Abstract
Antibiotic efflux is observed in both eukaryotic and prokaryotic cells, modulating accumulation and resistance. The present study examines whether eukaryotic and prokaryotic fluoroquinolone transporters can cooperate in the context of an intracellular infection. We have used (i) J774 macrophages (comparing a ciprofloxacin-resistant cell line overexpressing an MRP-like transporter with wild-type cells with basal expression), (ii) Listeria monocytogenes (comparing a clinical isolate [CLIP21369] displaying ciprofloxacin resistance associated with overexpression of the Lde efflux system with a wild-type strain [EGD]), (iii) ciprofloxacin (substrate of both Lde and MRP) and moxifloxacin (nonsubstrate), and (iv) probenecid and reserpine (preferential inhibitors of MRP and Lde, respectively). The ciprofloxacin MICs for EGD were unaffected by reserpine, while those for CLIP21369 were decreased approximately fourfold (and made similar to those of EGD). Neither probenecid nor reserpine affected the moxifloxacin MICs against EGD or CLIP21369. In dose-response studies (0.01× to 100× MIC) in broth, reserpine fully restored the susceptibility of CLIP21369 to ciprofloxacin (no effect on EGD) but did not influence the activity of moxifloxacin. In studies with intracellular bacteria, reserpine, probenecid, and their combination increased the activity of ciprofloxacin in wild-type and ciprofloxacin-resistant macrophages in parallel with an increase in ciprofloxacin accumulation in macrophages for EGD and an increase in accumulation and decrease in MIC (in broth) for CLIP21369. Moxifloxacin accumulation and intracellular activity were consistently not affected by the inhibitors. A bacterial efflux pump may thus actively cooperate with a eukaryotic efflux transporter to reduce the activity of a common substrate (ciprofloxacin) toward an intracellular bacterial target.
Active efflux is a very general mechanism of drug resistance present in almost all cell types (33). In this context, efflux transporters recognizing antibiotics have now been described in both eukaryotic and prokaryotic cells (34, 35). Their expression in phagocytic cells significantly reduces the activity of the corresponding substrates toward intracellular bacteria, as exemplified for azithromycin and daptomycin (both substrates of the P-glycoprotein) against Staphylococcus aureus (20, 30) or fluoroquinolones (substrates of MRP) against Listeria monocytogenes (30). Conversely, addition of transporter inhibitors favors the intracellular activity of these drugs in parallel with an increase of their intracellular concentrations (20, 30). More complex mechanisms may, however, also be envisaged, as recently demonstrated by the enhancement of the intracellular killing of Mycobacterium tuberculosis upon addition of reserpine, attributed to inhibition of K+ transport (2). In bacteria, efflux transporters are well recognized as a cause of resistance for almost all antibiotic classes, with quinolones, tetracyclines, and chloramphenicol being the most universal substrates (34). Data, however, are scarce on the intracellular expression of bacterial resistance by efflux, as well as on the potential cooperation between prokaryotic and eukaryotic efflux pumps having a common substrate and on the impact of such cooperation on antibiotic efficacy toward intracellular bacteria.
In the present study, we have examined the activities of two fluoroquinolones, ciprofloxacin and moxifloxacin, against L. monocytogenes, using bacterial strains and macrophage cell lines that express or overexpress efflux transporters against these antibiotics in comparison with their wild counterparts. Thus, we compared (i) a clinical isolate (CLIP21369, displaying a ciprofloxacin resistance phenotype associated with overexpression of Lde, a member of the major facilitator superfamily of secondary transporters encoded by the chromosomal lde gene) (10) to the wild-type strain EGD (widely used for the evaluation of antibiotic activity against L. monocytogenes) (5, 12, 13, 15), and (ii) ciprofloxacin-resistant J774 macrophages, a cell line created in our laboratory and in which an MRP-like transporter (member of the ATP-binding cassette superfamily) acting on ciprofloxacin (24) is overexpressed (22), to wild-type cells (with a basal-level expression of this transporter). In this system, we also compared ciprofloxacin to moxifloxacin, since the former is a substrate for both Lde and MRP-like transporters, whereas the latter remains largely unaffected by either transporter (10, 23).
MATERIALS AND METHODS
Bacterial strains and susceptibility testing.
Wild-type L. monocytogenes EGD (serotype 1/2a) was provided by P. Berche. Strain CLIP21369 (serotype 1/2b), isolated from a human case in France, is resistant to quinolones by overexpression of the Lde efflux pump (10). BM4497 (derived from CLIP21369 by insertional inactivation of lde [10]) was used as a control. Bacteria were maintained and characterized as described previously (27). MICs were determined in tryptic soy broth (TSB) by arithmetic or geometric dilution (5).
Killing curves in acellular media.
