Abstract
Aromatic diamidines are DNA minor groove-binding ligands that display excellent antimicrobial activity against fungi, bacteria, and protozoa. Due to the currently unsatisfactory chemotherapy for Chagas’ disease and in view of our previous reports regarding the effect of diamidines and analogues against both in vitro and in vivo Trypanosoma cruzi infection, this study evaluated the effects of a diarylthiophene diamidine (DB1362) against both amastigotes and bloodstream trypomastigotes of T. cruzi, the etiological agent of Chagas’ disease. The data show the potent in vitro activity of DB1362 against both parasite forms that are relevant for mammalian infection at doses which do not exhibit cytotoxicity. Ultrastructural analysis and flow cytometry studies show striking alterations in the nuclei and mitochondria of the bloodstream parasites. In vivo studies were performed at two different drug concentrations (25 and 50 mg/kg/day) using a 2-day or a 10-day regimen. The best results were obtained when acutely infected mice were treated with two doses at the lower concentration, resulting in 100% survival, compared to the infected and untreated mice. Although it did not display higher efficacy than benznidazole, DB1362 reduced both cardiac parasitism and inflammation, and in addition, it protected against the cardiac alterations (determined by measurements) common in T. cruzi infection. These results support further investigation of diamidines and related compounds as potential agents against Chagas’ disease.
Aromatic diamidines, such as pentamidine, have been studied since the 1930s, when significant activity against African trypanosomes was reported (25). Since then, several studies have demonstrated their excellent activity against different pathogens, such as bacteria, fungi and protozoa, which has been attributed, in part, to the association of the dicationic molecules to the DNA minor groove at AT-rich sites (28). Although recent data obtained with African trypanosomes show that neither the DNA affinity nor the distribution in the parasite can be directly related to diamidine (and analogue) activity (16, 17), the concentration of diamidines in parasite mitochondria (kinetoplast) appears to represent a pivotal step in their antitrypanosomal action, and thus the diamidine-kinetoplast DNA interaction seems to be an important part of their biological activity (13, 17). Chagas’ disease, caused by the protozoan Trypanosoma cruzi, is a neglected disease that affects 12 to 14 million people in areas of endemicity in Latin America, where approximately 100 million people are at risk (19). The disease is a major public health problem in the affected areas. The infection triggers an important cardiomyopathy, for which the pathophysiological mechanisms are not completely understood (20, 27). Current chemotherapy is based largely on nifurtimox and benznidazole (Bz), which are only partially effective and have considerable side effects (21, 29), and this clearly demonstrates the urgent need for new drugs.
Despite their well-known antimicrobial activity, diamidines often exhibit high toxicity, such as cardiotoxicity, nephrotoxicity, and pancreatic complications, and display poor oral bioavailability, which is likely due to their cationic character (30). To overcome these limitations, new aromatic dications and their prodrugs have been synthesized and screened both in vitro and in vivo against different pathogens (2, 26). Recent studies have shown good in vitro and in vivo activity for diamidines such as furamidine (DB75), its N-phenyl-substituted analogue (DB569), and reversed amidines (5, 7, 8, 23, 24) against T. cruzi. The trypanocidal effect of these dications led to the evaluation of in vitro and in vivo antiparasitic activity of the diarylthiophene diamidine DB1362 against T. cruzi.
MATERIALS AND METHODS
The synthesis of 3-bromo-4-methyl-2,5-bis(4-amidinophenyl)thiophene dihydrochloride (DB1362) is described in the supplemental material.
Drug solutions.
Stock solutions (5 mM) of DB1362 (Fig. 1) were prepared in dimethyl sulfoxide, with the final concentration of the latter in the experiments never exceeding 0.6%, which did not exhibit any toxicity for the parasite or mammalian host cells (data not shown).
FIG. 1.
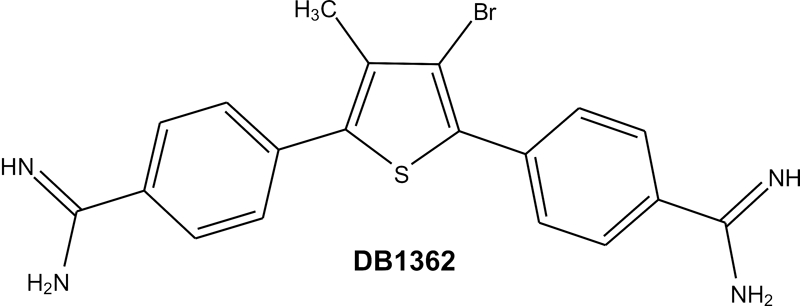
Chemical structure of DB1362.
Cell cultures.
