Abstract
The need to investigate novel dosing regimens and combinations is essential in combating poor treatment outcomes for Staphylococcus aureus bacteremia and endocarditis. We evaluated the impact of simulated standard- and high-dose daptomycin in combination with gentamicin or rifampin against daptomycin-susceptible and nonsusceptible matched strains of S. aureus. These strains were collected from the daptomycin bacteremia and endocarditis clinical trial and consisted of three susceptible strains (MIC, 0.25 mg/liter) and four nonsusceptible isolates (MICs, 2 to 4 mg/liter). Daptomycin regimens of 6 and 10 mg/kg of body weight daily alone and in combination with gentamicin at 5 mg/kg daily or rifampin at 300 mg every 8 h were evaluated using an in vitro model with simulated endocardial vegetations over 96 h. Rapid bactericidal activity, identified by time to 99.9% kill, was displayed in all regimens with the daptomycin-susceptible strains. Concentration-dependent activity was noted by more-rapid killing with the 10-mg/kg/day dose. The addition of gentamicin improved activity in the majority of susceptible isolates. Daptomycin 6-mg/kg/day monotherapy displayed bactericidal activity for only one of the nonsusceptible isolates and for only two isolates with increased doses of 10 mg/kg/day. Combination regimens demonstrated improvement with some but not all nonsusceptible isolates. Three isolates developed a reduction in daptomycin susceptibility with 6-mg/kg/day monotherapy, but this was suppressed with both combination therapy and high-dose daptomycin. These results suggest that high-dose daptomycin therapy and combination therapy may be reasonable treatment options for susceptible isolates; however, more investigations are needed to confirm the variability of these regimens with nonsusceptible isolates.
The poor treatment outcomes associated with serious methicillin-resistant Staphylococcus aureus (MRSA) infections reflect the need for alternative effective treatment options. For decades, the mainstay of therapy for MRSA has been vancomycin. However, increasing reports of clinical vancomycin treatment failure along with reduced vancomycin susceptibility and vancomycin tolerance may be consequences of its long-term use (6, 15, 16, 20, 22). Although S. aureus, including MRSA, continues to be a major cause of bacteremia and endocarditis, alternative treatment options to vancomycin with proven efficacy remain minimal. Several in vitro studies indicate the potential of synergy with agents such as aminoglycosides in combination with vancomycin, but this has not been clearly elucidated in the clinical setting.
Daptomycin is a lipopeptide antibiotic with potent activity against gram-positive organisms, including multidrug-resistant S. aureus (25, 26, 30). Multiple reports have displayed the bactericidal and concentration-dependent activity of daptomycin in vitro. In the advent of serious S. aureus infections, some studies advocate the importance of rapid inoculum reduction and bactericidal activity (9, 22). Daptomycin has been approved in the United States for the treatment of skin and soft-tissue infection and bacteremia and right-sided endocarditis. A recent study evaluated a daptomycin regimen of 6 mg/kg of body weight daily versus standard therapy of vancomycin or nafcillin in combination with gentamicin for the treatment of S. aureus bacteremia and endocarditis. In this study, a daptomycin dosage of 6 mg/kg/day displayed noninferiority compared to both standard therapies. Treatment failure rates were similar for both daptomycin and the comparator agent groups. A few select study patients failed daptomycin therapy, resulting in the recovery of S. aureus isolates with reduced daptomycin susceptibility (MIC of ≥1 mg/liter). Most treatment failures were attributed to deep-seated infections causing persistent bacteremia or a lack of surgical intervention to remove the source of infection (11).
The antimicrobial activities of various agents in combination with daptomycin have been evaluated in multiple in vitro studies. Previously we demonstrated increased bactericidal activity with short-course gentamicin 5-mg/kg once-daily regimens in combination with daptomycin dosages of 6 and 8 mg/kg daily in both daptomycin-susceptible MRSA and methicillin-susceptible S. aureus (MSSA) isolates. Improved bacterial killing was most pronounced in the first 4 h of combination therapy (29). Another study evaluated daptomycin in combination with gentamicin and rifampin for isolates with various daptomycin MICs. For six vancomycin-intermediate S. aureus isolates with daptomycin MICs of 2 to 8 mg/liter, gentamicin and daptomycin were synergistic for 67% of isolates and rifampin and daptomycin had 33% synergy (8). Although the current recommended dose for daptomycin is 6 mg/kg daily for S. aureus bacteremia, including right-sided endocarditis, concentrations greater than 6 mg/kg/day have demonstrated efficacy and safety in vitro and in vivo. In a clinical study of healthy volunteers, daptomycin doses up to 12 mg/kg daily were administered without any increase in adverse events (2). We previously reported the enhanced bactericidal in vitro activity with daptomycin doses of 10 mg/kg daily in a historical case with daptomycin-susceptible and nonsusceptible strains in vitro and in vivo (23). Although standard and high-dose daptomycin in combination with gentamicin or rifampin demonstrates promising results against daptomycin-nonsusceptible strains, the pharmacodynamic effects of these regimens are not completely understood.
