Abstract
Human immunodeficiency virus (HIV)-infected children and adolescents who are failing antiretrovirals may have a better virologic response when drug exposures are increased, using higher protease inhibitor doses or ritonavir boosting. We studied the pharmacokinetics and safety of high-dose lopinavir-ritonavir (LPV/r) in treatment-experienced patients, using an LPV/r dose of 400/100 mg/m2 orally every 12 h (p.o. q12h) (without nonnucleoside reverse transcriptase inhibitor [NNRTI]), or 480/120 mg/m2 p.o. q12h (with NNRTI). We calculated the LPV inhibitory quotient (IQ), and when the IQ was <15, saquinavir (SQV) 750 mg/m2 p.o. q12h was added to the regimen. We studied 26 HIV-infected patients. The median age was 15 years (range, 7 to 17), with 11.5 prior antiretroviral medications, 197 CD4 cells/ml, viral load of 75,577 copies/ml, and a 133-fold change in LPV resistance. By treatment week 2, 14 patients had a viral-load decrease of >0.75 log10, with a median maximal decrease in viral load of −1.57 log10 copies/ml at week 8. At week 2, 19 subjects showed a median LPV area under the concentration-time curve (AUC) of 157.2 (range, 62.8 to 305.5) μg·h/ml and median LPV trough concentration (Ctrough) of 10.8 (range, 4.1 to 25.3) μg/ml. In 16 subjects with SQV added, the SQV median AUC was 33.7 (range, 4.4 to 76.5) μg·h/ml and the median SQV Ctrough was 2.1 (range, 0.2 to 4.1) μg/ml. At week 24, 18 of 26 (69%) subjects remained in the study. Between weeks 24 and 48, one subject withdrew for nonadherence and nine withdrew for persistently high virus load. In antiretroviral-experienced children and adolescents with HIV, high doses of LPV/r with or without SQV offer safe options for salvage therapy, but the modest virologic response and the challenge of adherence to a regimen with a high pill burden may limit the usefulness of this approach.
Children and adolescents with human immunodeficiency virus (HIV) infection who fail at least one course of antiretroviral (ARV) therapy may have drug-resistant virus that constrains later treatment options. While for some drugs, higher doses are not expected to overcome viral resistance (e.g., lamivudine), for other drugs (especially protease inhibitors), the decrease in resistance is more relative than absolute, and a good therapeutic outcome may be possible in patients when higher protease inhibitor drug exposures are achieved either by ritonavir (RTV) boosting or by increasing the administered dose. The potential for added antiviral effect needs to be balanced against the potential for an increase in drug toxicity with higher drug exposures.
Combination lopinavir-ritonavir (LPV/r; Kaletra) therapy is approved for treatment of adolescents and adults with HIV infection at a dose of 400/100 mg every 12 h when used without a nonnucleoside reverse transcriptase inhibitor (NNRTI), with recommendations to consider increasing the dose to 600/150 mg every 12 h when administered with an NNRTI or amprenavir to treatment-experienced patients (Kaletra product label). The FDA-recommended dose in children from 6 months to 12 years is 230/57.5 mg/m2 every 12 h or 300/75 mg/m2 every 12 h for patients receiving concurrent NNRTI or amprenavir therapy.
For patients previously treated with ARVs, who may have HIV isolates with reduced susceptibility to LPV, successful LPV/r therapy has been associated less strongly with plasma drug concentrations alone and more strongly with measures that incorporate plasma drug concentrations (usually trough concentration [Ctrough]) and measures of drug resistance (50% inhibitory concentration [IC50] for viral replication in vitro, 50% effective concentration [EC50] for inhibition of viral replication in plasma, amount of change in level of resistance to wild-type HIV, or summary scores of genotype resistance mutations) (15, 24). The ratio of the Ctrough to the IC50 is the inhibitory quotient (IQ) (9), and variations of this ratio that have been applied to the interpretation of LPV kinetics include the genotype IQ (4), the protein-binding corrected IQ (15), and others (2, 11, 27). The “target IQ” that predicts therapeutic response depends on the drug in question and the method used to calculate the IQ. In previously treated adult and pediatric patients, who are expected to have HIV isolates that are more resistant to ARVs, higher ARV doses might be more effective than standard doses if they result in higher plasma Ctroughs and, consequently, higher IQ values.
LPV has been administered to adults at doses as high as 667 mg every 12 h (q12h) (16, 29), and once-a-day doses as high as 800 mg have been used without undue toxicity (10). In children and adolescents, doses as high as 467 mg/m2 have been administered twice daily (13) and doses of 460 mg/m2 once daily (35, 40) have been administered without undue toxicity.
Children given adult doses of saquinavir (SQV) normalized to body weight have lower-than-expected plasma concentrations because of higher clearance (CL) referenced to body size, and SQV administered without pharmacologic boosting results in inadequate efficacy in children (12). When SQV at a dose of 50 mg/kg of body weight/dose twice daily (approximately equivalent to 750 mg/m2/dose) was administered with LPV/r, CL of SQV was slowed, there was excellent efficacy, and the plasma concentrations in children (1) more closely approximated those found in adults (25). In previously treated patients with HIV, the combination of SQV and LPV/r showed potential benefit as salvage therapy (23, 34, 37), while combinations of LPV/r with other protease inhibitors have shown enhanced toxicity (indinavir) (8) or drug interactions that may lead to lower potency (5, 39).
This study was undertaken to measure the safety, efficacy, and pharmacokinetics (PK) of doses of LPV/r higher than those previously approved by the FDA in protease inhibitor-experienced children and adolescents. The study was restricted to those subjects with evidence of highly LPV-resistant HIV isolates(20, 26) to ensure that all children exposed to the study treatment were at risk of ARV failure if lower doses were used. In addition, to optimize the therapeutic outcome in these heavily pretreated patients, SQV was added to the regimen at study week 4 for those patients with a low LPV IQ (<15) (15).
MATERIALS AND METHODS
Subjects and design.
Protocol P1038 was a multicenter phase I/II open-label trial of high-dose LPV/r with or without SQV to assess the safety and tolerability of the regimen in HIV-infected children and adolescent subjects previously treated with protease inhibitors. The study subjects were HIV-infected children and adolescents ages 2 to 18 years who had been treated for at least 6 months with a protease inhibitor and were failing their current ARV regimen, with HIV RNA at >5,000 copies/ml. Phenotypic-resistance testing (Monogram Biosciences, South San Francisco, CA) was performed while patients were maintained on unchanged ARVs. Only patients with phenotypic resistance to LPV at least five times that of wild-type HIV were enrolled. All subjects were treated with at least three ARVs, one of which was LPV/r. Protease inhibitors other than LPV/r were not allowed in the first phase of the study (step 1; see below). The site investigator in consultation with the protocol chair (Peter Havens) chose the background NRTIs to maximize the number of active ARVs in the regimen based on prior history and the results of phenotypic-resistance testing. Because concurrent treatment with LPV/r and NNRTIs leads to increased LPV CL in children and adolescents (3, 6), LPV/r doses were increased in patients treated with NNRTIs (see below).