Killing curves were performed in TSB. Cultures in logarithmic growth phase (∼109 CFU/ml) were diluted in TSB to a density of 106 CFU/ml. At the end of the 5-h incubation at 37°C, aliquots were diluted, plated on tryptic soy agar, and incubated overnight. The number of viable bacteria was determined by colony counting using an automated detector (5).
Cells and cell culture.
Wild-type J774 macrophages and macrophages rendered resistant to ciprofloxacin by long-term exposure to this antibiotic (henceforth referred to as ciprofloxacin-resistant macrophages [22]) were used in parallel. Both cell lines were maintained and cultured as described previously (22).
Determination of cellular concentrations of quinolones.
A high-pressure liquid chromatography assay, based on a published method (1), was used since reserpine was found to interfere with the fluorometric assay used previously (23). In brief, cells incubated with fluoroquinolones were washed three times with ice-cold phosphate-buffered saline, scraped with a Teflon policeman, and collected in distilled water. An aliquot of each sample was withheld and used for determination of the total protein content (21). The remaining part was centrifuged at 14,000 rpm for 10 min, and 50 to 100 μl of the supernatant was used for chromatography (Alliance Waters 2690 chromatograph equipped with a Waters 996 photodiode array detector [set at 275 nm for ciprofloxacin and 298 nm for moxifloxacin] and an autoinjector), using a reversed-phase column in conjunction with a precolumn, both XTerra RP18 5-μm columns, and a mobile phase made of acetonitrile and 25 mM Na2HPO4 buffer, pH 3.0 (20:80, vol/vol; 1.0 ml/min). Data were collected using Millennium32 version 4.00 software (Waters Corporation). The limit of detection was ∼1.5 ng for both fluoroquinolones, with intraday and interday variation coefficients of 2.5 and 5.2% for ciprofloxacin and 2.6 and 6.5% for moxifloxacin, respectively. The cellular drug concentrations were expressed by reference to the corresponding total protein contents (22). In preliminary experiments, this method was validated by comparison with the fluorometric assay (23), with correlation slope factors of 1.13 (R2 = 0.963) and 0.92 (R2 = 0.975) for ciprofloxacin and moxifloxacin, respectively, using cells incubated at extracellular concentrations spanning from 10 to 50 mg/liter (n = 20).
Cell infection and determination of intracellular activity of quinolones.
Experiments were conducted as described earlier (22, 30) with a multiplicity of infection (bacterium-to-macrophage ratio) of 7.
Morphological studies.
Infections were carried out using a multiplicity of infection of 70 to allow for the observation of a sufficiently large number of intracellular bacteria. Electron microscopy was performed on cells fixed in situ, and samples were processed as previously described (32).
Materials.
Ciprofloxacin and moxifloxacin were provided by Bayer AG, Wuppertal, Germany, as microbiological standards, with potencies of 85.0% and 90.9%, respectively. Reserpine and probenecid were purchased from Sigma-Aldrich (St. Louis, MO). Cell culture medium and serum were from Invitrogen (Paisley, Scotland, United Kingdom). TSB and tryptic soy agar were from Difco, Becton Dickinson and Co. (Sparks, MD).
RESULTS
MICs and influence of pump inhibitors.
Table 1 shows the MICs of ciprofloxacin and moxifloxacin against L. monocytogenes EGD and CLIP21369, under control conditions or in the presence of pump inhibitors, used at the highest testable concentration (33 μM, concentration for which reserpine caused maximal effect and was soluble; 15 mM, maximal concentration at which probenecid did not affect macrophage viability). Values for EGD were not influenced by the addition of probenecid or reserpine. In contrast, CLIP21369 was approximately fourfold less susceptible to ciprofloxacin than was EGD, but the MIC was brought close to that against EGD in the presence of reserpine or probenecid. The MICs of moxifloxacin against CLIP21369 were close to those for EGD and were not influenced by the addition of either of the two efflux inhibitors.
TABLE 1.
Influence of efflux pump inhibitors on the MICs of ciprofloxacin and moxifloxacin against L. monocytogenes
| L. monocytogenes strain | MIC (mg/litera) of drug with efflux pump inhibitorb
|
|||||
|---|---|---|---|---|---|---|
| Ciprofloxacin
|
Moxifloxacin
|
|||||
| None (control) | Reserpine | Probenecid | None (control) | Reserpine | Probenecid | |
| EGD | 1.2 | 1.2 | 1 | 0.6 | 0.5 | 0.5 |
| CLIP21369 | 5 | 1 | 0.6 | 0.5 | 0.3 | 0.2 |
Arithmetic dilution.
Concentrations of inhibitors were as follows: reserpine, 33 μM (20 mg/liter); probenecid, 15 mM (4.3 g/liter).
Extracellular activity of fluoroquinolones.
