Abstract
Listeriosis is a rare but life-threatening infection. A favorable outcome is greatly aided by early administration of antibiotics with rapid bactericidal activity against Listeria monocytogenes. Moxifloxacin, a new-generation fluoroquinolone with extended activity against gram-positive bacteria, has proved its effectiveness in vitro against intracellular reservoirs of bacteria. The efficacies of moxifloxacin and amoxicillin were compared in vivo by survival curve assays and by studying the kinetics of bacterial growth in blood and organs in a murine model of central nervous system (CNS) listeriosis. We combined pharmacokinetic and pharmacodynamic approaches to correlate the observed efficacy in vivo with plasma and tissue moxifloxacin concentrations. Death was significantly delayed for animals treated with a single dose of moxifloxacin compared to a single dose of amoxicillin. We observed rapid bacterial clearance from blood and organs of animals treated with moxifloxacin. The decrease in the bacterial counts in blood and brain correlated with plasma and cerebral concentrations of antibiotic. Moxifloxacin peaked in the brain at 1.92 ± 0.32 μg/g 1 hour after intraperitoneal injection. This suggests that moxifloxacin rapidly crosses the blood-brain barrier and diffuses into the cerebral parenchyma. Moreover, no resistant strains were selected during in vivo experiments. Our results indicate that moxifloxacin combines useful pharmacokinetic properties and rapid bactericidal activity and that it may be a valuable alternative for the treatment of CNS listeriosis.
Listeria monocytogenes is a gram-positive bacterium that is widespread in the environment (10). It is a facultative intracellular food-borne pathogen that causes severe and life-threatening infections, especially septicemia, abortions, and central nervous system (CNS) infections (10). Listeriosis mainly occurs in high-risk groups, including individuals with severe underlying diseases or with impaired cellular immunity (13). In these cases, the outcome depends on early administration of antibiotics with rapid bactericidal activity against L. monocytogenes (13, 17, 31). Currently, the reference treatment is based on a synergistic combination of high-dose amoxicillin or ampicillin and gentamicin administrated intravenously (16, 40).
Nevertheless, despite effective treatment, listeriosis is associated with a high mortality rate, especially for CNS infections (30%), and sequels are frequent (13, 31). Indeed, treatment of CNS listeriosis is particularly complex because efficacy is limited by the abilities of antibiotics to diffuse extensively into tissues and cross the blood-brain barrier. Moreover, intracellular activity of the antibiotic is also required to eliminate intracytoplasmic reservoirs of bacteria (17, 18, 40). Consequently, there are few appropriate candidate molecules combining rapid bactericidal activity and high diffusion within host cells. Moreover, prospective clinical validations of the efficacies of antibiotics against L. monocytogenes infections are difficult to conduct because listeriosis is a rare disease in humans and occurs sporadically (10, 17).
Thus, a review of the literature reveals considerable diversity in the second-line treatment used in case of first-line failure or contraindication of beta-lactams because of intolerance, and also variable therapeutic success (2, 5, 10, 13, 17, 22, 31, 34, 39, 40). The classical use of cotrimoxazole, in which trimethoprim is the more active drug (16, 40), as the second-line treatment suffers various limitations because some strains isolated from the environment and, more recently, some strains isolated from humans are resistant to trimethoprim (8). Moreover, some episodes of hypersensitivity have been reported, leading to contraindication of its use (22, 40).
More recently, new-generation fluoroquinolones with extended activity against gram-positive bacteria have been suggested and tested against L. monocytogenes (7, 24, 32, 36). They share several useful pharmacokinetic properties, such as good bioavailability and the ability to penetrate and concentrate intracellularly, giving them activity against intracellular pathogens, including L. monocytogenes (4, 36, 41). A range of pharmacodynamic properties of the newer fluoroquinolones have been described in vitro (27, 43). Moxifloxacin, clinafloxacin, and gemifloxacin are the most effective against intracellular L. monocytogenes (28, 36). However, moxifloxacin is the only one of these antibiotics released on the market and still commercially available that combines useful pharmacokinetic and pharmacodynamic properties with rapid bactericidal activity in vitro against both extracellular forms of L. monocytogenes and intracellular reservoirs of bacteria (6, 14, 28, 36). Determination of MICs for a large collection of strains did not detect any resistant strains, whatever their origins (14). Moreover, no cross-resistance was observed with ciprofloxacin resistance expressed by clinical isolates due to an efflux mechanism (12, 14).
