Abstract
No effective approved drug therapy exists for Cryptosporidium infection of immunocompromised patients. Here we investigated the nonabsorbed anthelmintic drug pyrvinium pamoate for inhibition of the growth of the intestinal protozoan parasite Cryptosporidium parvum. The concentration of pyrvinium that effected 50% growth inhibition in human enterocytic HCT-8 cells by a quantitative alkaline phosphatase immunoassay was 354 nM. For comparison, in the same assay, 50% growth inhibition was obtained with 711 μM paromomycin or 27 μM chloroquine. We used a neonatal mouse model to measure the anti-Cryptosporidium activity of pyrvinium pamoate in vivo. Beginning 3 days after infection, pyrvinium at 5 or 12.5 mg/kg of body weight/day was administered to the treatment group mice for 4 or 6 consecutive days. Nine days after infection, the mice were sacrificed, and drug efficacy was determined by comparing the numbers of oocysts in the fecal smears of treated versus untreated mice. The intensities of trophozoite infection in the ileocecal intestinal regions were also compared using hematoxylin-and-eosin-stained histological slides. We observed a >90% reduction in infection intensity in pyrvinium-treated mice relative to that in untreated controls, along with a substantial reduction in tissue pathology. Based on these results, pyrvinium pamoate is a potential drug candidate for the treatment of cryptosporidiosis in both immunocompetent and immunocompromised individuals.
Cryptosporidium is an important apicomplexan protozoan pathogen that contributes significantly to diarrheal disease in both humans and animals throughout the world (9, 10, 21). In immunocompetent hosts, infections are generally restricted to the intestinal epithelium, causing an acute, self-limiting gastroenteritis. However, in AIDS patients and other immunocompromised individuals, infection can result in life-threatening, chronic diarrhea and may spread to extraintestinal locations (16, 26). Although the efficacies of numerous antimicrobial agents against Cryptosporidium infection have been tested using animal and cell culture models, there is currently no reliably effective therapeutic for the treatment of chronic cryptosporidiosis in immunocompromised patients (30).
Recently, nitazoxanide (NTZ), a nitrothiazole benzamide, was approved by the FDA for the treatment of cryptosporidiosis in immunocompetent adults and children aged >1 year (2). However, while clinical studies are ongoing, the efficacy of NTZ for the treatment of Cryptosporidium infection in immunocompromised patients has not yet been demonstrated (1). A 50% inhibitory concentration (IC50) of 3.8 μM has been reported for NTZ in cell culture (12). In a neonatal mouse model, oral administration of NTZ at 150 mg/kg of body weight reduced oocyst output to less than 5% of that seen in controls (6); however, NTZ at 100 or 200 mg/kg was ineffective at reducing parasite burdens in a SCID mouse model (24). Prior to the FDA approval of NTZ as an anti-Cryptosporidium therapeutic, the glycoside antibiotic paromomycin was one of the agents most widely used to treat Cryptosporidium infections, but it still was not reliably effective and was never approved by the FDA. Although paromomycin performs well against Cryptosporidium in animal and cell culture models (24), the results of human clinical trials of this drug have been equivocal (13, 15). Reported IC50s for paromomycin have varied, ranging from 83 μM (29) to >100 μM (17). In a neonatal mouse model, paromomycin at 50 mg/kg reduced oocyst shedding to less than 2% of that seen in controls (6).
Pyrvinium pamoate is a cyanine dye, a substituted quinoline, that has been used to treat pinworm (Enterobius vermicularis) infections (5) as well as strongyloidiasis in humans (27). In 1955, pyrvinium received FDA approval for enterobiasis treatment in adults and children (NDA-9582). The usual human dosage is 5 mg/kg/day, up to 350 mg; however, pyrvinium has been used safely for humans with doses as high as 35 mg/kg/day for 3 to 5 days. The drug has no measurable absorption across the gastrointestinal tract, and 90% is excreted in feces (23). With the discovery of more-effective, broad-spectrum agents for the treatment of helminth infections, the drug has been discontinued in the United States, but it is still available under the Parke-Davis label in Europe. In a recent screen of FDA-approved drugs for antimalarial activity, pyrvinium was determined to have an IC50 of 3 nM against the apicomplexan parasite Plasmodium falciparum (7). Despite potent in vitro activity, the drug was not pursued for malaria treatment, since there is no measurable absorption of pyrvinium into the bloodstream. However, because Cryptosporidium infection is generally confined to the gastrointestinal epithelium, we hypothesized that pyrvinium would be effective against this luminal apicomplexan protozoan, for which no effective therapy is currently approved for immunocompromised patients. Here we report the efficacy of pyrvinium pamoate against C. parvum in cell culture and in a neonatal mouse model.
MATERIALS AND METHODS
C. parvum oocysts.
C. parvum (Iowa isolate) oocysts were obtained through experimental infection of a female Holstein calf. The oocysts were extracted from the feces using continuous-flow centrifugation, purified by cesium chloride gradient centrifugation, and stored at 4°C in phosphate-buffered saline (PBS) (pH 7.4).
Drugs.
