Abstract
Antimicrobial photodynamic inactivation (APDI) combines a nontoxic photoactivatable dye or photosensitizer (PS) with harmless visible light to generate singlet oxygen and reactive oxygen species that kill microbial cells. Cationic phenothiazinium dyes, such as toluidine blue O (TBO), are the only PS used clinically for APDI, and we recently reported that this class of PS are substrates of multidrug efflux pumps in both gram-positive and gram-negative bacteria. We now report that APDI can be significantly potentiated by combining the PS with an efflux pump inhibitor (EPI). Killing of Staphylococcus aureus mediated by TBO and red light is greatly increased by coincubation with known inhibitors of the major facilitator pump (NorA): the diphenyl urea INF271, reserpine, 5′-methoxyhydnocarpin, and the polyacylated neohesperidoside, ADH7. The potentiation effect is greatest in the case of S. aureus mutants that overexpress NorA and least in NorA null cells. Addition of the EPI before TBO has a bigger effect than addition of the EPI after TBO. Cellular uptake of TBO is increased by EPI. EPI increased photodynamic inactivation killing mediated by other phenothiazinium dyes, such as methylene blue and dimethylmethylene blue, but not that mediated by nonphenothiazinium PS, such as Rose Bengal and benzoporphyrin derivative. Killing of Pseudomonas aeruginosa mediated by TBO and light was also potentiated by the resistance nodulation division pump (MexAB-OprM) inhibitor phenylalanine-arginine beta-naphthylamide but to a lesser extent than for S. aureus. These data suggest that EPI could be used in combination with phenothiazinium salts and light to enhance their antimicrobial effect against localized infections.
Photodynamic therapy (PDT) combines a nontoxic photoactivatable dye or photosensitizer (PS) with harmless visible light of the correct wavelength to excite the dye to its reactive triplet state, which will then generate reactive oxygen species, such as singlet oxygen and superoxide, that are toxic to cells (4). Although discovered more than 100 years ago by its killing effect on microorganisms (19), PDT has found most success as a treatment for cancer (6) and age-related macular degeneration (3). In recent years, antimicrobial photodynamic inactivation (APDI) has been proposed as an alternative treatment for localized infections in response to the ever-growing problem of antibiotic resistance. In this medical application, it is proposed that the PS is directly introduced into the infected tissue, rather than being injected intravenously as is common with PDT for cancer.
A frequently employed class of antimicrobial PS are the blue dyes known as phenothiazinium salts, such as toluidine blue O (TBO) (2), methylene blue (MB) (7), and azure dyes (37). Phenothiazinium salts are amphipathic planar molecules that possess one intrinsic quarternary nitrogen atom and have phototoxic efficiency against a broad range of microorganisms (25, 38), such as Escherichia coli, Staphylococcus aureus (25), streptococci (24), Listeria monocytogenes (27), and Vibrio vulnificus (39). At the present time, the only PS used clinically for antimicrobial treatments are phenothiazinium salts. For instance, the combination of MB or TBO together with red light is used to disinfect blood products and sterilize dental cavities and root canals and has been proposed for treatment of periodontitis (36).
Microbial efflux pumps (MEP) have become broadly recognized as major components of microbial resistance to many classes of antibiotics (26). Some MEP selectively extrude specific antibiotics, while others, referred to as multidrug resistance pumps, expel a variety of structurally diverse compounds with differing modes of action. Gram-positive species mainly have major facilitator-type MEP, typified by NorA in S. aureus, while gram-negative species tend to have three-component MEP, known as resistance nodulation division (RND) and typified by MexAB-OprM in Pseudomonas aeruginosa. It has been suggested that amphipathic cations represent the existing natural substrates of MEP (12), and these molecules have been frequently used to study MEP-mediated efflux. It has been established that disabling MEP by employing either MEP mutants or synthetic efflux pump inhibitors (EPI) leads to a striking increase in the activity a wide array of plant secondary metabolites, including natural MEP substrates (31).
We recently showed (33) that phenothiazinium salts, which are structurally characterized as amphipathic cations, were substrates of MEP. We studied MEP knockout and MEP-overexpressing mutants of the human pathogens S. aureus (NorA), E. coli (AcrAB-TolC), and P. aeruginosa (MexAB-OprM) and a range of phenothiazinium salts. The uptake of phenothiazinium dye by the cells and the extent of light-mediated bacterial killing were inversely proportional to the level of MEP expression. These observations suggest that specific inhibitors of MEP might be used to potentiate APDI. We now report that four different inhibitors of the NorA pump dramatically potentiate photodynamic inactivation (PDI) of S. aureus mediated by four different phenothiazinium dyes and an inhibitor of gram-negative RND pumps also potentiates light-mediated killing of P. aeruginosa by TBO.
MATERIALS AND METHODS
Microbial strains and culture conditions.