This study investigated the activities of daptomycin 6- and 10-mg/kg daily dosages with and without gentamicin or rifampin for daptomycin-susceptible S. aureus isolates and their nonsusceptible derivatives from the bacteremia and endocarditis clinical trial utilizing an in vitro pharmacodynamic model with simulated endocardial vegetations (SEVs).
MATERIALS AND METHODS
Bacterial strains.
A total of seven clinical isolates of S. aureus were evaluated: six MSSA isolates and one MRSA isolate. These S. aureus isolates are from the daptomycin bacteremia and endocarditis clinical trial and were supplied by Cubist Pharmaceuticals, Inc. (Lexington, MA).
Antibiotics.
Daptomycin (Cubist Pharmaceuticals, Inc.) analytical powder was provided by the manufacturer. Gentamicin and rifampin analytical powder was purchased from a commercial source (Sigma Chemical Company, St. Louis, MO).
Media.
Mueller-Hinton broth (Difco, Detroit, MI) supplemented with calcium adjusted to physiologic conditions of approximately 1.1 to 1.3 mM and 12.5 mg/liter magnesium was used for all in vitro pharmacodynamic models to evaluate daptomycin simulations alone and in combination with gentamicin or rifampin. Colony counts were determined using tryptic soy agar (TSA) (Difco) plates.
Susceptibility.
MICs of study antimicrobial agents were determined by broth microdilution and Etest methodology according to Clinical and Laboratory Standards Institute guidelines (7). Minimum bactericidal concentrations were determined by inspection of colony counts on wells displaying no visible growth. All samples were incubated at 35°C for 24 h.
In vitro pharmacodynamic infection model with SEVs.
A bacterial inoculum of approximately 109 CFU/g was achieved in the SEVs by combining 50 μl of a high-inoculum organism suspension, 500 μl of human cryoprecipitate antihemolytic factor from human volunteer donors (American Red Cross, Detroit, MI), and 2.5 μl of a platelet/saline suspension (250,000 to 500,000 platelets per clot) in 1.5-ml siliconized Eppendorf tubes. After vortex mixing to ensure a homogeneous mixture, a monofilament line followed by 50 μl bovine thrombin (5,000 U/ml) was added to each tube. The resulting SEVs were then removed from the Eppendorf tubes with a sterile 21-gauge needle and inserted into the model. This methodology results in SEVs consisting of approximately 3 to 3.5 g/dl of albumin and 6.8 to 7.4 g/dl of total protein (23).
An in vitro infection model consisting of a 250-ml one-compartment glass apparatus with ports, where the SEVs were suspended and utilized for all simulations, was used. The apparatus was prefilled with medium, and antibiotics were administered as boluses into the central compartment via an injection port. The model apparatus was placed in a 37°C water bath throughout the procedure, and a magnetic stir bar was placed in the medium for thorough mixing of the drug in the model. Fresh medium was continuously supplied and removed from the compartment along with the antibiotics via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL) set to simulate the half-lives of the antibiotics. The pH was monitored throughout all experiments with daptomycin due to the possible effects on its activity.
Antibiotic regimens were as follows: daptomycin, 6 mg/kg and 10 mg/kg every 24 h (peak, 98.6 and 164.3 mg/liter, respectively; average half-life, 8 h); gentamicin, 5 mg/kg every 24 h (peak, 15 mg/liter; average half-life, 3 h); and rifampin, 300 mg every 8 h (peak, 8 mg/liter; average half-life, 3 h) (1). The pump rate was at 0.4 ml/min to achieve an average half-life of 8 h for daptomycin and 1 ml/min to account for the half-lives when using combination therapy, as previously described (3).
Pharmacodynamic analysis.