Pyrvinium pamoate and paromomycin were purchased from MP Biomedicals (Solon, OH) and chloroquine from Sigma (St. Louis, MO). Paromomycin and chloroquine were diluted in water just prior to use. Pyrvinium was first dissolved in dimethyl sulfoxide (DMSO) or ethanol and then diluted in water prior to use.
Pyrvinium activity in cell culture.
HCT-8 cells (CCL-244) were obtained from the American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 medium supplemented with 10% Opti-MEM (GIBCO-BRL, Grand Island, NY), 2% fetal bovine serum, and 2 mM l-glutamine. To determine in vitro drug efficacy, a quantitative alkaline phosphatase immunoassay was used to measure parasite growth inhibition in cell culture as described previously (11, 29). Briefly, 96-well, flat-bottom microtiter plates were seeded with 5 × 104 HCT-8 cells 24 h prior to infection. For infection, the maintenance medium was removed and 5 × 103 oocysts were added to wells in 100 μl of RPMI 1640 supplemented with 10% fetal bovine serum and 0.05% bile salts. After incubation at 37°C for 90 min to induce excystation and to allow cell invasion, cells were washed once with warm PBS to remove unexcysted oocysts and free sporozoites. Negative-control wells to measure background absorbance received 5 × 103 nonviable oocysts subjected to five cycles of freezing in liquid nitrogen and thawing in a 37°C water bath. Drugs were diluted to appropriate concentrations and added to cells in 150 μl of parasite growth medium. For pyrvinium treatment, final DMSO levels were 0.5%. Vehicle control wells for the pyrvinium group received growth medium containing 0.5% DMSO. The glycoside antibiotic paromomycin and another quinoline, chloroquine, were used as comparison drugs. Each drug concentration was tested in triplicate wells in two independent experiments. Plates were first incubated for 48 h at 37°C in a 5% CO2-95% humidified-air incubator and then fixed in 8% formalin for 2 h at room temperature. After fixation, plates were washed three times with PBS and then blocked for 1 h with 300 μl of a blocking solution consisting of 5% bovine serum albumin (BSA) and 0.002% Tween 20 in PBS. Rat anti-Cryptosporidium polyclonal sera raised against washed sporozoite membrane proteins (29) (provided by Steve Upton at Kansas State University) were diluted 1:500 in a solution of 1% BSA-0.002% Tween 20 in PBS, and 50 μl was added to each well. After a 30-min incubation at room temperature, the wells were washed three times with PBS, and 50 μl of a horseradish peroxidase-conjugated goat anti-rat secondary antibody diluted 1:2,000 in 1% BSA-0.002% Tween 20-PBS was added to each well. After 20 min, plates were washed three times with PBS, and 100 μl of a 3,3′,5,5′-tetramethylbenzidine (TMB; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) solution was added. After 10 min, 100 μl of Stop Solution (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) was added to each well, and plates were read at 450 nm using an enzyme-linked immunosorbent assay (ELISA) microplate reader (Molecular Devices, Sunnyvale, CA). The background absorbance reading taken from wells receiving freeze-thawed oocysts was subtracted from all absorbance readings from drug-treated and control wells, and the percentage of growth inhibition was calculated as 1 − (mean A450 of infected wells with drug/mean A450 of infected wells without drug) × 100.
The cytotoxicity of pyrvinium pamoate for HCT-8 cells was determined using the CellTiter 96 AQueous One solution cell proliferation assay (Promega, Madison, WI), which measures the number of viable, metabolically active cells by their abilities to convert a tetrazolium compound, 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), to a colored formazan product. This assay has been described previously (12, 24) for use in determining the cytotoxicity of drugs tested against Cryptosporidium using multiple cell lines. Briefly, 96-well microtiter plates were seeded with 104 cells/well and incubated at 37°C for 24 h. Cells were then exposed to various drug concentrations or 0.5% DMSO for 48 h. Following addition of the CellTiter 96 AQueous One solution reagent to wells, plates were incubated for 1.5 h at 37°C, after which time absorbance was read at 490 nm. The background absorbance reading from wells receiving medium but no cells was subtracted from readings from drug-treated and solvent control wells, and the percentage of cytotoxicity was calculated as 1 − (mean A490 of drug-treated wells/mean A490 of solvent control wells) × 100. Chloroquine cytotoxicity was measured by the neutral red assay, which measures the number of viable cells by the accumulation of dye in lysosomes. For chloroquine, this gives more sensitive results (28).
Pyrvinium activity in neonatal mice.
