Abstract
HCV-796 selectively inhibits hepatitis C virus (HCV) NS5B RNA-dependent RNA polymerase. In hepatoma cells containing a genotype 1b HCV replicon, HCV-796 reduced HCV RNA levels by 3 to 4 log10 HCV copies/μg total RNA (the concentration of the compound that inhibited 50% of the HCV RNA level was 9 nM). Cells bearing replicon variants with reduced susceptibility to HCV-796 were generated in the presence of HCV-796, followed by G418 selection. Sequence analysis of the NS5B gene derived from the replicon variants revealed several amino acid changes within 5 Å of the drug-binding pocket. Specifically, mutations were observed at Leu314, Cys316, Ile363, Ser365, and Met414 of NS5B, which directly interact with HCV-796. The impacts of the amino acid substitutions on viral fitness and drug susceptibility were examined in recombinant replicons and NS5B enzymes with the single-amino-acid mutations. The replicon variants were 10- to 1,000-fold less efficient in forming colonies in cells than the wild-type replicon; the S365L variant failed to establish a stable cell line. Other variants (L314F, I363V, and M414V) had four- to ninefold-lower steady-state HCV RNA levels. Reduced binding affinity with HCV-796 was demonstrated in an enzyme harboring the C316Y mutation. The effects of these resistance mutations were structurally rationalized using X-ray crystallography data. While different levels of resistance to HCV-796 were observed in the replicon and enzyme variants, these variants retained their susceptibilities to pegylated interferon, ribavirin, and other HCV-specific inhibitors. The combined virological, biochemical, biophysical, and structural approaches revealed the mechanism of resistance in the variants selected by the potent polymerase inhibitor HCV-796.
Hepatitis C virus (HCV) is an enveloped, positive-sense, single-stranded RNA virus of approximately 9.6 kb that possesses an RNA-dependent RNA polymerase (RdRp), NS5B. Like that in many RNA viruses, this RNA replicase lacks a proofreading mechanism. The mutation rate of the HCV RdRp is estimated to be 10−3 to 10−4 mutations/nucleotide, or 1 mutation per genome replication (13a, 27). Genetic heterogeneity is further amplified by robust viral production of ∼1 × 1012 virions per day. As a consequence, quasispecies of viral variants have been found in HCV-infected patients (4, 6, 8). During chemotherapy, the high rates of viral replication and the high frequency of mutation lead to rapid generation of drug-resistant mutants. Emergence of resistant viruses is a major challenge in developing effective antiviral therapies against HCV infection.
NS5B RdRp, the principal catalytic enzyme for HCV replication, is a viable target for anti-HCV therapeutics (44). Recent research efforts have led to the discovery of many inhibitors that specifically target this enzyme (5, 7, 11, 19, 34, 35). Among all polymerase inhibitors reported to date, HCV-796 (Fig. 1) represents one of the most potent and selective antiviral agents demonstrating in vitro and in vivo activities (A. Y. M. Howe, S. K. Chunduru, D. C. Young, H. Cheng, D. Pevear, M. Collett, C. Burns, A. Del Vecchio, T. Bailey, B. Kulkarni, T. Faitg, S. Rippin, C. Blackledge, D. Rys, T. Lessen, J. Swestock, Y. Deng, T. Nitz, J. Reinhardt, H. Feng, A. Saha, T. Herbertz, T. Mansour, and J. F. O'Connell, presented at the 13th International Meeting on Hepatitis C Virus and Related Viruses, Cairns, Australia, 27 to 31 August 2006). Because of the high mutation rate during HCV replication, amino acid changes may accumulate in the NS5B RdRp, leading to decreased sensitivity to the polymerase inhibitors. Resistance studies using tissue culture systems help to validate the enzyme target, delineate the mechanism of action, and provide important information for optimizing second-generation inhibitors against HCV. In addition, mutations identified in the resistant viruses may serve as diagnostic and prognostic markers to screen for drug susceptibility in patients.
FIG. 1.
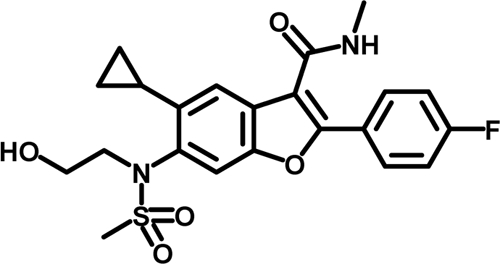
Structure of HCV-796, 5-cyclopropyl-2-(4-fluorophenyl)-6-[(2-hydroxyethyl)(methanesulfonyl)amino]-N-methyl-1-benzofuran-3-carboxamide.
Currently, cell cultures that support whole-virus replication are restricted to a few isolates, including a genotype 2a JFH-1 isolate derived from an individual with fulminant hepatitis (43, 49), a genotype 1a H77-S isolate that harbors five tissue culture-adaptive mutations (47), and chimeric constructs derived from genotype 1 and 2a JFH-1 (17). These infectious culture systems have not been widely used for resistance studies because of their restricted genotype specificities and limited infectivity after multiple cell passages. Several studies successfully demonstrated the HCV subgenomic replicon in selecting variants resistant to antiviral inhibitors (12, 16, 38, 46). A replicon is a subgenomic RNA that contains all essential elements and genes required for replication in the absence of structural genes (3, 18). The HCV replicon also contains a foreign gene encoding a drug-selectable marker (neomycin phosphotransferase) to allow antibiotic selection of cells that contain a functional replicon. Transfection of the HCV replicon into human hepatoma (Huh-7) cells leads to autonomous HCV RNA replication. This report describes the selection and characterization of replicon variants that have reduced susceptibility to HCV-796. Mapping of the amino acid changes encoded by the NS5B gene derived from the replicon variants identified the key mutations within the drug-binding pocket. Mutations in this pocket were responsible for the reduced susceptibilities of recombinant replicons and enzymes molecularly engineered with the single mutations to HCV-796. The drug susceptibilities of these replicon variants were evaluated in comparison to a panel of antiviral agents, including pegylated interferon and ribavirin.
MATERIALS AND METHODS
Materials.
All tissue culture reagents were purchased from Gibco BRL and HyClone. Clone A cells (licensed from Apath, LLC) were derived from Huh-7 cells, a human hepatoma cell line. The clone A cells contain approximately 1,000 genome copies of HCV genotype 1b replicon per cell when maintained in a subconfluent monolayer in the presence of 1 mg/ml geneticin (G418 sulfate; Gibco BRL). The sequence of the replicon in clone A cells is similar to that of the genotype 1b Con 1 strain of HCV (GenBank accession no. AJ238799), with the exception of two mutations in NS3 (Q1112R) and NS5A (S2204I). Clone A cells were propagated in Dulbecco's minimal essential medium (Gibco BRL) containing 10% fetal bovine serum (HyClone) supplemented with 1% penicillin/streptomycin (Gibco BRL), 1% nonessential amino acids (Gibco BRL), 1 mg/ml G418, and 0.66 mM HEPES buffer, pH 7.5.
The plasmid pBB7 containing the HCV genotype 1b BB7 replicon cDNA was also licensed from Apath, LLC. The coding sequence of pBB7 is similar to that of the genotype 1b Con 1 strain of HCV except for one nucleotide mutation resulting in an amino acid change of S2204I within NS5A. All other molecular biology reagents were obtained from suppliers as indicated.
Selection of drug-resistant HCV replicons in clone A cells.
Approximately 3 × 105 clone A cells were seeded in a T-25 tissue culture flask in triplicate and cultured in medium containing 2% fetal bovine serum without G418 and with 0.1 or 1 μM HCV-796 dissolved in dimethyl sulfoxide (DMSO) (final concentration, 0.5% [vol/vol]). As a control, clone A cells were passaged in parallel in the same medium containing 0.5% DMSO without compound. When the cell density reached approximately 80% confluence (about 2 to 3 days), the cells were split (1:3) in fresh medium containing HCV-796. An aliquot of the cells from each passage was collected to monitor HCV RNA levels. As the intracellular HCV load was reduced and reached a plateau (about 16 days), fresh medium containing HCV-796 and 0.5 mg/ml G418 was added to select for cells containing a functional replicon variant. Approximately 20 days after the selection, small colonies of cells resistant to the inhibitor and the antibiotic became visible and were pooled. The resistant cells (796R) generated from 0.1 and 1 μM HCV-796 were named 796R(0.1 μΜ) and 796R(1 μΜ), respectively. To generate resistant cells at a high compound concentration, cells from 796R(0.1 μΜ) and 796R(1 μΜ), respectively, were further incubated with 10 μΜ HCV-796 and 0.5 mg/ml G418 to generate pools of 796R(10 μΜ) cells. The selection of 796R(10 μΜ) cells from the 796R(0.1 μΜ) and the 796R(1 μΜ) pools required another 20 days after the addition of the drug. All resistant cells were cultured at the indicated drug concentrations in the presence of 0.5 mg/ml G418 for at least 3 weeks before analysis. To ascertain the reproducibility of the selection, genotype 1b (BB7 isolate) replicon-containing cells were cultured in the presence of 0.1 μM or 0.2 μM of HCV-796 with 0.5 mg/ml or 1 mg/ml G418, respectively, for six passages. As a control, genotype 1b (BB7 isolate) replicon-containing cells were passaged in parallel without HCV-796.