However, the results of in vitro efficacy studies do not necessarily predict in vivo efficacy with accuracy, as the concentrations of antibiotic in vivo depend on the pharmacokinetic parameters of the particular antibiotic (17, 18, 30, 43). Various experimental animal models of listeriosis (gerbil, rat, guinea pig, rabbit, and mouse) have been used to confirm the in vivo efficacies of antibiotics (3, 17, 26, 32, 38). Some results can even be misleading: fosfomycin has recently been shown to be effective against L. monocytogenes in vivo, whereas it is not in vitro (35).
We compared the efficacy of moxifloxacin with that of amoxicillin using survival curve assays and studies of the kinetics of bacterial growth in blood and organs in a murine model of CNS listeriosis. We combined pharmacokinetic and pharmacodynamic approaches to correlate the observed efficacy in vivo with plasma and tissue moxifloxacin concentrations.
MATERIALS AND METHODS
Antibiotics.
Moxifloxacin and amoxicillin were provided by Bayer Pharma (Bayer AG, Wuppertal, Germany) and GlaxoSmithKline (Marly-le-Roi, France), respectively. Antibiotics were diluted to the appropriate concentration with isotonic saline solution.
Bacterial strains.
The virulent strain L. monocytogenes EGD-e (11) was used for survival curve assays and studies of the kinetics of bacterial growth in organs and blood associated with the two antibiotics. The MICs of moxifloxacin and amoxicillin for the EGD-e strain were 0.5 and 0.125 mg/liter, respectively. Before each experiment, a calibrated suspension of bacteria was obtained by appropriate dilution in saline isotonic solution from a frozen stock.
Experimental CNS infection in mice. (i) Mice.
BALB/c female mice, 7 to 8 weeks old, purchased from Elevage Janvier (Le Genest-St-Isle, France) were used. The mice were housed in wire bottom cages, with free access to food and water, and held under these conditions for at least 72 h before infection. The animal experimentation protocol was approved by the Animal Welfare Committee of the Institut Pasteur.
(ii) Infection of mice.
The mice were weighed and then injected intravenously via the lateral tail vein with 1 × 105 L. monocytogenes organisms in 0.5 ml. All animals were examined daily.
(iii) Survival curves.
The animals were checked for death twice a day starting from the 36th hour after infection. The time of death was noted for five experimental treatment groups: a single injection of amoxicillin (n = 12), a single injection of moxifloxacin (n = 12), injections of amoxicillin every 12 h for 5 days (n = 20), injections of moxifloxacin every 12 h for 5 days (n = 20), and untreated control mice (n = 5). The surviving animals were followed for 2 months to detect any relapse of infection.
(iv) Kinetics of bacterial growth in organs.
At intervals after infection (1, 6, 24, 48, and 72 h), groups of three mice were anesthetized by intramuscular injection of 200 μl of a mixture of 200 mg·kg of body weight−1 ketamine (Imalgene 100; Merial, Lyon, France) and 10 mg·kg−1 xylazine hydrochloride (Rompun 2%; Bayer, Puteaux, France) and killed. The abdominal cavity was then aseptically opened, and each mouse was bled by intracardiac puncture with a previously heparinized syringe (Heparin sodic; Sanofi-Wintrop, France). We performed a whole-body wash using 40 ml of sterile isotonic saline injected into the right cardiac ventricle. Organs (liver, spleen, and brain) were aseptically removed and homogenized in isotonic saline. These organ homogenates were serially 10-fold diluted and plated on brain heart infusion (Becton Dickinson, Le Pont de Claix, France) agar plates and on brain heart infusion agar supplemented with 2 μg/ml of moxifloxacin and incubated for 24 and 48 h. Bacterial counts were determined after 24 h of incubation at 37°C. The results were expressed as the mean ± standard error of log10 CFU per organ.
(v) Treatment of mice.
Groups of infected BALB/c mice were treated 36 h after infection by a single intraperitoneal (i.p.) injection of moxifloxacin (50 mg·kg−1; volume, 0.1 ml), amoxicillin (50 mg·kg−1; volume 0.1 ml), or isotonic saline (0.1 ml) for untreated controls. Some mice were subjected to this treatment every 12 h for 5 days.
Analytical procedure.
During kinetics of bacterial growth in organ experiments, moxifloxacin was dosed in brain homogenates and plasma in which bacteria had been counted.