The in vivo efficacy of pyrvinium pamoate was tested using a neonatal mouse model for Cryptosporidium infection, which is well characterized and has been described previously (6). Briefly, 3-day-old BALB/c mice (National Cancer Institute, Frederick, MD), initially in groups of 4 to 6 and then in groups of 8 to 11 (Table 1) were inoculated orally with 105 C. parvum oocysts. Three days postinfection, treatment regimens were initiated. Pyrvinium was administered orally at 5 mg/kg/day or 12.5 mg/kg/day for 4 or 6 consecutive days. Five milligrams per kilogram per day is the dose of pyrvinium used for the treatment of Enterobius in humans. Pyrvinium was initially dissolved in 100% DMSO or ethanol. Prior to administration, drug stocks were diluted to the desired concentration in water so that the final solvent levels were 5% for DMSO and 5% or 10% for ethanol. Vehicle-treated mice received 5% DMSO in water or 5 or 10% ethanol in water. As a positive-control comparison drug, paromomycin was administered at 100 mg/kg/day for 4 or 6 consecutive days. Vehicle control mice received an equal volume of water. All mice were euthanized by cervical dislocation on day 9 postinfection, the time at which levels of oocyst shedding peak in infected neonatal mice, according to one study (20). To quantify oocyst shedding, fecal smears were made from 2 to 3 μl of stool removed from the distal colon. Thin smears were methanol fixed, and oocysts were stained with immunofluorescent antibodies (IFA) using a commercially available test kit (MeriFluor Cryptosporidium/Giardia; Meridian Bioscience Inc., Cincinnati, OH) according to the manufacturer's instructions. For each smear, oocyst counts were made from 21 microscopic fields that represented a vertical transect through the center of the IFA slide well at a magnification of ×400. This enabled an approximate standardization of counts despite slight variations in the density of fecal smears across the area of the slide wells. Levels of trophozoite-stage C. parvum in the intestinal epithelium were evaluated for mice treated for 6 days with 5 mg/kg pyrvinium or 100 mg/kg paromomycin and their respective controls. To quantify trophozoites, 1-cm-long intestinal sections were excised at points 1 cm, 2 cm, 3 cm, 4 cm, and 6 cm proximal to the appendix. The appendix and ileocecal junction with 1 cm of proximal colon were collected separately from the distal 2 cm of colon. Each tissue section was fixed separately in neutral buffered formalin, prepared by standard histological procedures, and stained with hematoxylin and eosin (H&E). Duplicate 1-cm-long sections from all mice were microscopically examined in a blinded fashion by two readers, and the number of trophozoites per 1,000 epithelial cells was determined by examining multiple fields on both ends of each tissue section. The mean number of trophozoites per 1,000 cells and the standard deviation was determined for each tissue section within each treatment group. An overall histology score for comparing trophozoites in treated versus untreated groups was derived for each mouse by summing the total number of trophozoites per 1,000 cells for each of the ileal sections as well as for the appendix, cecum, and colon.
TABLE 1.
Comparative efficacies of pyrvinium and paromomycin for reducing oocyst shedding from the distal colons of C. parvum-infected neonatal mice
| Trial | Days of treatment | Treatment | No. of mice | No. of oocysts
|
% Reduction in oocyst shedding | ||
|---|---|---|---|---|---|---|---|
| Meana | SD | 95% CI | |||||
| I | 4 | Pyrvinium vehicle | 4 | 2,980 | 1,000 | 2,000, 3,960 | |
| 5 mg/kg pyrvinium | 6 | 119 | 147 | 1.7, 237 | 96.0 | ||
| 100 mg/kg paromomycin | 5 | 148 | 102 | 59, 237 | 95.0 | ||
| II | 4 | Pyrvinium vehicle | 10 | 2,452 | 875 | 1,910, 3,327 | |
| 5 mg/kg pyrvinium | 10 | 180 | 267 | 15, 346 | 92.6 | ||
| 12.5 mg/kg pyrvinium | 8 | 261 | 216 | 112, 411 | 89.3 | ||
| III | 6 | Pyrvinium vehicle | 9 | 2,334 | 1,919 | 1,080, 3,588 | |
| 5 mg/kg pyrvinium | 10 | 83 | 87 | 29, 137 | 96.6 | ||
| Paromomycin vehicle | 11 | 2,567 | 1,781 | 1,514, 3,619 | |||
| 100 mg/kg paromomycin | 9 | 86 | 82 | 32, 140 | 96.4 | ||
Mean sum of oocysts counted in 21 microscopic fields (magnification, ×400) that represented a vertical transect through the center of the IFA slide well.
Statistical analysis.
Statistical analysis was performed using STATA, version 8.0 (copyright 1984-2003; Stata Corporation, College Station, TX). A Kruskal-Wallis nonparametric test of equality was used to determine if there were any significant differences in oocyst or trophozoite-stage parasite counts between the different treatment groups. Pairwise comparisons using a nonparametric two-sample Mann-Whitney test were used to determine whether reductions in numbers of oocysts and intestinal trophozoites for treated versus untreated control mice were statistically significant and whether levels of oocyst shedding and trophozoites were equivalent for paromomycin- and pyrvinium-treated mice. Results were considered to be significant at a P value of <0.05.
RESULTS
Drug activity in vitro.
The effects of pyrvinium, paromomycin, and chloroquine treatment on parasite growth in cell culture were investigated by exposure of infected cells to the antimicrobial agents for 48 h (Fig. 1). IC50s for pyrvinium, paromomycin, and chloroquine were calculated as 354 nM, 711 μM, and 27 μM, respectively, indicating that pyrvinium was ∼2,000 times more potent than paromomycin and ∼76 times more potent than chloroquine in vitro. Cytotoxic effects of pyrvinium on HCT-8 cells after 48 h of exposure were minimal (a <15% reduction in the number of cells) at all dose levels except the highest dose, 1.6 μM, at which a 40% reduction in cell numbers was observed. The cytotoxicity of chloroquine by the neutral red assay was quantified as a 15% reduction in cell numbers at 100 μM, a 12% reduction at 80 μM, and <5% reductions at all lower doses. The cytotoxicity of paromomycin in HCT-8 cells has previously been described as negligible by Gargala et al. (12).
