.37 | ||
| Compound 6 (300) | 115347 (0.39) | Lung | 7.64 | −0.06 |
| Spleen | 6.75 | −0.18 | ||
| Compound 7 (300) | 151985 (0.78) | Lung | 4.55 (±0.23) | 3.02 |
| Spleen | 3.95 (±0.10) | 2.62 | ||
| Compound 8 (300) | 149513 (0.39) | Lung | 8.73 | −0.68 |
| Spleen | 7.18 | −0.47 | ||
| Compound 9 (300) | 153731 (0.78) | Lung | 7.17 | 0.4 |
| Spleen | 6.63 | −0.016 | ||
| Compound 10 (300) | 150565 (0.10) | Lung | 7.41 | 0.16 |
| Spleen | 6.96 | −0.38 | ||
| Compound 11 (300) | 149514 (0.10) | Lung | 8.07 | −0.03 |
| Spleen | 7.17 | −0.46 | ||
| Compound 12 (300) | 153293 (0.10) | Lung | 7.21 | 0.36 |
| Spleen | 6.62 | −0.05 |
Results from experiment 1 are shown.
The MICs for M. tuberculosis strain Erdman (used in the in vivo efficacy testing) were determined for all compounds selected for in vivo efficacy testing and are given in parentheses.
However, the ethyl 7-chloroquinoxaline derivative (compound 7) afforded significant reductions of 3.2 and 2.62 log10 CFU in lungs and spleens, respectively. Generally, compounds are considered to be active if they yield at least a 0.75-log10 reduction in bacterial counts compared to day 24 counts. The protection shown by compound 7 is similar to that afforded by clinically available compounds and other compounds classified as very active (17). INH reduced the numbers of CFU by 3.10 and 4.17 log units in the same experiment. Since the majority of compounds showing good in vitro activity do not show in vivo efficacy in mice (23), the in vivo activity of ethyl 7-chloro-3-methylquinoxaline-2-carboxylate 1,4-di-N-oxide is promising. Bactericidal activity of compound 7 was observed, since the final lung CFU count following 9 days of treatment was below the starting lung CFU count on day 15, the day therapy was initiated (6.81 log CFU untreated lung, compared to 4.55 log CFU following 9 days of treatment) (Table 4). Similar evidence for bactericidal activity was obtained from the spleen data, with an initial count of 5.37 log CFU untreated spleen compared to 3.95 log CFU following 9 days of treatment (Table 4).
In a second experiment (Table 5), the dose-response relationship for compound 7 in vivo was determined using the GKO mouse model and doses of 25, 100, and 300 mg/kg (Table 5). Compound 7 was weakly active in the lung and spleen at a dose of 25 mg/kg (CFU readings for tissues from treated mice on day 24 were lower than those for tissues from the untreated controls on day 24, but the activity appeared to be bacteriostatic). At 100 and 300 mg/kg (P, <0.001 by a one-way analysis of variance followed by a multiple-comparison analysis of variance using a one-way Tukey test in the SigmaStat software program), compound 7 appeared to be bactericidal, since the numbers of CFU recovered from both the lungs and spleens were well below the CFU levels observed on day 15 (Table 5), except for the spleens from the 100-mg/kg-dose group, in which the mean number of CFU was reduced by only 0.28 logs, which was not significant. Activity at a dose of 300 mg/kg was striking in that it lowered the numbers of CFU by 5.58 and 5.51 logs, respectively, in the lungs and spleens. Although no clear toxicity was apparent in the single-dose MTD test and in the first in vivo experiment, some toxicity was observed in the second dose-response experiment; three mice in the 300-mg/kg group died during treatment, which was truncated to 7 days instead of the usual 9 days. No CFU were recovered from the lung and spleen of one of the surviving mice dosed at 300 mg/kg when the entire organs were plated for CFU enumeration. Although it appears that multiple dosing at 300 mg/kg may result in some toxicity in infected animals, data collected to date on this series (quinoxaline-2-carboxylate 1,4-di-N-oxide derivatives) indicate that both in vitro (cytotoxicity) and in vivo toxicity can be separated from the antitubercular activity, as determined by plotting MICs versus 50% inhibitory concentrations for over 100 analogs, wherein no correlation was observed. Although the MTD is high (>1,000 mg/kg for normal mice), we have observed that some compounds can show toxicity in infected GKO mice at lower levels, due likely to the added stress of disease. Thus, 300 mg/kg may be on the borderline for tolerability in GKO infected mice and may therefore show toxicity in some experiments. Experiments with normal C57BL/6 mice and longer treatment times are being scheduled.
TABLE 5.