(i) Preparation of samples and extraction.
Brains were washed to clean out the circulating blood, dried, weighed (0.5 ± 0.01 g), crushed, homogenized aseptically, and centrifuged. Plasma was obtained from the blood by centrifugation. The brain supernatants and plasma were frozen and stored at −80°C until moxifloxacin assays were performed.
The brain homogenates and plasma (100 μl) were mixed with 100 μl acetonitrile and 20 μl of methanolic ciprofloxacin (3.0 mg/liter) used as an internal standard; the samples were centrifuged at 4,000 rpm for 10 min and diluted with water (1:4), and the moxifloxacin concentration was determined by high-performance liquid chromatography (HPLC).
(ii) HPLC analysis.
The HPLC technique used for dosing moxifloxacin was as described by Grayo et al. (15). Briefly, chromatographic separation was carried out on an inverse-phase column coupled with fluorescence detection (excitation at 290 nm and emission at 550 nm). Analytical methods for quantification of moxifloxacin in plasma and brain were fully validated according to bioanalytical method validation (1). Moxifloxacin/ciprofloxacin area ratio data are linear functions of the moxifloxacin concentration in plasma and brain. The limits of quantification were 5 μg/liter of plasma and 5 ng/g of brain, respectively. The within-assay and between-assay precision levels (n = 6) were <6%.
(iii) Pharmacokinetic analysis of moxifloxacin in infected mice.
Moxifloxacin concentrations were determined as previously described by Grayo et al. (15) in the plasma and the cerebral parenchymas from groups of six mice killed 0, 5, 15, 30, 60, 120, 240, 360, and 480 min after single-dose treatment. The maximum concentrations (Cmax) in plasma and brain were determined directly by analysis of the experimental concentration-time curves. Areas under the curve at 24 h (AUC24) were estimated by using the trapezoidal rule up to the last concentration measured, and the area under the inhibition curve (AUIC) (or the AUC24/MIC ratio), Cmax/MIC ratio, and coefficient of diffusion (AUCbrain/AUCplasma ratio) were then calculated. The results were expressed as means ± standard errors in μg/g of brain or ng/ml of plasma.
Statistical analysis.
The survival of animals following infection was analyzed using Kaplan-Meier estimates. Survival rates across groups were compared using log rank tests. P values of <0.05 were considered to be statistically significant. Statistical analyses were performed using Stata 9 (Statacorp, College Station, TX).
RESULTS
Survival curves.
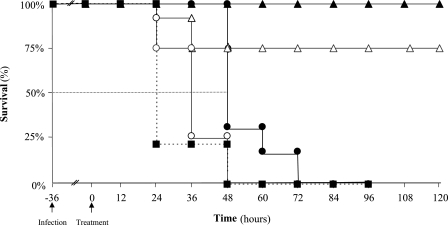
The survival of infected mice was scored following single or multiple injections of moxifloxacin or amoxicillin performed 36 h after infection with 1 × 105 bacteria. After that time, with no antibiotic treatment, 80% of the animals died within 60 h, and all had died within 84 h (Fig. 1).
FIG. 1.
Kaplan-Meier survival estimates for mice infected by L. monocytogenes according to various schemes of antibiotic administration. BALB/c mice were treated 36 h after intravenous infection by 1 × 105 L. monocytogenes (EGD-e) organisms with a single dose of amoxicillin (50 mg·kg−1 i.p.; n = 12) ○, a single dose of moxifloxacin (50 mg·kg−1 i.p.; n = 12) •, one dose of amoxicillin every 12 h for 5 days (50 mg·kg−1 i.p.; n = 20) ▵, one dose of moxifloxacin every 12 h for 5 days (50 mg·kg−1 i.p.; n = 20) ▴, or sterile isotonic saline (control; n = 5) ▪. The arrows indicate intravenous infection and the start of treatment.
Animals that received a single dose of antibiotics survived longer than untreated animals (P = 0.0001) (Fig. 1). Moreover, survival after a single dose of moxifloxacin was significantly longer than after a single dose of amoxicillin (24 h and 12 h, respectively, longer than controls, according to the 50% survival time; P = 0.0002) (Fig. 1). The behavior of animals treated with moxifloxacin changed rapidly after the first injection: they recovered from prostration and listlessness and started moving again. Multiple injections of antibiotics (twice a day for 5 days) led to better survival (75% with amoxicillin and 100% with moxifloxacin). None of the animals that survived for 5 days died during the 2-month follow-up period.