FIG. 1.
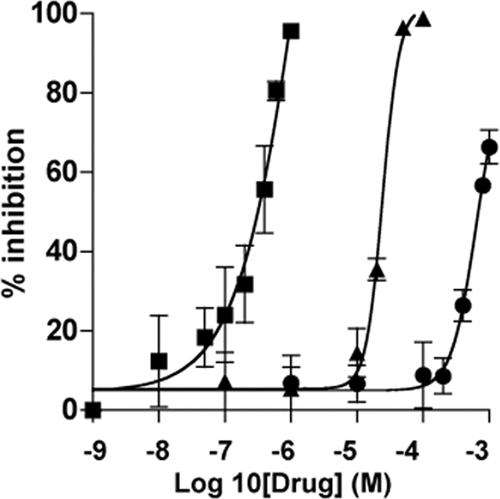
Effects of pyrvinium, chloroquine, and paromomycin on C. parvum growth in HCT-8 cells. Drugs were added to cultures 1.5 h after the addition of 5 × 103 oocysts/well and were incubated for 48 h. Parasite growth was determined by an ELISA using rat polyclonal anti-Cryptosporidium sera. The percentages of inhibition by pyrvinium (squares), chloroquine (triangles), and paromomycin (circles) are from biologic replicate experiments. Error bars, representing standard deviations, are from triplicate wells with the biologic duplicates.
Drug activity in neonatal mice.
The efficacy of pyrvinium, in comparison to that of paromomycin, was tested in a neonatal mouse model (20). Oocyst shedding by C. parvum-infected mice, as one indicator of drug efficacy, was quantified by IFA on distal colonic fecal smears made at the time of necropsy. The effects of various treatment regimens on oocyst shedding are presented in Table 1. At the 5-mg/kg/day dose, a >90% reduction in oocyst shedding was observed for the pyrvinium-treated mice compared to the vehicle control mice. Based on the confidence intervals, the level of oocyst shedding in mice treated with 5 mg/kg/day pyrvinium was equivalent to that in mice treated with 100 mg/kg/day paromomycin, though paromomycin was administered at a 20-fold-higher dose. Slightly higher levels of oocyst shedding were observed at the 12.5-mg/kg/day dose of pyrvinium, a finding that may be associated with drug-induced toxicity, since three mice in this treatment group died, though there were no deaths in any other group. However, after 4 days of pyrvinium treatment at either dose, there was no significant reduction in the weight of mice from that of controls (data not shown).
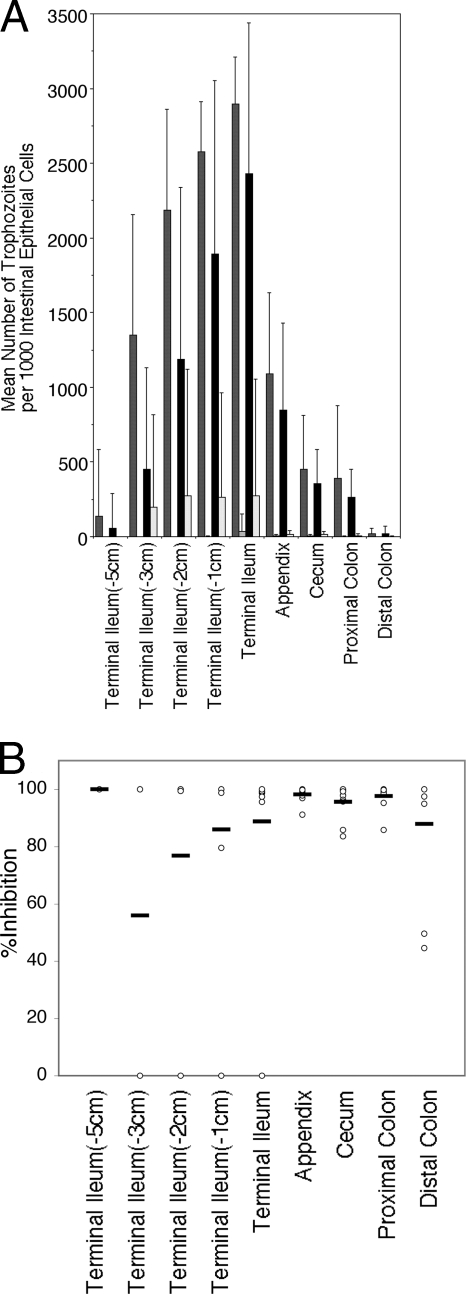
In addition to evaluation of oocyst shedding, levels of C. parvum trophozoites in the intestinal epithelium were determined by microscopic evaluation of H&E-stained histological sections from treated and untreated mice (Fig. 2). The comparative effects of treatment with pyrvinium or paromomycin for 6 consecutive days on levels of C. parvum trophozoites in individual intestinal sections are shown in Fig. 3. Parasite densities tended to be highest at the terminal ileum, adjacent to the appendix. Although parasite densities were generally lower in the appendix than in the ileum, we observed that low numbers of trophozoites could often be found in the appendix even when the ileum appeared to be clear of infection, indicating that the appendix could act as a reservoir for Cryptosporidium in the gastrointestinal tract, from which the ileum could be repopulated. Both drugs showed near 90% or greater reductions in the mean numbers of trophozoites in the terminal ileum, appendix, and colon.
