Abstract
Mutations in two specific regions of the Fks1 subunit of 1,3-β-d-glucan synthase are known to confer decreased caspofungin susceptibility on Candida spp. Clinical isolates of Candida spp. (404 Candida albicans, 62 C. tropicalis, and 21 C. krusei isolates) sent to the French National Reference Center were prospectively screened for susceptibility to caspofungin in vitro by the broth microdilution reference method of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing (AFST-EUCAST). Twenty-eight isolates (25 C. albicans, 2 C. tropicalis, and 1 C. krusei isolate) for which the caspofungin MIC was above the MIC that inhibited 90% of the isolates of the corresponding species (MIC90) were subjected to molecular analysis in order to identify mutations in the fks1 gene. Substitutions in the deduced protein sequence of Fks1 were found for 8 isolates, and 20 isolates had the wild-type sequence. Among the six C. albicans isolates harboring mutations, six patterns were observed involving amino acid changes at positions 641, 645, 649, and 1358. For C. tropicalis, one isolate showed an L644W mutation, and for one C. krusei isolate, two mutations, L658W and L701M, were found. Two media, RPMI medium and AM3, were tested for their abilities to distinguish between isolates with wild-type Fks1 and those with mutant Fks1. In RPMI medium, caspofungin MICs ranged from 0.25 to 2 μg/ml for wild-type isolates and from 1 to 8 μg/ml for mutant isolates. A sharper difference was observed in AM3: all wild-type isolates were inhibited by 0.25 μg/ml of caspofungin, while caspofungin MICs for all mutant isolates were ≥0.5 μg/ml. These data demonstrate that clinical isolates of C. albicans, C. tropicalis, and C. krusei with decreased susceptibility to caspofungin in vitro have diverse mutations in the fks1 gene and that AM3 is potentially a better medium than RPMI for distinguishing between mutant and wild-type isolates using the AFST-EUCAST method.
Caspofungin is an echinocandin that inhibits 1,3-β-d-glucan synthesis in several fungal species involved in human infections, including Candida spp. and Aspergillus spp. (10). Caspofungin is used for the treatment of invasive candidiasis and aspergillosis as well as for oropharyngeal and esophageal candidiasis (9). The target of caspofungin is the enzyme 1,3-β-d-glucan synthase, encoded by one or several fks genes, depending on the species (14). It has been shown that in laboratory mutants as well as in some clinical isolates, mutations in the fks1 gene resulting in amino acid changes in the protein were necessary and sufficient to confer reduced susceptibility to caspofungin (27, 28). These mutations, associated with reduced susceptibility to caspofungin, have been observed only within two “hot spot” (HS) regions of the Fks protein (located at amino acid positions 640 to 650 and 1345 to 1365 in Candida albicans) (22, 23, 27). Mutations in Fks2 have also been linked with echinocandin resistance in Candida glabrata (20).
A recent study failed to demonstrate a correlation between caspofungin MICs for Candida spp. isolates and clinical or microbiological outcomes for patients with esophageal or invasive candidiasis enrolled in four clinical trials (19). Nevertheless, cases of invasive and esophageal infections with isolates harboring decreased in vitro susceptibility to caspofungin have been reported (1, 7, 12, 15, 16, 20-24, 27, 32). In several instances, increased caspofungin MICs appeared during treatment with an echinocandin, and molecular studies of the pre- and posttreatment isolates demonstrated a genotypic identity suggesting the acquisition of resistance (1, 7, 12, 15, 16, 21, 22, 24, 27, 32). Elevated MICs were associated with mutations in the HS1 or HS2 region of the deduced Fks protein (1, 20, 22, 23, 27). A correlation between in vitro resistance and therapeutic failure was confirmed in vivo in an animal model of candidiasis (16, 21, 27). Furthermore, a comprehensive study including in vitro susceptibility, inhibition of glucan synthase activity, and response to antifungal therapy in an animal model of disseminated candidiasis demonstrated that mutations in the Fks1 protein are sufficient to confer reduced susceptibility to caspofungin (27).
If resistance correlates with therapeutic failure, it is of prime importance to be able to detect it routinely by an in vitro susceptibility testing method. However, the best methodological parameters to use for echinocandin susceptibility testing remain uncertain. It has been shown that antibiotic medium 3 (AM3) is superior to RPMI medium for testing caspofungin against Candida spp. (3). More recently, better discrimination between Candida spp. isolates of known high and low susceptibilities to caspofungin was achieved by using AM3 than by using RPMI medium (25).
The aims of the present study were (i) to identify isolates with mutations in the HS1 or HS2 region of the Fks protein among a large collection of clinical Candida spp. isolates and (ii) to test RPMI medium and AM3 for their abilities to distinguish between wild-type and Fks1 mutant isolates.