Dose-response data for compound 7 (TAACF 151985) in the GKO mouse model
| Treatment (dose [mg/kg] or time point) | Organ | Log CFU/organ (SD) | Log CFU decrease vs control | Comment |
|---|---|---|---|---|
| None (day 15) | Lung | 6.62 (±0.14) | NAa | Normal |
| Spleen | 4.42 (±0.52) | NA | Normal | |
| None (day 24) | Lung | 7.76 (±0.10) | NA | Normal |
| Spleen | 6.86 (±0.16) | NA | Normal | |
| INH (25) | Lung | 4.43 (±0.06) | 3.33 | Normal |
| Spleen | 1.82 (±0.31) | 5.04 | 1 Mouse without CFU | |
| TAACF 151985 (25) | Lung | 6.77 (±0.03) | 0.99 | Normal |
| Spleen | 5.25 (±0.48) | 1.61 | Normal | |
| TAACF 151985 (100) | Lung | 5.73 (±0.05) | 2.03 | Normal |
| Spleen | 4.14 (±0.22) | 2.72 | Normal | |
| TAACF 151985 (300) | Lung | 2.18 (±1.30) | 5.58 | Toxicityb |
| Spleen | 1.35 (±0.65) | 5.51 | Toxicityb |
NA, not applicable.
Data were obtained from only two mice. Three deaths occurred due to apparent drug toxicity. Only one surviving mouse had CFU; the other mouse was culture negative. Therapy stopped at 7 days. Mice were slightly lethargic, with a hunched posture.
In conclusion, an extended evaluation of the in vitro and in vivo antimycobacterial activities of quinoxaline 1,4-di-N-oxide derivatives was performed. Most of these compounds displayed good inhibitory activity against resistant strains, and only compound 2 was associated with apparent cross-resistance in EMB-resistant and KAM-resistant strains, although we have not yet confirmed these data. Compound 7 was the only analog active in vivo. This activity was clearly due to adequate oral bioavailability and levels in the blood (measured at 3, 6, and 9 μg/ml for the 25-, 100-, and 300-mg/kg doses, respectively) and to a terminal elimination half-life of 4 to 8 h (unpublished data). Finally, we did detect bactericidal activity when we tested the sera of animals dosed orally with 200 mg of compound 7/kg and bled at 30 min. Sera diluted twofold killed 2.51 logs, and when diluted fourfold, they still killed 1.15 logs after 7 days of incubation. Thus, compound 7 has all of the necessary characteristics of a validated lead that can serve as the starting point for more advancement in medicinal chemical and preclinical development.
Acknowledgments
Antimycobacterial data were provided by the TAACF through research and development contracts.
This research was funded in part by contracts N01-AI-95364 (Southern Research Institute/University of Illinois at Chicago) and N01-AI-95385 (Colorado State University) from the National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Published ahead of print on 14 July 2008.
REFERENCES
- 1.Abreu, F. C., M. O. Goulart, and A. M. Brett. 2002. Detection of the damage caused to DNA by niclosamide using an electrochemical DNA-biosensor. Biosens. Bioelectron. 17:913-919. [DOI] [PubMed] [Google Scholar]
- 2.Benatar, S. R. 2006. Extensively drug resistant tuberculosis: problem will get worse in South Africa unless poverty is alleviated. Br. Med. J. 333:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho, S. H., S. Warit, B. J. Wan, C. H. Hwang, G. F. Pauli, and S. G. Franzblau. 2007. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 51:1380-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, K. P., T. B. Bair, Y. M. Bae, and L. Daniels. 2001. Use of transposon Tn5367 mutagenesis and a nitroimidazopyran-based selection system to demonstrate a requirement for fbiA and fbiB in coenzyme F420 biosynthesis by Mycobacterium bovis BCG. J. Bacteriol. 183:7058-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi, K. P., N. Kendrick, and L. Daniels. 2002. Demonstration that fbiC is required by Mycobacterium bovis BCG for coenzyme F420 and FO biosynthesis. J. Bacteriol. 184:2420-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahle, U. R. 2006. Extensively drug resistant tuberculosis: beware patients lost to follow-up. Br. Med. J. 333:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye, C., and M. A. Espinal. 2001. Will tuberculosis become resistant to all antibiotics? Proc. Biol. Sci. 268:45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinal, M. A. 2003. The global situation of MDR-TB. Tuberculosis 83:44-51. [DOI] [PubMed] [Google Scholar]
- 10.Franzblau, S. G., R. S. Witzig, J. C. McLaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B. Cook, V. K. Quenzer, R. M. Ferguson, and R. H. Gilman. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 36:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi, N. R., A. Moll, A. W. Sturm, R. Pawinski, T. Govender, U. Lalloo, K. Zeller, J. Andrews, and G. Friedland. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575-1580. [DOI] [PubMed] [Google Scholar]