Kinetics of bacterial growth in organs and blood.
In the absence of antibiotic treatment, there was rapid and uncontrolled growth of L. monocytogenes in blood, liver, spleen, and brain until the mice died (Fig. 2).
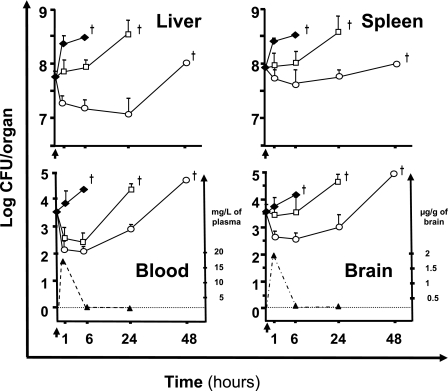
FIG. 2.
Kinetics of bacterial growth in blood and organs and correlation with concentrations of moxifloxacin. BALB/c mice were treated 36 h after intravenous infection by 1 × 105 L. monocytogenes (EGD-e) organisms with a single dose of amoxicillin (50 mg·kg−1 i.p.) □, moxifloxacin (50 mg·kg−1 i.p.) ○, or sterile isotonic saline (control) ⧫. Bacterial counts in blood and organs were determined after 1, 6, 24, and 48 h. The results shown are means plus standard errors of groups of three mice expressed as log10 CFU/organ, or log10 CFU/ml for blood. Concentrations of moxifloxacin determined in the same plasma and brain are represented by dotted lines, and the corresponding scale is on the right side of each panel. The results of moxifloxacin concentrations are expressed as means plus standard errors in ng/ml of plasma or μg/g of brain from six animals. The arrows represent the start of treatment 36 h after intravenous infection, and deaths are symbolized by crosses.
We then studied bacterial growth in organs and blood from groups of three mice treated with moxifloxacin or amoxicillin. A single dose of moxifloxacin or amoxicillin had a rapid but partial and temporary effect on the bacterial count in blood: the count declined by about 1 log CFU/ml within the first hour. This corresponded to the time when the peak plasma concentration of moxifloxacin was reached (Cmax = 17.3 ± 6.7 mg/liter) (Fig. 2). Thereafter, from 1 hour, the concentration of moxifloxacin decreased rapidly to become undetectable in the plasma after 6 hours. The AUC24 were 27.1 mg·h·liter−1 and 2.9 μg·h·g−1 for plasma and brain, respectively. The AUC24/MIC ratio (AUIC) for moxifloxacin was 54, and the Cmax /MIC ratio was 34. The bacterial counts then increased again. However, the increase occurred earlier in animals treated with amoxicillin than in animals treated with moxifloxacin (Fig. 2).
Moxifloxacin killed bacteria residing within the liver and spleen; there was a rapid reduction in the bacterial counts from the first hour following treatment. In contrast, amoxicillin inhibited the bacterial growth in these organs only during the first 6 hours following treatment (Fig. 2).
Moxifloxacin was rapidly detected in the brain and peaked at 1.96 ± 0.32 μg/g of brain tissue within 1 hour of i.p. administration (Fig. 2). After 1 hour, the cerebral concentrations of moxifloxacin decreased, but it was still detected 6 hours after administration (Fig. 2).
These results underlined the high capacity of moxifloxacin to cross the blood-brain barrier and to diffuse into the cerebral parenchyma. Moxifloxacin reduced the bacterial count in the brain by about 1 log CFU within the first hour, whereas amoxicillin did not. The effect of moxifloxacin on bacterial growth seemed to persist beyond the time when moxifloxacin could no longer be detected in the brain (Fig. 2). Untreated control mice died with bacterial counts higher than 1 × 104 CFU/brain. Bacterial counts in the brains of mice treated with a single dose of amoxicillin reached similar values, and the mice died between 24 and 48 h after treatment, whereas mice treated with single-dose moxifloxacin died between 48 and 72 h after treatment (Fig. 2).
Selection of strains resistant to moxifloxacin.
No strains resistant to moxifloxacin were selected from any blood or organ samples after 48 h of incubation. In addition, we did not detect increased MICs of moxifloxacin for strains isolated during the experiments with the kinetics of bacterial growth in organs and blood (data not shown).