The Division of AIDS (DAIDS) toxicity tables (1994 version) were used to grade adverse events and laboratory abnormalities. The planned treatment duration was 48 weeks. Study medications were stopped if a subject was noncompliant with medications, developed clinical or laboratory toxicity of ≥grade 3, failed to achieve and maintain a plasma viral-load reduction larger than a 0.75-log10 drop from baseline by week 24 and thereafter, or failed to achieve and maintain a CD4 count increase of ≥5% from baseline to week 24 and thereafter.
This study was approved by each site's Institutional Review Board, and written consent was obtained from each subject's parent or guardian before any study procedure was performed, with assent of the subject as appropriate for age and maturity. Department of Health and Human Service guidelines governing experimentation with human subjects were followed.
Drug administration.
Subjects not receiving NNRTI as part of their step 1 ARV regimen were designated group 1 and were administered LPV/r 400/100 mg/m2 q12h. Those receiving an NNRTI-containing regimen in step 1 were designated group 2, and a dose of LPV/r 480/120 mg/m2 q12h was used (Table 1). LPV/r was administered in either the gel capsule or liquid formulation. LPV/r tablets were not used for this study, since the study began prior to the availability of that dosing formulation. After 2 weeks of the initial step 1 regimen, a 12-h LPV- and RTV-intensive PK study was performed after an observed dose, without a standardized meal. The protein-binding-corrected LPV IQ (15) was calculated for each patient (see below). Subjects with an IQ of <15 proceeded to step 2, and at study week 4, SQV 750 mg/m2 q12h was added to their step 1 ARV regimen (1, 12). SQV was administered as 200-mg hard gel capsules or 500-mg tablets when they became available. For subjects unable to swallow either the capsule or tablet dosage form, SQV capsules were opened and added to food, milk, or liquid enteral feeding, a common practice in some of the participating centers. Subjects with an IQ of ≥15 or those who were unable to swallow SQV capsules remained on step 1 and did not have SQV added to their regimen. For subjects in step 2, after 2 weeks of combination SQV and LPV/r therapy (study week 6) another 12-h intensive PK study was performed to determine the SQV, LPV, and RTV plasma concentrations. If the SQV plasma concentrations 12 h after an administered dose were between 0.50 and 3.00 μg/ml, the patient continued on step 2. If the SQV plasma concentrations 12 h after an administered dose were <0.5 μg/ml, the SQV doses were increased to 1,200 mg/m2 q12h. If the SQV plasma concentrations 12 h after an administered dose were greater than 3.00 μg/ml with an SQV AUC of 100 μg·h/ml or higher, SQV was reduced to 500 mg/m2 q12h (18). If an SQV dose adjustment was made, the subject was moved to study step 3.
TABLE 1.
Study schema and disposition of subjects
| Study phase | Study wk | Treatment | No. of subjects | Subject disposition |
|---|---|---|---|---|
| Step 1, LPV/r plus >2 NRTIs | Baseline | Group 1 (n = 21), not on NNRTI, LPV/r 400/100 mg/m2 p.o. q12h plus >2 NRTIs, and group 2 (n = 5), on NNRTI, LPV/r 480/100 mg/m2 p.o. q12h plus >2 NRTIs | 26 | 6 discontinued study treatment in step 1 without obtaining LPV/r kinetics, 1 due to Hurricane Katrina (wk 4), 1 due to LPV/r hypersensitivity (wk 4), 1 due to abacavir hypersensitivity (wk 6), 1 due to opportunistic infection requiring treatment (wk 6), and 2 due to repeated nonadherence (wk 6 and wk 16) |
| Initial LPV/r PK | 2 | 19 | 18 performed at wk 2; 1 performed at wk 8, required intensive adherence support | |
| Calculate IQ | 2 to 4 | If IQ is <15, add SQV | 20 | 1 had no wk 2 LPV PK but LPV trough from wk 8 was used to calculate IQ |
| Step 2, add SQV | 4 | SQV 750 mg/m2 p.o. q12h added | 18 | 1 had IQ of >15 so no need for SQV and 1 could not swallow pills |
| SQV and LPV/r PK | 6 | 16 | 1 stopped SQV after 1 wk due to difficulty swallowing pills and 1 had kinetics that were not evaluable | |
| Step 2, reduce SQV dose based on kinetics | 8 | If SQV C12h is >3,000 ng/ml and SQV AUC is >100,000 ng · h/ml, then decrease SQV to 500 mg/m2 p.o. q12h | 3 | 3 had dose reduced |
| Step 3, increase SQV dose based on kinetics | 8 | If SQV C12h is <500 ng/ml in the absence of SQV-related toxicity, increase SQV to 1,200 mg/m2 p.o. q12h | 1 | 1 had dose increased |
| Follow to wk 24 (first study endpoint) | 24 | 18 | 1 discontinued study treatment for nonadherence (wk 6) and 1 discontinued study treatment due to high triglycerides (wk 13) | |
| Follow to study end | 48 | Continue after wk 24 if viral load decline is >0.75 log10 from baseline | 8 | 9 reached virologic endpoint and discontinued study treatment between wk 24 and 48 (at wk 26, 28, 32 [2], 33, 34, 35, 36, and 42), 1 had repeated nonadherence (wk 39), and 8 remained on study at wk 48 |
PK sample acquisition.
Intensive PK studies were scheduled at week 2 for all subjects (LPV and RTV), again at week 6 for step 2 subjects (LPV, RTV, and SQV), and two weeks after any dose adjustment for step 3 subjects. On the morning of an intensive PK study, a predose plasma sample was obtained prior to the morning dose and then the dose was administered under observation; subsequent plasma samples were obtained 1, 2, 3, 6, and 12 h postdose. All samples were collected in spray dry powdered EDTA tubes and analyzed in real time, including IQ calculation, and the results reported back to the sites in time for SQV to be added by study week 4 for those with IQ <15. In addition to these intensive PK visits, predose samples for LPV and RTV (and SQV for those on step 2) plasma concentrations were obtained at week 4, every 4 weeks until week 24, and then every 8 weeks until the end of the study.
Analytical methods.
Samples were analyzed by using a validated multianalyte reverse-phase high-performance liquid chromatography (HPLC)-UV assay. This method has been validated for the measurement of LPV, RTV, SQV, efavirenz, amprenavir, nelfinavir, and indinavir. An internal standard (A-86093, provided by Abbott Laboratories) was added to 125 μl of plasma sample and was extracted with 1 ml of tert-butylmethylether under basic conditions (plus 125 μl of 0.05 N NaOH). The organic layer was removed, transferred to a fresh tube, evaporated to dryness, and reconstituted with 100 ml of the mobile phase of 54% by volume 20 mM sodium acetate at pH 4.88 and 46% acetonitrile. The samples were transferred to HPLC autosampler vials, and 50 μl from each vial was acquired by the autosampler and injected into the HPLC. Separation was accomplished by using a YMC 100-mm by 4.6-mm C8 column with a 3-μm particle size, and sample absorbance was monitored at a wavelength of 212 nm. The range of the assay was 0.050 to 20 μg/ml for RTV and SQV and 0.100 to 40 μg/ml for LPV. The assay had percent coefficient of variation and percent E values of <15 over this range except at the limit of quantitation, where 20 was acceptable. The laboratory successfully completed Pediatric AIDS Clinical Trials Group (PACTG)/ACTG pharmacology proficiency testing every 6 months for this assay.
