Abstract
In preclinical studies, artemisone (BAY 44-9585), a new artemisinin derivative, was shown to possess enhanced efficacy over artesunate, and it does not possess the neurotoxicity characteristic of the current artemisinins. In a phase I program with double-blind, randomized, placebo-controlled, single and multiple ascending oral-dose studies, we evaluated the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of artemisone. Single doses (10, 20, 30, 40, and 80 mg) and multiple doses (40 and 80 mg daily for 3 days) of artemisone were administered orally to healthy subjects. Plasma concentrations of artemisone and its metabolites were measured by liquid chromatography/tandem mass spectrometry (LC/MS-MS). Artemisone was well tolerated, with no serious adverse events and no clinically relevant changes in laboratory and vital parameters. The pharmacokinetics of artemisone over the 10- to 80-mg range demonstrated dose linearity. After the single 80-mg dose, artemisone had a geometric mean maximum concentration of 140.2 ng/ml (range, 86.6 to 391.0), a short elimination half-life (t1/2) of 2.79 h (range, 1.56 to 4.88), a high oral clearance of 284.1 liters/h (range, 106.7 to 546.7), and a large volume of distribution of 14.50 liters/kg (range, 3.21 to 51.58). Due to artemisone's short t1/2, its pharmacokinetics were comparable after single and multiple dosing. Plasma samples taken after multiple dosing showed marked ex vivo pharmacodynamic antimalarial activities against two multidrug-resistant Plasmodium falciparum lines. Artemisone equivalent concentrations measured by bioassay revealed higher activity than artemisone measured by LC/MS-MS, confirming the presence of active metabolites. Comparable to those of other artemisinin's, artemisone's t1/2 is well suited for artemisinin-based combination therapy for the treatment of P. falciparum malaria.
Artemisinin and its derivatives, artesunate, artemether, and dihydroartemisinin, are the most potent and rapidly acting antimalarial drugs available today (1, 33). They have very high parasite kill rates with a broad stage specificity of antimalarial action and produce a faster clinical and parasitological response than any other class of antimalarial drug (24, 32, 33). However, a therapeutic drawback of the artemisinins is the high recrudescence rates associated with monotherapy (6). For artemisinins to be effective when given alone, they must be administered for 7 days, but adherence to 7-day regimens may be poor in patients (34).
The latest therapeutic approach to combat both malaria infections and the development of drug resistance has been the use of artemisinin-based combination therapy (ACT) administered over 3 days (1, 23, 24, 34). However, 3-day regimens with the rapidly eliminated artemisinins are usually not curative unless they are given in combination with a slowly eliminated drug, such as mefloquine (34). Although the artemisinins have become the drug of choice for the treatment of multidrug-resistant Plasmodium falciparum malaria (12, 31) financial considerations have limited the development of these compounds. Private-public partnerships between the pharmaceutical industry and nonprofit organizations should be able to reverse this situation and produce artemisinin compounds suitable for licensing by drug regulatory authorities.
Artemisone (BAY 44-9585), a 10-alkylaminoartemisinin, is a new artemisinin derivative that is being developed to international drug regulatory standards. It is a highly crystalline (no polymorphs), thermally stable compound that has an aqueous solubility at pH 7.2 of 89 mg/liter and a log P (partition coefficient n-octanol-water) at pH 7.4 of 2.49 (11). The low log P characterizes artemisone as a nonlipophilic drug, and its low lipophilicity attenuates to low neuro- and cytotoxicity in in vitro and in vivo assays (11). The lack of neurotoxicity of artemisone is crucial, since preclinical studies have shown artemisinins, and in particular dihydroartemisinin, to be neurotoxic in neuronal-cell cultures in vitro (8, 18, 30) and in animal models (5, 13, 22). We have shown that in direct comparison with the most widely used artemisinin derivative, artesunate, artemisone possesses superior in vitro antimalarial activity against chloroquine-sensitive and -resistant P. falciparum lines and greater in vivo activity in the rodent-Plasmodium berghei and Aotus monkey-P. falciparum models (11).
This report describes the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of artemisone after administration of single and multiple ascending oral doses to healthy subjects. The data obtained provide a rationale for safe and tolerable, as well as effective, dosing of artemisone in patients for the treatment of P. falciparum malaria. It also establishes a rationale for the design of clinical trials for the further development of artemisone.
(Part of this work was presented previously by J. Nagelschmitz, B. Voith, and A. Roemer as posters at the XVIth International Congress for Tropical Medicine and Malaria, Marseille, France, 11 to 15 September 2005.)
MATERIALS AND METHODS
Compounds.
Artemisone (BAY 44-9585; molecular weight, 401.5); M1 (BAY 78-1787; molecular weight, 399.5), M2 (BAY 78-1788; molecular weight, 417.5), and M3 (BAY 78-1789; molecular weight, 417.5) metabolites of artemisone; dihydroartemisinin; D3-BAY 44-9585; and D4-BAY 44-9585 were supplied by Bayer AG. For in vitro testing, stock solutions of the drugs were prepared in 100% methanol in glass vials coated with 0.2% (vol/vol) AquaSil/water solution (Pierce, Rockford, IL) to minimize adherence of the drug to the glass surface. Subsequently, twofold dilutions were made in plain RPMI 1640 (Gibco, Grand Island, NY) to obtain working drug concentrations.
Study design and subjects.
The treatments were performed in two studies in a double-blind, randomized, placebo-controlled, single- and multiple-oral-dose design. For single-dose treatments, 32 healthy Caucasian male subjects participated in a single ascending dose study of artemisone. The dose escalation was performed in consecutive order. After fulfilling the inclusion and exclusion criteria, subjects were recruited for one dose group only and then randomly assigned to active or placebo treatment. The next group was then recruited and again randomized separately. The mean age of the subjects was 32.5 years (range, 21 to 44 years), the mean body weight plus or minus standard deviation was 79.3 ± 9.4 kg, and the body mass index was 24.8 ± 2.3 kg/m2. Subjects who were to receive artemisone (n = 24) or the matched placebo (n = 8) were assigned to one of five single-dose treatment groups (10-, 20-, 30-, and 40-mg oral solutions and 80 mg of artemisone as immediate-release tablets [four 20-mg tablets]). The ratios of active to placebo subjects were 3 to 1 for 10 mg, 6 to 2 for 20 mg, 3 to 1 for 30 mg, 6 to 2 for 40 mg, and 6 to 2 for 80 mg. Safety and tolerability data were evaluated before continuing to the next dose level.