DISCUSSION
Recently, we showed rapid bactericidal activity of moxifloxacin in vitro against the intracellular reservoir of L. monocytogenes in infected BALB/c mouse bone marrow-derived macrophages (14). Here, we report a study of BALB/c mice experimentally infected with a virulent strain of L. monocytogenes (EGD-e), a model that is easily accessible and widely used (17, 19, 32).
The experimental conditions for infection (1 × 105 L. monocytogenes organisms) and treatment (50 mg·kg−1, i.p., 36 h postinfection) were chosen on the basis of a review of the literature (17, 19, 32, 37) and the results of preliminary studies with various sublethal inocula and doses of antibiotics (data not shown). We obtained a reproducible model of listeriosis associated with CNS infection confirmed by the observation of cerebral abscesses in histological examination of brain sections (19). The time chosen for treatment was before any deaths occurred.
To be effective, antibiotics have to be present at a sufficiently high concentration at the site of infection. Their ability to reach these sites depends on their pharmacokinetic properties (30, 43). Moxifloxacin is rapidly and completely absorbed (37). In our animal model, i.p. administration provided rapid and high bioavailability in the bloodstream and good diffusion into various tissues, as previously shown in both uninfected and infected mice (15, 20, 37). We observed rapid diffusion into the cerebral parenchyma in infected mice, probably enhanced by inflammation of the meninges, leading to prolonged detection of the drug in the brain beyond 6 hours after its administration (15, 44). This substantial diffusion into tissues, including the cerebral parenchyma, should be beneficial for patients by minimizing the occurrence of sequels and mortality. The administration of clinically relevant doses of moxifloxacin to humans twice a day is expected to lead to sufficiently high concentrations in tissues (20, 37).
However, the concentrations achieved in animal models are not in themselves predictive of clinical efficacy. The clinical outcome, antimicrobial effect in organs, and emergence of resistant strains are also important factors (30, 43).
In infected mice, a single dose of moxifloxacin rapidly and significantly decreased bacterial counts in blood and organs, associated with a rapid improvement in the behavior of infected animals, and significantly delayed death. Pharmacodynamic parameters clearly linked the antimicrobial activity in blood and brain to the concentrations of moxifloxacin obtained in vivo. Most investigators and drug companies have now adopted the AUC24/MIC ratio as a practical predictive parameter for in vivo efficacy (41). Concerning the AUIC, the question remaining unanswered is the minimal value of this parameter. For fluoroquinolones, a reviewed value that appears sufficient in vitro and in animal and clinical trials, and for other infections, is between 30 (21) and 125 (29). Moreover, moxifloxacin was rapidly bactericidal against Streptococcus pneumoniae in a rabbit model, with an AUIC of 1.3 to 102 (20).
Moxifloxacin diffuses into several cellular compartments (6, 28, 36). Thus, the in vivo efficacy of moxifloxacin is probably a consequence of its rapid bactericidal activity against intracellular reservoirs of bacteria, including those within macrophages (6, 14, 17) recruited in large numbers at infectious foci, especially in brain abscesses. We recently showed early inhibition by moxifloxacin of cell-to-cell spreading and prevention of the lysis of infected macrophages in vitro (14). This probably reduces bacterial release from infected cells and prevents bacterial dissemination within sites of infection. Moreover, by acting against intracellular bacteria, moxifloxacin most likely prevents bacterial dissemination within infected phagocytes that are able to cross the blood-brain barrier (19). These various effects all contribute to a subsequent decrease in the bacterial inoculum in organs, including the brain.
During the study, we failed to select for strains resistant to moxifloxacin, as we had failed during in vitro efficacy studies (14). Moxifloxacin has a weak intrinsic ability to select for resistant strains due to its original structure, an 8-methoxyquinolone that provides enhanced activity against DNA gyrase and topoisomerase IV, and reduced efflux from bacterial cells due to its 7-diazobicyclonyl group (33, 43, 45). Moreover, a Cmax/MIC ratio of >10 was shown to be sufficient to prevent the selection of strains resistant to fluoroquinolones (9, 43). This may explain why we did not detect resistant bacteria during bacterial growth in organs and blood despite concentrations of moxifloxacin that were below the detection threshold for longer times after administration. This may be due to the postantibiotic effect previously described for the newer fluoroquinolones, including moxifloxacin (4, 41, 43).