All plasma HIV type 1 (HIV-1) levels were determined by using a Roche ultrasensitive Amplicor HIV-1 Monitor test, version 1.5 (Roche Diagnostic Systems, Branchberg, NJ) in laboratories certified by the Division of AIDS Virology Quality Assurance program. CD4 cell counts were determined in Clinical Laboratory Improvement Act (CLIA)-certified laboratories.
IQ calculations.
To calculate the IQ, the IC50 was adjusted for plasma protein binding following the method of Hsu et al. (15). The protein-binding-corrected IC50 for wild-type HIV is assumed to be the same for all individuals, 0.07 μg/ml. Resistance is reported as the change in the level of resistance compared to the wild-type IC50, and therefore the LPV IQ is calculated as LPV C12h/[(n-fold change in resistance) × 0.07], where C12h is the concentration at 12 h. For a subject with an LPV C12h of 15.53 (the 75th percentile in adults treated with high-dose LPV/r [15]), a change in resistance of 15-fold or higher would result in an IQ of <15, and this IQ is associated with decreased treatment benefit (15). Step 2, with the addition of SQV, was designed to offer patients with a large change in their level of resistance (and low IQ even with high LPV exposure) an effective approach to controlling plasma viremia.
PK analysis.
Noncompartmental PK parameters were calculated, including the AUC, maximum concentration (Cmax), time to Cmax (Tmax), predose concentration (Cpre), C12h, CL/F (where F is bioavailability), and apparent half-life. The AUC and CL values were calculated for RTV, SQV, and LPV. WinNonlin Pro version 4.1 (Pharsight, MountainView, CA) was used to perform the PK analysis. For the purposes of IQ calculation, Ctrough was the average of Cpre and C12h. If Cpre was <1 μg/ml and C12h was >4 μg/ml, the patient was deemed nonadherent and the intensive PK study repeated.
Statistical analysis.
The Wilcoxon rank-sum test was used to measure the statistical significance of differences between group 1 (not on NNRTI) and group 2 (on NNRTI) subjects with respect to plasma viral RNA levels, CD4/CD8 counts, and PK parameters. For each subject, the median difference from baseline to later time points was calculated for CD4 count, viral load, and toxicity variables, and the Wilcoxon signed-rank test was used to measure the statistical significance of those differences.
Clinical trial accession number.
The clinical trials registration number is NCT00084058 (ClinicalTrials.gov).
RESULTS
Patients.
Twenty-six patients enrolled but six withdrew from the study (five for nonadherence) without obtaining LPV/r PK evaluation, and one subject who remained in the study never underwent the 12-h LPV/r PK (Table 1).
The median age of the study subjects was 15 years (range, 7 to 17); 50% were male, 54% were black (non-Hispanic), and 27% were Hispanic (regardless of race) (Table 2). The study subjects had all been treated with multiple ARVs (Table 2). The majority (20/26) were previously treated with LPV/r, and 10 of 26 were previously treated with SQV. Most had resistance to LPV, with a median change in resistance to LPV of 133-fold (range, 5.2 to 250) (Table 2). There were no statistically significant differences in demographic characteristics between the groups of subjects who entered step 1 (n = 26), those who had evaluable LPV/r PK (n = 19), and those who had evaluable SQV PK (n = 16) (Table 1).
TABLE 2.
Initial study population
| Parameter | Step 1, group 1 | Step 1, group 2 | Total |
|---|---|---|---|
| Number of patients | 21 | 5 | 26 |
| Male (%) | 10 (48) | 3 (60) | 13 (50) |
| Female (%) | 11 (52) | 2 (40) | 13 (50) |
| Mean age ± SD (yr) | 14.0 ± 2.7 | 14.0 ± 4.0 | 14.0 ± 2.9 |
| Median age (yr [range]) | 15 (7-17) | 15 (7-17) | 15 (7-17) |
| Median height (cm [range]) | 150.6 (116.0-170.8) | 151.8 (124.7-200.0) | 151.2 (116.0-200.0) |
| Median weight (kg [range]) | 41.2 (23.1-67.9) | 48.6 (25-69.8) | 43.6 (23.1-69.8) |
| Median BSA (m2 [range]) | 1.4 (0.9-1.8) | 1.5 (1.0-2.0) | 1.4 (0.8-2) |
| Median viral load (RNA copies/ml [range]) | 75,577 (3,345-896,373) | 77,992 (21,204-192,216) | 76,785 (3,345-896,373) |
| Median CD4 count (range) | 205 (12-1,416) | 286 (31-508) | 262 (12-1,416) |
| Median CD4 % (range) | 13 (1-24) | 17 (4-20) | 15 (1-24) |
| Median LPV change in resistance [fold (range)] | 152 (5.4-250) | 33 (5.2-238) | 133 (5.2-250) |
| Median no. of prior antiviral drugs (range) | 13 (8-17) | 9 (6-9) | 11.5 (6-17) |
| No. of patients previously exposed to LPV | 18 | 2 | 20 |
| No. of patients previously exposed to SQV | 8 | 2 | 10 |
PK results.
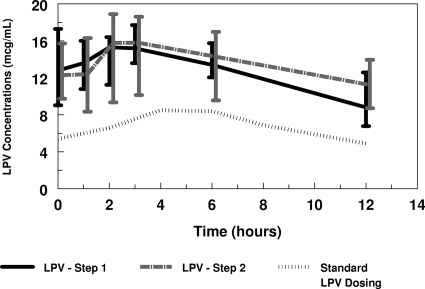
Table 3 summarizes the noncompartmental LPV parameters for this study. While the nominal LPV/r study dose was 400/100 mg/m2 for group 1 or 480/120 mg/m2 for group 2, due to constraints imposed by formulations the actual administered LPV doses were median (range) 392.8 (365.2 to 433.5) mg/m2, equivalent to 12.0 (10.0 to 14.8) mg/kg, for step 1, group 1, and 471.6 (469.4 to 487.7) mg/m2, equivalent to 15.1 (13.0 to 17.4) mg/kg, for step 1, group 2. Actual administered RTV doses were median (range) 98.2 (91.3 to 108.4) mg/m2, equivalent to 3.0 (2.5 to 3.7) mg/kg, for step 1, group 1, and 117.9 (117.3 to 121.9) mg/m2, equivalent to 3.8 (3.3 to 4.4 mg/kg), for step 1 group 2. Figure 1 shows the median LPV plasma concentrations over the 12 hours of the intensive PK studies, which were comparable for steps 1 and 2. The increase in administered LPV dose in group 2 resulted in comparable LPV exposure between groups 1 and 2 (Table 3). There was no significant difference in LPV kinetic parameters in the presence or absence of SQV (Table 3). The plasma predose LPV concentrations were higher than the concentrations measured at 12 h. Repeated plasma predose concentrations measured every 4 weeks from week 4 to week 24 and then every 8 weeks to week 48 suggested that the LPV plasma concentrations measured at weeks 2 and 4 are reasonable estimates of steady-state concentrations.