Isolation and sequencing of the NS5B gene from the replicon-containing cells.
Total cellular RNA was extracted from the replicon-containing cells using a Micro-to-Midi total-RNA purification system (Invitrogen). The NS5B-containing cDNA was generated in a two-step reverse transcription (RT)-PCR. The first-strand cDNA was generated by RT in a 10-μl reaction mixture containing 0.1 to 0.3 μg of total cellular RNA, 2 pmol of primer (7761R, 5′-CGTTCATCGGTTGGGGAGTA-3′) and 10 nmol each of deoxynucleoside triphosphates using the SuperScript first-strand synthesis system for RT-PCR (Invitrogen). The reaction mixture was mixed, heated at 65°C for 5 min, and placed on ice to allow the primer to anneal to the template RNA. Ten microliters of the RNA-primer mixture was added to 9 μl of the SuperScript II reaction mixture, which contained 10 mM dithiothreitol, 5 μM MgCl2, and 40 units of RNaseOut RNase inhibitor. After the reaction mixture (19 μl) was incubated at 42°C for 2 min, the RT reaction was initiated by adding 1 μl of the SuperScript II reverse transcriptase (50 units), followed by incubation at 42°C for 50 min. The reaction was terminated at 70°C for 15 min, followed by digestion with RNase H at 37°C for 20 min. To amplify the NS5B gene, 2 to 4 μl of the RT reaction products was mixed with 10 pmol each of the primers (5919F, 5′-GATCTCAGCGACGGGTCTT-3′, and 7761R), 10 nmol each of deoxynucleoside triphosphates, 2 units of Taq DNA polymerase, and 1× buffer supplemented with 1.5 mM MgCl2 provided by the supplier (Invitrogen). The reaction (final volume, 50 μl) was carried out at 95°C for 1 min, followed by 25 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 2 min and an extension at 72°C for 7 min. The PCR products were evaluated by agarose gel electrophoresis. The 1.8-kb fragment was excised and purified from the gel. The cDNA was ligated with the PCR4-TOPO vector, and the resulting recombinant DNA plasmid was transformed into One Shot chemical-competent Escherichia coli according to the manufacturer's instructions (Topo TA cloning kit for sequencing; Invitrogen). The presence of the HCV NS5B insert in the plasmids was verified by EcoRI digestion. Plasmids containing the HCV NS5B inserts were subjected to nucleotide sequencing using an ABI Prism BigDye terminator cycle-sequencing ready-reaction kit v3.0 (Applied Biosystems) according to the manufacturer's instructions. The sequenced products were gel purified using a DyeEx 96 kit (Qiagen), dried down, denatured with formaldehyde, and separated by electrophoresis using an ABI Prism 3700 DNA sequencer. Sequence data were analyzed using Sequencher v4.0.
Cloning and mutagenesis.
Standard recombinant DNA technology was used to construct and purify pBB7 replicon variant plasmids. All NS5B variants were initially generated using the plasmid NS5B-BB7dCT21-His as the input template (11). Single-nucleotide changes were introduced using the QuikChange XL Site Directed Mutagenesis kit (Stratagene) according to the manufacturer's procedure. Amino acid mutations were designated by the single-letter code of the parental amino acid, the amino acid position within NS5B, and the altered amino acid in the mutant constructs (e.g., L314F). The change in the nucleotide sequence in each mutant construct is indicated in Table 1. Individual clones were sequenced to confirm the presence of the desired mutations and the lack of other changes. To prepare the pBB7 replicon variant plasmids, the Bsu36I fragments from the mutant plasmid NS5B-BB7dCT21-His were cloned into pHCVrep1b.BB7 (licensed from Apath, LLC) backbones digested with Bsu36I. The pBB7 plasmids were sequenced to confirm the expected single-nucleotide changes in the coding sequence for NS5B.
TABLE 1.
Mutations in NS5B
| Mutation in 1b BB7 NS5Ba | Nucleotide positions | Nucleotide change |
|---|---|---|
| L314F | 940-942 | CTC→TTC |
| C316F | 947-949 | TGC→TTC |
| C316Y | 947-949 | TGC→TAC |
| C316N | 947-949 | TGC→AAC |
| C316S | 947-949 | TGC→AGC |
| I363V | 1087-2089 | ATA→GTA |
| S365A | 1094-1096 | TCA→GCA |
| S365T | 1094-1096 | TCA→ACA |
| S365L | 1094-1096 | TCA→TTA |
| S368F | 1102-1104 | TCC→TTC |
| M414I | 1240-1242 | ATG→ATC |
| M414T | 1240-1242 | ATG→ACG |
| M414V | 1240-1242 | ATG→GTG |
| N316Cb | 947-949 | AAC→TGC |
The numbering system for the amino acids and their corresponding coding nucleotide are based on the 1b, BB7 NS5B.
This construct was derived from the 1b BK isolate.
RNA transcription and electroporation of cultured cells.
pBB7 replicon variant DNAs were linearized with ScaI, and in vitro transcription was performed using Ambion's Megascript T7 High Yield Transcription kit. Purified RNA transcripts were electroporated into Huh-7 cells in quadruplicate using a Bio-Rad Gene Pulsar Electroporation System (settings, 270 V and 950 μF). Stably transfected replicon variant cell lines were initially selected with 0.25 mg/ml G418 and stepped up to 1 mg/ml before further testing. One cell plate was stained with crystal violet to visualize the number of colonies and to determine the colony formation efficiency. Individual cell clones from each plate were pooled and expanded for drug susceptibility testing. The NS5B gene of an early-passaged replicon variant was sequenced to confirm the presence of the expected nucleotide changes in the coding region for NS5B. No other changes affecting the amino acid sequence of NS5B were detected.
Expression and purification of NS5B enzyme variants.
All NS5B enzymes were expressed and purified according to the protocol for NS5B-BB7dCT21-His as previously described (11). Briefly, recombinant plasmids were transformed into E. coli cells, and the NS5B expression was initiated by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside). After 4 to 6 h of incubation, the cells were harvested and lysed. NS5B enzymes were purified by chromatography using a nickel affinity column (Talon; BD Biosciences Clontech), followed by a cation-exchange column (Poros HS; Applied Biosystems).
Evaluation of antiviral agents in replicon variants.
The drug susceptibility of the replicon-containing cells was evaluated as described previously (11). Briefly, the cells were treated with increasing concentrations of compounds in medium containing 2% fetal calf serum without G418 for 3 days at 37°C and 5% CO2. After incubation, total RNA from the replicon-containing cells was isolated. The levels of HCV, GAPDH (glyceraldehyde 3-phosphate dehydrogenase), and rRNAs were quantified using TaqMan reverse transcriptase PCRs. The amounts of HCV, 18S rRNA, and GAPDH RNA in each sample were estimated by comparing the number of cycles during the exponential phase of the PCR amplification with those in the corresponding standard curves. HCV RNA standards used for the construction of the standard curve were prepared by extracting the total RNA from clone A cells. The RNA sample was sent to the National Genetics Institute to quantify HCV RNA. The total RNA extracted from clone A cells was quantified by measurement of the optical density at 260 nm and used for construction of the standard curves of rRNA and GAPDH. The levels of HCV RNA and GAPDH were expressed as HCV RNA (copies) and GAPDH (ng) per μg of total RNA using rRNA as a marker for total-RNA measurement. The concentrations of the compounds that inhibited 50% of the HCV RNA level (EC50) were determined using the MDL LSW data analysis software in Microsoft Excel.
Binding of HCV-796 to NS5B.
Changes in the intrinsic fluorescence of HCV-796 were monitored by the excitation at 320 nm in the presence and absence of the NS5B polymerase. The emission maximum of the inhibitor occurred at 395 nm when free in solution and at 375 nm when bound to the enzyme. The shift in the emission maximum was accompanied by an increase in the fluorescence intensity of the bound inhibitor. To determine the binding affinity using these intensity changes, increasing concentrations of either the recombinant NS5B enzyme or the C316Y mutant enzyme were added to 200 nM of the inhibitor. The fluorescence spectra were recorded after incubation for about 40 min. The recombinant enzymes used in this study lacked 21 amino acids at the C terminus and were tagged with six histidines (11). In the control experiments, buffer instead of the enzyme was added to the inhibitor. The resulting changes in the fluorescence intensity at the emission maximum of 375 nm were used to generate binding isotherms and estimates of the affinity. The fluorescence spectra were recorded on a Fluoromax-3 (Jobin-Yvon, Edison, NJ). The fluorescence intensity changes were fitted to a single binding-site model to estimate KD values, using the following equation:
 |
where ΔF is the fluorescence intensity change at a fixed wavelength, KD is the binding affinity of the inhibitor for the protein, [Pt] is the total protein concentration, [It] is the total inhibitor concentration, and c is a constant that relates the fluorescence intensity to the concentration.
RESULTS
Selection of replicon variants with reduced susceptibility to HCV-796.
To select for HCV-796-associated replicon variants, cells bearing a genotype 1b HCV replicon were treated multiple times with 0.1 and 1 μM HCV-796 (an equivalent of 10- and 100-fold EC50, respectively, in a 3-day assay). At the end of the 16-day treatment, about 3.6 log10 and 4.2 log10 reductions of HCV RNA levels were observed in cells treated with 0.1 and 1 μM HCV-796, respectively (Fig. 2). The mRNA level of a housekeeping gene, the GAPDH gene, remained essentially unchanged throughout the 16-day period (data not shown). These results suggested that HCV-796 had a direct antiviral effect on HCV replication and that the compound was well tolerated by the cells.