Bacterial strains used in this study are listed in Table 1. Cells were cultured in brain heart infusion broth with aeration at 37°C. Cell growth (optical density) was assessed with a spectrophotometer (Mini 1240; Shimadzu) at 600 nm. Cells were used for experiments in mid-log growth phase (optical density at 600 nm, ∼0.4 to 0.8 or 108 per ml).
TABLE 1.
Bacterial strains used in this work
| Strain | Genotype | Phenotype (reference) |
|---|---|---|
| S. aureus 8325-4 | WTa | Wild type (11) |
| S. aureus 8325-4 (1758) | ΔnorA::erm | NorA knockout (11) |
| S. aureus 8325-4 (QT1) | norR::cat | NorA overexpressing (35) |
| P. aeruginosa PA767 | WT (PAO1 prototroph) | WT (14) |
| P. aeruginosa K1119 | ΔmexAB-oprM | MexAB knockout (14) |
| P. aeruginosa PAM1032 | nalB | MexAB overexpressing (15) |
WT, wild type.
Photosensitizers and light sources.
MB, TBO, and 1,9-dimethylmethylene blue (DMMB), all as chloride salts, were used as phenothiazinium-based PS and along with Rose Bengal (RB) were obtained from Sigma-Aldrich (St. Louis, MO). Stock solutions were prepared in water at a concentration of 2 mM and stored for a maximum of 2 weeks at 4°C in the dark before use. Liposomal benzoporphyrin derivative monoacid ring A (Verteporfin for injection) (BPD) was a kind gift from QLT Inc. (Vancouver, British Columbia, Canada) and was prepared by diluting the powder to a concentration of 0.3 mg/ml in sterile 5% dextrose. Spectra of stock solutions of PS diluted 140- to 280-fold in methanol were recorded. A noncoherent light source with interchangeable fiber bundles (LC122; LumaCare, London, United Kingdom) was employed. Thirty-nanometer-band-pass filters at ranges of 540 ± 15 nm for RB, 635 ± 15 nm for TBO and for DMMB, 660 ± 15 nm for MB, and 690 ± 15 nm for BPD were used. The total power output provided out of the fiber bundle ranged from 300 to 600 mW. The spot was arranged to give an irradiance of 100 mW/cm2.
Multidrug efflux pump inhibitors.
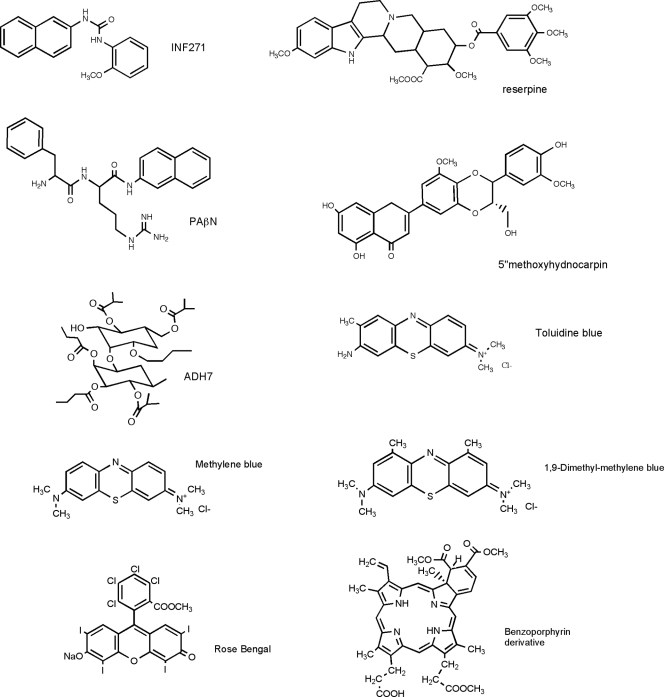
Reserpine and phenylalanine-arginine-β-naphthylamide (PAβN) were obtained from Sigma-Aldrich (St. Louis, MO), INF271 was from Chembridge (San Diego, CA), and both 5-methoxyhydnocarpine and polyacetylated neohesperidoside (ADH7) (Fig. 1) were isolated from primary botanical material, as previously described (28, 29). Stock solutions were prepared in dimethylsulfoxide or ethanol (PAβN) at a concentration of 10 mM and stored at −20°C.
FIG. 1.
Chemical structures of the EPI and PS used.
Incubation with compounds.
Bacterial suspensions in phosphate-buffered saline (PBS) (initial concentration, 108 CFU ml−1) were incubated with the appropriate PS or combination of PS and EPI in the dark at room temperature for 30 min at concentrations varying from 1 to 50 μM for the PS and 5 to 25 μM for the EPI. The cell suspensions were centrifuged at 12,000 rpm and then washed twice and resuspended in sterile PBS. In some cases the bacterial cells were incubated either with PS alone or with EPI alone for 30 min, followed by a washing step and a second 30-min incubation with either EPI alone or PS alone, respectively, followed by a second washing step and a final resuspension in PBS. These conditions were compared to the simultaneous incubation described above.