TABLE 3.
LPV PK parametersa
| LPV PK parameter (unit of measure) | Result [median (range)] for step:
|
|||||
|---|---|---|---|---|---|---|
| 1 in group:
|
2 in group:
|
|||||
| 1 (n = 16) | 2 (n = 3) | Overall (n = 19) | 1 (n = 13) | 2 (n = 3) | Overall (n = 16) | |
| AUC (μg · h/ml) | 155.4 (62.8-305.5) | 162.2 (63.8-185.7) | 157.2 (62.8-305.5) | 144.72 (36.4-388.5) | 206.8 (33.8-234) | 151.81 (33.8-388.5) |
| Cmax (μg/ml) | 16.8 (6.7-29.8) | 15.8 (8.28-17.2) | 17.2 (6.7-29.8) | 15.7 (5.86-39.0) | 19.6 (5.44-22.4) | 16.05 (5.44-39.0) |
| CL (liter/h/m2) | 2.53 (1.29-6.32) | 3.01 (2.53-7.39) | 2.58 (1.29-7.39) | 2.76 (1.23-10.5) | 2.11 (2.07-14.3) | 2.61 (1.23-14.3) |
| C0h (μg/ml) | 12.95 (4.56-28.0) | 8.98 (8.28-17.2) | 12.8 (4.56-28.0) | 11.4 (5.9-39.0) | 13.6 (2.43-16.6) | 12.1 (2.54-31.0) |
| C12h (μg/ml) | 8.40 (2.54-22.6) | 12.4 (2.88-12.6) | 8.80 (2.54-22.6) | 9.59 (0.32-20.4) | 13.8 (0.14-19.8) | 10.0 (0.14-20.8) |
| C0h and C12h avg (μg/ml) | 10.5 (4.1-25.3) | 10.8 (5.58-14.8) | 10.8 (4.1-25.3) | 11.6 (3.09-29.9) | 13.7 (1.28-18.2) | 11.9 (1.28-29.9) |
| Tmax (h) | 3.0 (predose to 6) | Predose (predose to 3) | 3.0 (predose to 6) | 2.05 (0-12) | 4.5 (2.0-6.08) | 2.15 (0-12) |
Step 1 in group 1 is LPV/r without NNRTI and in group 2 is with NNRTI; step 2 is addition of SQV with group 1 having no additional NNRTI and group 2 having additional NNRTI.
FIG. 1.
Lopinavir concentrations over 12 h. Values shown are medians ± interquartile ranges.
LPV exposure showed high intersubject variability (Table 3) that was not correlated with age, body surface area (BSA), or gender. RTV was coadministered with LPV, resulting in a median AUC of 9.72 for group 1 and 11.73 for group 2. The RTV AUC correlated with the LPV AUC in step 1 (r = 0.75) and step 2 (r = 0.66).
Calculation of the IQ was based on the week 2 12-h LPV PK analysis in 19 subjects and on the mean of the LPV Ctrough from weeks 8 and 11 in 1 subject. The median IQ was 1.3, with a range of 0.2 to 29.8; the mean IQ ± standard deviation was 3.63 ± 7.17.
Twenty patients had evaluable LPV IQs; one had an IQ of >15, leaving 19 eligible to move to step 2 and add SQV to their regimen. Of those, two were unable to swallow pills, so SQV could not be added. One of the 17 subjects taking SQV on step 2 had nonevaluable SQV kinetics due to sampling error. For the remaining 16 step 2 subjects (Table 1), 13 were in group 1 (no concurrent NNRTI) and 3 were in group 2. Two had their step 2 PK evaluations at week 16 because of adherence issues. The median (range) administered SQV dose for group 1 subjects in step 2 was 751.4 (683.8 to 892.9) mg/m2 [equivalent to 21.5 (18.6 to 31.3) mg/kg]. The median SQV dose for step 2, group 2, subjects was 740.7 (645.2 to 769.2) mg/m2 or 22.7 (21.1 to 24.3) mg/kg. The SQV PK parameters did not differ between groups 1 and 2 (Table 4). The SQV AUC correlated with the LPV AUC (r = 0.51). Based on predetermined criteria (see Materials and Methods), the SQV dose was reduced in three subjects and increased in one.
TABLE 4.
SQV PK parameters after SQV addition (step 2)
| Parameter (unit of measure) | Result [median (range)] for:
|
||
|---|---|---|---|
| Group 1 (n = 13) | Group 2 (n = 3) | Overall (n = 16) | |
| AUC (μg ·h/ml) | 33.9 (4.4-76.5) | 32.1 (4.4-33.3) | 33.7 (4.4-76.5) |
| Cmax (μg/ml) | 3.5 (0.8-8.3) | 3.6 (0.6-4.0) | 3.6 (0.6-8.3) |
| C0h (μg/ml) | 1.83 (0.11-4.66) | 2.05 (0.41-2.42) | 1.88 (0.11-4.66) |
| C12h (μg/ml) | 2.18 (0.05-4.86) | 1.45 (0.05-2.58) | 1.82 (0.05-4.86) |
| C0h and C12h avg (μg/ml) | 2.2 (0.4-4.1) | 1.9 (0.2-2.3) | 2.1 (0.2-4.1) |
| CL (liter/h/m2) | 22.8 (8.9-184.5) | 23.9 (22.2-147.6) | 23.1 (8.9-184.5) |
| Tmax | 3.0 (0-6.0) | 6.0 (2.0-6.0) | 3.0 (0-6.0) |
Treatment outcome.