Two simulated endocardial vegetations were removed from each model (total of four) over 96 h. The SEVs were homogenized and diluted in cold saline and were plated on TSA plates. Plates were incubated at 35°C for 24 h, at which time colony counts were performed. The total reduction in log10 CFU/g over 96 h was determined by plotting time-kill curves based on the number of remaining organisms over the model duration. Bactericidal activity (99.9% kill) was defined as a ≥3-log10 CFU/g reduction in colony count from the initial inoculum. Bacteriostatic activity was defined as a <3-log10 CFU/g reduction in colony count, while inactivity was defined as no observed reductions in initial inoculums. The time to achieve a 99.9% (T99) bacterial load reduction was determined by linear regression (if r2 was ≥0.95) or visual inspection.
Pharmacokinetic analysis.
Pharmacokinetic samples were obtained through the injection port of each model (duplicate samples) over 96 h for verification of target antibiotic concentrations. In addition, all simulated endocardial vegetations were assayed for antimicrobial concentrations after homogenizing and were compared to model concentrations to determine percent penetration over time. All samples were stored at −70°C until ready for analysis. Gentamicin concentrations were determined by fluorescence polarization immunoassay (Abbott Diagnostics TDx). This assay has a limit of detection of 0.27 mg/liter for gentamicin (r2 = 0.99; between-day CV for high medium and low standards, 7.2, 4.6, and 13.7%). Concentrations of daptomycin and rifampin were determined by microbioassay utilizing Micrococcus luteus ATCC 9341. Blank 1/4-in. disks were spotted with 20 μl of the standards or samples. Each standard was tested in triplicate by placing the disk on Mueller-Hinton agar plates, which were preswabbed with a 0.5 McFarland suspension of the test organism. Plates were incubated for 18 to 24 h at 37°C, at which time the zone sizes were measured. Concentrations of 150, 50, 10, and 5 mg/liter were used as standards for daptomycin, while standard concentrations of 10, 5, 1, and 0.5 mg/liter were used for rifampin. This assay for daptomycin was linear over the range of 2.5 to 150 mg/liter (r2 = 0.99; interday CV for high medium and low standards were 4.0, 7.8, and 6.9%, respectively). The rifampin microbioassay was linear over the range of 0.06 to 10 mg/liter, with interday CV for high medium and low standards of 9.3, 4.9, and 4.8%, respectively (r2 = 0.99). The half-lives, area under the curve, and peak concentrations of the antibiotics were determined by the trapezoidal method utilizing the PK Analyst software program (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
Nonsusceptibility.
Development of reduced susceptibility to daptomycin was evaluated at multiple time points throughout the simulation, at 0, 8, 24, 48, 72, and 96 h. One-hundred-microliter samples from each time point were plated on TSA plates containing three- and sixfold MICs of daptomycin to assess the development of reduced susceptibility. Plates were then examined for growth after 24 to 48 h of incubation at 35°C.
Statistical analysis.
Changes in CFU/g at 24, 48, 72, and 96 h were compared by two-way analysis of variance with Tukey's posthoc test. A P value of ≤0.05 was considered significant. All statistical analyses was performed using SPSS statistical software (Release 10.07; SPSS, Inc., Chicago, IL).
RESULTS
Susceptibility.
One MRSA isolate, CB 1734, and two MSSA isolates, CB 1740 and CB 1815, were susceptible to daptomycin, with MICs of 0.25 mg/liter. Four MSSA isolates, CB 1735, CB 1741, CB 1813, and CB 1814, displayed nonsusceptible daptomycin MICs of 2, 2, 2, and 4 mg/liter, respectively. Two nonsusceptible isolates, CB 1735 and CB 1741, contained mprF mutations (data on file; Cubist), which has been previously reported to occur in nonsusceptible isolates (12). All isolates were initially susceptible to both gentamicin (MIC, 1 to 2 mg/liter) and rifampin (MIC, <0.0625 mg/liter).
In vitro pharmacokinetics and pharmacodynamics.