In the multiple-dose study, 24 healthy Caucasian male subjects (mean age, 33.3 ± 7.3 years; weight, 80.7 ± 11.5 kg; body mass index, 25.0 ± 2.7 kg/m2) were treated over 3 days with multiple ascending doses of artemisone. Sixteen subjects were assigned to receive artemisone (40 mg [two 20-mg immediate-release tablets] or 80 mg [four 20-mg immediate-release tablets]) daily for 3 days, and eight subjects were assigned to receive matched placebo. The ratios of active to placebo subjects were 8 to 4 for 40 mg and 8 to 4 for the 80-mg dose. For both studies, all administrations were given with 240 ml water in the fasting state after a 10-h overnight fast. The studies were performed at the Institute of Clinical Pharmacology, Bayer Health Care, in Wuppertal, Germany, and at ClinPharmCologne, Cologne, Germany. The protocol was approved by the Ethics Committee of the North Rhine Medical Council (Dusseldorf, Germany). Before commencement of the study, written informed consent was obtained from each participating subject.
Safety assessments.
Physical examinations and clinical laboratory tests, including hematology, chemistries (electrolytes and liver function tests), and urinalysis, were conducted prior to and after drug administration. Supine blood pressure, heart rate, and a resting 12-lead electrocardiogram were measured before and after drug administration.
Tolerability assessments.
Adverse events were recorded in response to open questioning or to spontaneous reporting by the subjects and were graded according to the severity of the adverse event as either mild, not affecting daily activities; moderate, with some interference with daily activities; or severe, when daily activities could not be completed.
Blood collection.
In study 1, venous blood samples (6 ml) for pharmacokinetic assessment were collected in lithium heparin tubes immediately before drug administration and at 0.25, 0.5, 0.75, 1.0, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, and 14 h after single-oral-dose treatments of artemisone. In study 2, the same bleed schedule was used after the first and last doses of the multiple-dose regimens of artemisone for both pharmacokinetic and ex vivo pharmacodynamic antimalarial-activity assessments. Two additional blood samples were collected after the dose on the second treatment day. The blood samples were centrifuged at 4°C and 1,512 × g for 10 min, and the separated plasma samples were transferred to cryotubes and stored below −18°C prior to transport on dry ice to A&M GmbH (Bergheim, Germany) for liquid chromatography/tandem mass spectrometry (LC/MS-MS) analysis and to the Australian Army Malaria Institute for ex vivo pharmacodynamic antimalarial-activity analysis, where they were kept at −80°C until they were analyzed.
Parasites and drug preparation.
Two P. falciparum lines (K1 and TM90-C2A) were used in this study. K1 is chloroquine and pyrimethamine resistant but sensitive to mefloquine (28), and TM90-C2A is resistant to chloroquine, pyrimethamine, and mefloquine (17). The parasites were cultured continuously in RPMI 1640, and the cultures were synchronized with 5% sorbitol every 48 h to produce experimental cultures consisting of parasites 6 to 12 h into the schizogony cycle (16). For in vitro antimalarial testing, a stock solution of artemisone (1 mg/ml) was prepared in 100% methanol in glass vials coated with 0.2% (vol/vol) AquaSil/water solution (Pierce, Rockford, IL) to minimize drug adsorption to the glass surface. Subsequently, dilutions of the artemisone stock solution were made in drug-free human serum to obtain working artemisone concentrations covering the range from 0.04 to 40 ng/ml to obtain 50% inhibitory concentration (IC50) values.
In vitro drug susceptibility and ex vivo pharmacodynamic antimalarial activity.
The ex vivo pharmacodynamic antimalarial activity of artemisone in human plasma samples was assessed by bioassay (15). Drug-treated human plasma (25 μl) was serially diluted twofold on microtiter plates (range, 8 to 8,192) with drug-free human serum. All wells were then inoculated with 75 μl of infected erythrocyte suspension in plain culture medium. The sensitivities of the two lines to artemisone were determined in parallel with the bioassay analysis. Working drug solutions (20 μl) were serially diluted twofold on microtiter plates using plain RPMI 1640, followed by the addition of 80 μl of infected red blood cells suspended in culture medium supplemented with drug-free serum. The final cell suspension (100 μl) for bioassays and sensitivity tests had a hematocrit of 2%, of which 1% was infected erythrocytes (>95% rings). Tritiated-hypoxanthine incorporation was used to determine the extents to which parasite growth was inhibited by different drug concentrations or different dilutions of human plasma during 48 h of incubation. These data were used to generate concentration (or dilution)-response curves (TableCurve 2D; Jandal Scientific Software, California). IC50 and 50% inhibitory dilution (ID50) values were defined as the drug concentrations and dilutions, respectively, that produced a 50% inhibition of uptake of tritiated hypoxanthine by intraerythrocytic malaria parasites compared to drug-free serum samples (controls). The artemisone equivalent concentrations obtained by bioassay were estimated by multiplying the ID50 of the plasma samples collected from the subjects after artemisone administration by the IC50 value of artemisone obtained against the K1 and TM90-C2A lines.
LC/MS-MS.