PDI studies.
The bacterial suspensions were placed in wells of 48-well microtiter plates (Fisher Scientific) and illuminated using appropriate optical parameters. Fluences ranged from 0 to 40 J/cm2 at a fluence rate of 100 mW/cm2. During illumination, aliquots of 100 μl were taken to determine the CFU. The contents of the wells were constantly stirred during illumination (to ensure that bacteria did not settle to the bottom of the wells) and mixed before sampling. The aliquots were serially diluted 10-fold in PBS to give dilutions of 10−1 to 10−6 times the original concentrations and were streaked horizontally on square brain heart infusion agar plates as described by Jett et al. (10). This allowed a maximum of seven logs of killing to be measured. Plates were incubated at 37°C overnight. Two types of control conditions were used: illumination in the absence of PS and incubation with PS in the dark.
Uptake studies.
These were carried out using a previously validated extraction procedure (5, 33). Bacterial suspensions (108 CFU/ml−1) were incubated in PBS in the dark at room temperature for 30 min with the appropriate PS or combination of PS and EPI at the same concentrations that were used for the PDI experiments. Incubations were carried out in triplicate. The cell suspensions were centrifuged (9,000 × g, 1 min), the PS solution was aspirated, and bacteria were washed twice in 1 ml of sterile PBS and centrifuged as described above. Finally, the cell pellet was dissolved by digesting it in 3 ml of 0.1 M NaOH-1% sodium dodecyl sulfate (SDS) for 48 h to give the cell extract as a homogenous solution. Fluorescence in the extracts was measured on a spectrofluorimeter (model FluoroMax3; SPEX Industries, Edison, NJ). For TBO and DMMB, the excitation wavelength was 620 nm and the range for emission was 627 to 720 nm. For MB, the excitation wavelength was 650 nm and the range for emission was 655 to 720 nm. The fluorescence was calculated from the height of the peaks recorded. If necessary, the solution was diluted with 0.1 M NaOH-1% SDS to reach a concentration of the PS where the fluorescence response was linear. Calibration curves were made from pure PS dissolved in NaOH-SDS and used for determination of the PS concentration in the suspension. Uptake values were obtained by dividing the number of nmol of PS in the dissolved pellet by the number of CFU obtained by serial dilutions and the number of PS molecules/cell calculated by using Avogadro's number.
Statistics.
Values are means from three separate experiments, and bars are standard errors of the means (SEM). Differences between means were tested for significance by one-way analysis of variance (ANOVA) (Microsoft Excel). The significance level was set at a P value of <0.05.
RESULTS
Potentiation of APDI in S. aureus by NorA inhibitors.
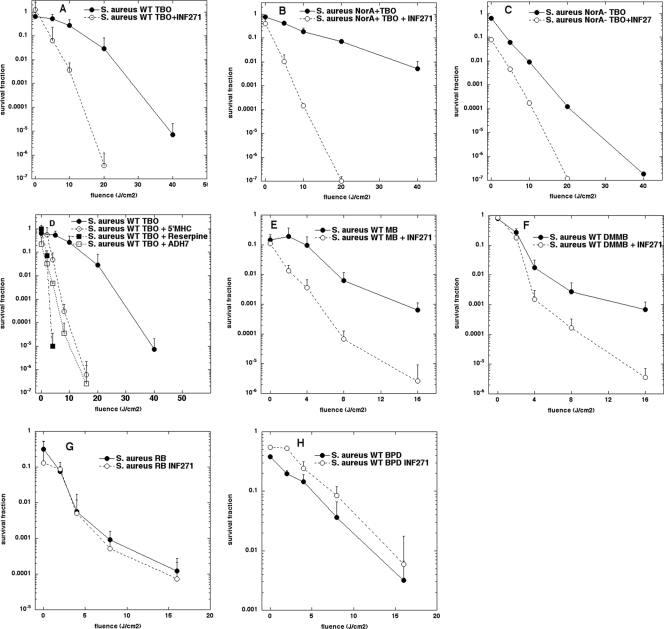
We initially tested the potentiation of TBO-mediated PDI by EPI in S. aureus. We previously showed (33) that the effectiveness of PDI mediated by phenothiazinium PS in S. aureus was inversely proportional to the level of NorA expression, as demonstrated by the comparison of strains that had been engineered to overexpress NorA, the isogenic strain with wild-type NorA, or the NorA knockout. We now reasoned that these same three strains could be usefully employed to test the ability of a NorA inhibitor to increase PDI-mediated killing. The first EPI compound we tested was the diphenylurea derivative INF271. This compound has been previously shown to lower the MIC of ethidium bromide and ciprofloxacin against S. aureus by at least eightfold (18). We incubated wild-type S. aureus with 10 μM TBO for 30 min in either the presence or absence of 5 μM INF271 and delivered increasing fluences of 635-nm light. Figure 2A shows that the light-dependent killing in the presence of INF271 was 2 to 5 logs more than the killing at the same fluence without INF271. There was no appreciable killing of S. aureus in the presence of INF271 alone, either in the presence or absence of 635-nm light (data not shown). Figure 2B shows the potentiation of TBO-PDI by INF271 in the NorA-overexpressing mutant of S. aureus. As expected, the killing by TBO-PDI is significantly less in the case of the strain overexpressing the drug efflux pump than for the wild-type strain, and consequently, the increase in the killing observed on adding INF271 was more pronounced than that seen with wild-type S. aureus. In the case of the NorA knockout mutant shown in Fig. 2C, there was still some potentiation observed when INF271 was added. This observation correlates with the fact that additional efflux systems are present in S. aureus that have not yet been extensively studied or characterized, including NorB, NorC, and AbcA (34).