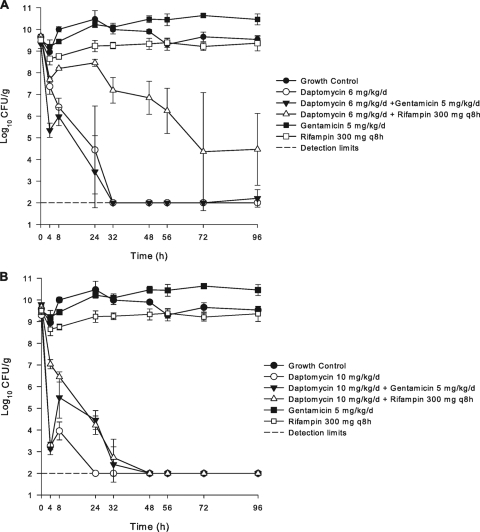
The pharmacokinetic parameters of the three antimicrobial agents utilized are displayed in Table 1. These values were within the targeted range. The in vitro activities of antibiotic regimens for the set of isolates evaluated are described in Table 2. Standard-dose daptomycin (6 mg/kg/day) simulated in the in vitro pharmacodynamic model displayed potent activities against all daptomycin-susceptible isolates. Rapid bactericidal activity was achieved for all susceptible strains, with a time to 99.9% kill of 6.6, 6.9, and 18.9 h for each strain. Bacterial regrowth occurred with only one of three isolates using a 6-mg/kg daily dosage and was consistent with a threefold increase in the MIC (0.75 mg/liter) with isolate 1815. High-dose daptomycin therapy (10 mg/kg daily) resulted in even greater bactericidal activity, with T99s of 1.8, 3.8, and 9.7 h. This activity of 10 mg/kg/day was maintained for most isolates throughout the 96-h evaluation, with only isolate 1740 displaying regrowth. The high-dose regimen prevented the increase in MIC that was noted for isolate 1815 with 6-mg/kg daily doses. Gentamicin and rifampin monotherapy resulted in no activity and resistance (MICs of >4 and >32 mg/liter, respectively) by the end of the treatment duration.
TABLE 1.
Pharmacokinetic parameters of daptomycin, gentamicin, and rifampin achieved using in vitro pharmacodynamic model with the SEVsa
| Drug, dosage | Cmax (mg/liter) | Cmin (mg/liter) | Half-life (h) | AUC0-24 (mg/liter·h) |
|---|---|---|---|---|
| Daptomycin, 6 mg/kg/day | 103.0 ± 7.6 | 19.4 ± 2.6 | 8.9 ± 1.9 | 1504.6 ± 149.9 |
| Daptomycin, 10 mg/kg/day | 162.3 ± 3.4 | 19.0 ± 8.5 | 7.8 ± 1.9 | 1736.8 ± 111.0 |
| Gentamicin, 5 mg/kg/day | 16.6 ± 0.6 | 0.04 ± 0.02c | 2.8 ± 0.3 | 60.8 ± 4.0 |
| Rifampin, 300 mg q8hb | 6.2 ± 0.8 | 1.3 ± 0.3 | 3.2 ± 0.5 | 107.7 ± 6.0 |
Cmax, maximum concentration; Cmin, minimum concentration; AUC0-24, area under the concentration-time curve from 0 to 24 h. Results are expressed as means ± standard deviations.
q8h, every 8 h.
Extrapolated data; limit of detection = 0.27.
TABLE 2.
In vitro activities of daptomycin alone and in combination with gentamicin or rifampin
| Isolate | MIC of daptomycin (mg/liter) | Drug(s) and dosea | T99b (h) | Regrowthc | Fold change in MIC of daptomycin |
|---|---|---|---|---|---|
| CB 1815 | 0.25 | D6 | 6.9 ± 0.2 | Y | 3 |
| D6 + G | 4.6 ± 0.3 | N | 0 | ||
| D6 + R | 40.6 ± 2.0 | N | 0 | ||
| D10 | 3.8 ± 0.2 | N | 0 | ||
| D10 + G | 10.2 ± 0.5 | N | 0 | ||
| D10 + R | 29.5 ± 2.3 | N | 0 | ||
| CB 1813 | 2 | D6 | NA | Y | 1.5 |
| D6 + G | NA | Y | 0 | ||
| D6 + R | NA | N | 0 | ||
| D10 | NA | N | 0 | ||
| D10 + G | 16.8 ± 0.7 | N | 0 | ||
| D10 + R | 50.9 ± 5.1 | N | 0 | ||
| CB 1814 | 4 | D6 | NA | Y | 1.5 |
| D6 + G | NA | Y | 0 | ||
| D6 + R | NA | N | 0 | ||
| D10 | NA | Y | 0 | ||
| D10 + G | NA | Y | 0 | ||
| D10 + R | 49.8 ± 1.4 | N | 0 | ||
| CB 1734 | 0.25 | D6 | 6.6 ± 0.3 | N | 0 |
| D6 + G | 3.0 ± 0.2 | N | 0 | ||
| D6 + R | 48.1 ± 1.2 | N | 0 | ||
| D10 | 2.0 ± 1.4 | N | 0 | ||
| D10 + G | 1.8 ± 0.1 | N | 0 | ||
| D10 + R | 7.4 ± 0.3 | N | 0 | ||