The measurement of artemisone and its three metabolites (M1, M2, and M3) in plasma using D4-BAY 44-9585 as the internal standard and of dihydroartemisinin using D3-BAY 44-9585 as the internal standard were performed by LC/MS-MS (multiple-reaction monitoring). Reference solutions for the artemisone metabolites were obtained with human liver microsomes and expressed CYP3A4, and each was separated and identified by MS/high-field one- and two-dimensional nuclear magnetic resonance spectroscopy. Briefly, artemisone, its three metabolites, dihydroartemisinin, and the internal standards were extracted from plasma samples (200 μl) using liquid-liquid extraction with diethyl ether. After being mixed, centrifuged, and deep frozen, the organic phase was transferred to a vial and evaporated to dryness. Following reconstitution with a methanol-acetic acid solution, samples were separated on a Prontosil C18 column (VDS Optilab) for artemisone and its metabolites and a Prontosil C8 SH column (VDS Optilab) for dihydroartemisinin. An ammonium acetate-methanol-acetonitrile-tetrahydrofuran-water gradient was used for artemisone and its metabolites, and an ammonium acetate-methanol-acetonitrile-formic acid-water gradient was used for dihydroartemisinin. The gradient was produced by an LC-10AD pump (Shimadzu) coupled to an HTS-PAL autosampler (CTC Analytics). Mass spectrometric detection was accomplished on an API 3000 (Applied Biosystems) triple-quadrupole mass spectrometer equipped with a TurboIon Spray in the positive-ion mode. The peak area ratios of artemisone (product at m/z 163.0 from parent ion at m/z 402.0), M1 (product at m/z 163.0 from parent ion at m/z 400.0), M2 (product at m/z 285.0 from parent ion at m/z 418.0), and M3 (product at m/z 161.0 from parent ion at m/z 418.0) to the D4-BAY 44-9585 internal standard (product at m/z 163.0 from parent ion at m/z 406.0) and of dihydroartemisinin (product at m/z 163.1 from parent ion at m/z 302.0) to the D3-BAY 44-9585 internal standard (product at m/z 166.1 from parent ion at m/z 405.0) were calculated for each sample from the measured peak areas obtained by multiple-reaction monitoring. Linear regression of the concentration data (artemisone range, 0.2 to 500 ng/ml; M1 range, 3.0 to 428 ng/ml; M2 range, 1.4 to 191 ng/ml; M3 range, 1.0 to 144 ng/ml; dihydroartemisinin range, 2 to 500 ng/ml) yielded a correlation coefficient of >0.99. The lower limits of quantitation were as follows: artemisone, 0.4 ng/ml; M1, 3.0 ng/ml; M2, 1.4 ng/ml; and M3, 1.0 ng/ml; dihydroartemisinin was 2.0 ng/ml using 200 μl of plasma. The overall precision and accuracy of the method, as defined by the percent coefficient of variation (%CV) of quality control samples at the lower limit of quantitation, were ≤6.8% for artemisone, ≤5.0% for M1, ≤6.0% for M2, ≤29.9% for M3, and ≤4.9% for dihydroartemisinin.
Pharmacokinetic analysis.
Plasma artemisone pharmacokinetic parameters were determined by noncompartmental analysis of individual concentration-time data. The maximum drug concentration (Cmax) and time of maximum concentration (Tmax) were obtained directly from the observed data. The elimination rate constant (kel) was determined by least-squares regression analysis of the plasma drug concentration-time curve in the terminal postdistribution phase. The elimination half-life (t1/2) was calculated from 0.693/kel. The area under the plasma drug concentration-time curve AUC0-∞ and the area under the first moment curve (AUMC0-∞) were calculated by the log-linear trapezoidal rule from the beginning of artemisone administration to the last data point (AUC0-last and AUCM0-last) and with extrapolation to infinity (dividing the last concentration by kel). The mean residence time (MRT) was calculated as follows: MRT = AUMC0-∞/AUC0-∞. Total systemic clearance (CL/F), expressed as a function of bioavailability (F), was calculated as the dose divided by AUC0-∞. The apparent volume of distribution (V/F) was calculated as the ratio of CL/F to kel. Drug accumulation (RACC) was calculated by dividing either the Cmax or AUC0-∞ after the last dose of the 3-day course by the Cmax or AUC0-∞ following the first dose of the regimen. The metabolic ratio (AUCmet/AUCparent) was calculated by dividing the AUC0-∞ of the metabolites (M1, M2, and M3) of artemisone by the AUC0-∞ of the parent drug.
Statistical analysis.
Data are summarized as geometric means (95% confidence interval [CI]) unless otherwise specified. A paired t test was used to compare metabolic ratios after the first and third doses of the 3-day courses of artemisone. A P value of <0.05 was taken as significant.
RESULTS
Subjects.
A total of 39 healthy subjects received the study medication and were evaluated for safety, tolerability, and pharmacokinetics on all study days. One subject, randomized to the 3-day course of 80 mg artemisone, discontinued the study prematurely before receiving any medication.
Safety assessment.
No serious adverse events were seen, and no withdrawals occurred following drug administration during the phase I study. No evidence of clinically relevant changes in vital signs or electrocardiogram parameters were observed in any subjects administered the single- or multiple-dose regimen. Monitoring of haptoglobin and free-hemoglobin levels revealed no hemolytic effects of artemisone. Clinical laboratory tests were normal, with the exception of one subject who had increases of alanine aminotransferase (ALT) (normal range, 0 to 41 units/liter) and glutamate dehydrogenase (GLDH) (normal range, 0 to 6.4 units/liter), which peaked 24 h after the last dose of the 3-day course of 80 mg (ALT, 16 units/liter before treatment versus 152 units/liter; GLDH, 2 units/liter versus 86.4 units/liter). These levels decreased further to 86 units/liter for ALT and 12 units/liter for GLDH 2 days later and normalized.
Tolerability assessment.
Overall, artemisone was well tolerated without specific adverse events after administration of either the single- or multiple-dose regimen. The only adverse events judged to be possibly drug related were two subjects with headaches following the 3-day course of 40 mg daily and one subject with gastrointestinal discomfort (abdominal pain, diarrhea, nausea, and intestinal hypermotility) following the 3-day course of 80 mg daily. The severity of their adverse events was mild.
Pharmacokinetic assessment.
For the single-dose escalation study, geometric mean plasma artemisone concentration-time profiles at each of the five dose levels are shown in Fig. 1, with the derived pharmacokinetic parameters summarized in Table 1. Artemsione was rapidly absorbed, with median Tmax values increasing from 0.25 to 0.87 h for the five dose groups. The Cmax of artemisone increased less than dose proportionally, with moderate to high intersubject variability in groups of three to six subjects. The normalized exposure (AUCnorm) was dose proportional, with a relative bioavailability for the 80-mg-tablet dose comparable to that of the 40-mg solution. The three metabolites of artemisone and the degradation product, dihydroartemisinin, were measured simultaneously with the parent drug. The highest plasma concentrations were found for M1, followed by M2 and M3, after administration of the solution and tablets, with lower exposures and slightly shorter half-lives than for the parent compound. The geometric mean values for Cmax, AUC0-∞, and t1/2 for the three metabolites after the 80-mg dose were, respectively, 121.1 ng/ml, 315.4 ng · h/ml, and 2.78 h for M1; 108.9 ng/ml, 379.9 ng · h/ml, and 1.36 h for M2; and 74.3 ng/ml, 294.8 ng · h/ml, and 1.59 h for M3. The plasma concentrations of dihydroartemisinin were low, with geometric mean Cmax values of 10 ng/ml after the 80-mg dose. The formation of low concentrations of dihydroartemisinin is attributed to artemisone, like artesunate, being unstable at low pH in the stomach.