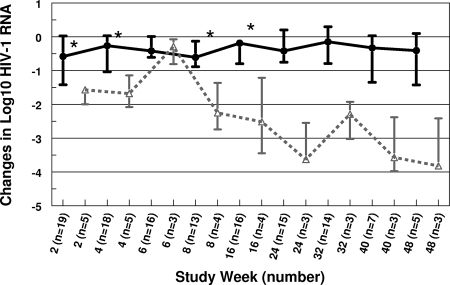
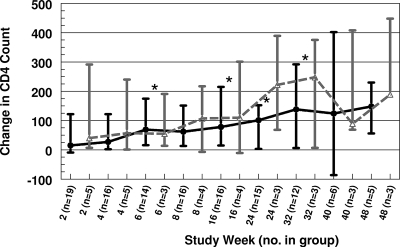
For all study subjects with evaluable viral loads, the median viral RNA decreased from 76,785 copies/ml at baseline (n = 26) to 28,395 copies/ml at week 24 (n = 18) and to 8,492 copies/ml at week 48 (n = 8). The median CD4 count increased from 262 cells/ml at baseline (n = 26) to 341 cells/ml at week 24 (n = 18) and to 572 cells/ml at week 48 (n = 8). For group 1 subjects, the viral load was statistically significantly lower than baseline at weeks 2, 4, 8, and 16 (Fig. 2) and the CD4 count was significantly higher than baseline at weeks 6, 8, 16, and 32 (Fig. 3). A reduction in viral load of more than 0.75 log10 occurred in 14 subjects by week 2 and 7 subjects by week 24, and 5 subjects maintained that reduction for the duration of their participation in the study (median 40 weeks). Five subjects had a viral load of <200 copies/ml at least once during the study, and three maintained that response to week 48. There was a modestly greater decrease in viral load with increase in IQ that did not reach statistical significance (Table 5).
FIG. 2.
Change in log10 HIV-1 RNA over time. Values are medians ± interquartile ranges. Black closed circles, group 1; gray open triangles, group 2; *, significantly different from baseline.
FIG. 3.
Change in CD4 count over time. Values are medians ± interquartile ranges. Black closed circles, group 1; gray open triangles, group 2; *, significantly different from baseline.
TABLE 5.
Relationship of IQ to change in virus load at week 2a
| Result for IQ of: | Median IQ (range) | Change in log10 virus load from baseline to wk 2
|
|
|---|---|---|---|
| Mean | Median | ||
| <1.0 (n = 7) | 0.76 (0.24-0.89) | −0.6 ± 0.8 | −0.4 (−1.6 to 0.4) |
| ≥1.0 (n = 13) | 1.72 (1.04-29.81) | −1.2 ± 0.8 | −1.4 (−2 to 0.6) |
The overall median IQ was 1.3 (range, 0.2-29.8), and the mean IQ was 3.63 ± 7.17. P = 0.24 for the comparison of IQ to virus load change. Based on a median trough of 10.8, a resistance of ≤10-fold achieves an IQ of ≥15. When IQ was calculated using C12h instead of the mean of C0h and C12h, there was no difference in the relationship of IQ and outcome.
Toxicity.
These doses were well tolerated, with no withdrawals for GI side effects and no GI toxicity greater than grade 2 except in subjects with diarrhea prior to starting the study drugs. There was an initial increase in cholesterol (median, 22 mg/dl at week 2) which remained statistically significant through weeks 8, 12, and 16 but no significant increase after week 16, and there was no other statistically significant change in cholesterol, triglycerides, or alanine aminotransferase. One subject, with a history of high triglycerides prior to study entry (triglyceride concentration initially 950 mg/dl and 259 mg/dl repeated before study entry) stopped the study medication because of high triglycerides (maximum of 3,951 mg/dl), which remained elevated even after high-dose LPV was discontinued. While two subjects had elevated Bazett-corrected QT (QTc) measurements (maximum of 522 in one and 479 in the other), they remained on the study drug and their follow-up QTc measurements were normal (431 and 456, respectively). Extensive analysis showed no relationship of plasma drug concentrations to heart rate or QTc.
One subject was withdrawn from the study for LPV hypersensitivity consisting of rash and fever which occurred on day 6, resolved when LPV/r was stopped, and recurred with rechallenge.
Adherence was a challenge for many in this study. Five subjects withdrew prior to the first PK evaluation at week 2, and 2 others showed evidence of nonadherence at PK evaluations. Only 11 of 20 (55%) subjects took >95% of LPV/r doses through the first 12 weeks of the study, and 8 of 15 (53%) reported no missed doses in the three days prior to the study visit through study week 24.
DISCUSSION
The results of this study show that doses of LPV/r higher than those currently approved by the FDA are safe and well tolerated for up to 48 weeks in children and adolescents with HIV infection. There were no significant GI problems except in subjects with preexisting diarrhea. There was no significant increase in plasma triglycerides, and while there was an initial statistically significant increase in cholesterol, this was of modest size and did not worsen over the course of the study. There was no evidence of hepatic or cardiac toxicity with these higher doses. For those subjects who moved to step 2, there was no added toxicity with SQV in the regimen.
These higher doses of LPV/r resulted in drug exposures only slightly lower than those in adults treated with 667/167 mg of the LPV capsule twice daily (Kaletra product label; 15, 28, 36). That LPV dose directly scaled for BSA (adult dose in mg/adult BSA, or 667 mg/1.73 m2) would have been 385 mg/m2 for subjects not concurrently treated with an NNRTI. Our target dose was somewhat higher (400 mg/m2) since the directly scaled pediatric dose has previously been shown to result in drug exposures lower than those in adults (36). The target dose for subjects concurrently treated with NNRTIs was 480 mg/m2, chosen to compensate for the higher LPV CL induced by NNRTI (3, 6). The actual administered doses were as high as 433 mg/m2 (14.8 mg/kg) and 487 mg/m2 (17.4 mg/kg) in group 1 and group 2, respectively, which were well tolerated by study subjects. While we found an NNRTI effect on LPV CL similar to that suggested by prior studies (17), our study did not show the age and gender difference in CL identified by those investigators, perhaps because of the small size of this cohort.
We found diurnal variation in the LPV plasma concentration, with higher concentrations in the morning than in the evening. This pattern of diurnal variation has been identified by other investigators for RTV (14) and other protease inhibitors (19) and is postulated to result from reduced hepatic blood flow during sleep or changes in the plasma lipid concentration during the overnight fast which may alter the rate of drug absorption or CL. It is possible that the diurnal variation found in this study is accentuated by the use of higher doses of LPV.
The dose of SQV chosen for this study is higher than the directly scaled adult dose (1,000 mg/1.73 m2 = 578 mg/m2) but was chosen because prior studies of SQV in children had suggested high oral CL (1, 12, 22, 34, 38). Our subjects had higher SQV exposures than adults treated with SQV and standard doses of LPV/r, which is perhaps from the higher doses of LPV/r used in this study. Based on predetermined PK criteria, the SQV dose was decreased in three subjects and increased in one. The three subjects with higher SQV exposures had no evidence of drug-related toxicity.
While this study showed a trend toward improved virus load response in subjects with higher IQs (Table 5), the enrolled subjects had failed many prior ARV regimens and had such a large change in their level of resistance to LPV prior to study entry (Table 2) that the achievable IQ was quite low for most enrollees despite higher LPV concentrations. Even so, the CD4 cell count rose for the first 32 weeks of the study (Fig. 3), and the virus load was statistically significantly lower than baseline through study week 16.