FIG. 2.
Potentiation of APDI in S. aureus. Phototoxicity of TBO (10 μM) with or without INF271 (5 μM) after incubation with the S. aureus wild type (WT) (A), NorA-overexpressing (NorA+) mutant (B), or NorA knockout (NorA−) mutant (C). Other NorA inhibitors (5 μM 5′MHC, 10 μM reserpine, or 5 μM ADH7) potentiate TBO-APDI of S. aureus WT (D). INF271 potentiates APDI of WT S. aureus mediated by other phenothiazinium dyes, 10 μM MB (E) or 10 μM DMMB (F), but not by nonphenothiazinium dyes, 1 μM RB (G) or 10 μM BPD (H). Light wavelengths were as follows: for TBO and DMMB, 635 nm; for MB, 660 nm; for RB, 540 nm; and for BPD, 690 nm. Values are means from three separate experiments, and bars are SEM.
To establish the generality of the potentiation of TBO-PDI in S. aureus by NorA inhibitors, we used three additional compounds that have been reported to enhance the susceptibility of S. aureus to antibiotics (Fig. 1). Reserpine (8) at 10 μM, 5-methoxyhydnocarpine (29) at 5 μM, and ADH7 (28) at 5 μM all showed remarkable potentiation (up to 5 logs more killing) of TBO-PDI, as shown in Fig. 2D. Of these three additional NorA inhibitors, reserpine was the most effective, but it was used at twice the concentration of the other compounds.
To test the generality of the approach by which INF271 increases killing of S. aureus incubated with phenothiazinium salts, we used two other PS that we had previously shown were substrates of the NorA pump in S. aureus. MB at 10 μM gave about 2 logs more killing of wild-type S. aureus after 16 J/cm2 when INF271 was present at 5 μM (Fig. 2E), and likewise the killing mediated by 10 μM DMMB and red light was also increased by about 2 logs at 16 J/cm2 when INF271 was present in the incubation mixture (Fig. 2F).
It was possible that the EPI could have other effects on the bacterial cells that could potentiate PDI by photosensitizers in general; for instance, the EPI might increase the permeability of the cells to dyes in general, or EPI might decrease the antioxidant defenses of the cells, rendering them more susceptible to the reactive oxygen species generated during PDI. To test this possibility, we used two PS, one of which (RB) we had previously shown was not a substrate of NorA and the other of which (BPD) as a lipophilic noncationic tetrapyrrole is not expected to be a substrate. It should be noted that it is well established that gram-positive cells, such as S. aureus cells, are relatively easy to kill using PS that do not have cationic charges in combination with the appropriate light, while gram-negative cells are resistant (9, 17, 22). Figure 2G shows no significant difference between the killing of wild-type S. aureus mediated by RB at 1 μM and 540-nm light, with or without INF271 at 5 μM. Similarly, INF271 at 5 μM did not increase the killing observed when BPD at 10 μM was used together with 690-nm light (Fig. 2H).
PAβN (an inhibitor of P. aeruginosa MexAB) potentiates TBO phototoxicity.
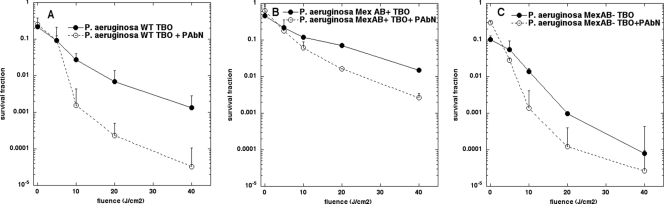
In our previous work, we used MexAB overexpression (MexAB+) and MexAB knockout (MexAB−) mutants of P. aeruginosa to show that the phenothiazinium dye MB was a substrate of the RND pump MexAB-OprM (33). The dipeptide derivative PAβN, formerly known as MC207,110, was reported to be a specific inhibitor of MexAB (15), and we now asked if this compound could also potentiate TBO-PDI in P. aeruginosa. Figure 3A to C depict the light-dependent killing of cells incubated with 50 μM TBO in the presence and absence of 25 μM PAβN. As shown in Fig. 3A, wild-type P. aeruginosa was killed 1 to 1.5 logs more when the inhibitor was present, while as shown in Fig. 3B, the MexAB-overexpressing mutant was also killed 1 to 1.5 logs more with the inhibitor, and as shown in Fig. 3C, the MexAB knockout cells were killed about 0.5 logs more when PAβN was employed. These data suggest that either TBO may not be a designated substrate of MexAB, as it is appears to be for NorA, or the inhibitory effects of PAβN on MexAB may not be as pronounced as those of INF271 on NorA.