FIG. 1.
Geometric mean plasma concentration-time curves following administration of four single doses of artemisone solutions and immediate-release (IR) tablets of artemisone to healthy subjects.
TABLE 1.
Pharmacokinetic parameters of artemisone in plasma following single ascending oral doses of artemisone administered to healthy subjects as solutions or immediate-release tablets
| Parameter | Value for solution (mg)a
|
Value for immediate-release tabletsa (80 mg) (n = 6) | |||
|---|---|---|---|---|---|
| 10 (n = 3) | 20 (n = 6) | 30 (n = 3) | 40 (n = 6) | ||
| Cmax (ng/ml) | 40.0/78.0 (19.6-77.6) | 57.3/32.7 (41.3-98.2) | 50.5/60.6 (26.5-70.4) | 83.0/28.1 (56.4-132.0) | 140.2/65.4 (86.6-391.0) |
| Cmax, norm (ng/ml) | 329.7/99.4 (145.0-760.5) | 229.7/39.6 (150.7-378.1) | 120.0/78.8 (53.9-187.7) | 160.9/32.6 (110.4-264.0) | 138.1/64.1 (74.69-366.6) |
| AUC (ng · h/ml) | 29.5/20.5 (23.6-34.9) | 65.7/26.8 (53.4-107.7) | 71.8/69.6 (35.0-112.5) | 118.3/31.2 (83.9-198.6) | 281.6/74.3 (146.3-749.6) |
| AUCnorm (ng · h/ml) | 243.2/30.2 (174.4-306.8) | 263.1/31.7 (194.9-414.5) | 170.4/88.1 (71.20-277.5) | 229.3/37.1 (143.7-397.2) | 277.4/72.8 (136.4-702.8) |
| AUCextrap (%)c | 2.0 (1.4-2.5) | 2.0 (1.0-4.8) | 2.7 (1.3-6.1) | 2.1 (0.8-3.5) | 1.6 (0.4-3.5) |
| Tmax (h) | 0.25 (0.25-0.50) | 0.50 (0.25-0.50) | 0.50 (0.50-0.75) | 0.625 (0.50-0.75) | 0.875 (0.75-1.50) |
| t1/2 (h)b | 1.51/43.2 (1.03-2.34) | 3.48/42.5 (1.89-6.54) | 3.14/61.8 (1.94-5.89) | 3.54/37.1 (1.87-5.23) | 2.79/46.7 (1.56-4.88) |
| MRT (h) | 0.98/30.9 (0.74-1.35) | 1.79/29.1 (1.36-2.70) | 2.29/33.2 (1.71-3.24) | 2.39/10.5 (2.08-2.78) | 2.64/16.8 (2.22-3.30) |
| CL/F (liters/h) | 338.6/20.5 (286.5-424.2) | 304.5/26.8 (185.8-374.6) | 417.8/69.6 (266.6-856.7) | 338.2/31.2 (201.4-476.8) | 284.1/74.3 (106.7-546.7) |
| V/F (liters/kg) | 8.93/43.5 (5.53-11.71) | 19.05/65.6 (7.99-46.98) | 26.59/60.2 (14.08-39.39) | 22.24/35.5 (14.61-35.06) | 14.50/142.1 (3.21-51.58) |
Values are presented as geometric mean/geometric %CV (range).
Median and range are listed for Tmax.
AUCextrap, AUCextrap (%) = [(AUC0-∞ − AUC0-last)/AUC0-∞ × 100].
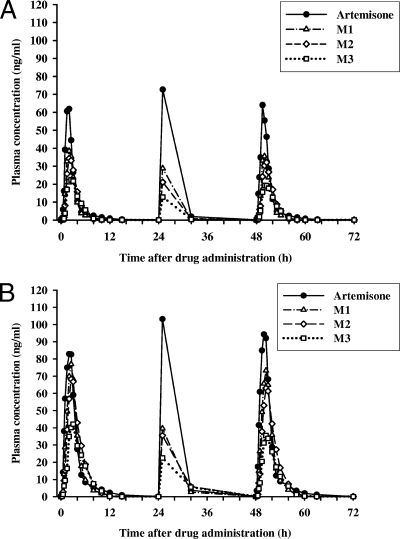
For the multiple-dose treatments, geometric mean plasma concentration-time profiles of artemisone and its three metabolites following the two 3-day treatment courses of artemisone are shown in Fig. 2A and B, with the derived pharmacokinetic parameters after the first and third doses summarized in Tables 2 and 3 . The plasma artemisone concentrations were virtually superimposed after the first and last doses of artemisone for both regimens. Intersubject variability of the Cmax and AUC0-∞ values was moderate for both treatment courses (the geometric CV values were 17.6% to 36.6% for Cmax and 26.8% to 41.3% for AUC0-∞). The Cmax and AUC0-∞ of artemisone and its metabolites increased in a nearly dose-proportional manner. With the exception of M3, the metabolic ratios of M1 and M2 were not significantly different after the first and third doses of artemisone. For the 40-mg daily dose, the mean metabolic ratio was slightly greater after the first dose than after the third dose, with values of 0.63 versus 0.62 (95% CI, −0.06 to 0.08; P = 0.75) for M1, 0.71 versus 0.65 (95% CI, −0.06 to 0.17; P = 0.32) for M2, and 0.54 versus 0.47 (95% CI, 0.03 to 0.12; P = 0.01) for M3. The corresponding values after the 80-mg daily dose were 0.89 versus 0.79 (95% CI, −0.02 to 0.22; P = 0.08) for M1, 0.91 versus 0.82 (95% CI, −0.05 to 0.23; P = 0.16) for M2, and 0.65 versus 0.52 (95% CI, 0.06 to 0.20; P = 0.005) for M3.