| CB 1735 | 2 | D6 | 8.3 ± 0.4 | Y | 2 |
| D6 + G | 7.7 ± 0.3 | N | 0 | ||
| D6 + R | 59.7 ± 4.1 | N | 0 | ||
| D10 | 3.0 ± 0.1 | N | 0 | ||
| D10 + G | 3.8 ± 0.2 | N | 0 | ||
| D10 + R | 60.8 ± 5.6 | N | 0 | ||
| CB 1740 | 0.25 | D6 | 18.9 ± 6.6 | N | 0 |
| D6 + G | 2.6 ± 0.2 | N | 0 | ||
| D6 + R | 37.9 ± 4.7 | N | 0 | ||
| D10 | 9.7 ± 0.6 | Y | 0 | ||
| D10 + G | 5.4 ± 0.2 | N | 0 | ||
| D10 + R | 8.9 ± 1.2 | N | 0 | ||
| CB 1741 | 2 | D6 | NA | Y | 0 |
| D6 + G | NA | Y | 0 | ||
| D6 + R | 61.7 ± 5.2 | N | 0 | ||
| D10 | 30.8 ± 2.4 | N | 0 | ||
| D10 + G | NA | N | 0 | ||
| D10 + R | 88.1 ± 5.9 | N | 0 |
D6, daptomycin at 6 mg/kg/day; D10, daptomycin at 10 mg/kg/day; G, gentamicin at 5 mg/kg daily; R, rifampin at 300 mg every 8 h.
NA, not achieved; T99, time to achieve a 99.9% bacterial load reduction.
Y, yes; N, no.
The addition of gentamicin to both standard and high-dose daptomycin therapy resulted in enhanced bactericidal activity for the daptomycin-susceptible isolates. Figure 1 graphically depicts the in vitro activities of all regimens evaluated against the daptomycin-susceptible isolate 1734. The enhanced bactericidal activity with the addition of gentamicin was especially pronounced in the first 4 to 8 h of simulated therapy and continued to kill to detection limits by 24 to 32 h. The gentamicin addition to the daptomycin 6-mg/kg/day regimen prevented the emergence of the MIC increase that was noted for one strain with daptomycin monotherapy. Daptomycin regimens in combination with rifampin against the susceptible isolates displayed antagonistic properties compared to daptomycin alone. This effect was most evident in the first 24 to 48 h of simulated therapy. However, bactericidal activity was still achieved in all rifampin combination regimens in addition to elimination of both regrowth and increases in MICs.
FIG. 1.
In vitro activity of daptomycin at 6 mg/kg/day (A) or daptomycin at 10 mg/kg/day (B) in combination with gentamicin or rifampin against daptomycin-susceptible strain CB 1734.
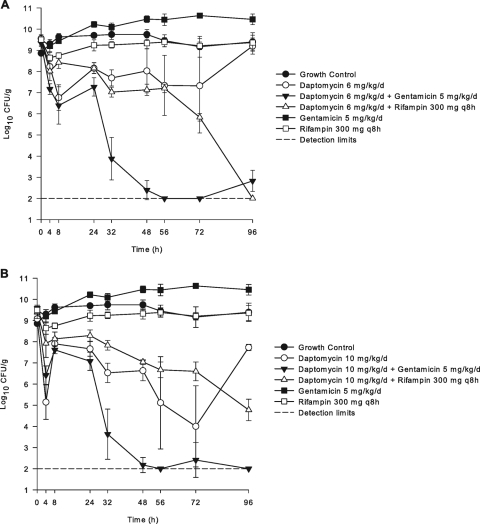
Four daptomycin-nonsusceptible strains were evaluated with the in vitro model with SEVs utilizing 6- and 10-mg/kg daily regimens. Daptomycin at 6 mg/kg/day displayed minimal activity and achieved bactericidal killing for only one strain (CB 1735), as displayed in Table 2 and Fig. 2. However, for this strain, regrowth was noted after only 8 h of therapy, resulting in a twofold MIC increase to 4 mg/liter by 96 h. High-dose daptomycin 10-mg/kg/day therapy also displayed minimal activity, with bactericidal killing for two of the four nonsusceptible strains (1735 and 1741) evaluated. For these two strains, daptomycin activity was maintained and continued to kill up to 96 h but never reached detection limits. Increases in baseline MICs occurred for CB 1735 (4 mg/liter), CB 1813 (3 mg/liter), and 1814 (6 mg/liter) when these isolates were dosed at 6 mg/kg/day, while no changes occurred for the one remaining nonsusceptible isolates at this dose. High-dose daptomycin (10 mg/kg/day) alone prevented the emergence of increased daptomycin MICs regardless of activity.