LPV/r was useful in other studies of ARV therapy for children who failed many prior regimens (7, 30-33), and the results of the current study suggest that higher doses might be helpful for some patients in that setting. The addition of SQV might further enhance the efficacy of salvage therapy for patients who had previously failed multiple ARV regimens (1). In this study, subjects treated with NNRTI in addition to LPV/r and SQV had better virologic (Fig. 2) and immunologic (Fig. 3) responses to therapy, arguing that in the presence of a large change in the level of resistance, the addition of active drugs to a regimen may have a bigger impact on outcome than intensifying a regimen by using higher drug doses. Other new drugs (e.g., darunavir, tipranavir, maraviroc, raltegravir, etravirine, and enfuvirtide) might also offer appropriate options for salvage therapy when there is adequate PK information on dosing in children.
Adherence was difficult, as the regimen included a high pill burden. There were early dropouts for nonadherence, and there was difficulty with adherence for subjects staying on the study. In addition, the lack of a liquid formulation of SQV further enhanced the complexity of the regimen. These factors may have contributed to the poor viral load response seen in this study, although the high baseline resistance is an important consideration in explaining the persistence of detectable viremia (7).
LPV/r was initially developed in capsule and liquid formulations. The liquid is still available, but the capsule has been discontinued and replaced with a tablet produced with a proprietary melt extrusion technology (21). Absorption of the tablet is less dependent on meal conditions, and drug exposures are more uniform with the tablet than the capsule formulation (21). This study was performed using the liquid or the capsule formulation of LPV/r. Drug exposures using the tablet formulation might be somewhat less variable than those found in this study.
This study was initially designed to enroll 48 subjects and have the statistical power to show improvement in virus load over 48 weeks of treatment. However, enrollees had very high levels of resistance to LPV, and therefore, the changes in virus load were less robust than anticipated. Study enrollment was therefore stopped early, when we had accumulated enough data to report accurate data on the PK and safety of high-dose LPV and SQV.
This study shows the safety of LPV/r when used at doses as high as 400/100 mg/m2/dose orally (p.o.) q12h and 480/120 mg/m2 p.o. q12h when combined with an NNRTI and also shows the safe addition of SQV to these high doses of LPV/r. This offers useful options for salvage ARV regimens for the treatment of children and adolescents who may have failed prior therapy, but the limited virologic response and the challenge of adherence to a regimen with a high pill burden may limit its usefulness since other ARVs are available for use. In addition, these higher doses identify a clearly safe “upper bound” to weight band dosing algorithms, an important consideration as LPV/r is used more widely in second-line regimens around the world.
Acknowledgments
We dedicate this article to the memory of John H. Rodman, our colleague, mentor and friend, who passed away suddenly in April, 2006. Through his hard work and dedication he made significant contributions to the field of HIV pharmacology.
This work was supported by Pediatric AIDS Clinical Trials Group (PACTG) grant U01 AI 41089 and International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group grants U01 AI 068632 and grant 1 U01 AI 068616, all of the National Institute of Allergy and Infectious Diseases; grant NO1-HD-3-3345 from the National Institute of Child Health and Development, National Institutes of Health; and by Abbott Laboratories and Roche Pharmaceuticals. The work was supported in part by ALSAC, the American Lebanese-Syrian Associated Charities.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
We acknowledge the technical expertise of Charles Rose, Michael Bates (Monogram Biosciences), Malte Schutz (Roche Pharmaceuticals), and Marisol Martinez (Abbott Laboratories). PACTG sites and personnel that made this study possible include, from Boston Children's Hospital, Kenneth McIntosh, Sandra K. Burchett, Nancy Karthas, and Catherine Kneut; from Long Beach Memorial Hospital, Audra Deveikis, Jagmohan Batra, and Susan Marks; from Johns Hopkins Medical Institute, Nancy Hutton, Andrea Ruff, and Mary Beth Griffith; from Duke University, Margaret Donnelly, Juliana Simonetti, Carole Mathison, and Opemipo Johnson; from Harlem Hospital, Elaine Abrams, Susan Champion, Maxine Frere, and Kamali Swaminathan; from St. Jude's Children's Research Hospital, Pat Flynn, Aditya Gaur, Nehali Patel, and Jill Utech; and Children's Hospital of Chicago, UCSF Medical Center, NYU Medical Center at Bellevue, Jacobi Medical Center, City Hospital of San Juan, the University of Puerto Rico, and Tulane University.
Footnotes
Published ahead of print on 14 July 2008.
This is PACTG study P1038.
REFERENCES
- 1.Ananworanich, J., P. Kosalaraksa, A. Hill, U. Siangphoe, A. Bergshoeff, C. Pancharoen, C. Engchanil, K. Ruxrungtham, D. Burger, and H.-N. S. Team. 2005. Pharmacokinetics and 24-week efficacy/safety of dual boosted saquinavir/lopinavir/ritonavir in nucleoside-pretreated children. Pediatr. Infect. Dis. J. 24:874-879. [DOI] [PubMed] [Google Scholar]
- 2.Back, D., S. Gibbons, and S. Khoo. 2006. An update on therapeutic drug monitoring for antiretroviral drugs. Ther. Drug Monit. 28:468-473. [DOI] [PubMed] [Google Scholar]
- 3.Bergshoeff, A. S., P. L. Fraaij, J. Ndagijimana, G. Verweel, N. G. Hartwig, T. Niehues, R. De Groot, and D. M. Burger. 2005. Increased dose of lopinavir/ritonavir compensates for efavirenz-induced drug-drug interaction in HIV-1-infected children. J. Acquir. Immune Defic. Syndr. 39:63-68. [DOI] [PubMed] [Google Scholar]