FIG. 2.
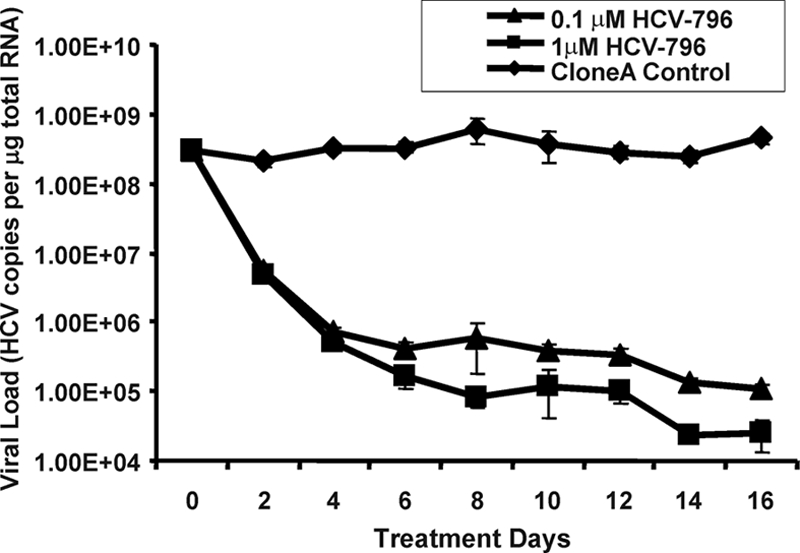
Multiple treatments of clone A cells with HCV-796. Clone A cells were treated with 0.1 μM and 1 μM of HCV-796 in Dulbecco's minimal essential medium containing 2% fetal calf serum, 0.5% DMSO, and no G418. Control cells were grown in the same medium without HCV-796. When the cell density reached about 80% confluence, the cells were split, and aliquots of cells were harvested for total cellular RNA extraction. The amounts of HCV RNA and rRNA were determined in a quantitative duplex TaqMan RT-PCR assay. The y axis represents HCV copies per μg of total cellular RNA (using rRNA as a marker for quantification). Each data point represents an average of three cell replicates. The error bars indicate standard deviations.
The HCV replicon encodes a drug-selectable (neomycin phosphotransferase) gene that allows selection of cells with a functional replicon in the presence of G418. During the course of drug selection, only cells that contained replicon variants with reduced susceptibility to HCV-796 survived and gave rise to colonies. These colonies of variant cells (796R), designated 796R(0.1 μM) and 796R(1 μM) cells, were pooled and expanded. A third pool of resistant cells [796R(10 μM)] was generated by further treating the 796R(0.1 μM) and 796R(1 μM) cells with 10 μM HCV-796.
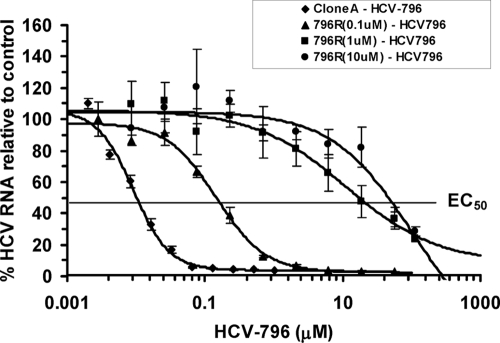
The susceptibility of the variant cells to HCV-796 was measured after treating the cells in the presence or absence of increasing concentrations of the compound for 72 h (Fig. 3). The levels of HCV RNA were determined using a quantitative TaqMan RT-PCR assay. All three 796R variant cell populations had similar steady-state HCV RNA levels compared to wild-type clone A cells (Table 2) and displayed different extents of susceptibility to the compound (Fig. 3). At the solubility limit (56 μM) of the compound in cell culture medium, HCV-796 reduced HCV RNA levels by 1.4 log10, 0.7 log10, and 0.5 log10 units in the 796R(0.1 μM), 796R(1 μM), and 796R(10 μM) cells, respectively. Control cells had a 2.1 log10 reduction in HCV RNA levels (Table 2). Comparison of the EC50 values for HCV-796 in the 796R cells with those in the control cells indicated that the replicon variants had 23- to >6,812-fold-reduced susceptibility to HCV-796 (Fig. 3 and Table 2). The resistant phenotypes were also confirmed by selecting replicon variants in the presence of 0.1 and 0.2 μM HCV-796. About 25- to 65-fold-reduced susceptibilities were observed among the variant cells in the second study (data not shown).
FIG. 3.
Effect of HCV-796 on variant cells selected by HCV-796. Seven thousand clone A or 796R cells were seeded per well in a 96-well tissue culture dish and treated with increasing concentrations of HCV-796 in the absence of G418. Cells were harvested 3 days after treatment and analyzed for HCV and rRNAs using a quantitative duplex TaqMan RT-PCR. The numbers of HCV RNA copies per μg total RNA were compared with those in the control cells. The data shown in the graph are the results from 1 of the 12 independent experiments. Each point represents an average of four replicates. The EC50 in the replicon-containing cells is indicated. The error bars indicate standard deviations.
TABLE 2.
Activities of HCV-796 against replicon variants
| Cellsa | EC50 (μM) ± SEb | Reduced susceptibility (n-fold) | Viral load (copies/μg total RNA) ± SEc | Mean log10 ± SE viral reductiond |
|---|---|---|---|---|
| Clone A | 0.013 ± 0.013 (n = 8) | 3.7 × 108 ± 2.4 × 108 | 2.1 ± 0.4 (0.7) | |
| 796R(0.1 μM) | 0.3 ± 0.2 (n = 4) | 23 | 2.9 × 108 ± 0.5 × 108 | 1.4 ± 0.3 (56) |
| 796R(1 μM) | 8.0 ± 5.2 (n = 12) | 618 | 2.4 × 108 ± 2.0 × 108 | 0.7 ± 0.2 (56) |
| 796R(10 μM) | >56.0 (n = 4) | >6,812 | 4.1 × 108 ± 1.7 × 108 | 0.5 ± 0.3 (56) |
796R represents cells that are less susceptible to HCV-796.
EC50 values were determined using MDL LSW data analysis. Inhibitory activity is expressed as mean EC50 ± standard error. n indicates the number of independent experiments.
Steady-state levels of HCV RNA after 3 days of incubation in tissue culture medium. The results represent at least three independent determinations.
Viral load reduction was determined at the indicated compound concentrations (μM, in parentheses) in a 3-day assay. The results represent at least three independent determinations.
Mapping of amino acid changes in HCV NS5B.
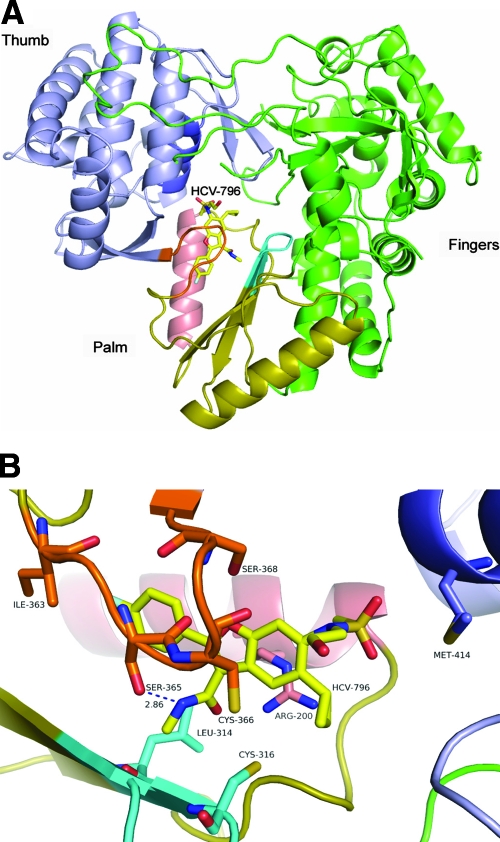
HCV-796 is a potent and selective inhibitor of HCV NS5B RdRp (A. Y. M. Howe, S. K. Chunduru, D. C. Young, H. Cheng, D. Pevear, M. Collett, C. Burns, A. Del Vecchio, T. Bailey, B. Kulkarni, T. Faitg, S. Rippin, C. Blackledge, D. Rys, T. Lessen, J. Swestock, Y. Deng, T. Nitz, J. Reinhardt, H. Feng, A. Saha, T. Herbertz, T. Mansour, and J. F. O'Connell, presented at the 13th International Meeting on Hepatitis C Virus and Related Viruses, Cairns, Australia, 27 to 31 August 2006). The crystal structure of NS5B in complex with HCV-796 showed that HCV-796 binds near the catalytic site in the palm domain of the enzyme (Fig. 4A). Therefore, it is likely that the resistance observed in the 796R cells was due to mutations within the NS5B gene. To map the nucleotide changes within NS5B, total cellular RNA was extracted from the 796R cells. The gene segment encoding NS5B was amplified by RT-PCR, followed by cloning and transformation into E. coli. Ninety-three bacterial clones containing a full-length NS5B gene derived from the pools of 796R cells were sequenced. In addition, 11 clones containing the NS5B gene derived from the control clone A cells were used as comparators.