FIG. 2.
In vitro activity of daptomycin at 6 mg/kg/day (A) or daptomycin at 10 mg/kg/day (B) in combination with gentamicin or rifampin against a daptomycin-nonsusceptible strain, CB 1735.
Combination therapy with the daptomycin-nonsusceptible isolates displayed variable activities. Gentamicin in combination with daptomycin at 6 mg/kg/day resulted in significantly increased killing against isolate CB 1735 (P < 0.001) but had no additional effects on isolates CB 1741, CB 1813, and CB 1814 compared to daptomycin (6 mg/kg/day) alone. The addition of rifampin to the daptomycin 6-mg regimen resulted in improved activity against CB 1741, lesser activity against CB 1735, and no change in killing for CB 1813 and CB 1814. Regrowth was demonstrated for three of the four isolates with the combination of gentamicin, while rifampin prevented regrowth for all nonsusceptible strains. Results with high-dose daptomycin in combination with gentamicin or rifampin were also highly variable. Significantly improved activity with the addition of gentamicin was noted for one of the four isolates. Rifampin also improved the killing activity compared to results with daptomycin (10 mg/kg/day) alone for only two of four isolates, with no regrowth for any strain. All combination regimens tested prevented the emergence of isolates with increased daptomycin MICs with the SEV model. When combined with daptomycin, no changes in gentamicin or rifampin MICs were found.
DISCUSSION
Although the historical drug of choice for MRSA infections has been vancomycin, high failure rates and increasing reports of vancomycin resistance have raised questions regarding the effectiveness of this antibiotic (6, 14-16, 18, 20). The approval of daptomycin as a treatment option for bacteremia and endocarditis has opened new treatment regimens for these serious infections. This study confirmed the rapid bactericidal activity and concentration-dependent effects of daptomycin that have been reported by others with its use against susceptible S. aureus isolates (17, 29).
The impact of combination therapy with daptomycin remains controversial. Although the exact mechanism is not well understood, previous in vitro studies have indicated synergistic activity with daptomycin and gentamicin tested against daptomycin-susceptible strains (8, 29). It is hypothesized that combining two rapidly bactericidal antibiotics will produce optimal bacterial killing. Limited clinical data are available, but this effect, especially early in the course of therapy, may be advantageous in initial inoculum reduction in patients with high-bacterial-load infections, such as endocarditis. In comparison, clinical evaluations of vancomycin in combination with gentamicin to treat S. aureus infections have produced conflicting results (5, 10). In the daptomycin bacteremia and endocarditis trial, the comparator arm for MRSA infections was treated with a vancomycin dosage of 1 g every 12 h combined with gentamicin at 1 mg/kg every 8 h for the first 4 days. In our study, we utilized a higher once-daily dose of gentamicin given over the 96-h model, which clinical studies have indicated as an effective and safe alternative to traditional aminoglycoside dosing (21). Rifampin is a concentration-dependent antibiotic and has been utilized in combination with vancomycin to treat MRSA infections. This regimen in vitro has displayed enhanced activity compared to vancomycin alone; however, clinical data and experience remain controversial (8, 18, 24). We utilized a dose of rifampin of 300 mg every 8 h, which is within the range of recommended daily doses in the treatment of endocarditis (1). The potential for enhanced activity and synergy with daptomycin and rifampin exists from the limited data available from in vitro studies (8).