For both drug toxicity and infection assays, primary cultures of peritoneal mouse macrophages were obtained as described previously (1), seeded at a density of 5 × 104 cells/well into 96-well culture plates or at 3 × 105 cells/well into 24-well culture plates, respectively, and sustained in Dulbecco's modified medium supplemented with 10% fetal bovine serum and 4 mM l-glutamine (DMES). All the cell cultures were maintained at 37°C in an atmosphere of 5% CO2 and air, and the assays were run three times at least in duplicate.
Parasites.
The Y strain of T. cruzi was used throughout the experiments. Cell culture-derived trypomastigotes were isolated from the supernatant of Vero lineage cells (from green monkey kidney) which had been previously infected with bloodstream trypomastigotes (5). Bloodstream forms were harvested by heart puncture from T. cruzi-infected Swiss mice at the parasitemia peak day (4).
Toxicity for mammalian cell cultures.
Uninfected peritoneal macrophages were incubated for 24 to 48 h at 37°C in the absence of DB1362 or the presence of increasing doses (10.6 to 96 μM) of DB1362, their viability was evaluated by light microscopy using the trypan blue exclusion assay (23), and the 50% lethal dose (drug concentration that reduces the number of viable cells 50%) was calculated.
Trypanocidal analysis.
Bloodstream trypomastigotes were incubated at 37°C for 24 h in the presence of increasing doses (0 to 32 μM) of DB1362 diluted in DMES (24). Alternatively, bloodstream parasites were incubated for 24 h at 4°C with DB1362 diluted in whole blood collected from T. cruzi-infected mice (23). After drug incubation, the death rates were determined by using light microscopy for direct quantification of the number of live parasites using a Neubauer chamber, and the 50% inhibitory concentration (IC50) (drug concentration that reduces the number of treated parasites 50%) was calculated (24). For the analysis of the effect on intracellular amastigotes, after initial host cell-parasite contact (24 h) with cell culture-derived trypomastigotes, the macrophages were washed to remove free parasites and treated at 37°C for 24 and 48 h with DB1362 (0.39 to 10.6 μM). Infected cultures not subjected to the drug treatment were used as controls. All cell cultures were maintained at 37°C in an atmosphere of 5% CO2 and air, and culture medium was replaced every 24 h. After the drug exposure, the untreated and treated infected cultures were fixed and stained with Giemsa solution and the mean numbers of infected host cells and of parasites per infected cell were then scored as reported previously (23). Only characteristic parasite nuclei and kinetoplasts were counted as surviving parasites, since irregular structures could indicate parasites undergoing death. The drug activity was estimated by calculating the endocytic index (percentage of infected cells times the average number of intracellular amastigotes per infected host cell) (23).
Flow cytometry analysis.
Bloodstream trypomastigotes (2 × 106 cells/ml) were briefly washed in phosphate-buffered saline (PBS) and treated for 24 h at 37°C with the respective IC50 (previously determined) of the compound diluted in dimethyl sulfoxide. After treatment, the parasite suspension was incubated for 15 min at 37°C with 10 μg/ml rhodamine 123 (Rh123) (24). Data acquisition and analysis were performed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) equipped with Cell Quest software (Joseph Trotter, Scripps Research Institute, San Diego, CA). A total of 10,000 events were acquired in the region established as that corresponding to bloodstream trypomastigotes, and the alterations in the Rh123 fluorescence were quantified by calculating the mean percentages of treated and untreated parasite populations that displayed depolarization of the mitochondrial membrane (designated M2). All assays were run three times at least in duplicate.
TEM analysis.
Bloodstream trypomastigotes and uninfected host cells treated for 24 h at 37°C with the corresponding IC50 of the drug or left untreated were fixed for 60 min at 4°C with 2.5% glutaraldehyde and 2.5 mM CaCl2 in 0.1 M cacodylate buffer, pH 7.2, and postfixed for 1 h at 4°C with 1% OsO4, 0.8% potassium ferricyanide, and 2.5 mM CaCl2 using the same buffer. Samples were routinely processed for transmission electron microscopy (TEM) and examined in a Zeiss EM10C electron microscope (Oberkochen, Germany) (5).
In vivo infection.
Male Swiss mice were obtained from the Fundação Oswaldo Cruz (FIOCRUZ) animal facilities (Rio de Janeiro, Brazil). Mice were housed at a maximum of eight per cage and kept in a conventional room at 20 to 24°C with a 12/12-h light/dark cycle. The animals were provided with sterilized water and chow ad libitum. Infection was performed by intraperitoneal (i.p.) injection of 104 bloodstream trypomastigotes. The animals (28 to 33 g) were divided into the following groups: uninfected (not infected and not treated); untreated (infected with T. cruzi but not treated); and treated (infected and treated with 25 and 50 mg of DB1362 per kg of body weight per day or with 100 mg/kg/day Bz). For DB1362 treatment, mice received a 0.1-ml i.p. injection at 5 and 8 days postinfection (dpi) or starting at 3 dpi for 10 consecutive days as described previously (7). For Bz treatment, infected mice received a 0.2-ml oral dose (gavage) according to the therapeutic schemes described above. At least eight mice from each group were used for analysis in each of two or three independent experiments that were performed.