MATERIALS AND METHODS
Strains.
C. albicans, Candida tropicalis, and Candida krusei isolates sent to the French National Reference Center for Mycoses and Antifungals from January 2005 to August 2007 were used for the present study. During that period, caspofungin MICs were prospectively determined by the method of the European Committee on Antibiotic Susceptibility Testing (EUCAST) in two different media, RPMI medium and AM3. Isolates with a caspofungin MIC above the MIC determined in the corresponding medium that inhibited 90% of the isolates of the same species (MIC90) were subjected to molecular analysis in order to identify mutations in the Fks protein.
All isolates were subcultured on CHROMagar Candida medium (Becton Dickinson GmbH, Heidelberg, Germany) to ensure purity and viability. Isolates were identified at the species level by standard mycological procedures including the assimilation patterns obtained with commercialized ID32C strips (bioMérieux, Marcy-l'Etoile, France). For all C. albicans isolates, a specific PCR amplification (13) was performed to distinguish this species from Candida dubliniensis.
In vitro susceptibility testing.
In vitro susceptibility was determined by a microdilution technique according to the guidelines of the reference procedure proposed by the Antifungal Susceptibility Testing Subcommittee of EUCAST (AFST-EUCAST) (31). A pure caspofungin powder of known potency (Merck and Co., Rahway, NJ) was used. Microplates were prepared by batch and stored frozen at −20°C. Briefly, testing was performed with a final inoculum size of 105 yeast cells/ml and a final concentration of echinocandin ranging from 0.015 to 8 μg/ml. Tests were performed in parallel in two media, RPMI 1640 (Sigma, Saint Quentin Fallavier, France) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) (Sigma) and AM3 (Difco, Becton Dickinson, Le Pont de Claix, France). Both media were supplemented with glucose to obtain a final concentration of 2% glucose. Two reference strains, C. krusei ATCC 6258 and Candida parapsilosis ATCC 22019, were included as quality controls. Microplates were incubated in ambient air in a humid environment for 24 h at 35°C. After shaking, fungal growth was determined by an automated microplate reader spectrophotometer (Multiscan RC-351; Labsystems Oy, Helsinki, Finland). The MIC end point was defined as a reduction of 50% or more in growth relative to that in the drug-free well. Microplates were also read after 48 h of incubation, and MICs with a 90% inhibition end point were determined at both times.
MIC data analysis.
For calculation, the high off-scale MICs were converted to the next highest concentration and the low off-scale MICs were left unchanged. Distributions of MICs obtained in RPMI medium and AM3 were compared by a paired test (Wilcoxon). MICs for wild-type and mutant isolates were compared by a nonparametric test (Mann-Whitney). Statistical analyses were performed using GraphPad Prism, version 3.00 for Windows (GraphPad Software, San Diego, CA). Statistical significance was defined as a P value of ≤0.05.
Genomic DNA extraction.
After 24 h of incubation at 27°C on Sabouraud agar plates, yeasts were discharged in 1 ml of distilled water in a microcentrifuge tube. Then DNA was extracted with the High Pure PCR template preparation kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer's instructions.
PCR primer design, amplification, and sequence determination.
Table 1 summarizes the nucleotide sequences of the primers used. Oligonucleotide primers were designed using Primer3, version 0.3.0 (http://frodo.wi.mit.edu/), and Oligonucleotide Properties Calculator software (http://www.basic.northwestern.edu/biotools/oligocalc.html). For C. albicans, primers described by Park and colleagues (27) were used for the amplification of the HS1 region of the gene coding for 1,3-β-glucan synthase (fks1). Specific primers were designed to amplify the sequence of the HS2 region based on the available sequence of the fks1 gene (GenBank accession no. D88815). The nucleotide and protein sequences of the fks1 genes of C. tropicalis and C. krusei were compared with the C. albicans sequences to determine the positions of the HS1 and HS2 regions. For C. krusei, primers were designed to amplify the sequences of the HS1 and HS2 regions by using the complete sequence of the fks1 gene (accession no. EF426563). For C. tropicalis, the partial sequence of the fks1 gene was used to design primers for the amplification of the HS1 and HS2 regions (http://www.broad.mit.edu/annotation/genome/candida_tropicalis/Home.html). Reaction volumes of 50 μl contained 3 μl of genomic DNA, 0.25 U of AmpliTaq Gold, 5 μl of 10× PCR buffer, 5 μl of 25 mM MgCl2, 5 μl of 2.5 mM deoxynucleoside triphosphates (Roche), and 1.25 μl of 20 μM primers. The PCR products were amplified in a iCycler thermocycler (Bio-Rad, Marnes-La-Coquette, France) set up with a first cycle of denaturation for 10 min at 95°C, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at the relevant temperature (Table 1) for 30 s, and elongation at 72°C for 30 s, with a final extension step of 10 min at 72°C. Both strands of purified amplified fragments were sequenced at the Genopole of the Pasteur Institute, on an ABI Prism 3700 DNA analyzer (Applied Biosystems, Courtaboeuf, France), with the same primers that were used in the PCR step. Sequences were edited with Chromas Pro software, version 1.33 (Technelysium Pty Ltd., Australia). Multiple sequence alignments were performed using Clustal W software, version 1.8. The sequences were translated with the standard genetic code (http://bioinformatics.org/sms/index.html). The resulting protein sequences were aligned with BioloMics software, version 7.2.5 (BioAware SA, Hannut, Belgium). Sequences of the two regions HS1 and HS2 for three reference strains (C. albicans B311 [ATCC 32354], C. tropicalis ATCC 750, and C. krusei ATCC 6258) were also amplified and sequenced. The positions of the nucleotides and amino acids for C. albicans and C. krusei were based on the complete fks1 genes (GenBank accession no. D88815 and EF426563, respectively). The positions of the nucleotides and amino acids for the C. tropicalis isolates were determined based on the coding sequence of the fks1 gene and on the sequence of the Fks1 protein of C. albicans, respectively.