To our knowledge, this is the first study to evaluate combination therapy with standard and high-dose daptomycin in combination with gentamicin or rifampin against daptomycin-susceptible and nonsusceptible derived clinical isolates. Combination antimicrobial therapy has many theorized benefits. Combining antibiotics with different mechanisms and sites of action may reduce the potential for subsequent isolates with reduced susceptibility during therapy. Also, in sequestered infections such as endocarditis and osteomyelitis, two antibiotics penetrating to the site of action may enhance activity and reduce the chance for resistance development. We evaluated this effect previously with daptomycin and rifampin, where the emergence of an isolate with reduced daptomycin susceptibility in the SEV model occurred with a daptomycin dosage of 6 mg/kg/day alone and was suppressed in combination with rifampin (27). In our current study, one daptomycin-susceptible isolate displayed a threefold increase in MIC, up to 0.75 mg/liter, while exposed to simulated doses of daptomycin at 6 mg/kg daily. Utilizing the same daptomycin dose in combination with gentamicin or rifampin, the MIC for this isolate remained stable during the 96-h simulation. Similar results with combination therapy were noted for three daptomycin-nonsusceptible isolates that had higher MICs after the 6-mg/kg/day regimen. Our study demonstrated that although the activity of combination regimens with daptomycin may be strain dependent, it may prove advantageous in select patients in suppressing the emergence of nonsusceptible isolates.
The concentration-dependent activity of daptomycin has been well established (4, 13, 23). Since daptomycin has been studied in safety trials up to 12 mg/kg/day, we decided to evaluate simulated high-dose daptomycin therapy at 10 mg/kg daily. Previously Tsuji et al. evaluated doses up to 8 mg/kg/day, which displayed improved bactericidal activity compared to 6-mg/kg/day doses in an in vitro pharmacokinetic/pharmacodynamic model (29). Our current study evaluated doses up to 10 mg/kg daily against both daptomycin-susceptible and nonsusceptible S. aureus isolates. The high-dose daptomycin regimen demonstrated a more-rapid T99 kill for all susceptible isolates and two of four nonsusceptible strains. We were surprised to discover that the bactericidal activity with combination regimens and high-dose daptomycin was highly variable. We attribute this effect to the heterogeneously susceptible nature of these particular S. aureus isolates, rather than an overall antagonistic effect of these combinations. It should be noted that daptomycin-nonsusceptible isolates continue to be relatively rare in clinical practice, with a select number of case reports from patient with deep-seated infections (19, 27, 28). More in vitro studies are necessary in order to fully characterize the pharmacodynamic effects of high-dose daptomycin in combination with gentamicin and rifampin.
In conclusion, we have demonstrated overall that combination daptomycin and gentamicin therapy provides the advantage of enhanced in vitro activity and suppression of resistance. While the majority of isolates tested did not display greater activity with the addition of rifampin, this regimen suppressed the emergence of reduced susceptibility that was noted with daptomycin 6-mg/kg/day monotherapy. Also, high-dose daptomycin (>6 mg/kg/day) may be a reasonable option for patients with serious infections and should be further explored.
Acknowledgments
This work was supported by a grant from Cubist Pharmaceuticals, Inc.
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Baddour, L. M., W. R. Wilson, A. S. Bayer, V. G. Fowler, Jr., A. F. Bolger, M. E. Levison, P. Ferrieri, M. A. Gerber, L. Y. Tani, M. H. Gewitz, D. C. Tong, J. M. Steckelberg, R. S. Baltimore, S. T. Shulman, J. C. Burns, D. A. Falace, J. W. Newburger, T. J. Pallasch, M. Takahashi, and K. A. Taubert. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394-e434. [DOI] [PubMed] [Google Scholar]
- 2.Benvenuto, M., D. P. Benziger, S. Yankelev, and G. Vigliani. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser, J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125-130. [DOI] [PubMed] [Google Scholar]
- 4.Cha, R., R. G. Grucz, Jr., and M. J. Rybak. 2003. Daptomycin dose-effect relationship against resistant gram-positive organisms. Antimicrob. Agents Chemother. 47:1598-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers, H. F., R. T. Miller, and M. D. Newman. 1988. Right-sided Staphylococcus aureus endocarditis in intravenous drug abusers: two-week combination therapy. Ann. Intern. Med. 109:619-624. [DOI] [PubMed] [Google Scholar]