Parasitemia, mortality rates, and ponderal curve analysis.
Parasitemia was individually checked by direct microscopic counting of parasites in 5 μl of blood, as described before (7). At 7, 14, and 21 dpi, body weight was evaluated, and mortality was checked daily until 60 dpi and expressed as a percentage of cumulative mortality (8).
ECG.
Electrocardiography (ECG) recording and analysis were performed in uninfected mice and in acutely T. cruzi-infected mice (14 dpi) receiving DB1362 and Bz therapy or not treated, as previously described (8). Briefly, mice were placed under stable sedation with diazepam (20 mg/kg, i.p.) and fixed in the supine position, and an eight-lead ECG was recorded from 18-gauge needle electrodes subcutaneously implanted in each limb and two electrodes at precordial position lead II. The electrocardiographic tracings were obtained with a standard lead (dipolar lead DII), recording with an amplitude set to give 2 mV/s. The ECG was recorded by using band-pass filtering (Bio Amp; AD Instruments, Hastings, United Kingdom) between 0.1 and 100 Hz. Supplementary amplification and analog-digital conversion was performed with a Powerlab 16S instrument (AD Instruments). Digital recordings (16 bit, 4 kHz/channel) were analyzed with the Scope (version 3.6.10) program (AD Instruments). The signal-averaged ECG was calculated by using the mouse signal-averaged ECG extension (version 1.2) program (AD Instruments) and a template-matching algorithm. ECG parameters were evaluated using the following standard criteria: (i) the heart rate (beats/minute) was monitored, and (ii) the variation at P wave and the PQ, QRS, and QT intervals were measured in milliseconds.
Histopathological analysis.
At 14 dpi, hearts were removed, cut longitudinally, rinsed in ice-cold PBS, and fixed in Millonig-Rosman solution (10% formaldehyde in PBS). The tissues were dehydrated and embedded in paraffin. Sections (3 μm) were stained with hematoxylin-eosin and were analyzed by light microscopy. The number of amastigote nests and of inflammatory infiltrates (more than 10 mononuclear cells) was determined in at least 30 fields (total magnification, ×40) for each slide. The mean number of amastigote nests or inflammatory infiltrates per field was obtained from at least three mice per group with three sections from each mouse.
Statistical analysis.
Statistical analysis was carried out using an analysis of variance program with the level of significance set at a P value of ≤0.05. The data are representative of two to four experiments run in duplicate.
All procedures were carried out in accordance with the guidelines established by the FIOCRUZ Committee of Ethics for the Use of Animals (CEUA 0099/01).
RESULTS
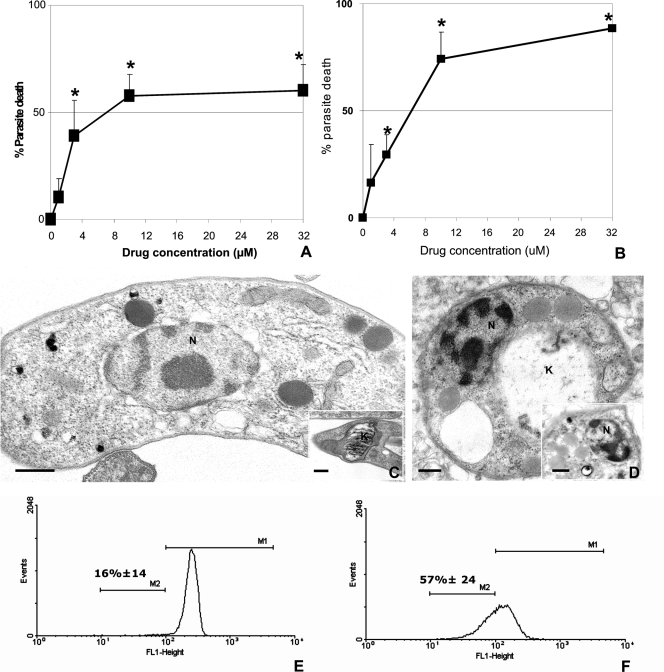
Treatment of bloodstream parasites with DB1362 for 24 h at 4°C in the presence of blood constituents resulted in an IC50 of 7.07 μM (Fig. 2A). Although treatment performed at 37°C showed an IC50 of 6.6 μM, about 90% of parasites died with the dose of 32 μM (Fig. 2B), while only 60% was with the same dose when mouse blood constituents were added (Fig. 2A). TEM studies showed that in untreated parasites typical organelles, such as nuclei and mitochondria, could be easily identified (Fig. 2C, inset). However, diamidine treatment induced profound alterations in the kinetoplast organization (Fig. 2D) and in the nucleus (Fig. 2D, inset). Due to the ultrastructural findings showing striking changes in the mitochondria, Rh123 was further used as a probe of the mitochondrial membrane potential (MMP) (4). Incubation of bloodstream trypomastigotes with the DB1362 caused an increase (P = 0.06) in the number of parasites that displayed interference in the proton electrochemical potential gradient of the mitochondrial membrane (Fig. 2F). Treatment reduced the MMP in 57% of the bloodstream forms exposed to DB1362 (Fig. 2F), in contrast to what occurred in the untreated group, which displayed a 14% reduction (Fig. 2E). The latter value corresponds to the percentage of bloodstream parasites that displayed apoptosis-like characteristics before drug incubation (4, 15).