FIG. 2.
Geometric mean plasma concentration-time curves of artemisone and its three major metabolites (M1, M2, and M3) following administration of multiple doses of artemisone to healthy subjects. (A) A 3-day course of 40 mg (two 20-mg immediate-release tablets) artemisone daily administered to eight healthy subjects. (B) A 3-day course of 80 mg (four 20-mg immediate-release tablets) artemisone daily administered to seven healthy subjects.
TABLE 2.
Pharmacokinetic parameters of artemisone in plasma following 3-day courses of artemisone in healthy subjects
| Parameter | Valuea
|
|||
|---|---|---|---|---|
| 40 mg daily for 3 days (n = 8)
|
80 mg daily for 3 days (n = 7)
|
|||
| Day 1 | Day 3 | Day 1 | Day 3 | |
| Cmax (ng/ml) | 96.4/27.1 (53.8-166.0) | 95.0/28.6 (58.0-207.0) | 155.1/17.6 (105.0-238.0) | 130.3/36.6 (67.8-219.0) |
| Cmax, norm (ng/ml) | 198.5/26.1 (118.4-373.5) | 195.4/33.7 (105.8-486.5) | 149.1/21.0 (102.4-220.1) | 125.2/28.8 (66.1-192.6) |
| AUC (ng · h/ml) | 183.5/30.6 (89.8-342.2) | 190.7/30.9 (88.5-312.7) | 343.2/26.8 (173.6-577.9) | 337.3/41.3 (156.1-646.7) |
| AUCnorm (ng · h/ml) | 376.8/30.6 (197.1-768.8) | 392.5/33.7 (194.6-734.9) | 328.8/27.3 (169.0-529.1) | 324.2/33.6 (152.2-573.9) |
| AUCextrapb (%) | 1.7 (1.1-2.4) | 2.3 (0.8-12.6) | 1.6 (0.6-3.8) | 1.7 (0.7-3.7) |
| Tmax (h) | 1.50 (0.50-2.50) | 1.13 (0.50-2.50) | 1.50 (0.50-2.00) | 1.50 (1.00-2.50) |
| t1/2 (h) | 3.36/14.7 (2.21-4.10) | 4.37/60.4 (1.44-15.53) | 2.97/22.4 (2.02-4.83) | 3.07/20.2 (1.83-3.73) |
| MRT (h) | 2.91/9.3 (2.32-3.34) | 3.74/32.4 (2.19-9.14) | 3.30/12.9 (2.43-4.01) | 3.53/13.3 (2.83-4.84) |
| CL/F (liters/h) | 217.9/30.6 (116.9-445.5) | 209.8/30.9 (127.9-452.2) | 233.1/26.8 (138.4-460.8) | 237.2/41.3 (123.7-512.4) |
| V/F (liters/kg) | 12.85/36.0 (5.75-25.8) | 16.06/87.8 (5.14-102.0) | 12.98/25.3 (8.72-20.06) | 13.65/24.9 (9.39-23.54) |
| RACC, Cmaxc (%) | 98.5/33.9 (68.2-273.1) | 84.0/28.3 (51.0-144.1) | ||
| RACC, AUCd (%) | 103.9/19.8 (74.2-181.50) | 98.3/26.8 (66.7-167.3) | ||
Values are presented as geometric mean/geometric %CV (range); median and range are listed for Tmax. Doses were as follows: 40 mg, two 20-mg immediate-release tablets; 80 mg, four 20-mg tablets.
AUCextrap, AUCextrap (%) = [(AUC0-∞ − AUC0-last)/AUC0-∞ × 100].
RCmax, RCmax (%) = [(Cmax on day 3/Cmax on day 1) × 100].
RAUC, RAUC (%) = [AUC0-∞ on day 3/AUC0-∞ on day 1) × 100].
TABLE 3.
Pharmacokinetic parameters of three major metabolites of artemisone (M1, M2, and M3) in plasma following 3-day courses of artemisone in healthy subjects
| Parameter | Valuea
|
|||
|---|---|---|---|---|
| 40 mg daily for 3 days (n = 8)
|
80 mg daily for 3 days (n = 7)
|
|||
| Day 1 | Day 3 | Day 1 | Day 3 | |
| M1 | ||||
| Cmax (ng/ml) | 51.7/16.2 (39.5-73.1) | 47.8/23.7 (31.3-89.8) | 112.6/16.4 (69.7-140.0) | 85.3/25.9 (45.6-136.0) |
| AUC (ng · h/ml) | 112.6/19.8 (83.6-171.0) | 112.3/17.6 (84.2-175.3) | 296.0/21.2 (173.8-404.5) | 255.2/31.9 (133.2-438.0) |
| Tmax (h) | 1.75 (1.0-2.5) | 1.75 (0.75-2.5) | 2.0 (1.0-3.0) | 1.5 (1.0-3.0) |
| t1/2 (h) | 1.90/19.7 (1.28-3.08) | 1.54/14.9 (1.20-2.12) | 2.13/34.7 (1.11-3.36) | 1.63/22.5 (1.01-2.42) |
| M2 | ||||
| Cmax (ng/ml) | 40.6/23.4 (25.4-60.3) | 36.4/20.5 (23.3-58.2) | 87.2/18.6 (51.2-111.0) | 66.5/22.8 (38.8-101.0) |
| AUC (ng · h/ml) | 117.3/23.5 (70.5-179.2) | 115.1/15.4 (88.6-164.5) | 302.4/20.9 (180.5-416.8) | 263.0/26.8 (145.4-409.5) |
| Tmax (h) | 1.75 (1.0-3.0) | 2.0 (0.75-2.5) | 2.0 (1.5-3.0) | 2.5 (1.5-3.0) |
| t1/2 (h) | 1.31/16.4 (1.02-1.99) | 1.28/15.6 (0.92-1.92) | 1.59/14.0 (1.22-2.18) | 1.44/10.0 (1.21-1.72) |
| M3 | ||||
| Cmax (ng/ml) | 27.8/18.2 (18.8-37.1) | 22.5/18.4 (15.8-34.7) | 51.9/22.9 (26.7-71.7) | 38.2/29.6 (17.6-60.4) |
| AUC (ng · h/ml) | 95.9/18.0 (74.0-146.1) | 84.9/17.1 (62.7-124.7) | 213.1/24.2 (113.1-296.1) | 165.1/31.2 (72.8-266.3) |
| Tmax (h) | 2.0 (1.0-3.0) | 2.5 (1.5-3.0) | 2.0 (1.5-3.0) | 2.5 (2.0-4.0) |
| t1/2 (h) | 1.52/16.4 (1.05-1.94) | 1.69/17.8 (1.13-2.17) | 1.83/7.0 (1.63-2.17) | 1.86/10.6 (1.45-2.25) |
Values are presented as geometric mean/geometric %CV (range); median and range are listed for Tmax. Doses were as follows: 40 mg, two 20-mg immediate-release tablets; 80 mg, four 20-mg tablets.