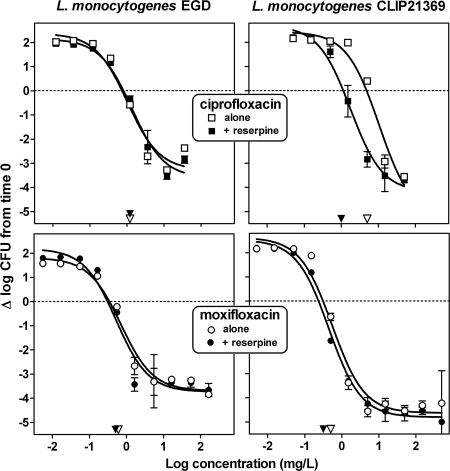
Figure 1 shows the results of dose-effect studies of the activities of ciprofloxacin and moxifloxacin against the EGD and CLIP21369 strains, using a wide range of concentrations (corresponding to approximately 0.01- to 100-fold the MIC for EGD) after 5 h of contact. In all conditions, a sigmoidal function could be fitted to the data, and the corresponding pertinent regression parameters are presented in Table 2. When the antibiotics were used alone, these data show (i) a static effect at concentrations close to the MIC in all cases (as expected); (ii) a larger relative maximal efficacy (Emax) for the CLIP21369 strain in comparison with EGD for both ciprofloxacin and moxifloxacin; and (iii) a larger relative potency (lower 50% effective concentrations [EC50s]) for moxifloxacin against both strains (reflecting its lower MICs), whereas the relative potency of ciprofloxacin (EC50) against CLIP21369 was 10 times lower than that against EGD, again reflecting the difference in MICs. When the antibiotics were tested in the presence of reserpine, this efflux inhibitor did not significantly affect the activity of ciprofloxacin against the EGD strain. In contrast, reserpine brought the activity of ciprofloxacin against CLIP21369 to values almost similar to those for EGD in the absence or in the presence of reserpine. Reserpine had no effect on moxifloxacin activity.
FIG. 1.
Killing curves of ciprofloxacin (top) or moxifloxacin (bottom) against L. monocytogenes EGD (left) or CLIP21369 (right). Bacteria in broth were incubated for 5 h in the presence of the antibiotic alone (open symbols) or combined with reserpine (33 μM; 20 mg/liter; closed symbols). The abscissas indicate the extracellular concentrations of the antibiotic in log scale; the ordinates show the changes in CFU (log10) per ml as observed after 5 h of incubation in comparison with the original inocula (horizontal dotted lines). The arrowheads point to the MIC of the strain (open arrowhead, antibiotic alone; closed arrowhead, antibiotic plus reserpine; values are indicated in Table 1). All values are the means of three independent determinations ± standard deviations (when not visible, error bars are smaller than the size of the symbol).
TABLE 2.
Pertinent regression parameters (with confidence intervals [CI]) and statistical analysis of the dose-response curves illustrated in Fig. 1 (killing curves of ciprofloxacin and moxifloxacin in broth)a,b
| Antibiotic and strain | Antibiotic alone
|
Antibiotic plus 20-mg/liter reserpine
|
ANCOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Emax (CI) (Δlog10 CFU) | EC50 (CI) (mg/liter) |
Cstatic
|
R2 | Emax (CI) (Δlog10 CFU) | EC50 (CI) (mg/liter) |
Cstatic
|
R2 | ||||
| mg/ liter | Multiple of MIC | mg/ liter | Multiple of MIC | ||||||||
| Ciprofloxacin | |||||||||||
| EGD | −3.28 (−4.47 to −2.10) a; A | 1.02 (0.34 to 3.07) a; A | 0.7 | 0.6 | 0.959 | −3.60 (−4.49 to −2.72) a; A | 1.35 (0.63 to 2.90) a; A | 0.8 | 0.7 | 0.981 | NS |
| CLIP21369 | −5.21 (−8.37 to −2.06) b; A | 10.3 (2.75 to 38.9) b; A | 4.8 | 1.0 | 0.963 | −4.19 (−5.12 to −3.26) b; B | 1.76 (0.85 to 3.63) a; B | 1.1 | 0.8 | 0.987 | P < 0.001 |
| Moxifloxacin | |||||||||||
| EGD | −3.74 (−4.44 to −3.04) a; A | 0.53 (0.23 to 1.19) c; A | 0.3 | 0.5 | 0.966 | −3.69 (−4.20 to −3.19) a; A | 0.70 (0.39 to 1.28) b; A | 0.3 | 0.5 | 0.981 | NS |
| CLIP21369 | −4.64 (−5.21 to −4.06) b; A | 0.55 (0.30 to 0.99) c; A | 0.3 | 0.5 | 0.979 | −4.81 (−5.11 to −4.51) c; A | 0.45 (0.32 to 0.61) c; A | 0.2 | 0.7 | 0.994 | NS |
Parameters are defined as follows: Emax, CFU decrease (in log10 units) at 5 h from the original inoculum, as extrapolated for antibiotic concentration = ∞; EC50, antibiotic concentration (mg/liter) causing a reduction of the inoculum halfway between initial (E0) and maximal (Emax) values, as obtained from the Hill equation (using a slope factor of 1); Cstatic, concentration (mg/liter or multiple of the MIC) as determined in the absence (antibiotic alone) or in the presence (antibiotic plus reserpine) of 20-mg/liter reserpine resulting in no apparent bacterial growth (number of CFU identical to the original inoculum), as determined by graphical intrapolation.