FIG. 2.
Effect of DB1362 on bloodstream trypomastigotes of T. cruzi (Y strain) in vitro. Activity was evaluated during treatment at 4°C with the drug diluted in whole mouse blood (A) and at 37°C with the drug diluted in culture medium (B). The percentage of dead parasites was measured after 24 h of treatment. Asterisks indicate significant differences for DB1362-treated parasites in relation to the untreated samples (P ≤ 0.05). (C to F) Transmission electron micrographs (C and D) and flow cytometry analysis (E and F) of trypomastigotes exposed to DB1362 for 24 h. Untreated parasites display typical mitochondria (C, inset) and nuclei (C), while DB1362-treated parasites show swelling mitochondria and damaged kinetoplasts (D) and alterations in nuclear morphology (D, inset). Bars = 2 μm (C and D) and 10 μm (insets). (E and F) Histograms showing results of a representative assay, displaying fluorescence intensities of untreated (E) and diamidine-treated parasites (F) after incubation with Rh123. M1, high-fluorescence-intensity peaks; M2, low-fluorescence-intensity peaks, which represent decreased MMP. k, kinetoplast; n, nucleus.
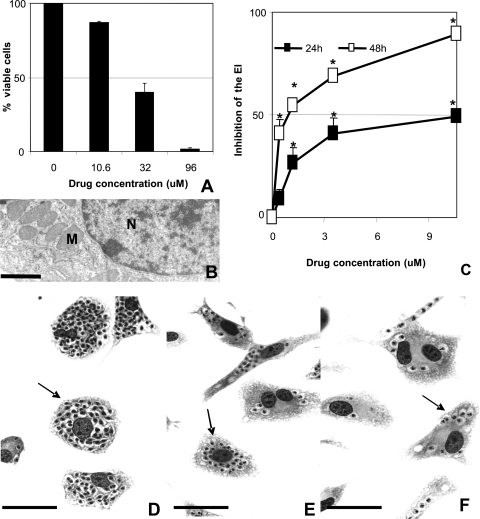
Treatment with DB1362 resulted in considerable loss of mammalian cell viability only when the cultures were incubated with 32 μM or higher doses, showing 50% lethal doses of 22.8 (Fig. 3A) and 20 (data not shown) μM after 24 and 48 h of incubation, respectively. TEM analysis performed in the DB1362-treated mammalian cells did not reveal ultrastructural damage in either nuclei or mitochondria of the host cells (Fig. 3B).
FIG. 3.
Cytotoxicity analysis of DB1362 in mammalian host cells (A and B) and diamidine activity against T. cruzi-infected peritoneal macrophages after 24 (C) and 48 (C to F) h of treatment. The effect of DB1362 on mammalian host cells was evaluated by both trypan blue exclusion assays (A) and TEM (B). Loss of cellular viability was noticed only when the cultures were incubated with 32 μM or higher doses (A). Ultrastructural analysis shows characteristic nuclei and mitochondria of the mammalian cell after exposure to the diamidine (B). N, nuclei; M, mitochondria. Bar = 10 μM. (C to F) DB1362 activity against intracellular amastigotes in T. cruzi-infected host cells. The activity of DB1362 after 24 and 48 h of drug incubation is shown by the inhibition of the endocytic index (EI) (C). Asterisks indicate significant differences between treated-T. cruzi-infected peritoneal macrophages and untreated cultures (P ≤ 0.05). (D to F) Light microscopy of untreated (D) and T. cruzi-infected (E and F) host cells exposed to 0.39 μM (E) and 1.18 μM (F) DB1362 for 48 h. Arrows indicate intracellular parasites. Bars = 1 μm.
Incubation of infected cultures with selected nontoxic doses (up to 10.6 μM) of DB1362 significantly reduced both the percentage of infected cells and the mean number of parasites per infected cells (Fig. 3C and E to F). The endocytic index (Fig. 3C) showed IC50s of 10.6 μM and 0.62 μM after 24 and 48 h of treatment, respectively.