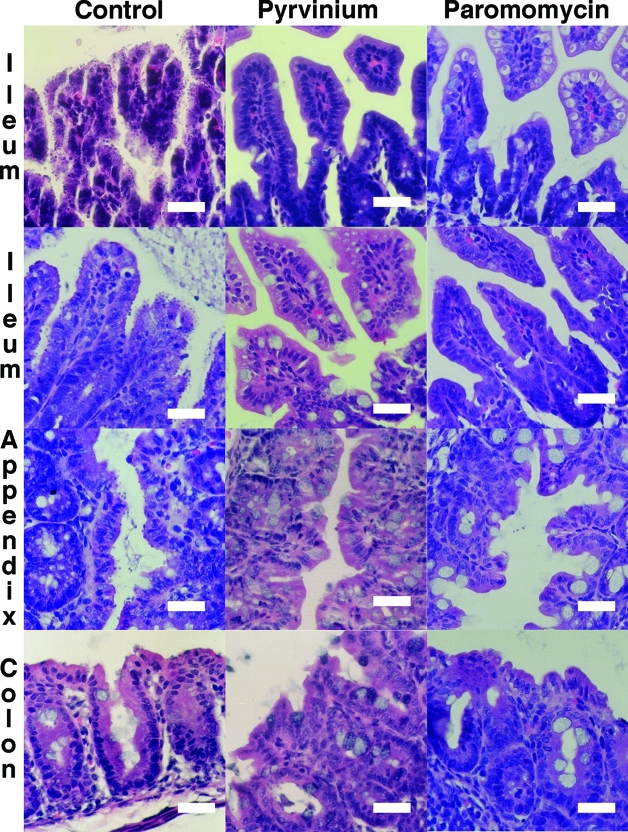
FIG. 2.
Representative H&E-stained intestinal sections from C. parvum-infected neonatal mice. Trophozoites are shown covering the intestinal epithelial cells in the ileum, appendix, and colon in control mice, while only a few trophozoites are present in pyrvinium- or paromomycin-treated mice. Size bars, 50 μm. Magnification, ×400.
FIG. 3.
Effects of drug treatment for 6 consecutive days on levels of C. parvum trophozoites in individual intestinal sections. (A) At necropsy, 1-cm-long intestinal sections were taken from the ileum, appendix, cecum, and colon and were stained with H&E for histological analysis to determine the extent of mucosal infection in drug-treated versus untreated mice. Results are mean numbers of trophozoites per 1,000 epithelial cells ± standard deviations (error bars). Experiments testing paromomycin at 100 mg/kg (n = 9) (light shaded bars) against its no-drug controls (n = 11) (dark shaded bars) and pyrvinium at 5 mg/kg (n = 10) (open bars) against its no-drug controls (n = 9) (filled bars) were performed at different times. Control mice had few trophozoites in the proximal ileum, with levels peaking in the terminal ileum and decreasing again in the colon. Oocysts in fecal matter were not counted. In paromomycin-treated mice, trophozoites were observed only in the terminal ileum, appendix, and colon. (B) Percentages of inhibition by pyrvinium in different intestinal sections for each of 10 individual mice (circles). Horizontal bars represent means. A single pyrvinium-treated mouse had little inhibition in the ileum but more-marked inhibition in the appendix and colon. Percentages of inhibition by paromomycin were greater than 90% in all sections (data not shown).
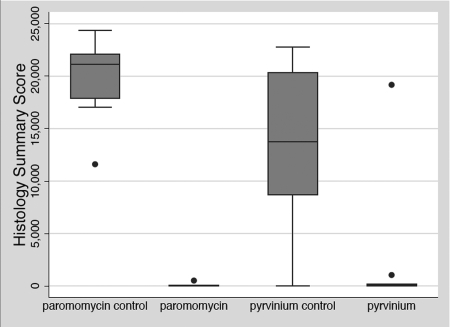
An overall score for comparing levels of trophozoites in treated versus untreated groups was derived by summing the total number of trophozoites per 1,000 cells over all of the tissue sections for each individual mouse. Box plots displaying the differences in this summary score between treatment groups are presented in Fig. 4. Based on mean summary scores, there was an 85% reduction in the number of trophozoites in the pyrvinium-treated group and a 99% reduction for the paromomycin-treated group compared to their respective controls. However, the mean summary score for the pyrvinium group was skewed by a single outlier mouse that had levels of trophozoites equivalent to those seen in control mice, though levels of oocyst shedding by this mouse were much lower than those seen in controls. The reductions in the numbers of trophozoites for both the pyrvinium and paromomycin treatment groups compared to their controls were statistically significant (P < 0.05), but there was no statistical difference in the numbers of trophozoites between paromomycin- and pyrvinium-treated mice (P = 0.2682).
FIG. 4.