FIG. 4.
Crystal structure showing HCV-796-associated amino acid mutations. (A) Overview of the HCV NS5b protein bound to HCV-796. The compound is shown with yellow carbons. The thumb domain is shown in blue, the palm in gold, and the fingers in green. The serine-rich loop is shown in orange (just in front of the compound), the active-site loop is shown in cyan, helix G is shown in salmon, and helix M is shown in dark blue. (B) Details of the HCV-796 binding site. The image is rotated 90° clockwise from panel A. HCV-796 is shown in yellow in the center of the model. The serine-rich loop is shown in orange, the active-site loop is shown in cyan, and helix G is shown in salmon. The hydrogen bond between the amide nitrogen of HCV-796 and the OH of Ser 365 is shown in blue. The positions of key wild-type amino acids are highlighted.
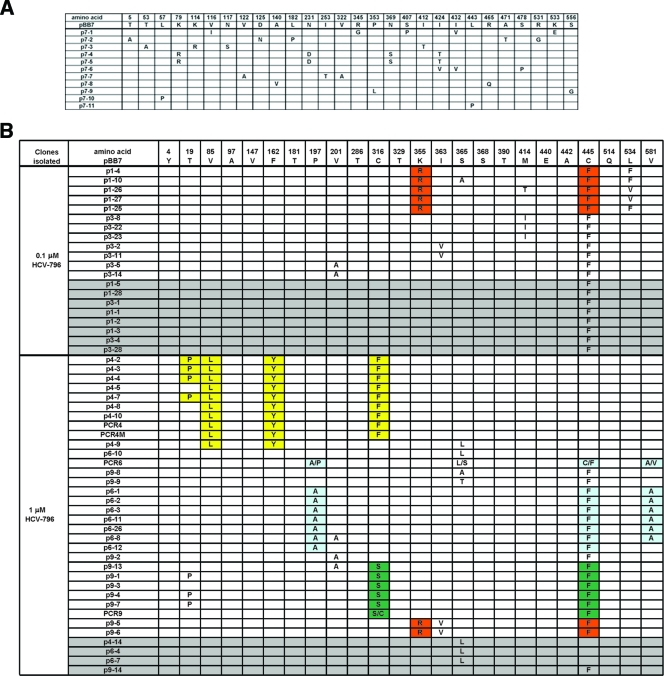
As shown in Fig. 5A, the NS5B genetic sequence from the control cells contained random amino acid changes with no specific patterns. A total of 32 amino acid changes were observed among the 11 clones, with an average of 3 amino acid changes per clone. All amino acid changes contained one nucleotide change per amino acid, resulting in a mutation rate of 1.6 × 10−3 mutations per nucleotide for the HCV replicon.
FIG. 5.
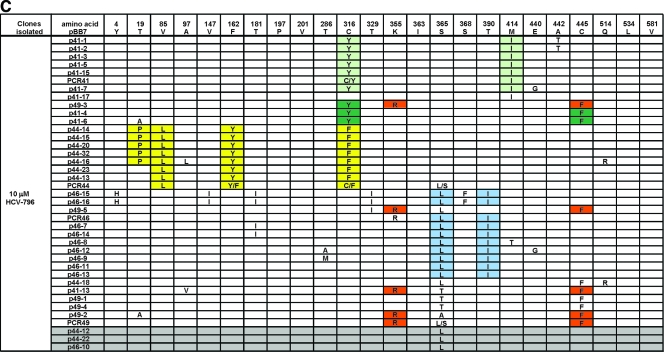
(A) Amino acid changes in NS5B derived from clone A control cells. (B) Amino acid changes in NS5B derived from 796R(0.1 μM) and 796R(1 μM) cells. (C) Amino acid changes in 796R(10 μM) cells. Some amino acids in the replicons are colored to illustrate the linkage of mutations. Replicon variants with only one mutation within NS5B are shaded in grey.
Several unique mutations within NS5B not found in the control cells were observed in the 93 clones derived from the 796R cells (Fig. 5B and C). Our attention was focused on changes in the vicinity of the HCV-796 binding site, which included C316Y, C316F, C316S, I363V, S365L, S365A, S365T, S368F, M414I, and M414T. An additional change, L314F, was observed in the second study. Cysteine 445 is located approximately 14 Å from the HCV-796 binding pocket. The C445F substitution was also frequently detected in replicon variants selected from other classes of HCV polymerase inhibitors. A luciferase replicon bearing the C445F mutation had over 10-fold-reduced susceptibility to HCV-796 (data not shown).
As illustrated in Fig. 4A and B, the key amino acid substitutions are distributed among four structural components within the drug-binding pocket. Amino acids Leu314 and Cys316 are within the active-site loop; Ile363, Ser365, and Ser368 are in the serine-rich loop; and M414 is in the α-helix M. All of these amino acids have direct interactions with HCV-796, as identified in the crystal structure of the NS5B-HCV-796 complex (Fig. 4B).
To assess if there was any pattern of mutations in the NS5B enzyme of replicon variants, combinations of amino acid substitutions were evaluated. Amino acid substitutions detected once in the DMSO-treated control cells were considered random mutations and were not included in the evaluation. Using these criteria, a total of 24 amino acid changes within NS5B were observed (Fig. 5B and C). Close examination of the amino acid changes revealed seven patterns of mutations. Of these, the double mutations (K355R plus C445F) were found in all three pools of 796R cells, and V85L plus F162Y plus C316F with or without T19P and C316S/Y plus C445F were found in replicon variants selected from 1 and 10 μM HCV-796. The rest of the combinations, P197A plus C445F plus V581A, C316Y plus M414I, and S365L plus T390I, were detected in either 796R(1 μM) or 796R(10 μM) variant cells. In some replicon variants, C445F and S365L existed as the sole amino acid change (Fig. 5B and C).
Characterization of the amino acid substitutions in replicon variants.
The contributions of amino acid substitutions located within the drug-binding pocket to drug susceptibility were assessed in replicon variants containing single-amino-acid mutations in NS5B in the background of the genotype 1b BB7 adaptive replicon (3). The replicon variants were tested in the presence or absence of increasing concentrations of HCV-796 in a 3-day assay. Within the active-site loop, the substitution of amino acid L314F did not alter the susceptibility of the replicon to HCV-796 (Table 3). In contrast, the replacement of cysteine 316 with phenylalanine, tyrosine, or serine (C316F/Y/S) resulted in EC50 values that were 130-, 166-, and 10-fold, respectively, greater than that of the wild-type 1b BB7 replicon (Table 3). Amino acid 316 within NS5B is highly polymorphic among natural isolates of genotype 1b (see Discussion). Although the C316N replicon variant was not detected in the replicon resistance selection, 316N makes up 40% of the NS5B sequences of natural isolates, as estimated from 249 sequences in the NIH genetic-sequence database (GenBank). A mutant replicon bearing the C316N substitution displayed over 26-fold-reduced susceptibility to HCV-796 (Table 3).
TABLE 3.
Activities of HCV-796 against HCV-796 replicon variants
| Replicon varianta | HCV RNA EC50 (nM) ± SDb | Resistance (n-fold) | Viral load (HCV copies/μg) ± SD | Viral load reductionc |
|---|---|---|---|---|
| 1b BB7 | 3.0 ± 1.0 (n = 11) | 1.8 × 108 ± 1.1 × 108 | 1.9 ± 0.3 | |
| 1b BB7-L314F | 4 ± 2 (n = 4) | 1 | 0.3 × 108 ± 0.1 × 108 | 1.6 ± 0.3 |
| 1b BB7-C316F | 392 ± 209 (n = 4) | 130 | 1.0 × 108 ± 0.2 × 108 | 0.8 ± 0.5 |
| 1b BB7-C316Y | 501 ± 291 (n = 4) | 166 | 1.3 × 108 ± 0.6 × 108 | 0.9 ± 0.2 |
| 1b BB7-C316Nd | 220 ± 110 (n = 4) | 26d | NAe | NA |
| 1b BB7-C316S | 30 ± 4 (n = 4) | 10 | 1.3 × 108 ± 0.7 × 108 | 1.3 ± 0.1 |
| 1b BB7-I363V | 16 ± 5 (n = 3) | 5 | 0.2 × 108 ± 0.1 × 108 | 1.4 ± 0.1 |
| 1b BB7-S365A | 124 ± 41 (n = 4) | 41 | 1.2 × 108 ± 0.3 × 108 | 1.7 ± 0.1 |
| 1b BB7-S365T | 643 ± 168 (n = 4) | 212 | 1.3 × 108 ± 0.6 × 108 | 0.6 ± 0.1 |
| 1b BB7-S365Lf | NA | NA | NA | NA |
| 1b BB7-S368F | 5 ± 2 (n = 4) | 2 | 2.6 × 108 ± 1.2 × 108 | 1.4 ± 0.3 |
| 1b BB7-M414I | 23 ± 3 (n = 5) | 8 | 1.3 × 108 ± 0.5 × 108 | 1.5 ± 0.2 |
| 1b BB7-M414T | 3 ± 1 (n = 4) | 1 | 1.5 × 108 ± 0.7 × 108 | 2.0 ± 0.2 |
| 1b BB7-M414V | 8 ± 1 (n = 3) | 3 | 0.4 × 108 ± 0.1 × 108 | 1.5 ± 0.1 |
1b BB7 represents the HCV genotype 1b BB7 isolate. The nomenclature of the replicon NS5B variants (e.g., L314F) is expressed as the amino acid of the input replicon, the amino acid position, and the amino acid substitution.