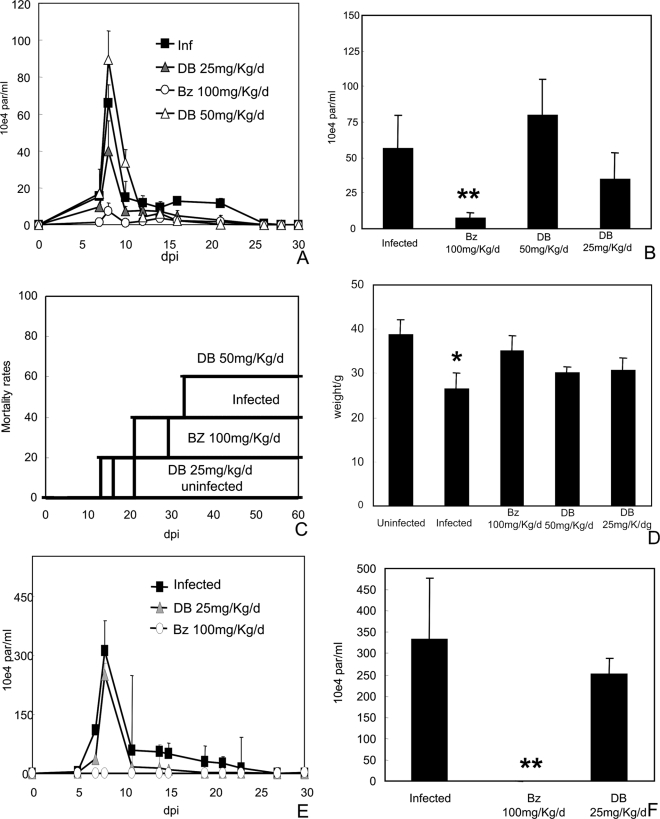
In vivo studies were performed using different drug concentrations and treatment schemes (doses up to 50 mg/kg/day, i.p. route) at doses which did not induce mortality in the control group (data not shown). The infected and untreated group presented high parasitemia levels, peaking at 8 dpi (Fig. 4A and B) and displayed 40 to 50% mortality at 60 dpi (Fig. 4C). As expected, T. cruzi infection caused loss of body weight (P = 0.014) compared to uninfected animals (Fig. 4D). When a 25-mg/kg/day dose of DB1362 was administered twice (at 5 dpi and 8 dpi), 100% survival was observed. However, only a 40% reduction (P = 0.1) in the bloodstream parasitemia levels compared to controls was noted. Furthermore, only a moderate improvement in the ponderal curve was observed (Fig. 4A to D). Administration of 50 mg/kg/day, following the protocol described above, resulted in a slight increase in both parasitemia peak levels (P = 0.15) and cumulative mortality compared to infected and untreated animals (Fig. 4A to D). The oral administration (gavage) of 100 mg/kg/day Bz (reference drug) at 5 and 8 dpi reduced both the parasitemia peak levels (P = 0.002) and mortality rates and led to a partial recovery of body weight compared to the uninfected group (Fig. 4A to D).
FIG. 4.
Parasitemia levels (A and E), parasitemia peak at 8 dpi (B and F), mortality rates (C), and mean weights at 21 dpi (D) of mice infected with the Y strain of T. cruzi and treated with DB1362 or Bz at 5 and 8 dpi (A to D) and 3 to 12 dpi (E and F). The single asterisk indicates significant differences between T. cruzi-infected mice and uninfected mice (P ≤ 0.05). Double asterisks indicate significant differences between T. cruzi-infected, treated mice and infected, untreated animals (P ≤ 0.05).
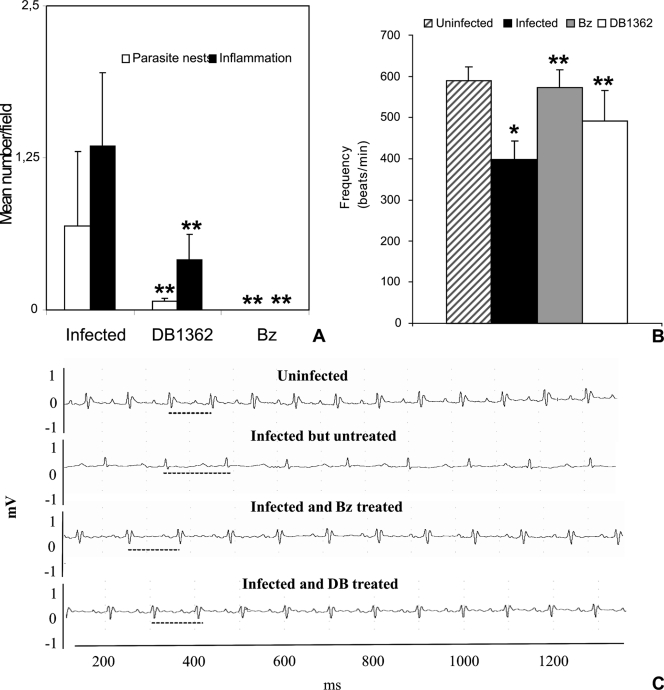
Administration of 25 mg/kg/day of DB1362 at 3 dpi for 10 consecutive days did not abolish circulating parasitemia peak levels (P = 0.2), as was observed for the Bz group also treated for 10 consecutive days (P = 0.0008) (Fig. 4E and F). However, DB1362 significantly decreased cardiac parasitism (P = 0.02), reducing by 90% the number of parasite nests at 14 dpi compared to untreated animals (Fig. 5A). Histopathological analysis of DB1362-treated animals also showed an important decrease (P = 0.0016) in cardiac inflammatory infiltration, reaching about 70% reduction, compared to nontreated mice (Fig. 5A). Cardiac parasitism and inflammation were completely eliminated in the Bz group after 10 days of treatment (Fig. 5A).