Statistical analyses: for analysis per column (one-way analysis of variance with Tukey test for multiple comparisons), values with different lowercase letters are significantly different from each other (P < 0.01); for analysis per row (unpaired, two-tailed t test between corresponding parameters for activity in the absence and in the presence of reserpine), values with different uppercase letters are significantly different from each other (P < 0.01); for global analysis of covariance (ANCOVA) with Tukey test for multiple comparison, curves are compared in the absence and in the presence of reserpine for each antibiotic toward each strain. NS, nonsignificant.
Intracellular accumulation and antibacterial activity of fluoroquinolones.
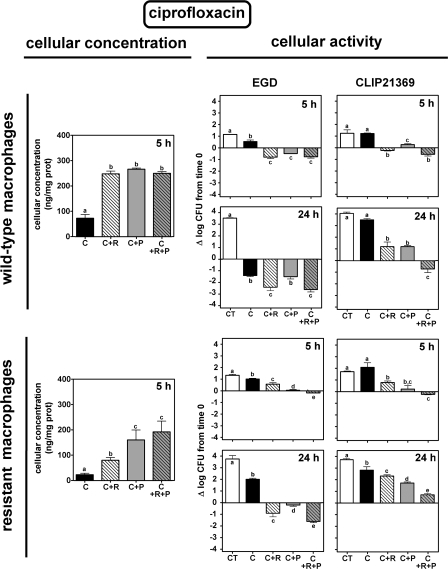
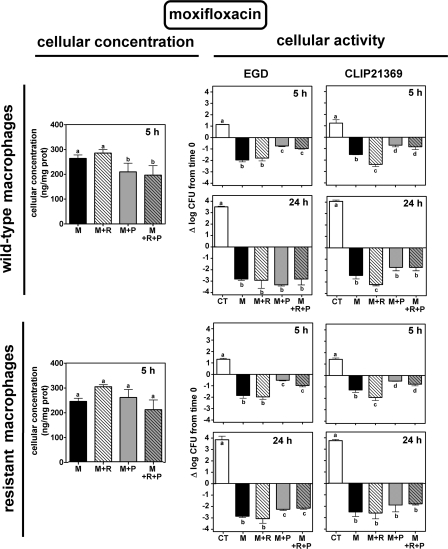
The cellular accumulation and intracellular activity of ciprofloxacin and moxifloxacin toward the EGD and the CLIP21369 strains were examined using wild-type J774 and ciprofloxacin-resistant J774 macrophages. Each cell line was infected by a bacterial strain and incubated for 5 h or 24 h without antibiotic (control) or in the presence of (i) ciprofloxacin or moxifloxacin alone; (ii) probenecid alone, reserpine alone, or both; and (iii) each fluoroquinolone plus probenecid, reserpine, or both. In all cases, drugs (fluoroquinolones and inhibitors) were added after infection of the cells and simultaneously when combined. Fluoroquinolones were used at concentrations mimicking the maximal concentration found in the serum of patients receiving conventional therapy (4.3 mg/liter for ciprofloxacin and 4 mg/liter for moxifloxacin [see reference 5]). In parallel, the cellular concentrations of the fluoroquinolones were determined (using the same experimental groups) in noninfected cells after 5 h (Fig. 2 and 3). Previous experiments have shown that (i) maximal accumulation of both fluoroquinolones is achieved at this period, (ii) the accumulation levels remain constant between 5 h and 24 h, and (iii) the accumulation levels do not differ between uninfected and infected cells (23, 29).
FIG. 2.
Concentration (left) and intracellular activity (right) of ciprofloxacin in J774 wild-type macrophages (top) or ciprofloxacin-resistant macrophages (bottom) exposed to 4.3 mg/liter of the antibiotic alone (C) or combined with reserpine (33 μM; 20 mg/liter [C+R]), probenecid (15 mM; 4.3 g/liter [C+P]), or both inhibitors (C+R+P). CT, control (no drug added). Cellular concentrations were measured in uninfected cells after 5 h of incubation and expressed as ng/mg cell protein; intracellular activities were compared in cells infected by L. monocytogenes EGD (middle panels) or CLIP21369 (right panels) and expressed as the change in CFU (log10) per mg cell protein after 5 h or 24 h of incubation in comparison with the original inocula. Values are the means of three independent determinations ± standard deviations (when not visible, error bars are smaller than the thickness of the bar border).
FIG. 3.