In vitro pharmacodynamic antimalarial-activity assessment.
The in vitro susceptibilities of the K1 and TM90-C2A lines to artemisone were comparable, with mean IC50 values of 0.60 ± 0.15 ng/ml (n = 10) for K1 and 0.64 ± 0.10 ng/ml (n = 15) for TM90-C2A. The three metabolites of artemisone all showed in vitro antimalarial activity, with mean IC50 values of 1.7 ± 0.5 ng/ml (n = 3) for M1, 25.7 ± 5.0 ng/ml (n = 3) for M2, and 2.3 ± 0.2 ng/ml (n = 3) for M3 against the K1 line.
The geometric mean Cmax artemisone equivalent concentration measured by bioassay using the K1 line was 231 ng/ml (range, 166 to 324 ng/ml) and 225 ng/ml (range, 132 to 388 ng/ml) after the first and third doses, respectively, after the 3-day course of 40 mg. Corresponding values for the 3-day course of 80 mg were 352 ng/ml (range, 260 to 546 ng/ml) and 296 ng/ml (range, 142 to 518 ng/ml). Compared to Cmax values of artemisone measured by LC/MS-MS, the artemisone equivalent concentrations were about 2.4-fold and 2.3-fold higher after the 40- and 80-mg daily regimens, respectively. The extra antimalarial activity demonstrated by the bioassay was presumably due to the presence of the active metabolites. The median Tmax using the bioassay was about 1.5 h for both treatment courses. Similar artemisone equivalent concentrations were obtained when the TM90-C2A line was used (data not shown).
DISCUSSION
The single- and multiple-ascending-dose trials were designed to investigate the safety, tolerability, and pharmacokinetics of the new antimalarial drug artemisone in healthy male subjects with increasing single doses (10 mg to 80 mg) and multiple doses of 40 and 80 mg given once daily for 3 days. We also evaluated the ex vivo pharmacodynamic antimalarial activities in plasma samples collected from the subjects after drug treatment. No dose-related changes in clinical laboratory, vital-sign, or electrocardiogram parameters were observed in the subjects following administration of single ascending doses from 10 to 80 mg. One subject (of seven), however, had elevations in some liver function tests after the last dose of the 3-day course of 80 mg, indicating the need for liver function tests during future trials with artemisone.
In this initial human study, artemisone was well tolerated by all subjects receiving single doses up to 80 mg. Multiple dosing was associated with some adverse events, possibly related to artemisone, in 3 of 16 recipients, which requires further assessment in future clinical trials, as events like headache are very common in healthy subjects and may be related to caffeine abstinence. The gastrointestinal discomfort experienced by one subject during the 3-day course of 80 mg might have been caused by an acute viral infection. Of particular note, none of the subjects developed a serious adverse event or required discontinuation of artemisone as a result of a drug-related adverse event.
The pharmacokinetics of artemisinins (artemisinin, artemether, artesunate, and dihydroartemisinin) in healthy subjects is characterized by considerable intersubject variability in plasma drug concentrations, high oral clearance (150 to 300 liters/h), a low to moderate apparent volume of distribution (6 to 20 liters/kg), and elimination half-lives up to 5 h (4, 7, 19, 21, 26, 27, 35). Artemisone, like other artemisinin compounds, is absorbed rapidly, with Tmax values attained about 1.5 h after dosing. Oral clearance, apparent volume of distribution, and elimination half-life values of 237 liters/h, 13.7 liters/kg, and 3.1 h, respectively, were obtained after the last dose of the 3-day course of 80 mg. A dose-proportional increase in the AUC was found following the five single-dose levels. No accumulation of artemisone and its three metabolites was seen after the 3-day course, which is in accord with the data obtained from the single-dose study. Plasma concentrations of artemisone and its metabolites increased in a nearly dose-proportional manner after the two 3-day courses.
After oral administration, artesunate and artemether, but not artemisinin, are rapidly biotransformed to dihydroartemisinin, the major active metabolite (reviewed in references 24 and 35). Artemisone metabolism in human liver microsomes is mediated primarily by CYP3A4 with the production of at least six metabolites, with M1, M2, and M3 being the predominant forms (11). Artemisone was rapidly and extensively biotransformed into its three major metabolites, with metabolic ratios, based on AUCs, ranging from 0.45 to 0.88 following multiple dosing.
The present study shows that the three metabolites possess intrinsic antimalarial activities, with M1 and M3 being the most potent. Although the metabolites are not as active as the parent drug, the relatively high concentrations of these metabolites obtained after artemisone administration would make an additional contribution to the overall parasiticidal effect of artemisone. Previous in vitro bioassay methods were developed that allowed the quantitative determination of antimalarial activity and provided a pharmacodynamic profile of artemisinins (20, 26). In this study, bioassay of subject plasma samples showed artemisone equivalent concentrations to be about 2.3-fold higher at Tmax than that measured by LC/MS-MS for artemisone. The extra antimalarial activity demonstrated by the bioassay was due to the presence of active metabolites of artemisone, including M1, M2, and M3. The overall activities of these metabolites could differ somewhat at other time points after artemisone administration.