Although amoxicillin also delayed death, the delay was shorter. Amoxicillin has nearly the same activity as moxifloxacin against the easily accessible bacteria in blood after a single injection. However, it is less effective against tissue reservoirs of bacteria, only inhibiting bacterial growth, whereas moxifloxacin kills bacteria, thereby decreasing bacterial counts. Moxifloxacin has the advantage of reducing L. monocytogenes organisms residing and multiplying intracellularly, predominantly within macrophages. This leads to delay in bacterial recirculation in blood observed after antibiotic clearance (26, 32). In contrast, amoxicillin has only slow and weak activity against intracytoplasmic reservoirs of bacteria, as only a small proportion of the drug reaches the cytoplasmic compartments of infected cells (7, 14, 25). Moreover, this may also have been due to the poor diffusion of amoxicillin into tissues, particularly into the cerebral parenchyma (17, 23). Thus, high doses of amoxicillin are required to ensure sufficient concentrations at the sites of infection, as the in vivo efficacy of beta-lactams largely depends on the dose and the duration of treatment (16, 23, 40).
The results obtained for treatment with multiple doses of moxifloxacin and amoxicillin are difficult to compare because these antibiotics do not share the same characteristics and the same rhythm of administration. However, all animals treated with repeated doses of moxifloxacin were cured, with no relapse after 2 months of follow-up. Similar results have been reported for other new-generation fluoroquinolones, including clinafloxacin, which unfortunately cannot be used clinically because of its toxicity (32).
Successful treatment of L. monocytogenes meningitis with amoxicillin may well be due to the fact that in this setting most of the bacteria are located extracellularly and therefore are accessible to antibiotics (16, 31, 42). Nevertheless, amoxicillin is also able to cure meningoencephalitis (3, 17, 26), but it seems to achieve this task rather late and only in cooperation with the immune system or in association with gentamicin, which is rapidly bactericidal in vitro even though it does not penetrate infected cells (16, 40). Indeed, for immunocompromised mice, there is no apparent cure, as in these cases the role of antibiotics is much more decisive, especially rapid bactericidal activity (17, 23, 32).
Conclusions.
We show for the first time that moxifloxacin has rapid bactericidal activity against L. monocytogenes in BALB/c mice. Moreover, this nontoxic and well-tolerated fluoroquinolone diffuses well into tissues, including in particular the cerebral parenchyma. Thus, moxifloxacin is a promising alternative for the treatment of CNS listeriosis, especially in cases of first-line treatment failure or contraindication for the use of beta-lactams or cotrimoxazole. However, care is required when extrapolating results in mice to clinical applications. Careful analysis of CNS listeriosis cases treated with moxifloxacin will provide information that will be helpful in further use of the molecule.
Acknowledgments
We thank Bayer Pharma (Wuppertal, Germany) for the generous gift of standard powder of moxifloxacin and Alex Edelman for careful reading of the manuscript.
This study was supported by Institut Pasteur (Paris, France) and Institut de Veille Sanitaire (Saint Maurice, France).
Footnotes
Published ahead of print on 23 June 2008.
REFERENCES
- 1.Anonymous. 2001. Guidance for industry bioanalytical method validation. U.S. Department of Health and Human Services, Washington, DC.