FIG. 3.
PAβN potentiates APDI of TBO against P. aeruginosa. Phototoxicity of TBO (300 μM) incubated with or without PAβN (25 μM) (added together for 30 min) with the P. aeruginosa wild type (A), MexAB+ mutant (B), or MexAB− mutant (C). Other conditions as described in the legend for Fig. 2.
MEP inhibitors are more effective when added before PS.
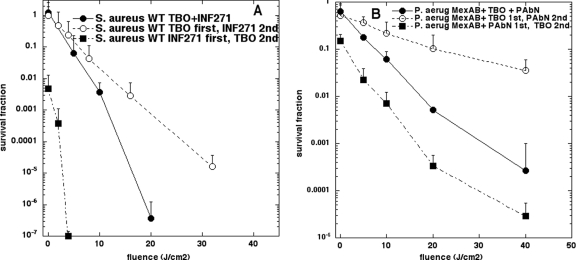
We asked whether the MEP inhibitors were as effective when added before the TBO, so that they could disable the pump before the TBO was added and increase uptake, or if they were more effective when added after the TBO, so that they could disable the pump that was extruding the TBO from the cells. Figure 4A shows the light-dependent killing curves for wild-type S. aureus when the INF271 was added before TBO or after TBO, in comparison with the curve for simultaneous addition from Fig. 2A. The phototoxicity (and the dark toxicity) was significantly higher when INF271 was added first, intermediate toxicity levels were obtained for simultaneous addition, and the least toxicity was seen for addition of INF271 after TBO. Very similar results were obtained when we investigated the effect of changing the order of addition of TBO and PAβN in MexAB-overexpressing P. aeruginosa, as can be seen in Fig. 4B. It was necessary to use the MexAB+ mutant because the degree of potentiation was not as great as that seen with S. aureus. Again the phototoxicity and dark toxicity were highest for addition of PAβN before TBO, intermediate toxicity for simultaneous addition, and least toxicity for addition of PAβN after TBO.
FIG. 4.
Relative order of addition of PS and EPI. (A) The S. aureus WT strain was incubated with TBO alone (10 μM) or with INF271 (5 μM) alone for 30 min, followed by a washing step and a second 30-min incubation with either EPI alone or PS alone, respectively, followed by a second washing step and a final resuspension in PBS. These conditions were compared to the simultaneous incubation described in the legend for Fig. 2. (B) The experiment was repeated with a P. aeruginosa Mex AB+ strain and TBO at 300 μM and with PAβN (25 μM).
MEP inhibitors increase cellular uptake of TBO, as determined by extraction.
To confirm that the increase in phototoxicity observed on combining phenothiazinium dyes with MEP inhibitors was really due to increased cellular uptake of the PS by the cells, we were able to measure the cell content of dye by extraction of the cell pellet and fluorescence spectrofluorimetry. The cellular uptake of dye can be expressed as molecules per cell by correlation of the extracted TBO concentration with the cell numbers in the pellet determined from CFU counting. Table 2 shows the TBO uptake for the three S. aureus NorA phenotypes with and without simultaneous addition of INF271. In each case the addition of INF271 approximately doubled the uptake of TBO by the cells, and these differences were statistically significant. A similar set of results was obtained when the uptake of TBO by the three P. aeruginosa MexAB phenotypes was measured with and without simultaneous addition of PAβN.
TABLE 2.
Cellular uptake of TBO is increased by EPI
| Species, phenotype | Uptake of TBOb
|
P valuec | |
|---|---|---|---|
| TBO alone | TBO + EPI | ||
| S. aureus, WTa | 4.01 ± 0.61 × 106 | 9.43 ± 0.2 × 106 | <0.001 |
| S. aureus, NorA− | 29.8 ± 1.52 × 106 | 47.2 ± 4.26 × 106 | <0.001 |
| S. aureus, NorA+ | 0.81 ± 0.06 × 106 | 1.91 ± 0.24 × 106 | <0.05 |
| P. aeruginosa, WT | 0.40 ± 0.07 × 106 | 0.68 ± 0.11 × 106 | <0.05 |
| P. aeruginosa, MexAB− | 1.00 ± 0.13 × 106 | 1.83 ± 0.31 × 106 | <0.01 |
| P. aeruginosa, Mex AB+ | 0.23 ± 0.03 × 106 | 0.43 ± 0.06 × 106 | <0.01 |
WT, wild type.