Time-dependent pharmacokinetics has been well described for artemisinin in healthy subjects and malaria patients (4, 10), with a decrease in dose-normalized artemisinin concentrations over 5 days of drug administration due to autoinduction of metabolism (9, 25). Declining plasma concentrations for artemether (29) and, less convincingly, for artesunate and dihydroartemisinin (14, 35) have been reported following multiple doses. In contrast to artemisinin and artemether, the present study shows that artemisone appears to be devoid of time-dependent pharmacokinetics, with comparable Cmax, AUC, and t1/2 values after the first and third doses following the 3-day courses of artemisone. Since current recommended ACTs, such as artesunate plus mefloquine (3) and dihydroartemisinin plus piperaquine (2), are administered over 3 days, the lack of autoinduction and its associated reduction in bioavailability suggest that artemisone would be a good candidate for ACT. In a preliminary study, 2- and 3-day courses of 80 mg daily combined with mefloquine (total dose, 25 mg/kg of body weight) was found to be safe and effective in the treatment of uncomplicated P. falciparum malaria with no recrudescences over a 28-day follow-up period (S. Looareesuwan, S Krudsood, P. Wilairatana, K. Chalermrut, W. Leowattana, B. Voith, and B. Hample, presented at the XVIth International Congress for Tropical Medicine and Malaria, Marseille, France, 11 to 15 September 2005).
In summary, these studies represent the first assessment of the safety, tolerability, and pharmacokinetics of artemisone in healthy subjects. Artemisone was found to be safe and well tolerated in healthy subjects who were administered the drug as either a single dose or multiple doses. Dose-proportional linear pharmacokinetics was demonstrated for artemisone. The results of this study can serve as the basis for the selection of an artemisone dosing regimen for future clinical trials of this promising new artemisinin derivative.
Acknowledgments
We are most grateful to Anthony Kotecki and Kerryn Rowcliffe for bioassay analysis. We also thank the Australian Red Cross Blood Service (Brisbane) for providing human erythrocytes and serum for the in vitro cultivation of P. falciparum. We thank the Hong Kong University of Science and Technology (HKUST) for synthesizing D3-BAY 44-9585 and D4-BAY 44-9585 for Bayer AG. J.N., B.V., G.W., and B.F. are employees of Bayer, Wuppertal, Germany, and A.R. is an employee of A&M, Bergheim, Germany.
Work at HKUST was carried out in the Open Laboratory of Chemical Biology of the Institute of Molecular Technology for Drug Discovery and Synthesis through financial support from the government and the HKSAR University Grants Committee Areas of Excellence Fund, Project No. AoE P/10 to 01, and University Grants Council Grants No. HKUST 6091/02P and HKUST 6493/06 M. This work was financially supported by Medicines for Malaria Ventures, Geneva, Switzerland.
We declare no conflict of interest, as in 2006, Bayer Health Care made the strategic decision to return the assets and transfer the future development of artemisone to HKUST.
The opinions expressed are those of the authors and do not necessarily reflect those of the Australian Defense Health Service or any extant Australian Defense Force policy.
Footnotes
Published ahead of print on 16 June 2008.
REFERENCES
- 1.Ashley, E. A., and N. J. White. 2005. Artemisinin-based combinations. Curr. Opin. Infect. Dis. 18:531-536. [DOI] [PubMed] [Google Scholar]
- 2.Ashley, E. A., R. McGready, R. Hutagalung, L. Phaiphun, T. Slight, S. Proux, K. L. Thwai, M. Barends, S. Looareesuwan, N. J. White, and F. Nosten. 2005. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin. Infect. Dis. 41:425-432. [DOI] [PubMed] [Google Scholar]
- 3.Ashley, E. A., K. Stepniewska, N. Lindegardh, R. McGready, R. Hutagalung, R. Hae, P. Singhasivanon, N. J. White, and F. Nosten. 2006. Population pharmacokinetic assessment of a new regimen of mefloquine used in combination treatment of uncomplicated falciparum malaria. Antimicrob. Agents Chemother. 50:2281-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashton, M., T. N. Hai, N. D. Sy, D. X. Huong, N. Van Huong, N. T. Nieu, and L. D. Cong. 1998. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab. Dispos. 26:25-27. [PubMed] [Google Scholar]
- 5.Brewer, T. G., S. J. Grate, J. O. Peggins, P. J. Weina, J. M. Petras, B. S. Levine, M. H. Heiffer, and B. G. Schuster. 1994. Fatal neurotoxicity of arteether and artemether. Am. J. Trop. Med. Hyg. 51:251-259. [DOI] [PubMed] [Google Scholar]
- 6.Bunnag, D., C. Viravan, S. Looareesuwan, J. Karbwang, and T. Harinasuta. 1991. Clinical trial of artesunate and artemether on multidrug resistant falciparum malaria in Thailand. A preliminary report. Southeast Asian J. Trop. Med. Public Health 22:380-385. [PubMed] [Google Scholar]
- 7.Duc, D. D., P. J. de Vries, X. K. Nguyen, B. Le Nguyen, P. A. Kager, and C. J. van Boxtel. 1994. The pharmacokinetics of a single dose of artemisinin in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 51:785-790. [DOI] [PubMed] [Google Scholar]
- 8.Fishwick, J., W. G. McLean, G. Edwards, and S. A. Ward. 1995. The toxicity of artemisinin and related compounds on neuronal and glial cells in culture. Chem. Biol. Interact. 96:263-271. [DOI] [PubMed] [Google Scholar]
- 9.Gordi, T., D. X. Huong, T. N. Hai, N. T. Nieu, and M. Ashton. 2002. Artemisinin pharmacokinetics and efficacy in uncomplicated-malaria patients treated with two different dosage regimens. Antimicrob. Agents Chemother. 46:1026-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan Alin, M., M. Ashton, C. M. Kihamia, G. J. Mtey, and A. Bjorkman. 1996. Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans. R. Soc. Trop. Med. Hyg. 90:61-65. [DOI] [PubMed] [Google Scholar]