- 2.Benes, J., J. Viechova, M. Kabelkova, and B. Horova. 2002. Listerial endocarditis in a penicillin-allergic woman successfully treated with a combination of 4 drugs. Scand. J. Infect. Dis. 34:383-384. [DOI] [PubMed] [Google Scholar]
- 3.Blanot, S., C. Boumaila, and P. Berche. 1999. Intracerebral activity of antibiotics against Listeria monocytogenes during experimental rhombencephalitis. J. Antimicrob. Chemother. 44:565-568. [DOI] [PubMed] [Google Scholar]
- 4.Boswell, F. J., J. M. Andrews, R. Wise, and A. Dalhoff. 1999. Bactericidal properties of moxifloxacin and post-antibiotic effect. J. Antimicrob. Chemother. 43(Suppl. B):43-49. [DOI] [PubMed] [Google Scholar]
- 5.Callapina, M., M. Kretschmar, A. Dietz, C. Mosbach, H. Hof, and T. Nichterlein. 2001. Systemic and intracerebral infections of mice with Listeria monocytogenes successfully treated with linezolid. J. Chemother. 13:265-269. [DOI] [PubMed] [Google Scholar]
- 6.Carryn, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2003. Activity of beta-lactams (ampicillin, meropenem), gentamicin, azithromycin and moxifloxacin against intracellular Listeria monocytogenes in a 24 h THP-1 human macrophage model. J. Antimicrob. Chemother. 51:1051-1052. [DOI] [PubMed] [Google Scholar]
- 7.Carryn, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2002. Comparative intracellular (THP-1 macrophage) and extracellular activities of beta-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob. Agents Chemother. 46:2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charpentier, E., and P. Courvalin. 1999. Antibiotic resistance in Listeria spp. Antimicrob. Agents Chemother. 43:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalhoff, A., and F. J. Schmitz. 2003. In vitro antibacterial activity and pharmacodynamics of new quinolones. Eur. J. Clin. Microbiol. Infect. Dis. 22:203-221. [DOI] [PubMed] [Google Scholar]
- 10.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 12.Godreuil, S., M. Galimand, G. Gerbaud, C. Jacquet, and P. Courvalin. 2003. Efflux pump Lde is associated with fluoroquinolone resistance in Listeria monocytogenes. Antimicrob. Agents Chemother. 47:704-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulet, V., and P. Marchetti. 1996. Listeriosis in 225 non-pregnant patients in 1992: clinical aspects and outcome in relation to predisposing conditions. Scand. J. Infect. Dis. 28:367-374. [DOI] [PubMed] [Google Scholar]
- 14.Grayo, S., O. Join-Lambert, M. C. Desroches, and A. Le Monnier. 2008. Comparison of the in vitro efficacies of moxifloxacin and amoxicillin against Listeria monocytogenes. Antimicrob. Agents Chemother. 52:1697-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grayo, S., R. Respaud, M. C. Desroches, S. Dubouch, A. Le Monnier, and E. Singlas. 2007. Modification of antibiotic pharmacokinetics due to Listeria monocytogenes infection, abstr. P05. Progr. Abstr. 16th Int. Symp. Problems Listeriosis, Savannah, GA.
- 16.Hof, H. 2004. An update on the medical management of listeriosis. Exp. Opin. Pharmacother. 5:1727-1735. [DOI] [PubMed] [Google Scholar]
- 17.Hof, H., T. Nichterlein, and M. Kretschmar. 1997. Management of listeriosis. Clin. Microbiol. Rev. 10:345-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hof, H., and G. Waldenmeier. 1988. Therapy of experimental listeriosis—an evaluation of different antibiotics. Infection 16(Suppl. 2):S171-S174. [DOI] [PubMed] [Google Scholar]
- 19.Join-Lambert, O. F., S. Ezine, A. Le Monnier, F. Jaubert, M. Okabe, P. Berche, and S. Kayal. 2005. Listeria monocytogenes-infected bone marrow myeloid cells promote bacterial invasion of the central nervous system. Cell Microbiol. 7:167-180. [DOI] [PubMed] [Google Scholar]
- 20.Keating, G. M., and L. J. Scott. 2004. Moxifloxacin: a review of its use in the management of bacterial infections. Drugs 64:2347-2377. [DOI] [PubMed] [Google Scholar]
- 21.Krays, M. B., and G. Denys. 2001. Fluoroquinolone susceptibility, resistance, and pharmacodynamics versus clinical isolates of Streptococcus pneumoniae from Indiana. Diagn. Microbiol. Infect. Dis. 40:193-198. [DOI] [PubMed] [Google Scholar]
- 22.Leiti, O., J. W. Gross, and C. U. Tuazon. 2005. Treatment of brain abscess caused by Listeria monocytogenes in a patient with allergy to penicillin and trimethoprim-sulfamethoxazole. Clin. Infect. Dis. 40:907-908. [DOI] [PubMed] [Google Scholar]
- 23.Marget, W., and H. P. Seeliger. 1988. Listeria monocytogenes infections—therapeutic possibilities and problems. Infection 16(Suppl. 2):S175-S177. [DOI] [PubMed] [Google Scholar]