FIG. 5.
Parasitism and inflammatory infiltrates in the heart (A) and ECG findings (B and C) for mice infected with T. cruzi and treated with DB1362 or Bz or left untreated. (A) Parasitism and inflammatory infiltration were decreased by diamidine and Bz treatment. (B) Mean cardiac frequencies were determined by ECG analysis in uninfected, infected but untreated, Bz-treated, and DB1362-treated mice. The single asterisk indicates significant differences between T. cruzi-infected mice and uninfected mice (P ≤ 0.05). Double asterisks indicate significant differences between T. cruzi-infected, treated mice and infected, untreated animals (P ≤ 0.05). (C) Representative electrocardiographic tracing of uninfected, infected but untreated, Bz-treated, and DB1362-treated mice. Note the normal patterns in uninfected mice and the variations in the heart rate (traced lines) for infected but untreated animals, which were partially recovered in both drug-treated groups.
The analysis of ECG showed that sinus bradycardia, detected by low heart rates, was the prevailing disorder found in untreated mice compared to the uninfected group (Fig. 5B and C). At 14 dpi, infected mice displayed a 32% reduction in heart rate (P = 0.000005) compared to uninfected animals (Fig. 5B). DB1362 treatment partially reversed (P = 0.037) this ECG alteration, and diamidine-treated mice displayed rate reductions of only 16% compared to uninfected mice (Fig. 5B and C). Treatment with Bz also blocked the ECG alteration, giving values similar to those for the counterpart uninfected group (Fig. 5B and C).
DISCUSSION
In view of the long history of aromatic diamidines as antiparasitic agents along with the promising activity of diamidines and analogues against T. cruzi (5, 8, 23, 24), the present study evaluated both in vitro and in vivo effects of DB1362 against this protozoan parasite. The present results show that DB1362 displays a trypanocidal effect against both bloodstream trypomastigotes and amastigotes localized within host cells in vitro, corroborating previous data that showed good dose-dependent activity of dicationic molecules against T. cruzi at noncytotoxic doses (5). The effect of DB1362 against bloodstream forms in the presence of mouse blood was reduced, possibly due to the association of the diamidine with serum components, as demonstrated with other drugs (22).
Ultrastructural analysis showed that the parasite mitochondria (kinetoplasts) were significantly affected by diamidine treatment without similar alterations in the mammalian host cells. This type of damage has been reported with other diamidines (3, 5, 9, 10, 12) and reversed amidines (24). Significantly, the flow cytometry studies corroborated the TEM results showing that DB1362 targeted the mitochondrion-kinetoplast complex. The use of Rh123 suggested interference with the proton electrochemical potential gradient of the mitochondrial membrane of T. cruzi similar to that shown upon treatment of trypomastigotes with other diamidines and reversed amidines (6, 24). Additional biochemical, ultrastructural, and biophysical studies are needed to more fully understand the mechanism of action of such diamidines.
Due to the trypanocidal effect of DB1362 in vitro, we performed in vivo studies to evaluate the activity of this diamidine against T. cruzi infection in mice. Since preliminary data showed that the intravenous dosing route resulted in high mortality rates, we opted for the i.p. route, using drug concentrations (up to 50 mg/kg) that did not result in any animal mortality (data not shown). In these studies, we employed different protocols for the administration of DB1362: 2 or 10 consecutive injections before or at the onset of parasitemia, respectively. The best results were obtained when acutely infected mice were treated with two doses of 25 mg/kg. DB1362 resulted in animal protection against T. cruzi infection, with 100% survival in this group, compared to 40 to 50% mortality in the control group at 60 dpi. These trypanocidal data for aromatic diamidines against T. cruzi in vivo corroborate previous studies in the same mouse model, which demonstrated the protective role of the diamidine DB569 (7). Importantly, although a 10-dose regimen did not eliminate the circulating parasites, histological analysis of heart samples at 14 dpi, corresponding to the peak of both cardiac parasite load and inflammation in our experimental model (7), showed a striking statistically significant decline of both of these factors in the DB1362 group compared to untreated mice. Similar results were found with DB569, which did not entirely reduce all of the circulating bloodstream trypomastigotes; however, it did clear cardiac parasitism and protected against heart rate alterations characteristic of T. cruzi infection (8).