EC50 values were determined using MDL LSW data analysis. Inhibitory activity is expressed as mean EC50 ± standard deviation. n indicates the number of determinations.
The viral load reduction was determined at 2,240 nM HCV-796 in a 3-day assay. The data represent the mean log reduction of viral RNA ± standard deviation. The results represent at least three determinations.
The evaluation of 1b BB7-C316N was performed in a separate laboratory. The EC50 for HCV-796 in 1b BB7 was 8.6 ± 4 (n = 14), which was used to calculate the resistance for 1b BB7-C316N.
NA, not applicable.
Replicon variant S365L failed to establish a stable cell line upon selection with G418.
While changes in residues 363 (I363V) and 368 (S368F) within the serine-rich loop had a modest effect on susceptibility to HCV-796, replacement of serine 365 with alanine or threonine (S365A/T) led to 41- and 212-fold, respectively, reduced susceptibility to the compound (Table 3).
In α-helix M, the replacement of methionine 414 with isoleucine or valine (M414I/V) resulted in low to moderate increases in replicon EC50 values leading to three- to eightfold-reduced susceptibility to HCV-796 (Table 3). The change of methionine 414 to threonine did not change susceptibility to HCV-796 in the replicon.
The effects of the amino acid substitutions beyond the vicinity of the drug-binding pocket have not been evaluated. Given that the pools of 796R cells displayed a substantially higher resistance than the individual mutations examined (compare Table 2 and Table 3), these additional mutations might serve important functions, such as adaptation to cell culture replication or compensation for the impaired fitness induced by the primary mutations (see below), in addition to direct effects on drug susceptibility.
The impact of amino acid substitutions on viral fitness and growth kinetics was estimated based on the colony formation efficiency and steady-state HCV RNA levels in the replicon-containing cells. Transfection of the replicon RNAs into Huh-7 cells resulted in colony formation in the presence of G418 within 20 days after transfection. No colonies were obtained from Huh-7 cells transfected with the RNAs containing a GAA (polymerase-negative) mutation of NS5B or mock transfected (result not shown). As shown in Table 4, the colony formation efficiencies for the replicon variants were 10- to 1,000-fold less than for the wild-type BB7 replicon, suggesting that the amino acid substitution in NS5B adversely affected viral fitness. In particular, the amino acid substitutions within the serine-rich loop (I363V and S365T/L) produced the most severe impairment of colony formation (Table 4). The replicon variant bearing S365L formed small colonies initially but could not sustain G418 selection and failed to establish a stable cell line. Reduced fitness was not as apparent once the cell lines were established. HCV RNA levels comparable to those of the wild-type replicon were observed in the replicon variants L314F, I363V, and M414V (compare Table 4 to Table 3). Even though the S365L/T replicon variants exhibited impaired colony formation efficiencies (Table 4), the replicon RNA level in the S365T variant was comparable to that in the control 1b BB7 cells (Table 3). It should be noted that the HCV RNA levels in the pools of 796R cells were comparable to that in the wild-type clone A cells (Table 2). It is possible that compensatory mutations present in NS5B and/or other regions of the replicon genome might have restored the viral RNA to wild-type levels.
TABLE 4.
Colony formation efficiencies of replicon variants in Huh-7 cells
| Replicon variant | No. of CFU/μg RNA |
|---|---|
| 1b BB7 control | 20,000 |
| 1b BB7-L314F | 1,500 |
| 1b BB7-C316F | 3,000-5,000 |
| 1b BB7-C316S | 3,000-5,000 |
| 1b BB7-I363V | 100 |
| 1b BB7-S365A | 1,000 |
| 1b BB7-S365T | 120 |
| 1b BB7-S365L | 20a |
| 1b BB7-M414V | 500 |
| 1b BB7-M414T | 3,000 |
Did not survive G418 selection.
Inhibitory activity of HCV-796 in mutant NS5B enzymes.
To assess the effects of HCV-796 on polymerase activity in the replicon variants, recombinant genotype 1b BB7 NS5B enzymes molecularly engineered with single mutations were cloned and expressed in E. coli. The polymerase activities of the purified mutant enzymes were evaluated in a biochemical assay in the presence or absence of increasing concentrations of HCV-796. Similar to the replicon variants, the polymerase variants displayed reduced susceptibility to HCV-796 compared with the wild-type enzyme; although the levels of resistance were substantially attenuated (Table 5). Among the enzyme variants, the C316N/Y/F substitution resulted in 2- to 124-fold-reduced susceptibility to HCV-796, whereas the M414V/I and the I363V substitutions showed little difference in susceptibility to the compound (Table 5).
TABLE 5.
Activities of HCV-796 on HCV NS5B enzyme variants
| Enzyme | IC50a (nM) ± SD | Susceptibility relative to wild-type enzymeb |
|---|---|---|
| 1b BB7 (C316) | 40 ± 20 (n = 35) | |
| 1b BB7-C316N | 81 ± 42 (n = 4) | −2 |
| 1b BB7-C316Y | 320 ± 10 (n = 3) | −8 |
| 1b BB7-C316F | 1,508 ± 419 (n = 3) | −124 |
| 1b BB7-M414V | 28 ± 2 (n = 3) | +1.4 |
| 1b BB7-M414I | 24 ± 6 (n = 3) | +1.7 |
| 1b BB7-I363V | 60 ± 10 (n = 3) | −1.5 |
| 1b BK (N316) | 140 ± 50 (n = 33) | |
| 1b BK-N316C | 31 ± 4 (n = 3) | +4.5 |
IC50, 50% inhibitory concentration.
n-fold more (+) or less (−).
In the biochemical assay, the recombinant HCV NS5B enzymes derived from the natural genotype 1b isolates BK and J4 (26), which contain asparagine at position 316, are less susceptible to HCV-796 than enzymes with a cysteine at this position (A. Y. M Howe, S. K. Chunduru, and D. C. Young, presented at the 13th International Meeting on Hepatitis C Virus and Related Viruses, Cairns, Australia, 27 to 31 August, 2006). To ascertain the effects of asparagine and cysteine at position 316 in NS5B on susceptibility to HCV-796, the NS5B enzyme derived from the genotype 1b BK isolate (BK N316) was engineered with a single asparagine-to-cysteine change at amino acid 316 (BK-N316C). This enzyme variant was 4.5-fold more susceptible to HCV-796 than the wild-type BK enzyme (Table 5), confirming the importance of this residue for drug susceptibility in HCV-796.
Biophysical binding of HCV-796 to mutant NS5B protein.
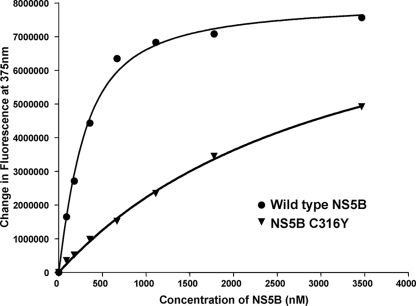
Sequence analysis of the NS5B gene derived from the 796R cells led to the identification of several amino acid changes within NS5B, including L314F, C316Y/F/S, I363V, S365L/A/T, S368F, and M414I/T/V. The X-ray crystal structure of HCV-796 in complex with HCV NS5B revealed that all these amino acids have direct interactions with HCV-796 (Fig. 4B). The mutations at cysteine 316 and serine 365 exhibited the strongest impact on drug susceptibility in both the replicon and the enzyme assays. To understand the mechanism of resistance of one of these mutations, the binding affinities of HCV-796 with the wild-type 1b BB7 NS5B and the mutant NS5B bearing the C316Y substitution were evaluated. The changes in the intrinsic fluorescence of HCV-796 were used to evaluate the binding affinity of the compound with the two enzymes. The emission maximum of HCV-796 occurred at 395 nm when free in solution and 375 nm when bound to enzyme. This emission maximum shift was accompanied by an increase in the fluorescence intensity of the bound inhibitor. Titration of HCV-796 with increasing concentrations of the enzymes resulted in an increase in the intrinsic fluorescence of the compound, which was used to generate the binding isotherms and to estimate the binding constants (Fig. 6). The results of the binding studies showed that the NS5B 1b BB7 enzyme bound HCV-796 tightly with a KD value of 180 nM. The mutant enzyme containing the C316Y substitution bound weakly with no evidence of saturation within the same enzyme concentration range, suggesting that the binding affinity was at least 1 log unit weaker. These results demonstrated that in the absence of RNA, NS5B with a tyrosine at position 316 binds HCV-796 much more weakly than with a cysteine at position 316, consistent with the reduced susceptibility in the C316Y replicon variant.
FIG. 6.