Concentration (left) and intracellular activity (right) of moxifloxacin in J774 wild-type macrophages (top) or ciprofloxacin-resistant macrophages (bottom) exposed for 5 h to 4 mg/liter of the antibiotic alone (M) or combined with reserpine (33 μM; 20 mg/liter [M+R]), probenecid (15 mM; 4.3 g/liter [M+P]), or both inhibitors (M+R+P). CT, control (no drug added). Cellular concentrations were measured in uninfected cells after 5 h of incubation and expressed as ng/mg cell protein; intracellular activity was compared in cells infected by L. monocytogenes EGD (middle panels) or CLIP21369 (right panels) and expressed as the change in CFU (log10) per mg cell protein after 5 h or 24 h of incubation in comparison with the original inocula. Values are the means of three independent determinations ± standard deviations (when not visible, error bars are smaller than the thickness of the bar border).
Intracellular bacterial growth in the absence of drugs was slightly slower for EGD than for CLIP21369 (1.24 ± 0.11 versus 1.47 ± 0.32 log10 at 5 h [P > 0.05] and 3.64 ± 0.14 versus 3.93 ± 0.18 log10 at 24 h [P < 0.01]). Growth was not significantly affected by the presence of reserpine, probenecid, or their combination for EGD but was significantly slowed down for CLIP21369 (10 to 15% reduction compared to control in three independent experiments). To ascertain whether this difference in intracellular growth and the specific effect of inhibitors on CLIP21369 intracellular multiplication rate were not related to the use of genetically unrelated strains, but rather to its specific phenotype, we also studied BM4497 (obtained by disruption of the lde gene in CLIP21369 [10]). It behaved like EGD, with very similar growth rates and no reduction of intracellular growth in the presence of inhibitors. In all cases, intracellular L. monocytogenes EGD and CLIP21369 were found by electron microscopy to multiply in the cytosol of both types of macrophages (not shown).
Accumulation of ciprofloxacin in wild-type macrophages was increased about 2.5-fold in the presence of reserpine, probenecid, or their combination (Fig. 2, upper panels). Ciprofloxacin alone only slowed down the growth of EGD at 5 h but caused a 1.4-log10-CFU decrease at 24 h. Reserpine, probenecid, or their combination allowed ciprofloxacin to cause a net reduction in bacterial counts (about 0.5 to 1 log10 CFU) at 5 h (with a linear relationship [R2 = 0.97] between activity and cellular accumulation in both wild-type and ciprofloxacin-resistant macrophages). At 24 h, reserpine alone or combined with probenecid significantly increased the activity of ciprofloxacin. Ciprofloxacin alone was inactive against CLIP21369 at both 5 h and 24 h. At 5 h, however, ciprofloxacin became static in the presence of probenecid or reserpine alone and caused a 0.5-log10-CFU decrease in the presence of the two combined inhibitors. At 24 h, the inhibitors decreased the bacterial growth seen in the presence of ciprofloxacin and caused a 0.5-log10-CFU decrease when used in combination. In ciprofloxacin-resistant macrophages (lower panels), cellular accumulation of ciprofloxacin was (i) markedly lower than that in the wild-type cells, (ii) increased in the presence of reserpine, and (iii) further increased in the presence of probenecid and still further if the two inhibitors were combined (however, not reaching the same level as that in wild-type macrophages). Intracellular activity of ciprofloxacin against EGD in these cells globally mirrored the changes in cellular accumulation. Thus, ciprofloxacin alone was essentially unable to prevent growth at either 5 h or 24 h. Addition of reserpine or probenecid allowed for a modest decrease in bacterial counts at 24 h, which was further increased by their combination, but not to the level observed with EGD. The overall effect of inhibitors on ciprofloxacin activity at 5 h against CLIP21369 was similar to that seen with EGD. At 24 h, ciprofloxacin was inactive under all conditions, with bacterial counts mirroring again the drug cell content.
The contribution of each transporter in reducing ciprofloxacin intracellular activity was estimated by comparing the degree of potentiation obtained in the presence of inhibitors (i) against EGD infecting wild-type or ciprofloxacin-resistant macrophages (disclosing their effect on the macrophage efflux pump [MRP]) and (ii) against EGD or CLIP21369 infecting wild-type macrophages (disclosing their effect on the Listeria efflux pump [Lde]). These calculations indicated that (i) inhibition of the macrophage transporter produced a gain of about 1 and 3 logs of intracellular activity in wild-type and ciprofloxacin-resistant cells, respectively, and (ii) inhibition of the Listeria transporter increased the intracellular activity of ciprofloxacin by about 2 to 2.5 logs (see Fig. S1 in the supplemental material). We also found a much weaker potentiation in ciprofloxacin-resistant cells infected by CLIP21369 than in the other situations tested, indicating that the inhibitors used were unable to cope with the concomitant overexpression of the two efflux pumps.