- 12.Goldman, R. C., K. V. Plumley, and B. E. Laughon. 2007. The evolution of extensively drug resistant tuberculosis (XDR-TB): history, status and issues for global control. Infect. Disord. Drug Targets 7:73-91. [DOI] [PubMed] [Google Scholar]
- 13.Gruppo, V., C. M. Johnson, K. S. Marietta, H. Scherman, E. E. Zink, D. C. Crick, L. B. Adams, I. M. Orme, and A. J. Lenaerts. 2006. Rapid microbiologic and pharmacologic evaluation of experimental compounds against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 50:1245-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaso, A., B. Zarranz, I. Aldana, and A. Monge. 2003. Synthesis of new 2-acetyl and 2-benzoyl quinoxaline 1,4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents. Eur. J. Med. Chem. 38:791-800. [DOI] [PubMed] [Google Scholar]
- 15.Jaso, A., B. Zarranz, I. Aldana, and A. Monge. 2005. Synthesis of new quinoxaline-2-carboxylate 1,4-dioxide derivatives as anti-Mycobacterium tuberculosis agents. J. Med. Chem. 48:2019-2025. [DOI] [PubMed] [Google Scholar]
- 16.Lawn, S. D., and R. Wilkinson. 2006. Extensively drug resistant tuberculosis: a serious wake-up call for global health. Br. Med. J. 333:559-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenaerts, A. J., V. Gruppo, K. S. Marietta, C. M. Johnson, D. K. Driscoll, N. M. Tompkins, J. D. Rose, R. C. Reynolds, and I. M. Orme. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenaerts, A. J. M., V. Gruppo, J. V. Brooks, and I. M. Orme. 2003. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 47:783-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manissero, D., and K. Fernandez de la Hoz. 2006. Extensive drug-resistant TB: a threat for Europe? Euro Surveill. 11:E060928. [DOI] [PubMed] [Google Scholar]
- 20.Manjunatha, U. H., H. Boshoff, C. S. Dowd, L. Zhang, T. J. Albert, J. E. Norton, L. Daniels, T. Dickl, S. S. Pang, and C. E. Barry. 2006. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 103:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migliori, G. B., G. De Iaco, G. Besozzi, R. Centis, and D. M. Cirillo. 2007. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill. 12:E070517.1. [DOI] [PubMed] [Google Scholar]
- 22.Montoya, M. E., Y. Sainz, M. A. Ortega, A. L. De Cerain, and A. Monge. 1998. Synthesis and antituberculosis activity of some new 2-quinoxalinecarbonitriles. Farmaco 53:570-573. [DOI] [PubMed] [Google Scholar]
- 23.Orme, I., J. Secrist, S. Anathan, C. Kwong, J. Maddry, R. Reynolds, A. Poffenberger, M. Michael, L. Miller, J. Krahenbuh, L. Adams, A. Biswas, S. Franzblau, D. Rouse, D. Winfield, and J. Brooks. 2001. Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 45:1943-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega, M. A., M. E. Montoya, A. Jaso, B. Zarranz, I. Tirapu, I. Aldana, and A. Monge. 2001. Antimycobacterial activity of new quinoxaline-2-carbonitrile and quinoxaline-2-carbonitrile 1,4-di-N-oxide derivatives. Pharmazie 56:205-207. [PubMed] [Google Scholar]
- 25.Ortega, M. A., Y. Sainz, M. E. Montoya, A. L. De Cerain, and A. Monge. 1999. Synthesis and antituberculosis activity of new 2-quinoxalinecarbonitrile 1,4-di-N-oxides. Pharmazie 54:24-25. [PubMed] [Google Scholar]
- 26.Ortega, M. A., Y. Sainz, M. E. Montoya, A. Jaso, B. Zarranz, I. Aldana, and A. Monge. 2002. Anti-Mycobacterium tuberculosis agents derived from quinoxaline-2-carbonitrile and quinoxaline-2-carbonitrile 1,4-di-N-oxide. Arzneimittel-Forsch. 52:113-119. [DOI] [PubMed] [Google Scholar]
- 27.Sainz, Y., M. E. Montoya, F. J. Martinez-Crespo, M. A. Ortega, A. L. de Cerain, and A. Monge. 1999. New quinoxaline 1,4-di-N-oxides for treatment of tuberculosis. Arzneimittel-Forsch. 49:55-59. [DOI] [PubMed] [Google Scholar]
- 28.Shah, N. S., R. Pratt, S. Althomsons, and J. P. Cegielski. 2007. Extensively drug-resistant tuberculosis, United States, 1993-2006. JAMA 297:1871-1873. [Reprinted from MMWR Morb. Mortal. Wkly. Rep. 56:250-253, 2007.] [PubMed] [Google Scholar]
- 29.Suter, W., A. Rosselet, and F. Knusel. 1978. Mode of action of quindoxin and substituted quinoxaline-di-N-oxides on Escherichia coli. Antimicrob. Agents Chemother. 13:770-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White, E. L., W. J. Suling, L. J. Ross, L. E. Seitz, and R. C. Reynolds. 2002. 2-Alkoxycarbonylaminopyridines: inhibitors of Mycobacterium tuberculosis FtsZ. J. Antimicrob. Chemother. 50:111-114. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. March 2006, posting date. Strategic and technical meeting on intensified control of neglected tropical diseases. A renewed effort to combat entrenched communicable diseases of the poor. World Health Organization, Geneva, Switzerland. http://www.popline.org/docs/312474.
- 32.Zarranz, B., A. Jaso, I. Aldana, and A. Monge. 2003. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Bioorg. Med. Chem. 11:2149-2156. [DOI] [PubMed] [Google Scholar]