TABLE 1.
Primer sequences used in this study
| Species | Region | Primer | Sequence (5′-3′) | Amplicon size (bp) | Annealing temp (°C) |
|---|---|---|---|---|---|
| Candida albicans | HS1a | GSC1f | GAAATCGGCATATGCTGTGTC | 450 | 50 |
| GSC1r | AATGAACGACCAATGGAGAAG | ||||
| HS2 | CAS2f | ACCACCAAGATTGGTGCTG | 497 | 58 | |
| CAS2r | TATCTAGCACCACCAACGG | ||||
| Candida krusei | HS1 | CKS1f | ACTGCATCGTTTGCTCCTCT | 500 | 50 |
| CKS1r | GAACATGATCAATTGCCAAC | ||||
| HS2 | CKS2f | CCGGTATGGGAGAACAAATG | 474 | 58 | |
| CKS2r | CACCACCAATGGAAACATCA | ||||
| Candida tropicalis | HS1 | CTS1-1f | ATGGTTCAGTATAGGTGGATG | 221 | 50 |
| CTS1-1r | AAGGAACGACCAATGGAGAAG | ||||
| HS2 | CTS1-2f | ACTACCAAGATTGGTGCTG | 497 | 56 | |
| CTS1-2r | TATCTAGCACCACCAACAG |
HS1 primers were from Park et al. (27).
RESULTS
Distribution of caspofungin MICs.
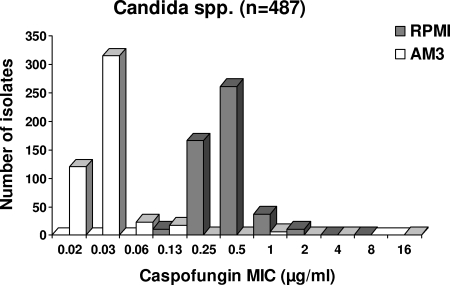
A total of 487 isolates (404 C. albicans, 62 C. tropicalis, and 21 C. krusei isolates) were analyzed. The caspofungin MIC distributions in RPMI medium and AM3 are shown in Fig. 1. Overall, MICs ranged from 0.125 to 8 μg/ml in RPMI medium and from 0.015 to 16 μg/ml in AM3 (Table 2). MICs were significantly (P < 0.0001) higher in RPMI medium (geometric mean MIC [GMIC], 0.42 μg/ml) than in AM3 (GMIC, 0.03 μg/ml). This difference between the two media was noted for all three species analyzed (Table 2), as well as for other species, such as C. glabrata and Candida kefyr (data not shown). In C. albicans, caspofungin MICs above the MIC90 were observed only in RPMI medium for 5 isolates, only in AM3 for 14 isolates, and in both media for 6 isolates. One C. tropicalis isolate had a MIC above the MIC90 only in RPMI medium, and one other isolate had a MIC above the MIC90 in both media. A caspofungin MIC above the MIC90 was detected (in both media) for one C. krusei isolate. These 28 isolates were recovered from blood (n = 17), oral cavities (n = 4), and other sites (n = 7) of 24 patients.
FIG. 1.
Distribution of caspofungin MICs for 487 isolates of Candida spp., determined by the EUCAST reference technique in RPMI medium and AM3.
TABLE 2.