Results of similar experiments with moxifloxacin are illustrated in Fig. 3. Moxifloxacin accumulated at high and similar levels in the two cell types, with no marked effect of any inhibitor (the level of cellular accumulation of moxifloxacin being similar to that seen with ciprofloxacin in the presence of reserpine or probenecid in wild-type cells). Under all conditions, moxifloxacin was able to reduce the intracellular bacterial counts, and this effect increased over time. No consistent effect of the inhibitors was seen, except that probenecid exerted a slightly depressing effect on moxifloxacin activity at 5 h under all conditions while reserpine increased it toward CLIP21369.
DISCUSSION
The key finding of the present study is that a bacterial efflux pump, such as Lde, may actively cooperate with a eukaryotic transporter, such as MRP, to reduce the activity of a common substrate (ciprofloxacin) toward an intracellular bacterial target (L. monocytogenes). The data comparing CLIP21369 with EGD and ciprofloxacin-resistant with wild-type J774 macrophages show that the expression of Lde and MRP transporters has distinct but additive negative effects on ciprofloxacin activity. Addition of transport inhibitors allows restoration of the activity of ciprofloxacin to an extent essentially commensurate with the increases of (i) its cellular accumulation (through modulation of the MRP activity) and (ii) its antibacterial potency (through impairment of Lde). The behavior of moxifloxacin supports this conclusion since no or only a minimal effect of inhibitors was observed with this antibiotic, which is substrate for neither MRP (this study; see also reference 23) nor Lde (based on MIC determinations in this study [Table 1] and in reference 10).
Probenecid, a well-known inhibitor of organic anions (4, 6) and of MRP transporters (11, 16) in mammalian cells, increases the cellular accumulation of ciprofloxacin in J774 macrophages (24) and its activity against intracellular L. monocytogenes (29). The present study suggests that it is also an inhibitor of Lde, which has not been reported so far. Reserpine, commonly used as an inhibitor of efflux in gram-positive bacteria (10, 19, 25, 28, 36), is also an inhibitor of the P-glycoprotein and of the breast cancer resistance protein (8, 38, 39). Our study shows that reserpine also impairs the activity of the ciprofloxacin MRP-like transporter in J774 macrophages. This is so far undescribed, but reserpine has been shown to inhibit the transport of methotrexate (14), another known MRP substrate (17). The sharing of substrates and inhibitors among phylogenetically remote transporters is not a surprise since (i) most inhibitors act through a competitive mechanism (18, 26, 37), (ii) substrates can also be inhibitors (3, 24), and (iii) common pharmacophores have been described for substrates and inhibitors of multidrug transporters (7, 9). Further studies, however, will need to better characterize the properties common to the prokaryotic Lde and the eukaryotic MRP. The faster intracellular growth of CLIP21369 than of EGD or the lde disruptant isogenic strain (partially prevented by the addition of efflux pump inhibitors) may also suggest a role of Lde in intracellular growth.
In a broader context, the observations reported in this study may have important implications for the treatment of intracellular infections. They underline the importance of active efflux in modulation of antibiotic efficacy, not only at the bacterial but also at the host cell level, strongly pleading for an early evaluation of the interactions between antibiotics and transporters expressed both in prokaryotic and in eukaryotic cells. This may ultimately lead to the selection of drugs which, like moxifloxacin, are substrates for neither of these transporters. The alternative approach, consisting in the development of broad-spectrum pump inhibitors, could indeed face major difficulties, related to their intrinsic but unwanted pharmacological activities, the impairment of the physiological functions exerted by the transporters in mammalian cells (31), the multiplicity of the efflux systems, and the potential risk of the rapid emergence of resistance.
Supplementary Material
Acknowledgments
We are grateful to M. C. Cambier, N. Couwenbergh, C. Misson, and M. Vergauwen for skillful technical assistance. We also thank Bayer for the gift of the fluoroquinolones.
F.V.B. is Maître de Recherches of the Belgian Fonds de la Recherche Scientifique (FRS-FNRS). This work was supported by the Belgian Fonds de la Recherche Scientifique Médicale (grants no. 3.4549.00 and 3.4542.02) and the Belgian Federal Science Policy Office (research project P5/33-P6/19; research action P5-P6) and through a grant-in-aid from Bayer AG, Leverkusen, Germany.