- 4.Breilh, D., I. Pellegrin, A. Rouzes, K. Berthoin, F. Xuereb, H. Budzinski, M. Munck, H. J. Fleury, M. C. Saux, and J. L. Pellegrin. 2004. Virological, intracellular and plasma pharmacological parameters predicting response to lopinavir/ritonavir (KALEPHAR study). AIDS 18:1305-1310. [DOI] [PubMed] [Google Scholar]
- 5.Colombo, S., T. Buclin, C. Franc, N. Guignard, M. Khonkarly, P. E. Tarr, B. Rochat, J. Biollaz, A. Telenti, L. A. Decosterd, and M. Cavassini. 2006. Ritonavir-boosted atazanavir-lopinavir combination: a pharmacokinetic interaction study of total, unbound plasma and cellular exposures. Antivir. Ther. 11:53-62. [PubMed] [Google Scholar]
- 6.Dailly, E., V. Reliquet, F. Raffi, and P. Jolliet. 2005. A population approach to study the influence of nevirapine administration on lopinavir pharmacokinetics in HIV-1 infected patients. Eur. J. Clin. Pharmacol. 61:153-156. [DOI] [PubMed] [Google Scholar]
- 7.Delaugerre, C., J. P. Teglas, J. M. Treluyer, P. Vaz, V. Jullien, F. Veber, C. Rouzioux, M. L. Chaix, and S. Blanche. 2004. Predictive factors of virologic success in HIV-1-infected children treated with lopinavir/ritonavir. J. Acquir. Immune Defic. Syndr. 37:1269-1275. [DOI] [PubMed] [Google Scholar]
- 8.Dragsted, U. B., J. Gerstoft, C. Pedersen, B. Peters, A. Duran, N. Obel, A. Castagna, P. Cahn, N. Clumeck, J. N. Bruun, J. Benetucci, A. Hill, I. Cassetti, P. Vernazza, M. Youle, Z. Fox, J. D. Lundgren, and MaxCmin1 Trial Group. 2003. Randomized trial to evaluate indinavir/ritonavir versus saquinavir/ritonavir in human immunodeficiency virus type 1-infected patients: the MaxCmin1 Trial. J. Infect. Dis. 188:635-642. [DOI] [PubMed] [Google Scholar]
- 9.Ellner, P. D., and H. C. Neu. 1981. The inhibitory quotient. A method for interpreting minimum inhibitory concentration data. JAMA 246:1575-1578. [DOI] [PubMed] [Google Scholar]
- 10.Eron, J. J., J. Feinberg, H. A. Kessler, H. W. Horowitz, M. D. Witt, F. F. Carpio, D. A. Wheeler, P. Ruane, D. Mildvan, B. G. Yangco, R. Bertz, B. Bernstein, M. S. King, and E. Sun. 2004. Once-daily versus twice-daily lopinavir/ritonavir in antiretroviral-naive HIV-positive patients: a 48-week randomized clinical trial. J. Infect. Dis. 189:265-272. [DOI] [PubMed] [Google Scholar]
- 11.Gianotti, N., L. Galli, A. Danise, H. Hasson, E. Boeri, A. Lazzarin, and A. Castagna. 2006. Ability of different lopinavir genotypic inhibitory quotients to predict 48-week virological response in highly treatment-experienced HIV-infected patients receiving lopinavir/ritonavir. J. Med. Virol. 78:1537-1541. [DOI] [PubMed] [Google Scholar]
- 12.Grub, S., P. Delora, E. Ludin, F. Duff, C. V. Fletcher, R. C. Brundage, M. W. Kline, N. R. Calles, H. Schwarzwald, and K. Jorga. 2002. Pharmacokinetics and pharmacodynamics of saquinavir in pediatric patients with human immunodeficiency virus infection. Clin. Pharmacol. Ther. 71:122-130. [DOI] [PubMed] [Google Scholar]
- 13.Havens, P. L., M. Frank, B. Cuene, V. Decker, R. Kohler, A. Wolfe, and M. Yenter. 2004. Pharmacokinetics and safety of lopinavir/ritonavir doses greater than 300 mg/m2/dose in children and adolescents with HIV infection, abstr. 937. 11th Conf. Retrovir. Opportun. Infect., San Francisco, CA, 8 to 11 February 2004.
- 14.Hsu, A., G. R. Granneman, G. Witt, C. Locke, J. Denissen, A. Molla, J. Valdes, J. Smith, K. Erdman, N. Lyons, P. Niu, J. P. Decourt, J. B. Fourtillan, J. Girault, and J. M. Leonard. 1997. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 41:898-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu, A., J. Isaacson, S. Brun, B. Bernstein, W. Lam, R. Bertz, C. Foit, K. Rynkiewicz, B. Richards, M. King, R. Rode, D. J. Kempf, G. R. Granneman, and E. Sun. 2003. Pharmacokinetic-pharmacodynamic analysis of lopinavir-ritonavir in combination with efavirenz and two nucleoside reverse transcriptase inhibitors in extensively pretreated human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 47:350-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu, A., J. Isaacson, D. Kempf, M. King, R. Rode, W. Lam, S. Bruni, B. Bernstein, E. Sun, and G. R. Granneman. 2000. Trough concentration-EC50 relationship as a predictor of viral response for ABT-378/ritonavir (ABT-378/r) in treatment-experienced patients, abstr. 171. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., Toronto, Canada.
- 17.Jullien, V., S. Urien, D. Hirt, C. Delaugerre, E. Rey, J.-P. Teglas, P. Vaz, C. Rouzioux, M.-L. Chaix, E. Macassa, G. Firtion, G. Pons, S. Blanche, and J.-M. Treluyer. 2006. Population analysis of weight-, age-, and sex-related differences in the pharmacokinetics of lopinavir in children from birth to 18 years. Antimicrob. Agents Chemother. 50:3548-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Justesen, U. S., Z. Fox, C. Pedersen, P. Cahn, J. Gerstoft, N. Clumeck, M. Losso, B. Peters, N. Obel, A. Castagna, U. B. Dragsted, J. D. Lundgren, and MaxCmin Trial Group. 2007. Pharmacokinetics of two randomized trials evaluating the safety and efficacy of indinavir, saquinavir and lopinavir in combination with low-dose ritonavir: the MaxCmin1 and 2 trials. Basic Clin. Pharmacol. Toxicol. 101:339-344. [DOI] [PubMed] [Google Scholar]
- 19.Justesen, U. S., and C. Pedersen. 2002. Diurnal variation of plasma protease inhibitor concentrations. AIDS 16:2487-2489. [DOI] [PubMed] [Google Scholar]
- 20.Kempf, D. J., J. D. Isaacson, M. S. King, S. C. Brun, J. Sylte, B. Richards, B. Bernstein, R. Rode, and E. Sun. 2002. Analysis of the virological response with respect to baseline viral phenotype and genotype in protease inhibitor-experienced HIV-1-infected patients receiving lopinavir/ritonavir therapy. Antivir. Ther. 7:165-174. [PubMed] [Google Scholar]
- 21.Klein, C. E., Y.-L. Chiu, W. Awni, T. Zhu, R. S. Heuser, T. Doan, J. Breitenbach, J. B. Morris, S. C. Brun, and G. J. Hanna. 2007. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J. Acquir. Immune Defic. Syndr. 44:401-410. [DOI] [PubMed] [Google Scholar]
- 22.Kline, M. W., R. C. Brundage, C. V. Fletcher, H. Schwarzwald, N. R. Calles, N. E. Buss, P. Snell, P. DeLora, M. Eason, K. Jorga, C. Craig, and F. Duff. 2001. Combination therapy with saquinavir soft gelatin capsules in children with human immunodeficiency virus infection. Pediatr. Infect. Dis. J. 20:666-671. [DOI] [PubMed] [Google Scholar]