- 11.Haynes, R. K., B. Fugmann, J. Stetter, K. Rieckmann, H. D. Heilmann, H. W. Chan, M. K. Cheung, W. L. Lam, H. N. Wong, S. L. Croft, L. Vivas, L. Rattray, L. Stewart, W. Peters, B. L. Robinson, M. D. Edstein, B. Kotecka, D. E. Kyle, M. Gerisch, M. Radtke, G. Schmuck, W. Steinke, U. Wollborn, K. Schmeer, and A. Romer. 2006. Artemisone—a highly active antimalarial drug of the artemisinin class. Angew. Chem. Int. Ed. Engl. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 12.Hien, T. T., and N. J. White. 1993. Qinghaosu. Lancet 341:603-608. [DOI] [PubMed] [Google Scholar]
- 13.Kamchonwongpaisan, S., P. McKeever, P. Hossler, H. Ziffer, and S. R. Meshnick. 1997. Artemisinin neurotoxicity: neuropathology in rats and mechanistic studies in vitro. Am. J. Trop. Med. Hyg. 56:7-12. [DOI] [PubMed] [Google Scholar]
- 14.Khanh, N. X., P. J. de Vries, L. D. Ha, C. J. van Boxtel, R. Koopmans, and P. A. Kager. 1999. Declining concentrations of dihydroartemisinin in plasma during 5-day oral treatment with artesunate for falciparum malaria. Antimicrob. Agents Chemother. 43:690-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotecka, B. M., M. D. Edstein, and K. H. Rieckmann. 1996. Chloroquine bioassay of plasma specimens obtained from soldiers on chloroquine plus doxycycline for malaria prophylaxis. Int. J. Parasitol. 26:1325-1329. [DOI] [PubMed] [Google Scholar]
- 16.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 17.Looareesuwan, S., C. Viravan, H. K. Webster, D. E. Kyle, D. B. Hutchinson, and C. J. Canfield. 1996. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am. J. Trop. Med. Hyg. 54:62-66. [DOI] [PubMed] [Google Scholar]
- 18.McLean, W. G., and S. A. Ward. 1998. In vitro neurotoxicity of artemisinin derivatives. Med. Trop. 58(Suppl. 3):28-31. [PubMed] [Google Scholar]
- 19.Mordi, M. N., S. M. Mansor, V. Navaratnam, and W. H. Wernsdorfer. 1997. Single dose pharmacokinetics of oral artemether in healthy Malaysian volunteers. Br. J. Clin. Pharmacol. 43:363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Na-Bangchang, K., K. Congpoung, R. Ubalee, A. Thanavibul, P. Tan-Anya, and J. Karbwang. 1997. Pharmacokinetics and ex vivo anti-malarial activity of sera following a single oral dose of dihydroartemisinin in healthy Thai males. Southeast Asian J. Trop. Med. Public Health 28:731-735. [PubMed] [Google Scholar]
- 21.Na-Bangchang, K., S. Krudsood, U. Silachamroon, P. Molunto, O. Tasanor, K. Chalermrut, N. Tangpukdee, O. Matangkasombut, S. Kano, and S. Looareesuwan. 2004. The pharmacokinetics of oral dihydroartemisinin and artesunate in healthy Thai volunteers. Southeast Asian J. Trop. Med. Public Health 35:575-582. [PubMed] [Google Scholar]
- 22.Nontprasert, A., M. Nosten-Bertrand, S. Pukrittayakamee, S. Vanijanonta, B. J. Angus, and N. J. White. 1998. Assessment of the neurotoxicity of parenteral artemisinin derivatives in mice. Am. J. Trop. Med. Hyg. 59:519-522. [DOI] [PubMed] [Google Scholar]
- 23.Olliaro, P. L., and W. R. Taylor. 2004. Developing artemisinin based combinations for the treatment of drug resistant falciparum malaria: a review. J. Postgrad. Med. 50:40-44. [PubMed] [Google Scholar]
- 24.Price, R. N. 2000. Artemisinin drugs: novel antimalarial agents. Exp. Opin. Investig. Drugs 9:1815-1827. [DOI] [PubMed] [Google Scholar]
- 25.Simonsson, U. S., B. Jansson, T. N. Hai, D. X. Huong, G. Tybring, and M. Ashton. 2003. Artemisinin autoinduction is caused by involvement of cytochrome P450 2B6 but not 2C9. Clin. Pharmacol. Ther. 74:32-43. [DOI] [PubMed] [Google Scholar]
- 26.Teja-Isavadharm, P., F. Nosten, D. E. Kyle, C. Luxemburger, F. Ter Kuile, J. O. Peggins, T. G. Brewer, and N. J. White. 1996. Comparative bioavailability of oral, rectal, and intramuscular artemether in healthy subjects: use of simultaneous measurement by high performance liquid chromatography and bioassay. Br. J. Clin. Pharmacol. 42:599-604. [DOI] [PubMed] [Google Scholar]
- 27.Teja-Isavadharm, P., G. Watt, C. Eamsila, K. Jongsakul, Q. Li, G. Keeratithakul, N. Sirisopana, L. Luesutthiviboon, T. G. Brewer, and D. E. Kyle. 2001. Comparative pharmacokinetics and effect kinetics of orally administered artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65:717-721. [DOI] [PubMed] [Google Scholar]
- 28.Thaithong, S., and G. H. Beale. 1981. Resistance of ten Thai isolates of Plasmodium falciparum to chloroquine and pyrimethamine by in vitro tests. Trans. R. Soc. Trop. Med. Hyg. 75:271-273. [DOI] [PubMed] [Google Scholar]
- 29.van Agtmael, M. A., S. Cheng-Qi, J. X. Qing, R. Mull, and C. J. van Boxtel. 1999. Multiple dose pharmacokinetics of artemether in Chinese patients with uncomplicated falciparum malaria. Int. J. Antimicrob. Agents 12:151-158. [DOI] [PubMed] [Google Scholar]
- 30.Wesche, D. L., M. A. DeCoster, F. C. Tortella, and T. G. Brewer. 1994. Neurotoxicity of artemisinin analogs in vitro. Antimicrob. Agents Chemother. 38:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White, N. J. 1994. Artemisinin: current status. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):3-4. [DOI] [PubMed] [Google Scholar]
- 32.White, N. J. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 41:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White, N. J. 2002. The assessment of antimalarial drug efficacy. Trends Parasitol. 18:458-464. [DOI] [PubMed] [Google Scholar]
- 34.White, N. J. 2004. Antimalarial drug resistance. J. Clin. Investig. 113:1084-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, S. Q., T. N. Hai, K. F. Ilett, D. X. Huong, T. M. Davis, and M. Ashton. 2001. Multiple dose study of interactions between artesunate and artemisinin in healthy volunteers. Br. J. Clin. Pharmacol. 52:377-385. [DOI] [PMC free article] [PubMed] [Google Scholar]