Binding of HCV-796 with NS5B. Shown is the binding isotherm of the interaction of HCV-796 with the wild-type NS5B and mutant NS5B bearing the C316Y substitution (NS5B C316Y). HCV-796 was incubated with the enzyme according to the procedure described in Materials and Methods. The change in the intrinsic fluorescence of HCV-796 at 375 nm, F0-F, in the presence of increasing concentrations of either the wild-type or mutant NS5B enzyme is proportional to the bound inhibitor concentration. The solid line corresponds to the curve fit to the quadratic equation.
Activities of antiviral agents in HCV-796-resistant replicon-containing cells.
The antiviral activities of a panel of antiviral agents, including two broad-spectrum antiviral agents and four HCV-specific inhibitors, were evaluated with the C316Y replicon variant and a pool of variant cells selected from HCV-796. Pegylated interferon and ribavirin both have demonstrated antiviral activities against many viruses (2, 10, 15, 22, 23, 40, 48). Pegylated interferon inhibited the HCV replication in the C316Y replicon variant and the 796R cells as efficiently as in the wild-type replicon (Table 6). Ribavirin had similar potencies against the C316Y and the wild-type replicons and was fivefold more active in the pool of resistant cells selected from 10 μM of HCV-796. The reasons for hypersensitivity against 796R are not clear; it might be due to metabolic variability in different cell lines and/or direct antiviral activities. Ribavirin also inhibited the replicon variants bearing the other HCV-796-associated mutations listed in Table 3 (data not shown).
TABLE 6.
Activities of antiviral agents against HCV-796-associated resistant replicon variants
| Compound | Replicon | EC50 (μM or pg/ml) ± SD | Resistance (n-fold) |
|---|---|---|---|
| Pegylated IFN-α-2ba | 1b BB7 (WT)b | 22 ± 29 (n = 3) | |
| 796R(1 μM) | 15 ± 13 (n = 7) | 0.7 | |
| Ribavirin | 1b BB7 (WT) | 123 ± 41 (n = 5) | |
| 796R(10 μM) | 29 ± 17 (n = 3) | 0.2 | |
| C316Y | 201 ± 37 (n = 3) | 1.6 | |
| HCV-086 | 1b BB7 (WT) | 0.3 ± 0.1 (n = 4) | |
| 796R(10 μM) | >80 (n = 4) | >40 | |
| C316Y | >80 (n = 2) | >40 | |
| Anthranilate | 1b BB7 (WT) | 0.7 (n = 2) | |
| 796R(10 μM) | 13 ± 1 (n = 3) | >19 | |
| HCV-371 | 1b BB7 (WT) | 12 ± 2 (n = 2) | |
| 796R(10 μM) | 12 ± 5 (n = 3) | 1 | |
| C316Y | 11 ± 1 (n = 2) | 0.8 | |
| 2′-C-methylcytidine | 1b BB7 (WT) | 1.0 (n = 2) | |
| 796R(10 μM) | 0.4 ± 0.1 (n = 4) | 0.4 |
IFN, interferon. EC50 is expressed in pg/ml.
WT, wild type.
The antiviral activities of four inhibitors that bind to different sites of the NS5B polymerase were evaluated. HCV-086, a benzofuran inhibitor structurally similar to HCV-796, showed reduced inhibitory activities against the C316Y replicon and 796R cells, suggesting a general resistance of the mutations to this series of inhibitors (Table 6). Interestingly, an anthranilate compound, an allosteric inhibitor that binds to the palm subdomain of the polymerase (25), also displayed reduced activities in the HCV-796R cells (Table 6). HCV-371, a pyranoindole inhibitor that binds in the lower thumb domain of the polymerase (11), inhibited both the wild-type and HCV-796-associated resistant replicons with similar activities (Table 6). 2′-C-Methylcytidine, an active nucleoside inhibitor of valopicitabine (NM 283) (39), which has demonstrated clinical efficacy, inhibited HCV-796-resistant replicons as effectively as the wild-type replicon.
DISCUSSION
HCV infection is the leading cause of cirrhosis and liver cancer affecting 170 million people worldwide (9, 45). The current standard of care, a combination of interferon products and ribavirin, has limited efficacy in less than 50% of patients infected with genotype 1 of the virus. Severe side effects leading to ∼10% to 20% treatment discontinuation further limit its utility in the clinic. Several HCV-specific protease and polymerase inhibitors, including BILN2061, telaprevir and boceprevir, valopicitabine, and HCV-796 (14, 21, 28, 29), have been evaluated in the clinic. In monotherapy studies, these agents reduced HCV RNA levels in patients chronically infected with HCV by 1 to 4 log units (1, 14, 31, 33, 39, 42). Resistant viruses were isolated in patients treated with all these agents (32, 41). Some mutations present in resistant clinical isolates were previously selected in the replicon system, underscoring the important utility of the replicon for preclinical resistance studies (16, 38).
In anticipation of resistance that might arise in the clinic, we sought to identify and characterize the HCV-796 resistance profile in vitro. We have done so using a comprehensive range of assays, from a cell-based replicon system to protein biophysical assays. Replicon variants, selected after exposure to HCV-796, displayed 23- to 6,812-fold-reduced susceptibility to the compound (Table 2). In a total of 93 clones that were sequenced in the NS5B region, several key mutations were identified. The frequencies of mutations varied, with C316Y/F/S, S365A/T/L, and C445F the most prevalent substitutions observed (Fig. 5B and C). The resistant phenotype of the replicon variants suggested that these amino acids played an important role in determining susceptibility to HCV-796 (Table 3). Based on low colony formation efficiency (Table 4) and the reduced HCV RNA levels in the established replicon cell lines (Table 3), these variants were less fit than the wild-type replicon. The binding of HCV-796 to apo-HCV NS5B has been characterized by an X-ray crystallography study of the complex (R. Chopra, G. Krishnamurthy and A. Y. M. Howe, unpublished observations). Using this information, the sites of mutations were mapped to the three-dimensional model, and their effects were evaluated. Ser365 forms a hydrogen bond from the side chain oxygen to the amide nitrogen of HCV-796. The S365A, S365T, and S365L mutations perturb this key hydrogen bond, providing a structural understanding of the 41- to 212-fold-reduced susceptibility to HCV-796 in the S365A/T/L replicon variants (Table 3). Similarly, the mutation on α-helix M at position 414 results in a direct perturbation of a side chain interaction with the compound HCV-796 and highlights a region of the protein that has been observed to generate resistance to other inhibitor classes that are located in close proximity to the HCV-796 binding site (20). Cys316 is immediately adjacent to the catalytic triad (GDD motif; G317, D318, and D319) of the NS5B RdRp, which coordinates the metal ions and nucleotide triphosphate during HCV RNA synthesis (18). Replacement of Cys316 with a larger hydrophobic side chain, such as phenylalanine or tyrosine, would result in a substantially different binding mode for HCV-796. Indeed, measured direct binding of HCV-796 to the mutant C316Y NS5B protein revealed an order-of-magnitude-weaker binding relative to wild-type NS5B (Fig. 6). Cys316 in NS5B is highly conserved in HCV genotype 1a isolates, whereas the position is rather polymorphic among isolates in genotypes 1b and 4. Of 117 genotype 1b sequences reported in GenBank, 40% contain asparagine, 57% contain cysteine, and 4% contain tyrosine at amino acid 316 of NS5B. Five percent of the natural isolates in genotype 4 contain asparagine at this position. In the 1b replicon, the C316Y mutation was selected after multiple treatments with HCV-796; however, the Cys316Asn mutation was not. The C316Y mutation requires one nucleotide change (underlined) (TGC→TAC), whereas the mutation of C316N requires two simultaneous or sequential substitutions (TGC→AAC) (Table 1). Although the C316N mutation might be more difficult to generate due to two nucleotide substitutions, the presence of Asn316 variants in the natural genotype 1b isolates might affect the efficacy of HCV-796. Both the Tyr316 and Asn316 replicon variants have reduced susceptibility to HCV-796 (Table 3).
A combination cocktail containing HCV-796 and other antiviral agents might help to combat viral resistance. The combination of a polymerase inhibitor, HCV-796, and a protease inhibitor, boceprevir, has been demonstrated to enhance antiviral activities in replicons and reduce the emergence of resistant variants (13). In addition to the nucleoside analogues, all nonnucleoside inhibitors reported to date were shown to bind at one of the four regions in the HCV NS5B: two at the thumb subdomain (36) and two at the palm subdomain. With the exception of anthranilate, the resistant variants selected by HCV-796 remained sensitive to HCV polymerase inhibitors that bind to different sites of the polymerase (Table 6), supporting the use of different polymerase inhibitors in a combination therapy. The reason for cross-resistance between the benzofuran and anthranilate classes of inhibitors is unclear. A structural comparison of the anthranilate and HCV-796 binding sites showed an overlap of amino acid residues in the palm subdomain of the polymerase (unpublished data). Several inhibitors from different chemical classes were shown to bind NS5B at or proximal to the anthranilate binding site (20, 24, 37); it is prudent to examine the potential cross-resistances of these inhibitors before combination therapy.