- 6.Charles, P. G., P. B. Ward, P. D. Johnson, B. P. Howden, and M. L. Grayson. 2004. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 38:448-451. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Credito, K., G. Lin, and P. C. Appelbaum. 2007. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob. Agents Chemother. 51:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finberg, R. W., R. C. Moellering, F. P. Tally, W. A. Craig, G. A. Pankey, E. P. Dellinger, M. A. West, M. Joshi, P. K. Linden, K. V. Rolston, J. C. Rotschafer, and M. J. Rybak. 2004. The importance of bactericidal drugs: future directions in infectious disease. Clin. Infect. Dis. 39:1314-1320. [DOI] [PubMed] [Google Scholar]
- 10.Fortun, J., E. Navas, J. Martinez-Beltran, J. Perez-Molina, P. Martin-Davila, A. Guerrero, and S. Moreno. 2001. Short-course therapy for right-side endocarditis due to Staphylococcus aureus in drug abusers: cloxacillin versus glycopeptides in combination with gentamicin. Clin. Infect. Dis. 33:120-125. [DOI] [PubMed] [Google Scholar]
- 11.Fowler, V. G., Jr., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, S. G. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fatkenheuer, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, and S. E. Cosgrove. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653-665. [DOI] [PubMed] [Google Scholar]
- 12.Friedman, L., J. D. Alder, and J. A. Silverman. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanberger, H., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 15.Howden, B. P., P. D. Johnson, P. B. Ward, T. P. Stinear, and J. K. Davies. 2006. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 50:3039-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howden, B. P., P. B. Ward, P. G. Charles, T. M. Korman, A. Fuller, P. du Cros, E. A. Grabsch, S. A. Roberts, J. Robson, K. Read, N. Bak, J. Hurley, P. D. Johnson, A. J. Morris, B. C. Mayall, and M. L. Grayson. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 38:521-528. [DOI] [PubMed] [Google Scholar]
- 17.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine, D. P., B. S. Fromm, and B. R. Reddy. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 115:674-680. [DOI] [PubMed] [Google Scholar]
- 19.Marty, F. M., W. W. Yeh, C. B. Wennersten, L. Venkataraman, E. Albano, E. P. Alyea, H. S. Gold, L. R. Baden, and S. K. Pillai. 2006. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J. Clin. Microbiol. 44:595-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moise, P. A., D. S. Smyth, N. El-Fawal, A. D. Robinson, P. N. Holden, A. Forrest, and G. Sakoulas. 2008. Microbiological effects of prior vancomycin use in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J. Antimicrob. Chemother. 61:85-90. [DOI] [PubMed] [Google Scholar]
- 21.Nicolau, D. P., C. D. Freeman, P. P. Belliveau, C. H. Nightingale, J. W. Ross, and R. Quintiliani. 1995. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob. Agents Chemother. 39:650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pankey, G. A., and L. D. Sabath. 2004. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 38:864-870. [DOI] [PubMed] [Google Scholar]
- 23.Rose, W. E., M. J. Rybak, and G. W. Kaatz. 2007. Evaluation of daptomycin treatment of Staphylococcus aureus bacterial endocarditis: an in vitro and in vivo simulation using historical and current dosing strategies. J. Antimicrob. Chemother. 60:334-340. [DOI] [PubMed] [Google Scholar]
- 24.Rose, W. E., M. J. Rybak, B. T. Tsuji, G. W. Kaatz, and G. Sakoulas. 2007. Correlation of vancomycin and daptomycin susceptibility in Staphylococcus aureus in reference to accessory gene regulator (agr) polymorphism and function. J. Antimicrob. Chemother. 59:1190-1193. [DOI] [PubMed] [Google Scholar]
- 25.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sader, H. S., J. M. Streit, T. R. Fritsche, and R. N. Jones. 2004. Antimicrobial activity of daptomycin against multidrug-resistant Gram-positive strains collected worldwide. Diagn. Microbiol. Infect. Dis. 50:201-204. [DOI] [PubMed] [Google Scholar]
- 27.Sakoulas, G., W. Rose, M. J. Rybak, S. Pillai, J. Alder, R. C. Moellering, Jr., and G. M. Eliopoulos. 2008. Evaluation of endocarditis caused by methicillin-susceptible Staphylococcus aureus developing nonsusceptibility to daptomycin. J. Clin. Microbiol. 46:220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheth, A., C. F. Carpenter, and B. Robinson-Dunn. 2006. Reduced vancomycin susceptibility and daptomycin nonsusceptibility associated with treatment failure in 2 cases of MRSA bacteremia, abstr. C2-1159. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 29.Tsuji, B. T., and M. J. Rybak. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 49:2735-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wootton, M., A. P. Macgowan, and T. R. Walsh. 2006. Comparative bactericidal activities of daptomycin and vancomycin against glycopeptide-intermediate Staphylococcus aureus (GISA) and heterogeneous GISA Isolates. Antimicrob. Agents Chemother. 50:4195-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]