Footnotes
Published ahead of print on 23 June 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Al Dgither, S., S. N. Alvi, and M. M. Hammami. 2006. Development and validation of an HPLC method for the determination of gatifloxacin stability in human plasma. J. Pharm. Biomed. Anal. 41:251-255. [DOI] [PubMed] [Google Scholar]
- 2.Amaral, L., M. Martins, and M. Viveiros. 2007. Enhanced killing of intracellular multidrug-resistant Mycobacterium tuberculosis by compounds that affect the activity of efflux pumps. J. Antimicrob. Chemother. 59:1239-1246. [DOI] [PubMed] [Google Scholar]
- 3.Asakura, E., H. Nakayama, M. Sugie, Y. L. Zhao, M. Nadai, K. Kitaichi, A. Shimizu, M. Miyoshi, K. Takagi, K. Takagi, and T. Hasegawa. 2004. Azithromycin reverses anticancer drug resistance and modifies hepatobiliary excretion of doxorubicin in rats. Eur. J. Pharmacol. 484:333-339. [DOI] [PubMed] [Google Scholar]
- 4.Cao, C. X., S. C. Silverstein, H. C. Neu, and T. H. Steinberg. 1992. J774 macrophages secrete antibiotics via organic anion transporters. J. Infect. Dis. 165:322-328. [DOI] [PubMed] [Google Scholar]
- 5.Carryn, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2002. Comparative intracellular (THP-1 macrophage) and extracellular activities of β-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob. Agents Chemother. 46:2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham, R. F., Z. H. Israili, and P. G. Dayton. 1981. Clinical pharmacokinetics of probenecid. Clin. Pharmacokinet. 6:135-151. [DOI] [PubMed] [Google Scholar]
- 7.Deeley, R. G., and S. P. Cole. 2006. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 580:1103-1111. [DOI] [PubMed] [Google Scholar]
- 8.Ebert, B., A. Seidel, and A. Lampen. 2005. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis 26:1754-1763. [DOI] [PubMed] [Google Scholar]
- 9.Ekins, S., R. B. Kim, B. F. Leake, A. H. Dantzig, E. G. Schuetz, L. B. Lan, K. Yasuda, R. L. Shepard, M. A. Winter, J. D. Schuetz, J. H. Wikel, and S. A. Wrighton. 2002. Application of three-dimensional quantitative structure-activity relationships of P-glycoprotein inhibitors and substrates. Mol. Pharmacol. 61:974-981. [DOI] [PubMed] [Google Scholar]
- 10.Godreuil, S., M. Galimand, G. Gerbaud, C. Jacquet, and P. Courvalin. 2003. Efflux pump Lde is associated with fluoroquinolone resistance in Listeria monocytogenes. Antimicrob. Agents Chemother. 47:704-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gollapudi, S., C. H. Kim, B. N. Tran, S. Sangha, and S. Gupta. 1997. Probenecid reverses multidrug resistance in multidrug resistance-associated protein-overexpressing HL60/AR and H69/AR cells but not in P-glycoprotein-overexpressing HL60/Tax and P388/ADR cells. Cancer Chemother. Pharmacol. 40:150-158. [DOI] [PubMed] [Google Scholar]
- 12.Grayo, S., O. Join-Lambert, M. C. Desroches, and A. Le Monnier. 2008. Comparison of the in vitro efficacies of moxifloxacin and amoxicillin against Listeria monocytogenes. Antimicrob. Agents Chemother. 52:1697-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guinane, C. M., P. D. Cotter, R. P. Ross, and C. Hill. 2006. Contribution of penicillin-binding protein homologs to antibiotic resistance, cell morphology, and virulence of Listeria monocytogenes EGDe. Antimicrob. Agents Chemother. 50:2824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson, G. B., T. R. Hughes, and M. Saxena. 1995. Distinct systems mediate the unidirectional efflux of methotrexate and cholate in human CCRF-CEM cells. Arch. Biochem. Biophys. 316:77-82. [DOI] [PubMed] [Google Scholar]
- 15.Kohda, C., Y. Yanagawa, and T. Shimamura. 2008. Epigallocatechin gallate inhibits intracellular survival of Listeria monocytogenes in macrophages. Biochem. Biophys. Res. Commun. 365:310-315. [DOI] [PubMed] [Google Scholar]
- 16.Krishna, R., and L. D. Mayer. 2000. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 11:265-283. [DOI] [PubMed] [Google Scholar]
- 17.Kruh, G. D., H. Zeng, P. A. Rea, G. Liu, Z. S. Chen, K. Lee, and M. G. Belinsky. 2001. MRP subfamily transporters and resistance to anticancer agents. J. Bioenerg. Biomembr. 33:493-501. [DOI] [PubMed] [Google Scholar]
- 18.Lee, C. H. 2004. Reversing agents for ATP-binding cassette (ABC) transporters: application in modulating multidrug resistance (MDR). Curr. Med. Chem. Anticancer Agents 4:43-52. [DOI] [PubMed] [Google Scholar]