Caspofungin MIC ranges and geometric mean MICs for 487 Candida sp. isolates tested in RPMI medium and AM3
| Species (no. of isolates) | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| In RPMI
|
In AM3
|
|||
| Range | Geometric mean | Range | Geometric mean | |
| C. albicans (404) | 0.125-4 | 0.38 | 0.015-16 | 0.03 |
| C. krusei (21) | 0.5-8 | 1.10 | 0.03-4 | 0.12 |
| C. tropicalis (62) | 0.25-2 | 0.60 | 0.015-1 | 0.03 |
| All (487) | 0.125-8 | 0.42 | 0.015-16 | 0.03 |
Molecular analysis of fks1.
The nucleotide and corresponding deduced protein sequences of the HS1 and HS2 regions of 1,3-β-glucan synthase were determined for the 28 clinical isolates with MICs above the MIC90 (25 C. albicans, 2 C. tropicalis, and 1 C. krusei isolate) as well as for 1 reference strain for each species (Table 3). Overall, mutations conferring amino acid substitutions were found in 8 isolates (6 C. albicans, 1 C. tropicalis, and 1 C. krusei isolate), whereas no amino acid changes were found for 19 C. albicans isolates and 1 C. tropicalis isolate. Among the six C. albicans isolates harboring amino acid substitutions, six mutation patterns were observed involving amino acid changes at positions 641, 645, 649, and 1358. Homozygous mutations were observed for four isolates (in HS1 for three isolates and in HS2 for one isolate). Of the three isolates with homozygous mutations in HS1, one had T1922C and C1923T point mutations, resulting in the protein modification F641S; one had a T1933C mutation, resulting in an S645P substitution in the deduced protein sequence; and one had a C1934A mutation, leading to an S645Y substitution in the deduced protein sequence. In HS2, a T4072C mutation induced a W1358R amino acid change for one isolate. Heterozygous mutations were observed for two isolates. In one of these isolates, two amino acid changes in HS1 were associated (F641S and S645P, due to point mutations T1922Y and T1933Y, respectively); in the other, a heterozygous P649H change (due to a C1946M point mutation) in HS1 was associated with a heterozygous W1358R change (due to a T4072Y point mutation) in HS2. Additionally, for C. albicans isolates, two silent mutations in HS1 were noted. First, a mutation at position 1923 that was homozygous (C1923T) for one isolate and heterozygous (C1923Y) for two other isolates was observed. Second, mutation T1929W was observed in four isolates. In the HS2 region, there was only one isolate harboring a silent mutation (T4062W).
TABLE 3.
Nucleotide and deduced protein sequences of the HS1 and HS2 regions of the fks1 gene for 28 clinical isolates of Candida spp. and 3 reference strains
| Isolate | Caspofungin MIC (μg/ml) at 24 h in:
|
HS1 region (amino acid positions 641-649)a
|
HS2 region (amino acid positions 1351-1358)a
|
Interpretation | |||
|---|---|---|---|---|---|---|---|
| RPMI | AM3 | Nucleotide sequence | Protein sequence | Nucleotide sequence | Protein sequence | ||
| C. albicans | |||||||
| B311 | 0.5 | 0.06 | TTCTTGACTTTGTCTTTAAGAGATCCT | FLTLSLRDP | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Wild type |
| 1, 2, 15, 25 | 0.25-1 | 0.03-0.25 | TTCTTGACWTTGTCTTTAAGAGATCCT | FLTLSLRDP | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Wild type |
| 3 | 0.25 | 0.06 | TTCTTGACTTTGTCTTTAAGAGATCCT | FLTLSLRDP | AATATTGCTCCWGCCGTTGATTGG | NIAPAVDW | Wild type |
| 4-6, 8, 9, 11-13, 16, 17 | 0.5-1 | 0.03-0.125 | TTCTTGACTTTGTCTTTAAGAGATCCT | FLTLSLRDP | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Wild type |
| 7, 10 | 0.5 | 0.06-0.125 | TTYTTGACTTTGTCTTTAAGAGATCCT | FLTLSLRDP | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Wild type |
| 14 | 1 | 0.03 | TTTTTGACTTTGTCTTTAAGAGATCCT | FLTLSLRDP | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Wild type |
| 18 | 1 | 0.015 | TTCTTGACTTTGTCTTTAAGAGATCCT | FLTLSLRDP | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Wild type |
| 19 | 1 | 0.5 | TTCTTGACTTTGTCTTTAAGAGATCMT | FLTLSLRDHc | AATATTGCTCCTGCCGTTGATYGG | NIAPAVDRc | Mutant |