Intestinal epithelium infection summary scores for C. parvum-infected neonatal mice in different treatment groups. The cumulative score for the extent of intestinal infection was calculated as the sum of the numbers of trophozoites per 1,000 epithelial cells from all of the tissue sections for each mouse. Treatment group data are presented in box plots displaying the median values (21,159, 10, 13,750, and 63 for the paromomycin no-drug control, paromomycin, the pyrvinium no-drug control, and pyrvinium, respectively). A Kruskal-Wallis nonparametric test of equality and pairwise comparisons using a nonparametric two-sample Mann-Whitney test showed that the levels of trophozoites in both the pyrvinium and the paromomycin treatment group were significantly (P < 0.05) lower than those in their respective controls but not significantly different from each other (P = 0.2682).
DISCUSSION
In light of the severe consequences of Cryptosporidium infection in human immunodeficiency virus/AIDS patients and other immune-compromised individuals, there is an urgent need for an anti-Cryptosporidium therapeutic that is reliably effective in these high-risk populations. Although numerous other drugs have demonstrated some activity against Cryptosporidium in preclinical studies, the safety of many of these drugs for human treatment has not been established. Taking into consideration the urgent need for a therapeutic and the considerable time and cost required to move a drug through the FDA approval process, one strategy to reduce the time required for an effective drug to reach the market is to test drugs previously approved by the FDA for other uses for activity against Cryptosporidium. In this report, we assessed the in vitro and in vivo efficacies of pyrvinium pamoate, an anthelmintic drug approved for the treatment of enterobiasis.
In this study we demonstrated that pyrvinium pamoate is a potent inhibitor of C. parvum growth, both in an in vitro cell culture system and in neonatal mice. In C. parvum-infected HCT-8 cells, the IC50 for pyrvinium (354 nM) was ∼2,000 times lower than the observed IC50 for paromomycin and ∼76 times lower than that of chloroquine. In previous studies, an IC50 for paromomycin as low as 83 μM has been reported (29), in contrast to the IC50 of 711 μM reported here, but variability in IC50s for paromomycin have been noted in the literature. In one study, paromomycin treatment at 100 μM resulted in only 40% inhibition of growth (17), and in another study, a concentration of 500 μM failed to achieve a measurable decrease in C. parvum levels in vitro (3). Concentrations of paromomycin as high as 3,200 μM have been needed to achieve a >80% reduction in parasite numbers (24). Several in vitro systems for testing the anti-Cryptosporidium activities of drugs have been described in the literature, and differences between the different procedures, along with variability in oocyst infectivity and excystation rates, can make the comparison of results between laboratories difficult, as is seen with the IC50 results for paromomycin. In contrast to our observations for paromomycin treatment, the in vitro activity of chloroquine against C. parvum in this study was consistent with previous findings, where a 20 μM dose produced 33% growth inhibition (3).
Pyrvinium was also a potent inhibitor of parasite growth in neonatal mice. A dose of 5 mg/day was sufficient to reduce oocyst shedding to 4 to 7% of that seen in controls. This level of reduction was equivalent to that seen for mice treated with 100 mg/kg/day paromomycin, a 20-fold-higher drug dose. A 2.5-fold increase in the pyrvinium dose failed to further reduce oocyst shedding levels below those seen for the 5-mg/kg group. Unexpectedly, oocyst shedding increased slightly at the 12.5-mg/kg dose, possibly due to drug-induced diarrhea, which was more severe in this treatment group than at the 5-mg/kg dose. Three deaths provided further evidence of drug toxicity at the 12.5-mg/kg dose, which may have resulted from increased absorption of pyrvinium from the gastrointestinal tract in neonatal mice. Acute oral toxicity studies have shown that higher doses of pyrvinium pamoate are tolerated in adult mice. In one report, doses as high as 125 mg/kg were well tolerated (5). In another study, treatment with 128 mg/kg pyrvinium resulted in >50% mortality, though a dose of 64 mg/kg was associated with a 93.5% survival rate (25). Results from drug studies with other animals, including rats, dogs, and monkeys, demonstrate the relatively low toxicity of more than 100 mg/kg/day of pyrvinium pamoate following oral administration (25), and doses as high as 35 mg/kg have been safely used in humans for the treatment of strongyloidiasis (27). However, the observed low-dose toxicity of pyrvinium pamoate in neonatal mice precludes dose escalation studies using this animal model for cryptosporidiosis. Such studies will have to be pursued using an alternative model system, such as a piglet diarrhea model (24).
Although the intensity of oocyst shedding was equivalent for the pyrvinium- and paromomycin-treated groups, the reduction in the mean levels of trophozoites in the intestinal epithelium was greater for paromomycin-treated mice than for pyrvinium-treated mice compared to their respective controls (99% and 85% reductions, respectively). However, this difference between the two drug treatment groups was not statistically significant. The mean histology score for the pyrvinium group was significantly skewed by a single outlier mouse that had levels of trophozoites in the ileum equivalent to those seen in controls, though levels were reduced in the appendix, cecum, and colon. As a result, the mean histology score for this group was ∼33 times higher than the median score and was associated with very large standard deviations. Surprisingly, the oocyst count from the fecal smear of this outlier mouse was lower than oocyst counts from several other mice in the treatment group. To verify the IFA results, stool remaining in the colon section on the histology slide from the outlier mouse was examined for the presence of oocysts, but no evidence of large numbers of oocysts was seen anywhere in the colon. The reasons for the discrepancy between oocyst shedding levels and the histology results for this mouse are unclear. It is possible that different mechanisms of action are responsible for the effects of pyrvinium on trophozoites and oocyst production. Without a better understanding of the mechanism of Cryptosporidium inhibition by pyrvinium, it is difficult to explain these results. It is also noteworthy that the two littermates of the outlier mouse had the second and third highest numbers of trophozoites in the group, indicating that some shared exposure, such as the nursing habits of the dam, may have affected the responses of these mice to drug treatment. One possible explanation is that the dam was providing a suboptimal amount of milk, which, along with drug-induced diarrhea, could have contributed to the malnourishment of these mice. The effects of malnourishment on the severity of Cryptosporidium infection are well known (19).