Mean uptake of TBO, measured in molecules per cell, by the S. aureus wild type or NorA knockout (NorA−) or NorA-overexpressing (NorA+) mutant incubated with TBO (10 μM) with or without INF271 (5 μM) or by the P. aeruginosa wild type or MexAB− or MexAB+ mutant incubated with TBO (300 μM) with or without PAβN (25 μM). Values are means ± SEM of three separate determinations.
P value comparing result for TBO alone with result for TBO plus EPI, determined by one-way ANOVA.
DISCUSSION
These studies have demonstrated that inhibitors of microbial efflux pumps can have dramatic effects on increasing microbial killing upon illumination when phenothiazinium salts are employed as antibacterial PS. We previously showed (33) that phenothiazinium PS, such as TBO, MB, and DMMB, were substrates of major facilitator pumps, such as NorA in S. aureus, as well as being substrates of RND pumps in E. coli (AcrAB-TolC) and P. aeruginosa (MexAB-OprM). This discovery prompted us to ask whether some of the compounds that have been reported to be inhibitors of these pumps might be used to potentiate PDI of these species.
Reserpine, an alkaloid from Rauwolfia plants with antihypertensive and neuroleptic properties, was one of the first bacterial EPI identified. Sequence similarity between the mammalian amine transporters known to be inhibited by reserpine and the DNA sequence for B. subtilis Bmr and S. aureus NorA pumps suggested that reserpine would also function as a bacterial EPI. Reserpine was found to potentiate bacterial killing by ethidium bromide and ciprofloxacin, among other compounds (1, 20), by inhibition of S. aureus NorA (21).
The NorA EPI, known as INF271 (a biphenyl urea), was originally identified by screening a synthetic chemical library using ethidium bromide as a substrate in a subinhibitory concentration, and the 28 hits included (along with INF271) the compounds INF-55 (an indole) and INF-392 (a thiobarbituric acid derivative) (18). The diverse group of compounds was thought to possess chemical substructures similar to the core nucleus of reserpine, but 11 of them were much better at increasing the antimicrobial effect of ciprofloxacin than reserpine (18).
The EPI 5′-methoxyhydnocarpin (5′-MHC), a flavonolignan, was previously identified in Hydnocarpus wightiana (Flacourtaceae) and described as a minor component of chaulmoogra oil, a traditional therapy for leprosy (23). It was found to be present in berberis plants together with the natural plant antimicrobial compound, berberine (29). 5′-MHC is an amphipathic weak acid and is distinctly different from the cationic substrates of NorA, such as phenothiazinium salts. 5′-MHC had no antimicrobial activity alone but strongly potentiated the action of berberine and other NorA substrates against S. aureus. MEP-dependent efflux of EtBr and berberine from S. aureus cells was completely inhibited by 5′-MHC. This was held to be a clear example of synergy between components of a medicinal plant described at a molecular level (29, 30). This proof-of-principle study inspired the quest for natural inhibitors of MEP (32) and raised the issue of whether the combination of weakly active phytochemicals could produce new antibacterial treatments (13).
Bioassay-directed fractionation of plant extracts for S. aureus EPI resulted in isolation of novel acylated neohesperidosides from Geranium caespitosum. The more highly acylated compound ADH7 had no direct activity against S. aureus but potentiated activity of the antibiotics berberine, rhein, ciprofloxacin, and norfloxacin (28).
In the case of RND pumps, an array of synthetic compounds and natural product extracts was assayed using strains of P. aeruginosa overexpressing each of the three pumps (MexAB, MexCD, and MexEF) in the presence of levofloxacin (15). Hit compounds enhanced the activities of levofloxacin in strains containing functioning pumps but not in a strain that lacked efflux pumps. One early lead compound, a low-molecular-weight dipeptide amide, PAβN (originally designated MC-207110), showed minimal intrinsic antibacterial activity but potentiated the in vitro activity of levofloxacin by eightfold at 10 μg/ml (15).
Our results showed an overall better potentiation effect of the EPI (four different compounds) on PDI with TBO against S. aureus than the increase in killing seen when PAβN was used in combination with TBO against P. aeruginosa. This observation is also consistent with the relative killing rates seen with the wild-type, pump overexpression, and pump knockout strains. In other words, the effect of NorA expression on the extent of PDI killing of S. aureus seems to be greater than the size of the effect seen with MexAB expression in P. aeruginosa. We interpret these data to mean that phenothiazinium salts are not designated substrates of RND pumps in P. aeruginosa as they are substrates of NorA in S. aureus. The literature suggests that PAβN is at least as good an inhibitor of P. aeruginosa RND pumps as INF271 is of S. aureus MFS pumps for more traditional antibiotics. We found that EPI were more effective when added before the phenothiazinium PS than when the EPI and PS were added simultaneously and least effective when the EPI was added after the PS. These data suggest that the inhibitor can more effectively disable the pump in the absence of a substrate, whereas when the inhibitor and PS are added simultaneously, the two compounds compete for access to the pump and the inhibition is less effective, while when the inhibitor is added after TBO, the inhibition is least effective, presumably because the inhibitor is prevented from accessing the pump protein components. If the EPI associates with the pump to exert its inhibitory action, the inhibition can continue when the EPI is washed from the medium. When the EPI is added after the PS has been incubated and washed from the medium, then presumably most of the efflux that is going to take place has already happened and the EPI cannot easily inhibit the pump because the PS may be blocking its site of action. It does appear that pump inhibition increases PS uptake rather than inhibiting efflux that takes place after the PS has already been taken up by the cells.