This study shows that while displaying in vivo activity superior to that of DB569 by resulting in 100% survival of mice, the diarylthiophene diamidine did not clear the circulating parasites as did Bz. The latter fact may be attributed to parasite burden in noncardiac tissues. Overall, the in vitro and in vivo data support further investigation of this class of compounds against T. cruzi and other trypanosomatids.
Supplementary Material
Acknowledgments
The present study was supported by grants from Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ and Pensa Rio/FAPERJ), Conselho Nacional Desenvolvimento Científico e Tecnológico (CNPq), DECIT/SCTIE/MS and MCT by CNPq, and PAPES/FIOCRUZ. Funding to D.W.B. by the Bill and Melinda Gates Foundation is gratefully acknowledged.
Footnotes
Published ahead of print on 14 July 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Araujo-Jorge, T. C., E. P. Sampaio, W. De Souza, and M. N. Meirelles. 1989. Trypanosoma cruzi: the effect of variations in experimental conditions on the levels of macrophage infection in vitro. Parasitol. Res. 75:257-263. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, M. P., D. W. Boykin, R. Brun, and R. R. Tidwell. 2007. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. Br. J. Pharmacol. 152:1155-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft, S. L., and R. P. Brazil. 1982. Effect of pentamidine isethionate on the ultrastructure and morphology of Leishmania mexicana amazonensis in vitro. Ann. Trop. Med. Parasitol. 76:37-43. [DOI] [PubMed] [Google Scholar]
- 4.de Meirelles, M. N., T. C. Araújo-Jorge, and W. de Souza. 1982. Interaction of Trypanosoma cruzi with macrophages in vitro: dissociation of the attachment and internalization phases by low temperature and cytochalasin B. Z. Parasitenkd. 68:7-14. [DOI] [PubMed] [Google Scholar]
- 5.De Souza, E. M., A. Lansiaux, C. Bailly, W. D. Wilson, Q. Hu, D. W. Boykin, M. M. Batista, T. C. Araujo-Jorge, and M. N. Soeiro. 2004. Phenyl substitution of furamidine markedly potentates its anti-parasitic activity against Trypanosoma cruzi and Leishmania amazonensis. Biochem. Pharmacol. 68:593-600. [DOI] [PubMed] [Google Scholar]
- 6.De Souza, E. M., R. Menna-Barreto, T. C. Araujo-Jorge, A. Kumar, Q. Hu, D. W. Boykin, and M. N. Soeiro. 2006. Antiparasitic activity of aromatic diamidines is related to apoptosis-like death in Trypanosoma cruzi. Parasitology 133:75-79. [DOI] [PubMed] [Google Scholar]
- 7.de Souza, E. M., G. M. Oliveira, D. W. Boykin, A. Kumar, Q. Hu, and M. N. Soeiro. 2006. Trypanocidal activity of the phenyl-substituted analogue of furamidine DB569 against Trypanosoma cruzi infection in vivo. J. Antimicrob. Chemother. 58:610-614. [DOI] [PubMed] [Google Scholar]
- 8.De Souza, E. M., G. Melo, M. N. Soeiro, and D. W. Boykin. 2007. Electrocardiographic findings in acutely and chronically Trypanosoma cruzi-infected mice treated by a phenyl-substituted analogue of furamidine DB569. Drug Target Insights 2:61-69. [PMC free article] [PubMed] [Google Scholar]
- 9.Fusai, T., Y. Boulard, R. Durand, M. Paul, C. Bories, D. Rivollet, A. Astier, R. Houin, and M. Deniau. 1997. Ultrastructural changes in parasites induced by nanoparticle-bound pentamidine in a Leishmania major/mouse model. Parasite 4:133-139. [DOI] [PubMed] [Google Scholar]
- 10.Hentzer, B., and T. Kobayasi. 1977. The ultrastructural changes of Leishmania tropica after treatment with pentamidine. Ann. Trop. Med. Parasitol. 71:157-166. [DOI] [PubMed] [Google Scholar]
- 11.Reference deleted.