The antiparasitic mechanism of action of pyrvinium has not been studied in depth and consequently is not well understood. The proposed mechanism of action in intestinal helminths has been inhibition of respiration in aerobes or interference with exogenous glucose utilization (8, 22). Despite mutagenic activity in bacteria and yeast, there has been no evidence of genotoxicity in mammalian cell lines (18) or in the colons of mice administered pyrvinium at doses as high as 12.5 times the recommended human dose (14). In fact, antitumor activity has been reported for pyrvinium under glucose starvation conditions (8). The lack of pyrvinium absorption probably plays a role in the absence of genotoxicity in vivo.
In summary, we found oral administration of pyrvinium pamoate to be highly effective at reducing C. parvum growth in vitro and at reducing both oocyst shedding and the number of intestinal trophozoites in infected neonatal mice. Based on the efficacy of this drug against C. parvum and the distantly related malaria parasite, P. falciparum, we postulate that pyrvinium will be effective against other Cryptosporidium species of medical and veterinary importance, including C. hominis and C. andersoni, respectively. Although trials with humans will be necessary to determine the minimum effective dose and tolerable doses, the safety of pyrvinium for treatment of humans has already been established (4, 27), which will significantly reduce the time and costs required for clinical trials. Based on these results, we believe that pyrvinium pamoate is a potential drug candidate for the treatment of cryptosporidiosis in immunocompetent and also immunocompromised individuals, for whom no effective therapy is currently approved.
Acknowledgments
We thank Steve J. Upton at Kansas State University, Manhattan, for providing the polyclonal rat anti-Cryptosporidium antiserum and infection medium reagents, and we thank Dwight D. Bowman of Cornell University College of Veterinary Medicine, Ithaca, NY, for providing C. parvum oocysts.
Funding was provided by the Johns Hopkins Center in Urban Environmental Health (grant P30 ES03819). The authors have applied for a provisional patent for the use of pyrvinium.
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Abubakar, I., S. H. Aliyu, C. Arumugam, N. K. Usman, and P. R. Hunter. 2007. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br. J. Clin. Pharmacol. 63:387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, V. R., and M. P. Curran. 2007. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs 67:1947-1967. [DOI] [PubMed] [Google Scholar]
- 3.Armson, A., B. P. Meloni, J. A. Reynoldson, and R. C. Thompson. 1999. Assessment of drugs against Cryptosporidium parvum using a simple in vitro screening method. FEMS Microbiol. Lett. 178:227-233. [DOI] [PubMed] [Google Scholar]
- 4.Beck, J. W. 1964. Treatment of pinworm infections with reduced single dose of pyrvinium pamoate. JAMA 189:511. [DOI] [PubMed] [Google Scholar]
- 5.Beck, J. W., D. Saavedra, G. J. Antell, and B. Tejeiro. 1959. The treatment of pinworm infections in humans (enterobiasis) with pyrvinium chloride and pyrvinium pamoate. Am. J. Trop. Med. Hyg. 8:349-352. [DOI] [PubMed] [Google Scholar]
- 6.Blagburn, B. L., K. L. Drain, T. M. Land, R. G. Kinard, P. H. Moore, D. S. Lindsay, D. A. Patrick, D. W. Boykin, and R. R. Tidwell. 1998. Comparative efficacy evaluation of dicationic carbazole compounds, nitazoxanide, and paromomycin against Cryptosporidium parvum infections in a neonatal mouse model. Antimicrob. Agents Chemother. 42:2877-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong, C. R., X. Chen, L. Shi, J. O. Liu, and D. J. Sullivan, Jr. 2006. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat. Chem. Biol. 2:415-416. [DOI] [PubMed] [Google Scholar]
- 8.Esumi, H., J. Lu, Y. Kurashima, and T. Hanaoka. 2004. Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-methyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation. Cancer Sci. 95:685-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fayer, R. 2004. Cryptosporidium: a water-borne zoonotic parasite. Vet. Parasitol. 126:37-56. [DOI] [PubMed] [Google Scholar]
- 10.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30:1305-1322. [DOI] [PubMed] [Google Scholar]