The discovery that these EPI have a dramatic effect in potentiating the killing effect of antimicrobial PDI with phenothiazinium dyes suggests that EPI may have some clinical application in this field. Despite much work on discovering and optimizing the structures and activity of EPI, there have as yet not been any clinical applications. One of the reasons for this lack of progress to the clinic has been the unacceptable toxicity of some of these compounds in rodent infection models (16). It is generally accepted that most proposed applications of antimicrobial PDT as a therapeutic approach for infections will be as a localized therapy where both the PS and light will be delivered into the infected area (9). The few existing clinical applications of antimicrobial PDT use phenothiazinium salts as PS administered topically to infected areas, in particular for sterilizing dental cavities, root canals, and periodontitis in dental pockets. It is less likely that EPI would have unacceptable toxicity problems when delivered topically to infected areas. It may be questioned whether the concentration of the PS could not simply be increased to compensate for the MEP-mediated PS efflux. However, there is an intrinsic limit to increasing the concentration of a topically applied PS that is also a dye. This limit happens because a very high concentration of dye acts as an optical shield, absorbing the light to no effect, because most of the dye is not bound to bacteria. Therefore, we believe that consideration should be given to adding the appropriate EPI to the PS formulation, depending on the likely identity of the causative bacterial species.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases (grant AI050875 to M.R.H.).
We are grateful to Kim Lewis (Northeastern University, Boston, MA), Olga Lomovskaya (Mpex Pharmaceuticals, Inc., San Diego, CA), and David C. Hooper (Massachusetts General Hospital, Boston, MA) for gifts of bacterial strains. We are grateful to QLT Inc. (Vancouver, Canada) for the generous gift of BPD. We thank Tatiana N. Demidova-Rice for helpful suggestions and a critical reading of the manuscript.
Footnotes
Published ahead of print on 12 May 2008.
REFERENCES
- 1.Ahmed, M., C. M. Borsch, A. A. Neyfakh, and S. Schuldiner. 1993. Mutants of the Bacillus subtilis multidrug transporter Bmr with altered sensitivity to the antihypertensive alkaloid reserpine. J. Biol. Chem. 268:11086-11089. [PubMed] [Google Scholar]
- 2.Bhatti, M., A. MacRobert, S. Meghji, B. Henderson, and M. Wilson. 1998. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochem. Photobiol. 68:370-376. [PubMed] [Google Scholar]
- 3.Bressler, N. M., and S. B. Bressler. 2000. Photodynamic therapy with verteporfin (Visudyne): impact on ophthalmology and visual sciences. Investig. Ophthalmol. Vis. Sci. 41:624-628. [PubMed] [Google Scholar]
- 4.Castano, A. P., T. N. Demidova, and M. R. Hamblin. 2004. Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 1:279-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demidova, T. N., and M. R. Hamblin. 2005. Photodynamic inactivation of bacillus spores, mediated by phenothiazinium dyes. Appl. Environ. Microbiol. 71:6918-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolmans, D. E., D. Fukumura, and R. K. Jain. 2003. Photodynamic therapy for cancer. Nat. Rev. Cancer 3:380-387. [DOI] [PubMed] [Google Scholar]
- 7.Gad, F., T. Zahra, T. Hasan, and M. R. Hamblin. 2004. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob. Agents Chemother. 48:2173-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons, S., and E. E. Udo. 2000. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother. Res. 14:139-140. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 11.Kaatz, G. W., S. M. Seo, L. O'Brien, M. Wahiduzzaman, and T. J. Foster. 2000. Evidence for the existence of a multidrug efflux transporter distinct from NorA in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis, K. 1999. Versatile drug sensors of bacterial cells. Curr. Biol. 9:403-407. [DOI] [PubMed] [Google Scholar]
- 13.Lewis, K., and F. M. Ausubel. 2006. Prospects for plant-derived antibacterials. Nat. Biotechnol. 24:1504-1507. [DOI] [PubMed] [Google Scholar]
- 14.Li, X. Z., L. Zhang, R. Srikumar, and K. Poole. 1998. Beta-lactamase inhibitors are substrates for the multidrug efflux pumps of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch, A. S. 2006. Efflux systems in bacterial pathogens: an opportunity for therapeutic intervention? An industry view. Biochem. Pharmacol. 71:949-956. [DOI] [PubMed] [Google Scholar]
- 17.Malik, Z., H. Ladan, and Y. Nitzan. 1992. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J. Photochem. Photobiol. B 14:262-266. [DOI] [PubMed] [Google Scholar]