- 19.Lee, E. W., M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis. Antimicrob. Agents Chemother. 47:3733-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaire, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2007. Modulation of the cellular accumulation and intracellular activity of daptomycin towards phagocytized Staphylococcus aureus by the P-glycoprotein (MDR1) efflux transporter in human THP-1 macrophages and Madin-Darby canine kidney cells. Antimicrob. Agents Chemother. 51:2748-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 22.Michot, J. M., M. F. Heremans, N. E. Caceres, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Cellular accumulation and activity of quinolones in ciprofloxacin-resistant J774 macrophages. Antimicrob. Agents Chemother. 50:1689-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michot, J. M., C. Seral, F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2005. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob. Agents Chemother. 49:2429-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michot, J. M., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2004. Active efflux of ciprofloxacin from J774 macrophages through an MRP-like transporter. Antimicrob. Agents Chemother. 48:2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neyfakh, A. A., C. M. Borsch, and G. W. Kaatz. 1993. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 37:128-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connor, R., M. O'Leary, J. Ballot, C. D. Collins, P. Kinsella, D. E. Mager, R. D. Arnold, L. O'Driscoll, A. Larkin, S. Kennedy, D. Fennelly, M. Clynes, and J. Crown. 2007. A phase I clinical and pharmacokinetic study of the multi-drug resistance protein-1 (MRP-1) inhibitor sulindac, in combination with epirubicin in patients with advanced cancer. Cancer Chemother. Pharmacol. 59:79-87. [DOI] [PubMed] [Google Scholar]
- 27.Ouadrhiri, Y., B. Scorneaux, Y. Sibille, and P. M. Tulkens. 1999. Mechanism of the intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gamma interferon. Antimicrob. Agents Chemother. 43:1242-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piddock, L. J., M. M. Johnson, S. Simjee, and L. Pumbwe. 2002. Expression of efflux pump gene pmrA in fluoroquinolone-resistant and -susceptible clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:808-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seral, C., M. Barcia-Macay, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2005. Comparative activity of quinolones (ciprofloxacin, levofloxacin, moxifloxacin and garenoxacin) against extracellular and intracellular infection by Listeria monocytogenes and Staphylococcus aureus in J774 macrophages. J. Antimicrob. Chemother. 55:511-517. [DOI] [PubMed] [Google Scholar]
- 30.Seral, C., S. Carryn, P. M. Tulkens, and F. Van Bambeke. 2003. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J. Antimicrob. Chemother. 51:1167-1173. [DOI] [PubMed] [Google Scholar]
- 31.Teodori, E., S. Dei, C. Martelli, S. Scapecchi, and F. Gualtieri. 2006. The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR). Curr. Drug Targets 7:893-909. [DOI] [PubMed] [Google Scholar]
- 32.Tyteca, D., P. Van Der Smissen, M. Mettlen, F. Van Bambeke, P. M. Tulkens, M. P. Mingeot-Leclercq, and P. J. Courtoy. 2002. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp. Cell Res. 281:86-100. [DOI] [PubMed] [Google Scholar]
- 33.Van Bambeke, F., E. Balzi, and P. M. Tulkens. 2000. Antibiotic efflux pumps. Biochem. Pharmacol. 60:457-470. [DOI] [PubMed] [Google Scholar]
- 34.Van Bambeke, F., Y. Glupczynski, P. Plesiat, J. C. Pechere, and P. M. Tulkens. 2003. Antibiotic efflux pumps in prokaryotic cells: occurrence, impact on resistance and strategies for the future of antimicrobial therapy. J. Antimicrob. Chemother. 51:1055-1065. [DOI] [PubMed] [Google Scholar]
- 35.Van Bambeke, F., J. M. Michot, and P. M. Tulkens. 2003. Antibiotic efflux pumps in eukaryotic cells: occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodynamics. J. Antimicrob. Chemother. 51:1067-1077. [DOI] [PubMed] [Google Scholar]
- 36.Van Bambeke, F., J. M. Pages, and V. J. Lee. 2006. Inhibitors of bacterial efflux pumps as adjuvants in antibiotic treatments and diagnostic tools for detection of resistance by efflux. Recent Patents Anti-Infect. Drug Discov. 1:157-175. [DOI] [PubMed] [Google Scholar]
- 37.Versantvoort, C. H., H. J. Broxterman, J. Lankelma, N. Feller, and H. M. Pinedo. 1994. Competitive inhibition by genistein and ATP dependence of daunorubicin transport in intact MRP overexpressing human small cell lung cancer cells. Biochem. Pharmacol. 48:1129-1136. [DOI] [PubMed] [Google Scholar]
- 38.Wang, E. J., C. N. Casciano, R. P. Clement, and W. W. Johnson. 2001. Active transport of fluorescent P-glycoprotein substrates: evaluation as markers and interaction with inhibitors. Biochem. Biophys. Res. Commun. 289:580-585. [DOI] [PubMed] [Google Scholar]
- 39.Yasuda, K., L. B. Lan, D. Sanglard, K. Furuya, J. D. Schuetz, and E. G. Schuetz. 2002. Interaction of cytochrome P450 3A inhibitors with P-glycoprotein. J. Pharmacol. Exp. Ther. 303:323-332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.