In vitro resistance data have been relatively easy to generate but difficult to interpret. We have delineated the important amino acids (Cys316, Ser365, and Met414) as markers for HCV-796 resistance generated either by exposure to the compound or by selection from preexisting pools of replicons. In addition to identifying and characterizing resistant variants selected by HCV-796 in the replicon and enzyme systems, the studies described here were unique in relating the HCV polymerase resistance to the site of inhibition through structural and biophysical analyses. At present, it is unclear if the resistant replicons selected by HCV-796 in vitro predict selection of resistant viruses in vivo. Some replicon variants with reduced replicative capacities were stabilized only under selective pressure from G418. These variants may not survive or may represent a minority of the HCV population in vivo. Nevertheless, the selection pressure exerted by an immune response in vivo is predicted to significantly affect the genetic evolution of the virus. To assess the impacts of resistance on chemotherapy, the mutation frequency, population size, temporal profile, and replication fitness of the resistant variants in patients should be evaluated or monitored. In the phase 1b proof-of-concept studies, patients treated with HCV-796 alone or in combination with pegylated interferon had mean viral-load reductions of 1.4 and 3.3 log10 units, respectively. The clinical testing of HCV-796 was halted due to the liver enzyme elevation observed in some patients after 8 to 10 weeks of dosing during the phase 2 studies. Population sequencing of the clinical isolates derived from the phase 1 patient samples showed that C316Y was the predominant mutation associated with viral rebound. At present, we do not know if other mutations identified in the replicon selection might also exist as minor variants in these clinical samples. The combined use of virological, biochemical, and structural methods has enhanced our understanding of the molecular mechanism of resistance, which may help us to design more successful treatment strategies and develop second-generation inhibitors with improved potencies and resistance profiles. In spite of a setback in clinical development, HCV-796 represents the only compound at this binding site that has demonstrated clinical efficacy. Efforts to eliminate the clinical side effects in this class of inhibitors will likely yield an optimal compound that will prove to have clinical utility.
Acknowledgments
We thank Anne Deatly and Mike Flint for their critical review and helpful discussion of the manuscript. Special thanks are extended to the Wyeth Core Sequencing Facility for their technical assistance. We are also grateful to Susan Nastasee for editorial support with manuscript preparation.
Footnotes
Published ahead of print on 16 June 2008.
REFERENCES
- 1.Afdhal, N., E. Godofsky, J. Dienstag, V. Rustgi, L. Schick, D. McEnlry, X. S. Zhou, G. Chao, C. Fang, B. Fielman, and M. Myers. 2004. Final Phase I/II trial results for NM283, a new polymerase inhibitor for hepatitis C: Antiviral efficacy and tolerance in patients with HCV-1 infection, including previous interferon failures. Hepatology 40(Suppl. 1):726. (Abstract.) [Google Scholar]
- 2.Akahane, Y., M. Sakamoto, Y. Miyazaki, S. Okada, T. Inoue, M. Ukita, H. Okamoto, Y. Miyakawa, and M. Mayumi. 1999. Effect of interferon on a nonenveloped DNA virus (TT virus) associated with acute and chronic hepatitis of unknown etiology. J. Med. Virol. 58:196-200. [DOI] [PubMed] [Google Scholar]
- 3.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 4.Cabot, B., M. Martell, J. I. Esteban, S. Sauleda, T. Otero, R. Esteban, J. Guardia, and J. Gomez. 2000. Nucleotide and amino acid complexity of hepatitis C virus quasispecies in serum and liver. J. Virol. 74:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll, S. S., J. E. Tomassini, M. Bosserman, K. Getty, M. W. Stahlhut, A. B. Eldrup, B. Bhat, D. Hall, A. L. Simcoe, R. LaFemina, C. A. Rutkowski, B. Wolanski, Z. C. Yang, G. Migliaccio, R. De Francesco, L. C. Kuo, M. MacCoss, and D. B. Olsen. 2003. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 278:11979-11984. [DOI] [PubMed] [Google Scholar]
- 6.Davis, G. L. 1999. Hepatitis C virus genotypes and quasispecies. Am. J. Med. 107:21S-26S. [DOI] [PubMed] [Google Scholar]
- 7.Dhanak, D., K. J. Duffy, V. K. Johnston, J. Lin-Goerke, M. Darcy, A. N. Shaw, B. Gu, C. Silverman, A. T. Gates, M. R. Nonnemacher, D. L. Earnshaw, D. J. Casper, A. Kaura, A. Baker, C. Greenwood, L. L. Gutshall, D. Maley, A. DelVecchio, R. Macarron, G. A. Hofmann, Z. Alnoah, H. Y. Cheng, G. Chan, S. Khandekar, R. M. Keenan, and R. T. Sarisky. 2002. Identification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277:38322-38327. [DOI] [PubMed] [Google Scholar]
- 8.Farci, P., and R. H. Purcell. 2000. Clinical significance of hepatitis C virus genotypes and quasispecies. Semin. Liver Dis. 20:103-126. [PubMed] [Google Scholar]
- 9.Global Burden Of Hepatitis C Working Group. 2004. Global burden of disease (GBD) for hepatitis C. J. Clin. Pharmacol. 44:20-29. [DOI] [PubMed] [Google Scholar]
- 10.Hartman, C., D. Berkowitz, D. Shouval, O. Eshach-Adiv, B. Hino, N. Rimon, I. Satinger, T. Kra-Oz, N. Daudi, and R. Shamir. 2003. Lamivudine treatment for chronic hepatitis B infection in children unresponsive to interferon. Pediatr. Infect. Dis. J. 22:224-229. [DOI] [PubMed] [Google Scholar]
- 11.Howe, A. Y., J. Bloom, C. Baldick, J. Christophers, H.-M. Cheng, J. S. Christensen, S. K. Chunduru, G. A. Coburn, B. Feld, A. Gopalsamy, W. P. Gorczyca, S. Herrman, S. Johann, X. Jiang, M. L. Kimberland, G. Krishnamurthy, M. W. Olson, M. Orlowski, S. Swanberg, I. Thompson, M. Thorn, A. M. Del Vecchio, D. C. Young, M. van Zeijl, J. W. Ellingboe, J. Upeslacis, M. S. Collett, T. S. Mansour, and J. O'Connell. 2004. Indentification of a novel non-nucleoside inhibitor of hepatitis C virus RNA-dependent RNA polymerase. Antimicrob. Agents Chemother. 48:4813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe, A. Y., H. Cheng, I. Thompson, S. K. Chunduru, S. Herrmann, J. O'Connell, A. Agarwal, R. Chopra, and A. M. Del Vecchio. 2006. Molecular mechanism of a thumb domain hepatitis C virus nonnucleoside RNA-dependent RNA polymerase inhibitor. Antimicrob. Agents Chemother. 50:4103-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe, A. Y., R. Ralston, R. Chase, X. Tong, A. Skelton, M. Flint, S. Mullen, B. Malcolm, C. Broom, and E. A. Emini. 2007. Favorable cross-resistance profile of two novel hepatitis C virus inhibitors, SCH 503034 and HCV-796, and enhanced anti-replicon activity mediated by the combined use of both compounds. Hepatology 46(Suppl. 1):S165. [Google Scholar]
- 13a.Kato, N., T. Nakamura, H. Dansako, K. Namba, K. Abe, A. Nozaki, K. Naka, M. Ikeda, and K. Shimotohno. 2005. Genetic variation and dynamics of hepatitis C virus replicons in long-term cell culture. J. Gen. Virol. 86:645-656. [DOI] [PubMed] [Google Scholar]
- 14.Lamarre, D., P. C. Anderson, M. Bailey, P. Beaulieu, G. Bolger, P. Bonneau, M. Bos, D. R. Cameron, M. Cartier, M. G. Cordingley, A. M. Faucher, N. Goudreau, S. H. Kawai, G. Kukolj, L. Lagace, S. R. LaPlante, H. Narjes, M. A. Poupart, J. Rancourt, R. E. Sentjens, R. St George, B. Simoneau, G. Steinmann, D. Thibeault, Y. S. Tsantrizos, S. M. Weldon, C. L. Yong, and M. Llinas-Brunet. 2003. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426:186-189. (Erratum, 426:246.) [DOI] [PubMed] [Google Scholar]
- 15.Lanford, R. E., B. Guerra, H. Lee, D. R. Averett, B. Pfeiffer, D. Chavez, L. Notvall, and C. Bigger. 2003. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(I)-poly(C), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J. Virol. 77:1092-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, C., K. Lin, Y. P. Luong, B. G. Rao, Y. Y. Wei, D. L. Brennan, J. R. Fulghum, H. M. Hsiao, S. Ma, J. P. Maxwell, K. M. Cottrell, R. B. Perni, C. A. Gates, and A. D. Kwong. 2004. In vitro resistance studies of hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061: structural analysis indicates different resistance mechanisms. J. Biol. Chem. 279:17508-17514. [DOI] [PubMed] [Google Scholar]