| 20 | 2 | 2 | TYCTTGACTTTGYCTTTAAGAGATCCT | SLTLPLRDPc | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Mutant |
| 21 | 2 | 1 | TTCTTGACTTTGTATTTAAGAGATCCT | FLTLYLRDP | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Mutant |
| 22b | 2 | 1 | TCTTTGACTTTGTCTTTAAGAGATCCT | SLTLSLRDP | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Mutant |
| 23 | 2 | 0.5 | TTCTTGACTTTGTCTTTAAGAGATCCT | FLTLSLRDP | AATATTGCTCCTGCCGTTGATCGG | NIAPAVDR | Mutant |
| 24 | 4 | 16 | TTCTTGACTTTGCCTTTAAGAGATCCT | FLTLPLRDP | AATATTGCTCCTGCCGTTGATTGG | NIAPAVDW | Mutant |
| C. tropicalis | |||||||
| ATCC 750 | 0.5 | 0.06 | TTCTTGACTTTGTCTTTAAGAGATCCA | FLTLSLRDP | AATCTTTCTCCAGCTGTTGATTGG | NLSPAVDW | Wild type |
| 1 | 2 | 0.06 | TTCTTGACTTTGTCTTTAAGAGATCCA | FLTLSLRDP | AATCTTTCTCCAGCTGTTGATTGG | NLSPAVDW | Wild type |
| 2 | 2 | 1 | TTCTTGACTTGGTCTTTAAGAGATCCA | FLTWSLRDP | AATCTTTCTCCAGCTGTTGATTGG | NLSPAVDW | Mutant |
| C. krusei | |||||||
| ATCC 6258 | 1 | 0.06 | TTCCTTATTTTGTCCATTAGAGATCCA(...)ACTGATCTG | FLILSIRDP(...)TDL | AATTTAGCTCCAGCAATTGATTGG | NLAPAIDW | Wild type |
| 1 | 8 | 4 | TTCCTTATTTGGTCCATTAGAGATCCA(...)ACTGATATG | FLIWSIRDP(...)TDM | AATTTAGCTCCAGCAATTGATTGG | NLAPAIDW | Mutant |
Positions for C. albicans and C. tropicalis (positions for C. tropicalis are defined as equivalent to those for C. albicans). The whole HS1 and HS2 regions were analyzed, but only the regions harboring mutations are shown (according to Park et al. [27], HS1 and HS2 are defined as the regions between positions 640 and 650 and positions 1345 and 1365, respectively). For C. krusei, the amino acid positions shown are 655 to 701 and 1358 to 1365 (based on the complete sequences of the fks1 gene [accession no. EF426563]) for HS1 and HS2, respectively. Boldfaced nucleotides and amino acids indicate changes from the consensus sequence.
The HS1 sequence of this isolate was published previously (1).
The mutation(s) is heterozygous. In the corresponding nucleotide sequence, W represents A or T; Y represents T or C; M represents A or C.
The C. tropicalis isolate showed a T-to-G point mutation in the HS1 region (equivalent to T1931G in C. albicans fks1), resulting in an L-to-W amino acid change (equivalent to position 644 in C. albicans Fks1p). Two missense mutations, T1973G and C2101A, were found in the HS1 region of the C. krusei isolate, resulting in the L658W and L701M amino acid changes, respectively. For C. tropicalis and C. krusei, there were no mutations in the HS2 region.
The eight resistant isolates were recovered from six patients and were isolated from blood culture (n = 2), urine (n = 2), bronchoalveolar lavage fluid (n = 1), and the oropharynx (n = 3). All eight isolates with missense mutations, either in HS1 or in HS2, exhibited caspofungin MICs of ≥1 μg/ml in RPMI medium (Table 3). Among the 20 isolates with a wild-type deduced Fks1 sequence, 5 C. albicans isolates and 1 C. tropicalis isolate had caspofungin MICs of 1 and 2 μg/ml in RPMI medium, respectively.
Comparison of RPMI medium and AM3 for detection of isolates with mutations.
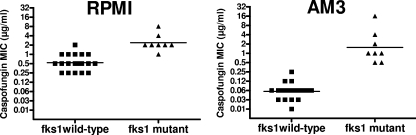
A comparison of caspofungin MIC distributions for isolates with a wild-type fks1 gene and isolates with mutant fks1 genes, according to the medium used, is shown in Fig. 2. Caspofungin MICs were significantly higher for mutant isolates than for wild-type isolates both in RPMI medium (P < 0.0002) and in AM3 (P < 0.0001). In RPMI medium, caspofungin MICs ranged from 0.25 to 2 μg/ml (GMIC, 0.54 μg/ml) for wild-type isolates and from 1 to 8 μg/ml (GMIC, 2.38 μg/ml) for mutant isolates. A sharper difference was observed in AM3, where all wild-type isolates were inhibited by 0.25 μg/ml of caspofungin (MIC range, 0.015 to 0.25 μg/ml; GMIC, 0.06 μg/ml) while all mutant isolates had caspofungin MICs of ≥0.5 μg/ml (MIC range, 0.5 to 16 μg/ml; GMIC, 1.54 μg/ml). MICs obtained either in RPMI medium or in AM3 by using a more stringent end point (90%) or an extended incubation time (48 h) did not distinguish better between resistant and susceptible isolates (data not shown).