- 23.la Porte, C. J., J. C. Wasmuth, K. Schneider, J. K. Rockstroh, and D. M. Burger. 2003. Lopinavir/ritonavir plus saquinavir in salvage therapy; pharmacokinetics, tolerability and efficacy. AIDS 17:1700-1702. [DOI] [PubMed] [Google Scholar]
- 24.Maillard, A., J.-M. Chapplain, O. Tribut, D. Bentue-Ferrer, P. Tattevin, C. Arvieux, C. Michelet, and A. Ruffault. 2007. The use of drug resistance algorithms and genotypic inhibitory quotient in prediction of lopinavir-ritonavir treatment response in human immunodeficiency virus type 1 protease inhibitor-experienced patients. J. Clin. Virol. 38:131-138. [DOI] [PubMed] [Google Scholar]
- 25.Merry, C., M. G. Barry, F. Mulcahy, M. Ryan, J. Heavey, J. F. Tjia, S. E. Gibbons, A. M. Breckenridge, and D. J. Back. 1997. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS 11:F29-F33. [DOI] [PubMed] [Google Scholar]
- 26.Molla, A., M. Korneyeva, Q. Gao, S. Vasavanonda, P. J. Schipper, H. M. Mo, M. Markowitz, T. Chernyavskiy, P. Niu, N. Lyons, A. Hsu, G. R. Granneman, D. D. Ho, C. A. Boucher, J. M. Leonard, D. W. Norbeck, and D. J. Kempf. 1996. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat. Med. 2:760-766. [DOI] [PubMed] [Google Scholar]
- 27.Morse, G. D., L. M. Catanzaro, and E. P. Acosta. 2006. Clinical pharmacodynamics of HIV-1 protease inhibitors: use of inhibitory quotients to optimise pharmacotherapy. Lancet Infect. Dis. 6:215-225. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, R. L., S. Brun, C. Hicks, J. J. Eron, R. Gulick, M. King, A. C. White, Jr., C. Benson, M. Thompson, H. A. Kessler, S. Hammer, R. Bertz, A. Hsu, A. Japour, and E. Sun. 2001. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS 15:F1-F9. [DOI] [PubMed] [Google Scholar]
- 29.Podzamczer, D., M. S. King, C. E. Klein, C. Flexner, C. Katlama, D. V. Havlir, S. L. Letendre, J. J. Eron, S. C. Brun, and B. Bernstein. 2007. High-dose lopinavir/ritonavir in highly treatment-experienced HIV-1 patients: efficacy, safety, and predictors of response. HIV Clin. Trials 8:193-204. [DOI] [PubMed] [Google Scholar]
- 30.Ramos, J. T., M. I. De Jose, J. Duenas, C. Fortuny, R. Gonzalez-Montero, M. J. Mellado, A. Mur, M. Navarro, C. Otero, I. Pocheville, M. A. Munoz-Fernandez, and E. Cabrero on behalf of the Spanish Collaborative Group on HIV Infection in Children. 2005. Safety and antiviral response at 12 months of lopinavir/ritonavir therapy in human immunodeficiency virus-1-infected children experienced with three classes of antiretrovirals. Pediatr. Infect. Dis. J. 24:867-873. [DOI] [PubMed] [Google Scholar]
- 31.Resino, S., J. M. Bellon, and M. A. Munoz-Fernandez on behalf of the Spanish Group of HIV Infection. 2006. Antiretroviral activity and safety of lopinavir/ritonavir in protease inhibitor-experienced HIV-infected children with severe-moderate immunodeficiency. J. Antimicrob. Chemother. 57:579-582. [DOI] [PubMed] [Google Scholar]
- 32.Resino, S., J. M. Bellon, J. T. Ramos, M. L. Navarro, P. Martin-Fontelos, E. Cabrero, and M. A. Munoz-Fernandez. 2004. Salvage lopinavir-ritonavir therapy in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 23:923-930. [DOI] [PubMed] [Google Scholar]
- 33.Resino, S., I. Galan, A. Perez, J. T. Ramos, J. M. Bellon, P. M. Fontelos, M. I. de Jose, M. D. Gutierrez, E. Cabrero, and M. A. Munoz-Fernandez. 2005. Immunological changes after highly active antiretroviral therapy with lopinavir-ritonavir in heavily pretreated HIV-infected children. AIDS Res. Hum. Retrovir. 21:398-406. [DOI] [PubMed] [Google Scholar]
- 34.Ribera, E., R. M. Lopez, M. Diaz, L. Pou, L. Ruiz, V. Falco, M. Crespo, C. Azuaje, I. Ruiz, I. Ocana, B. Clotet, and A. Pahissa. 2004. Steady-state pharmacokinetics of a double-boosting regimen of saquinavir soft gel plus lopinavir plus minidose ritonavir in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 48:4256-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosso, R., A. Di Biagio, C. Dentone, G. C. Gattinara, A. M. Martino, A. Vigano, M. Merlo, C. Giaquinto, O. Rampon, M. Bassetti, G. Gatti, and C. Viscoli. 2006. Lopinavir/ritonavir exposure in treatment-naive HIV-infected children following twice or once daily administration. J. Antimicrob. Chemother. 57:1168-1171. [DOI] [PubMed] [Google Scholar]
- 36.Saez-Llorens, X., A. Violari, C. O. Deetz, R. A. Rode, P. Gomez, E. Handelsman, S. Pelton, O. Ramilo, P. Cahn, E. Chadwick, U. Allen, S. Arpadi, M. M. Castrejon, R. S. Heuser, D. J. Kempf, R. J. Bertz, A. F. Hsu, B. Bernstein, C. L. Renz, and E. Sun. 2003. Forty-eight-week evaluation of lopinavir/ritonavir, a new protease inhibitor, in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 22:216-224. [DOI] [PubMed] [Google Scholar]
- 37.Staszewski, S., E. Babacan, C. Stephan, A. Haberl, A. Carlebach, P. Gute, S. Klauke, Y. Hermschulte, M. Stuermer, B. Dauer, and the Frankfurt HIV Cohort. 2006. The LOPSAQ study: 48 week analysis of a boosted double protease inhibitor regimen containing lopinavir/ritonavir plus saquinavir without additional antiretroviral therapy. J. Antimicrob. Chemother. 58:1024-1030. [DOI] [PubMed] [Google Scholar]
- 38.Stephan, C., N. Hentig, I. Kourbeti, B. Dauer, M. Mosch, T. Lutz, S. Klauke, S. Harder, M. Kurowski, and S. Staszewski. 2004. Saquinavir drug exposure is not impaired by the boosted double protease inhibitor combination of lopinavir/ritonavir. AIDS 18:503-508. [DOI] [PubMed] [Google Scholar]
- 39.Taburet, A. M., G. Raguin, C. Le Tiec, C. Droz, A. Barrail, I. Vincent, L. Morand-Joubert, G. Chene, F. Clavel, and P. M. Girard. 2004. Interactions between amprenavir and the lopinavir-ritonavir combination in heavily pretreated patients infected with human immunodeficiency virus. Clin. Pharmacol. Ther. 75:310-323. [DOI] [PubMed] [Google Scholar]
- 40.van der Lee, M., G. Verweel, R. de Groot, and D. Burger. 2006. Pharmacokinetics of a once-daily regimen of lopinavir/ritonavir in HIV-1-infected children. Antivir. Ther. 11:439-445. [PubMed] [Google Scholar]