- 17.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 18.Lohmann, V., S. Hoffmann, U. Herian, F. Penin, and R. Bartenschlager. 2003. Viral and cellular determinants of hepatitis C virus RNA replication in cell culture. J. Virol. 77:3007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love, R. A., H. E. Parge, X. Yu, M. J. Hickey, W. Diehl, J. Gao, H. Wriggers, A. Ekker, L. Wang, J. A. Thomson, P. S. Dragovich, and S. A. Fuhrman. 2003. Crystallographic identification of a noncompetitive inhibitor binding site on the hepatitis C virus NS5B RNA polymerase enzyme. J. Virol. 77:7575-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu, L., H. Mo, T. J. Pilot-Matias, and A. Molla. 2007. Evolution of resistant M414T mutants among hepatitis C virus replicon cells treated with polymerase inhibitor A-782759. Antimicrob. Agents Chemother. 51:1889-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malcolm, B. A., R. Liu, F. Lahser, S. Agrawal, B. Belanger, N. Butkiewicz, R. Chase, F. Gheyas, A. Hart, D. Hesk, P. Ingravallo, C. Jiang, R. Kong, J. Lu, J. Pichardo, A. Prongay, A. Skelton, X. Tong, S. Venkatraman, E. Xia, V. Girijavallabhan, and F. G. Njoroge. 2006. SCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrob. Agents Chemother. 50:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick, J. B., J. P. Getchell, S. W. Mitchell, and D. R. Hicks. 1984. Ribavirin suppresses replication of lymphadenopathy-associated virus in cultures of human adult T lymphocytes. Lancet ii:1367-1369. [DOI] [PubMed] [Google Scholar]
- 23.McCormick, J. B., I. J. King, P. A. Webb, C. L. Scribner, R. B. Craven, K. M. Johnson, L. H. Elliott, and R. Belmont-Williams. 1986. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 314:20-26. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen, T. T., A. T. Gates, L. L. Gutshall, V. K. Johnston, B. Gu, K. J. Duffy, and R. T. Sarisky. 2003. Resistance profile of a hepatitis C virus RNA-dependent RNA polymerase benzothiadiazine inhibitor. Antimicrob. Agents Chemother. 47:3525-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nittoli, T., K. Curran, S. Insaf, M. DiGrandi, M. Orlowski, R. Chopra, A. Agarwal, A. Y. Howe, A. Prashad, M. B. Floyd, B. Johnson, A. Sutherland, K. Wheless, B. Feld, J. O'Connell, T. S. Mansour, and J. Bloom. 2007. Identification of anthranilic acid derivatives as a novel class of allosteric inhibitors of hepatitis C NS5B polymerase. J. Med. Chem. 50:2108-2116. [DOI] [PubMed] [Google Scholar]
- 26.O'Farrell, D., R. Trowbridge, D. Rowlands, and J. Jager. 2003. Substrate complexes of hepatitis C virus RNA polymerase (HC-J4): structural evidence for nucleotide import and de-novo initiation. J. Mol. Biol. 326:1025-1035. [DOI] [PubMed] [Google Scholar]
- 27.Ogata, N., H. J. Alter, R. H. Miller, and R. H. Purcell. 1991. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc. Natl. Acad. Sci. USA 88:3392-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perni, R. B., S. J. Almquist, R. A. Byrn, G. Chandorkar, P. R. Chaturvedi, L. F. Courtney, C. J. Decker, K. Dinehart, C. A. Gates, S. L. Harbeson, A. Heiser, G. Kalkeri, E. Kolaczkowski, K. Lin, Y. P. Luong, B. G. Rao, W. P. Taylor, J. A. Thomson, R. D. Tung, Y. Wei, A. D. Kwong, and C. Lin. 2006. Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease. Antimicrob. Agents Chemother. 50:899-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierra, C., S. Benzaria, A. Amador, A. Moussa, S. Mathieu, R. Storer, and G. Gosselin. 2005. Nm 283, an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. Nucleosides Nucleotides Nucleic Acids 24:767-770. [DOI] [PubMed] [Google Scholar]
- 30.Reference deleted.
- 31.Reesink, H. W., S. Zeuzem, C. J. Weegink, N. Forestier, A. van Vliet, J. van de Wetering de Rooij, L. McNair, S. Purdy, R. Kauffman, J. Alam, and P. L. Jansen. 2006. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase Ib, placebo-controlled, randomized study. Gastroenterology 131:997-1002. [DOI] [PubMed] [Google Scholar]
- 32.Sarrazin, C., T. L. Kieffer, D. Bartels, B. Hanzelka, U. Muh, M. Welker, D. Wincheringer, Y. Zhou, H. M. Chu, C. Lin, C. Weegink, H. Reesink, S. Zeuzem, and A. D. Kwong. 2007. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 132:1767-1777. [DOI] [PubMed] [Google Scholar]
- 33.Sarrazin, C., R. Rouzier, F. Wagner, N. Forestier, D. Larrey, S. K. Gupta, M. Hussain, A. Shah, D. Cutler, J. Zhang, and S. Zeuzem. 2007. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonresponders. Gastroenterology 132:1270-1278. [DOI] [PubMed] [Google Scholar]
- 34.Shim, J., G. Larson, V. Lai, S. Naim, and J. Z. Wu. 2003. Canonical 3′-deoxyribonucleotides as a chain terminator for HCV NS5B RNA-dependent RNA polymerase. Antivir. Res. 58:243-251. [DOI] [PubMed] [Google Scholar]
- 35.Summa, V., A. Petrocchi, P. Pace, V. G. Matassa, R. De Francesco, S. Altamura, L. Tomei, U. Koch, and P. Neuner. 2004. Discovery of alpha, gamma-diketo acids as potent selective and reversible inhibitors of hepatitis C virus NS5b RNA-dependent RNA polymerase. J. Med. Chem. 47:14-17. [DOI] [PubMed] [Google Scholar]
- 36.Tomei, L., S. Altamura, L. Bartholomew, A. Biroccio, A. Ceccacci, L. Pacini, F. Narjes, N. Gennari, M. Bisbocci, I. Incitti, L. Orsatti, S. Harper, I. Stansfield, M. Rowley, R. De Francesco, and G. Migliaccio. 2003. Mechanism of action and antiviral activity of benzimidazole-based allosteric inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 77:13225-13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomei, L., S. Altamura, L. Bartholomew, M. Bisbocci, C. Bailey, M. Bosserman, A. Cellucci, E. Forte, I. Incitti, L. Orsatti, U. Koch, R. De Francesco, D. B. Olsen, S. S. Carroll, and G. Migliaccio. 2004. Characterization of the inhibition of hepatitis C virus RNA replication by nonnucleosides. J. Virol. 78:938-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong, X., R. Chase, A. Skelton, T. Chen, J. Wright-Minogue, and B. A. Malcolm. 2006. Identification and analysis of fitness of resistance mutations against the HCV protease inhibitor SCH 503034. Antivir. Res. 70:28-38. [DOI] [PubMed] [Google Scholar]
- 39.Toniutto, P., C. Fabris, D. Bitetto, E. Fornasiere, R. Rapetti, and M. Pirisi. 2007. Valopicitabine dihydrochloride: a specific polymerase inhibitor of hepatitis C virus. Curr. Opin. Investig. Drugs 8:150-158. [PubMed] [Google Scholar]
- 40.Umemura, T., H. J. Alter, E. Tanaka, K. Orii, A. E. Yeo, J. W. Shih, A. Matsumoto, K. Yoshizawa, and K. Kiyosawa. 2002. SEN virus: response to interferon alfa and influence on the severity and treatment response of coexistent hepatitis C. Hepatology 35:953-959. [DOI] [PubMed] [Google Scholar]
- 41.Villano, S., A. Howe, D. Raible, D. Harper, J. Speth, and G. Bichier. 2006. Analysis of HCV NS5B genetic variants following monotherapy with HCV-796, a non-nucleoside polymerase inhibitor, in treatment-naive HCV-infected patients. Hepatology 44(Suppl. 1):607A-608A. [Google Scholar]
- 42.Villano, S., D. Raible, D. Harper, J. Speth, P. Chandra, P. Shaw, and G. Bichier. 2007. Antiviral activity of the non-nucleoside polymerase inhibitor, HCV-796, in combination with pegylated interferon alfa-2b in treatment-naive patients with chronic HCV J. Hepatology 46(Suppl. 1):S24. [Google Scholar]
- 43.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. (Erratum, 11:905.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker, M. P., and Z. Hong. 2002. HCV RNA-dependent RNA polymerase as a target for antiviral development. Curr. Opin. Pharmacol. 2:534-540. [DOI] [PubMed] [Google Scholar]
- 45.WHO. 1999. Global surveillance and control of hepatitis C. Report of a WHO consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J. Viral Hepat. 6:35-47. [PubMed] [Google Scholar]
- 46.Yi, M., X. Tong, A. Skelton, R. Chase, T. Chen, A. Prongay, S. L. Bogen, A. K. Saksena, F. G. Njoroge, R. L. Veselenak, R. B. Pyles, N. Bourne, B. A. Malcolm, and S. M. Lemon. 2006. Mutations conferring resistance to SCH6, a novel hepatitis C virus NS3/4A protease inhibitor. Reduced RNA replication fitness and partial rescue by second-site mutations. J. Biol. Chem. 281:8205-8215. [DOI] [PubMed] [Google Scholar]
- 47.Yi, M., R. A. Villanueva, D. L. Thomas, T. Wakita, and S. M. Lemon. 2006. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc. Natl. Acad. Sci. USA 103:2310-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, M. L., W. L. Chuang, C. Y. Dai, S. C. Chen, Z. Y. Lin, M. Y. Hsieh, J. F. Tsai, L. Y. Wang, and W. Y. Chang. 2001. GB virus C/hepatitis G virus infection in chronic hepatitis C patients with and without interferon-alpha therapy. Antivir. Res. 52:241-249. [DOI] [PubMed] [Google Scholar]
- 49.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]