FIG. 2.
Caspofungin susceptibilities of 8 Candida spp. isolates with mutations in the fks1 gene and 20 wild-type isolates. MICs were determined by the EUCAST reference technique in RPMI medium (A) and AM3 (B). Horizontal lines represent GMICs.
DISCUSSION
A large collection of clinical isolates of C. albicans, C. tropicalis, and C. krusei was screened for caspofungin susceptibility by the AFST-EUCAST broth microdilution reference method. For the 28 isolates for which MICs were above the MIC90, sequencing of the fks1 gene coding for the 1,3-β-glucan synthase showed missense mutations in 8 isolates. These mutant isolates were recovered from different samples, including samples from deep and superficial body sites. Diverse mutations were found, including mutations that have never been reported previously. For C. albicans, mutations were found in HS1 at amino acid positions 641 (F641S), 645 (S645P or S645Y), and 649 (P649H) and in HS2 at position 1358 (W1358R). Most of the mutations described previously, both in clinical isolates and in laboratory mutants of C. albicans with reduced susceptibility to caspofungin, involved the serine residue at position 645 (2, 22, 23, 27). Another mutation involved phenylalanine at position 641 in three clinical isolates (1, 20) (one of these isolates is included in the present study) and in some laboratory mutants (2). Two additional mutations involving either leucine at position 644 in a laboratory mutant (2) or arginine at position 1361 in a clinical isolate (22) have been reported, associated with a substitution of Ser645. In a recent study, a large collection of 85 caspofungin-resistant laboratory mutants derived from two distinct parent strains were analyzed for fks1 mutations, and substitutions were found at positions 645, 641, and 644 in 93, 6, and 1% of the mutants, respectively (2). The results of the present study and previous reports demonstrate that alteration of several amino acids in HS1 or HS2 can be associated with elevated caspofungin MICs for C. albicans, and this was observed with both the CLSI and the EUCAST methods. Here we also found an L-to-W mutation (equivalent to position 644 in C. albicans Fks1p) in C. tropicalis. To our knowledge, mutations in fks1 associated with decreased in vitro susceptibility to caspofungin have never been reported for C. tropicalis. In the single C. krusei isolate with an elevated caspofungin MIC in our collection, two amino acid substitutions that had not been described to date, L658W and L701M, were found in HS1. Two C. krusei clinical isolates with mutations in fks1 have been reported previously. In the first isolate, an R1361G mutation in HS2 was associated with a marked decrease in susceptibility to caspofungin in vitro (27). For the second isolate, no mutation was found at first (15), but a subsequent analysis showed a mutation in HS1 involving a phenylalanine residue (18). These results showed that, as in C. albicans, diverse mutations can be associated with reduced caspofungin susceptibility in C. krusei. It should be noted that although the new mutations reported in the present study were associated with elevated caspofungin MICs, the role of these mutations in the phenotype has to be confirmed by further analyses. In particular, it could be of interest to test the glucan synthase inhibition in vitro and to transform a susceptible strain with the mutant fks1 gene. Moreover, mutant strains could be characterized in vivo in animal models to establish the important link between in vitro susceptibility and in vivo response to therapy. For the 20 wild-type isolates, only fks1 has been sequenced, and the presence of mutations in fks homologue genes cannot be ruled out.
It is noteworthy that diploid organisms such as C. albicans and C. krusei (17) can harbor both homozygous and heterozygous mutations in fks1 (1, 2, 18, 20, 27). Both heterozygous and homozygous laboratory mutant isolates showed high caspofungin MICs (27), but they differed by their abilities to inhibit glucan synthesis in vitro and by their responses to caspofungin treatment in a mouse model of candidiasis. Indeed, the dose of caspofungin that reduced the fungal burden in the kidneys by 90% was 0.07 to 0.14 mg/kg of body weight for mice infected with heterozygous mutants compared to 3.2 mg/kg for mice infected with the homozygous mutant (27). In the present study, we found heterozygous mutations for only two isolates, but for each one, two mutations at different locations were associated. The relationships between the homozygous/heterozygous state and the level of reduced caspofungin susceptibility in clinical isolates remain to be explored with a larger number of strains.