- 12.Hu, L., R. K. Arafa, M. A. Ismail, T. Wenzler, R. Brun, M. Munde, W. D. Wilson, S. Nzimiro, S. Samyesudhas, K. A. Werbovetz, and D. W. Boykin. 2008. Azaterphenyl diamidines as antileishmanial agents. Bioorg. Med. Chem. Lett. 18:247-251. [DOI] [PubMed] [Google Scholar]
- 13.Ismail, M. A., R. K. Arafa, R. Brun, T. Wenzler, Y. Miao, W. D. Wilson, C. Generaux, A. Bridges, J. E. Hall, and D. W. Boykin. 2006. Synthesis, DNA affinity, and antiprotozoal activity of linear dications: terphenyl diamidines and analogues. J. Med. Chem. 49:5324-5332. [DOI] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Marin-Neto, J. A., E. Cunha-Neto, B. C. Maciel, and M. V. Simões. 2007. Pathogenesis of chronic Chagas heart disease. Circulation 115:1109-1123. [DOI] [PubMed] [Google Scholar]
- 16.Mathis, A. M., J. L. Holman, L. M. Sturk, M. A. Ismail, D. W. Boykin, R. R. Tidwell, and J. E. Hall. 2006. Accumulation and intracellular distribution of antitrypanosomal diamidine compounds DB75 and DB820 in African trypanosomes. Antimicrob. Agents Chemother. 50:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathis, A. M., A. S. Bridges, M. A. Ismail, A. Kumar, I. Francesconi, M. Anbazhagan, Q. Hu, F. A. Tanious, T. Wenzler, J. Saulter, W. D. Wilson, R. Brun, D. W. Boykin, R. R. Tidwell, and J. E. Hall. 2007. Diphenyl furans and Aza analogs: effects of structural modification on in vitro activity, DNA binding, and accumulation and distribution in trypanosomes. Antimicrob. Agents Chemother. 51:2801-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19.Pinto Dias, J. C. 2006. The treatment of Chagas disease (South American trypanosomiasis). Ann. Intern. Med. 144:772-774. [DOI] [PubMed] [Google Scholar]
- 20.Rocha, M. O., M. M. Teixeira, and A. L. Ribeiro. 2007. An update on the management of Chagas cardiomyopathy. Expert Rev. Anti-Infect. Ther. 5:727-743. [DOI] [PubMed] [Google Scholar]
- 21.Rodriques Coura, J., and S. L. de Castro. 2002. A critical review on Chagas’ disease chemotherapy. Mem. Inst. Oswaldo Cruz 97:3-24. [DOI] [PubMed] [Google Scholar]
- 22.Santa-Rita, R. M., H. S. Barbosa, and S. L. de Castro. 2006. Ultrastructural analysis of edelfosine-treated trypomastigotes and amastigotes of Trypanosoma cruzi. Parasitol. Res. 100:187-190. [DOI] [PubMed] [Google Scholar]
- 23.Silva, C. F., M. M. Batista, R. A. Mota, E. M. De Souza, C. E. Stephens, D. W. Boykin, and M. N. Soeiro. 2007. Activity of “reversed” diamidines against Trypanosoma cruzi in vitro. Biochem. Pharmacol. 73:1939-1946. [DOI] [PubMed] [Google Scholar]
- 24.Silva, C. F., M. B. Meuser, E. M. De Souza, M. N. Meirelles, C. E. Stephens, P. Som, D. W. Boykin, and M. N. Soeiro. 2007. Cellular effects of reversed amidines on Trypanosoma cruzi. Antimicrob. Agents Chemother. 51:3803-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soeiro, M. N., E. M. De Souza, C. E. Stephens, and D. W. Boykin. 2005. Aromatic diamidines as antiparasitic agents. Expert Opin. Investig. Drugs 14:957-972. [DOI] [PubMed] [Google Scholar]
- 26.Soeiro, M. N. C., E. M. De Souza, and D. W. Boykin. 2007. Anti-parasitic activity of aromatic diamidines and their patented literature. Expert Opin. Ther. Patents 17:1-13. [Google Scholar]
- 27.Teixeira, A. R. L., N. Nitz, M. C. Guimaro, C. Gomes, and C. A. Santos-Buch. 2006. Chagas disease. Postgrad. Med. J. 82:788-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tidwell, R. R., and D. W. Boykin. 2003. Dicationic DNA minor groove binders as antimicrobial agents, p. 414-460. In M. Demeunynck, C. Bailly, and W. D. Wilson (ed.), Small molecule DNA and RNA binders: from synthesis to nucleic acid complexes. Wiley-VCH, New York, NY.
- 29.Villa, L., S. Morote, O. Bernal, D. Bulla, and P. Albajar-Vinas. 2007. Access to diagnosis and treatment of Chagas disease/infection in endemic and non-endemic countries in the XXI century. Mem. Inst. Oswaldo Cruz 102:87-94. [DOI] [PubMed] [Google Scholar]
- 30.Werbovetz, K. 2006. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr. Opin. Investig. Drugs 7:147-157. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.