- 11.Gargala, G., A. Delaunay, L. Favennec, P. Brasseur, and J. J. Ballet. 1999. Enzyme immunoassay detection of Cryptosporidium parvum inhibition by sinefungin in sporozoite infected HCT-8 enterocytic cells. Int. J. Parasitol. 29:703-709. [DOI] [PubMed] [Google Scholar]
- 12.Gargala, G., A. Delaunay, X. Li, P. Brasseur, L. Favennec, and J. J. Ballet. 2000. Efficacy of nitazoxanide, tizoxanide and tizoxanide glucuronide against Cryptosporidium parvum development in sporozoite-infected HCT-8 enterocytic cells. J. Antimicrob. Chemother. 46:57-60. [DOI] [PubMed] [Google Scholar]
- 13.Giacometti, A., F. Burzacchini, O. Cirioni, F. Barchiesi, M. Dini, and G. Scalise. 1999. Efficacy of treatment with paromomycin, azithromycin, and nitazoxanide in a patient with disseminated cryptosporidiosis. Eur. J. Clin. Microbiol. Infect. Dis. 18:885-889. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg, M. T. 1984. Pyrvinium pamoate lacks in vivo genotoxicity in the colon. Toxicol. Appl. Pharmacol. 74:293-295. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt, R. G., C. T. Yiannoutsos, E. S. Higgs, J. T. Carey, P. J. Geiseler, R. Soave, R. Rosenberg, G. J. Vazquez, L. J. Wheat, R. J. Fass, Z. Antoninievic, A. L. Walawander, T. P. Flanigan, and J. F. Bender for the AIDS Clinical Trials Group. 2000. Paromomycin: no more effective than placebo for treatment of cryptosporidiosis in patients with advanced human immunodeficiency virus infection. Clin. Infect. Dis. 31:1084-1092. [DOI] [PubMed] [Google Scholar]
- 16.Hoepelman, I. M. 1996. Human cryptosporidiosis. Int. J. STD AIDS 7(Suppl. 1):28-33. [DOI] [PubMed] [Google Scholar]
- 17.Kayser, O., W. R. Waters, K. M. Woods, S. J. Upton, J. S. Keithly, H. Laatsch, and A. F. Kiderlen. 2002. Evaluation of in vitro and in vivo activity of benzindazole-4,9-quinones against Cryptosporidium parvum. J. Antimicrob. Chemother. 50:975-980. [DOI] [PubMed] [Google Scholar]
- 18.Lake, R. S., M. L. Kropko, and F. A. de la Iglesia. 1982. Absence of in vitro genotoxicity of pyrvinium pamoate in sister-chromatid exchange, chromosome aberration, and HGPRT-locus mutation bioassays. J. Toxicol. Environ. Health 10:255-266. [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane, D. E., and J. Horner-Bryce. 1987. Cryptosporidiosis in well-nourished and malnourished children. Acta Paediatr. Scand. 76:474-477. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Gomez, S., M. Alvarez-Sanchez, and F. Rojo-Vazquez. 2006. A newborn mouse Cryptosporidium parvum infection model: its application to the study of therapeutic and prophylactic measures for controlling cryptosporidiosis in ruminants. Parasitol. Res. 99:1-6. [DOI] [PubMed] [Google Scholar]
- 21.O'Donoghue, P. J. 1995. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 25:139-195. [DOI] [PubMed] [Google Scholar]
- 22.Sheth, U. K. 1975. Mechanisms of anthelmintic action. Prog. Drug Res. 19:147-157. [DOI] [PubMed] [Google Scholar]
- 23.Smith, T. C., A. W. Kinkel, C. M. Gryczko, and J. R. Goulet. 1976. Absorption of pyrvinium pamoate. Clin. Pharmacol. Ther. 19:802-806. [DOI] [PubMed] [Google Scholar]
- 24.Theodos, C. M., J. K. Griffiths, J. D'Onfro, A. Fairfield, and S. Tzipori. 1998. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 42:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson, P. E., D. E. Worley, and J. E. Meisenhelder. 1962. Anthelmintic studies on pyrvinium pamoate (Povan) and other drugs in rodents, dogs, and monkeys. Am. J. Trop. Med. Hyg. 11:89-95. [DOI] [PubMed] [Google Scholar]
- 26.Ventura, G., R. Cauda, L. M. Larocca, M. E. Riccioni, M. Tumbarello, and M. B. Lucia. 1997. Gastric cryptosporidiosis complicating HIV infection: case report and review of the literature. Eur. J. Gastroenterol. Hepatol. 9:307-310. [DOI] [PubMed] [Google Scholar]
- 27.Wagner, E. D. 1963. Pyrvinium pamoate in the treatment of strongyloidiasis. Am. J. Trop. Med. Hyg. 12:60-61. [DOI] [PubMed] [Google Scholar]
- 28.Weyermann, J., D. Lochmann, and A. Zimmer. 2005. A practical note on the use of cytotoxicity assays. Int. J. Pharm. 288:369-376. [DOI] [PubMed] [Google Scholar]
- 29.Woods, K. M., M. V. Nesterenko, and S. J. Upton. 1995. Development of a microtitre ELISA to quantify development of Cryptosporidium parvum in vitro. FEMS Microbiol. Lett. 128:89-94. [DOI] [PubMed] [Google Scholar]
- 30.Zardi, E. M., A. Picardi, and A. Afeltra. 2005. Treatment of cryptosporidiosis in immunocompromised hosts. Chemotherapy 51:193-196. [DOI] [PubMed] [Google Scholar]