The best technique for testing the susceptibility of yeasts to caspofungin in vitro is still under scrutiny. Large surveillance studies of in vitro caspofungin susceptibility have been performed by the standardized broth microdilution techniques of the CLSI (26, 29) and ASFT-EUCAST (5, 6, 8) using RPMI medium as the test medium. These techniques have proven to be reproducible, and a good correlation between the results obtained by these two methodologies has been demonstrated (4). Nevertheless, results from a large multicenter study show better interlaboratory reproducibility for the CLSI method (with readings taken at 24 h of incubation) than for the EUCAST technique (25). This study also suggests that AM3 could be a better medium than RPMI for distinguishing between Candida spp. with normal and reduced susceptibilities to caspofungin (25). The components of AM3 are not completely defined, and lot-to-lot variations have been reported, but interlaboratory variabilities for caspofungin susceptibility testing were similar for AM3 and RPMI medium when the CLSI technique was used (25). Because the influence of the culture medium has not been evaluated for the reference broth microdilution technique developed by EUCAST, we prospectively determined MICs by this technique in two media, RPMI medium and AM3. We first showed that the MICs were indeed lower in AM3 than in RPMI medium, as reported with the CLSI method (25). More importantly, we showed by testing wild-type clinical isolates and a set of well-characterized clinical isolates harboring diverse mutations in the fks1 gene that the two media differ in their abilities to detect Candida spp. isolates with reduced caspofungin susceptibility. In both media, Fks1 mutant isolates had higher MICs than wild-type isolates, but some of the mutant isolates were not distinguishable from wild-type isolates when tested in RPMI medium. In contrast, in AM3, very good discrimination was obtained: 100% of mutant isolates had MICs higher than the highest MIC for a wild-type isolate. Although the present study was not designed to determine a breakpoint, it is interesting that a MIC of ≥0.5 μg/ml in AM3 characterized all mutant isolates. These results have to be confirmed with other species, other types of mutations, and even other echinocandin drugs. In a recent study, we demonstrated that the Etest, which has been shown to be useful for caspofungin susceptibility testing (30) and for the detection of two caspofungin-resistant clinical isolates of C. albicans in one patient (1), was able to distinguish between isolates with wild-type and mutant fks1 genes (11).
In conclusion, we have demonstrated that (i) clinical isolates of C. albicans, C. tropicalis, and C. krusei with decreased in vitro susceptibilities to caspofungin have missense mutations in the HS1 or HS2 region of the fks1 gene; (ii) these mutations are even more diverse than currently reported; and (iii) AM3 is potentially a better medium than RPMI for distinguishing between mutant and wild-type isolates by using the AFST-EUCAST method.
Acknowledgments
We thank the members of the French Mycosis Study Group, who collaborate actively with the National Reference Center for the epidemiological and microbiological surveys of invasive mycoses in France. Those outside Paris are as follows: Claire Bouges-Michel (Bobigny), Isabelle Poilane (Bondy), Jean Dunand (Boulogne), Chantal Duhamel (Colombes), Guy Galeazzi (Colombes), Nathalie Fauchet (Créteil), Elisabeth Forget (Clichy), Françoise Botterel and Christine Bonnal (Kremlin Bicêtre), Odile Eloy (Le Chesnay), Christine Lawrence (Garches), Sophie Cassaing (Toulouse), Marie-Françoise David and Liliana Mihaila (Hôpital Paul Brousse, Villejuif), and Elisabeth Chachaty and Olivier Adam (Institut Gustave Roussy, Villejuif). Those in Paris are as follows: Christian Chochillon (Hôpital Bichat), André Paugam and Marie-Thérèse Baixench (Hôpital Cochin), Muriel Cornet (Hôpital de l'Hôtel Dieu), Marie-Christine Escande (Curie), Svetlana Challier, Marie-Elisabeth Bougnoux, and Yvon Sterkers (Necker Enfants Malades), Annick Datry, Houria Laklache, Bader Lmimouni, and Sophie Brun (Hôpital de la Pitié-Salpétrière), Jean-Louis Poirot (Hôpital Saint Antoine), Claire Lacroix (Hôpital Saint Louis), Didier Moissenet (Hôpital Trousseau), Michel Develoux (Hôpital Tenon), and Stéphane Bonacorsi (Hôpital Robert Debré).