- 18.Markham, P. N., E. Westhaus, K. Klyachko, M. E. Johnson, and A. A. Neyfakh. 1999. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moan, J., and Q. Peng. 2003. An outline of the hundred-year history of PDT. Anticancer Res. 23:3591-3600. [PubMed] [Google Scholar]
- 20.Neyfakh, A. A. 1992. The multidrug efflux transporter of Bacillus subtilis is a structural and functional homolog of the Staphylococcus NorA protein. Antimicrob. Agents Chemother. 36:484-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neyfakh, A. A., C. M. Borsch, and G. W. Kaatz. 1993. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 37:128-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitzan, Y., M. Gutterman, Z. Malik, and B. Ehrenberg. 1992. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem. Photobiol. 55:89-96. [DOI] [PubMed] [Google Scholar]
- 23.Norton, S. A. 1994. Useful plants of dermatology. I. Hydnocarpus and chaulmoogra. J. Am. Acad. Dermatol. 31:683-686. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill, J., M. Wilson, and M. Wainwright. 2003. Comparative antistreptococcal activity of photobactericidal agents. J. Chemother. 15:329-334. [DOI] [PubMed] [Google Scholar]
- 25.Phoenix, D. A., Z. Sayed, S. Hussain, F. Harris, and M. Wainwright. 2003. The phototoxicity of phenothiazinium derivatives against Escherichia coli and Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 39:17-22. [DOI] [PubMed] [Google Scholar]
- 26.Poole, K. 2005. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 56:20-51. [DOI] [PubMed] [Google Scholar]
- 27.Romanova, N. A., L. Y. Brovko, L. Moore, E. Pometun, A. P. Savitsky, N. N. Ugarova, and M. W. Griffiths. 2003. Assessment of photodynamic destruction of Escherichia coli O157:H7 and Listeria monocytogenes by using ATP bioluminescence. Appl. Environ. Microbiol. 69:6393-6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stermitz, F. R., K. K. Cashman, K. M. Halligan, C. Morel, G. P. Tegos, and K. Lewis. 2003. Polyacylated neohesperidosides from Geranium caespitosum: bacterial multidrug resistance pump inhibitors. Bioorg. Med. Chem. Lett. 13:1915-1918. [DOI] [PubMed] [Google Scholar]
- 29.Stermitz, F. R., P. Lorenz, J. N. Tawara, L. A. Zenewicz, and K. Lewis. 2000. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 97:1433-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stermitz, F. R., J. Tawara-Matsuda, P. Lorenz, P. Mueller, L. Zenewicz, and K. Lewis. 2000. 5′-Methoxyhydnocarpin-D and pheophorbide A: Berberis species components that potentiate berberine growth inhibition of resistant Staphylococcus aureus. J. Nat. Prod. 63:1146-1149. [DOI] [PubMed] [Google Scholar]
- 31.Tegos, G., F. R. Stermitz, O. Lomovskaya, and K. Lewis. 2002. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 46:3133-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tegos, G. P. 2006. Natural substrates and inhibitors of multidrug resistant pumps (MDRs) redefine the plant antimicrobials. In C. Carpinella and M. Rai (ed.), Naturally occurring bioactive compounds: a new and safe alternative for control of pests and microbial diseases. Cambridge University Press, Cambridge, United Kingdom.
- 33.Tegos, G. P., and M. R. Hamblin. 2006. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob. Agents Chemother. 50:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truong-Bolduc, Q. C., and D. C. Hooper. 2007. The transcriptional regulators NorG and MgrA modulate resistance to both quinolones and beta-lactams in Staphylococcus aureus. J. Bacteriol. 189:2996-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truong-Bolduc, Q. C., X. Zhang, and D. C. Hooper. 2003. Characterization of NorR protein, a multifunctional regulator of norA expression in Staphylococcus aureus. J. Bacteriol. 185:3127-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wainwright, M. 2003. The use of dyes in modern biomedicine. Biotech. Histochem. 78:147-155. [DOI] [PubMed] [Google Scholar]
- 37.Wainwright, M., D. A. Phoenix, S. L. Laycock, D. R. Wareing, and P. A. Wright. 1998. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol. Lett. 160:177-181. [DOI] [PubMed] [Google Scholar]
- 38.Wainwright, M., D. A. Phoenix, J. Marland, D. R. Wareing, and F. J. Bolton. 1997. A study of photobactericidal activity in the phenothiazinium series. FEMS Immunol. Med. Microbiol. 19:75-80. [DOI] [PubMed] [Google Scholar]
- 39.Wong, T. W., Y. Y. Wang, H. M. Sheu, and Y. C. Chuang. 2005. Bactericidal effects of toluidine blue-mediated photodynamic action on Vibrio vulnificus. Antimicrob. Agents Chemother. 49:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]