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Baixench, M. T., N. Aoun, M. Desnos-Ollivier, D. Garcia-Hermoso, S. Bretagne, S. Ramires, C. Piketty, and E. Dannaoui. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 59:1076-1083. [DOI] [PubMed] [Google Scholar]
- 2.Balashov, S. V., S. Park, and D. S. Perlin. 2006. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob. Agents Chemother. 50:2058-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartizal, C., and F. C. Odds. 2003. Influences of methodological variables on susceptibility testing of caspofungin against Candida species and Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chryssanthou, E., and M. Cuenca-Estrella. 2002. Comparison of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing proposed standard and the E-test with the NCCLS broth microdilution method for voriconazole and caspofungin susceptibility testing of yeast species. J. Clin. Microbiol. 40:3841-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, M. J. Buitrago, A. Monzon, and J. L. Rodriguez-Tudela. 2006. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob. Agents Chemother. 50:917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuenca-Estrella, M., D. Rodriguez, B. Almirante, J. Morgan, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, M. Salvado, D. W. Warnock, A. Pahissa, and J. L. Rodriguez-Tudela. 2005. In vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population-based active surveillance programme, Barcelona, Spain, 2002-2003. J. Antimicrob. Chemother. 55:194-199. [DOI] [PubMed] [Google Scholar]
- 7.Daneman, N., A. K. Chan, R. Rennie, C. Sand, S. Porter, and S. M. Poutanen. 2006. The emergence of caspofungin resistance during treatment of recurrent Candida glabrata candidemia. Clin. Microbiol. Infect. 12(Suppl. 4):P1204. [Google Scholar]
- 8.Dannaoui, E., O. Lortholary, D. Raoux, M. E. Bougnoux, G. Galeazzi, C. Lawrence, D. Moissenet, I. Poilane, D. Hoinard, F. Dromer, and the YEASTS Group. 2008. Comparative in vitro activities of caspofungin and micafungin, determined using the method of the European Committee on Antimicrobial Susceptibility Testing, against yeast isolates obtained in France in 2005-2006. Antimicrob. Agents Chemother. 52:778-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 10.Deresinski, S. C., and D. A. Stevens. 2003. Caspofungin. Clin. Infect. Dis. 36:1445-1457. [DOI] [PubMed] [Google Scholar]
- 11.Desnos-Ollivier, M., F. Dromer, and E. Dannaoui. 2008. Detection of caspofungin resistance in Candida spp. by Etest. J. Clin. Microbiol. 46:2389-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodgson, K. J., A. R. Dodgson, C. Pujol, S. A. Messer, D. R. Soll, and M. A. Pfaller. 2005. Caspofungin resistant C. glabrata. Clin. Microbiol. Infect. 11(Suppl. 2):P1158. [Google Scholar]
- 13.Donnelly, S. M., D. J. Sullivan, D. B. Shanley, and D. C. Coleman. 1999. Phylogenetic analysis and rapid identification of Candida dubliniensis based on analysis of ACT1 intron and exon sequences. Microbiology 145:1871-1882. [DOI] [PubMed] [Google Scholar]
- 14.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakki, M., J. F. Staab, and K. A. Marr. 2006. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 50:2522-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez, S., J. L. Lopez-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobsen, M. D., N. A. Gow, M. C. Maiden, D. J. Shaw, and F. C. Odds. 2007. Strain typing and determination of population structure of Candida krusei by multilocus sequence typing. J. Clin. Microbiol. 45:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn, J. N., G. Garcia-Effron, M. J. Hsu, S. Park, K. A. Marr, and D. S. Perlin. 2007. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob. Agents Chemother. 51:1876-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kartsonis, N., J. Killar, L. Mixson, C. M. Hoe, C. Sable, K. Bartizal, and M. Motyl. 2005. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob. Agents Chemother. 49:3616-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katiyar, S., M. Pfaller, and T. Edlind. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krogh-Madsen, M., M. C. Arendrup, L. Heslet, and J. D. Knudsen. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938-944. [DOI] [PubMed] [Google Scholar]
- 22.Laverdiere, M., R. G. Lalonde, J. G. Baril, D. C. Sheppard, S. Park, and D. S. Perlin. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705-708. [DOI] [PubMed] [Google Scholar]
- 23.Miller, C. D., B. W. Lomaestro, S. Park, and D. S. Perlin. 2006. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy 26:877-880. [DOI] [PubMed] [Google Scholar]
- 24.Moudgal, V., T. Little, D. Boikov, and J. A. Vazquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdiere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., D. J. Diekema, S. A. Messer, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 47:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., S. A. Messer, K. Mills, A. Bolmstrom, and R. N. Jones. 2001. Evaluation of Etest method for determining caspofungin (MK-0991) susceptibilities of 726 clinical isolates of Candida species. J. Clin. Microbiol. 39:4387-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398-405. [DOI] [PubMed] [Google Scholar]
- 32.Villareal, N. C., A. W. Fother-Gill, C. Kelly, J. E. Patterson, M. G. Rinaldi, and T. F. Patterson. 2004. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1034.