- 24.Michelet, C., J. L. Avril, C. Arvieux, C. Jacquelinet, N. Vu, and F. Cartier. 1997. Comparative activities of new fluoroquinolones, alone or in combination with amoxicillin, trimethoprim-sulfamethoxazole, or rifampin, against intracellular Listeria monocytogenes. Antimicrob. Agents Chemother. 41:60-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michelet, C., J. L. Avril, F. Cartier, and P. Berche. 1994. Inhibition of intracellular growth of Listeria monocytogenes by antibiotics. Antimicrob. Agents Chemother. 38:438-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michelet, C., S. L. Leib, D. Bentue-Ferrer, and M. G. Tauber. 1999. Comparative efficacies of antibiotics in a rat model of meningoencephalitis due to Listeria monocytogenes. Antimicrob. Agents Chemother. 43:1651-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michot, J. M., M. F. Heremans, N. E. Caceres, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Cellular accumulation and activity of quinolones in ciprofloxacin-resistant J774 macrophages. Antimicrob. Agents Chemother. 50:1689-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michot, J. M., C. Seral, F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2005. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob. Agents Chemother. 49:2429-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montgomery, M. J., P. M. Beringer, A. Aminimanizani, S. G. Louie, B. J. Shapiro, R. Jelliffe, and M. A. Gill. 2001. Population pharmacokinetics and use of Monte Carlo simulation to evaluate currently recommended dosing regimens of ciprofloxacin in adult patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:3468-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouton, J. W., M. L. van Ogtrop, D. Andes, and W. A. Craig. 1999. Use of pharmacodynamic indices to predict efficacy of combination therapy in vivo. Antimicrob. Agents Chemother. 43:2473-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mylonakis, E., E. L. Hohmann, and S. B. Calderwood. 1998. Central nervous system infection with Listeria monocytogenes. 33 years' experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 77:313-336. [DOI] [PubMed] [Google Scholar]
- 32.Nichterlein, T., M. Kretschmar, C. Budeanu, J. Bauer, W. Linss, and H. Hof. 1994. Bay Y 3118, a new quinolone derivative, rapidly eradicates Listeria monocytogenes from infected mice and L929 cells. Antimicrob. Agents Chemother. 38:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pestova, E., J. J. Millichap, G. A. Noskin, and L. R. Peterson. 2000. Intracellular targets of moxifloxacin: a comparison with other fluoroquinolones. J. Antimicrob. Chemother. 45:583-590. [DOI] [PubMed] [Google Scholar]
- 34.Safdar, A., and D. Armstrong. 2003. Antimicrobial activities against 84 Listeria monocytogenes isolates from patients with systemic listeriosis at a comprehensive cancer center (1955-1997). J. Clin. Microbiol. 41:483-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scortti, M., L. Lacharme-Lora, M. Wagner, I. Chico-Calero, P. Losito, and J. A. Vazquez-Boland. 2006. Coexpression of virulence and fosfomycin susceptibility in Listeria: molecular basis of an antimicrobial in vitro-in vivo paradox. Nat. Med. 12:515-517. [DOI] [PubMed] [Google Scholar]
- 36.Seral, C., M. Barcia-Macay, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2005. Comparative activity of quinolones (ciprofloxacin, levofloxacin, moxifloxacin and garenoxacin) against extracellular and intracellular infection by Listeria monocytogenes and Staphylococcus aureus in J774 macrophages. J. Antimicrob. Chemother. 55:511-517. [DOI] [PubMed] [Google Scholar]
- 37.Siefert, H. M., A. Domdey-Bette, K. Henninger, F. Hucke, C. Kohlsdorfer, and H. H. Stass. 1999. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J. Antimicrob. Chemother. 43(Suppl. B):69-76. [DOI] [PubMed] [Google Scholar]
- 38.Sipahi, O. R., T. Turhan, H. Pullukcu, S. Calik, M. Tasbakan, H. Sipahi, B. Arda, T. Yamazhan, and S. Ulusoy. 2008. Moxifloxacin versus ampicillin + gentamicin in the therapy of experimental Listeria monocytogenes meningitis. J. Antimicrob. Chemother. 61:670-673. [DOI] [PubMed] [Google Scholar]
- 39.Stepanovic, S., G. Lazarevic, M. Jesic, and R. Kos. 2004. Meropenem therapy failure in Listeria monocytogenes infection. Eur. J. Clin. Microbiol. Infect. Dis. 23:484-486. [DOI] [PubMed] [Google Scholar]
- 40.Temple, M. E., and M. C. Nahata. 2000. Treatment of listeriosis. Ann. Pharmacother. 34:656-661. [DOI] [PubMed] [Google Scholar]
- 41.Van Bambeke, F., J. M. Michot, J. Van Eldere, and P. M. Tulkens. 2005. Quinolones in 2005: an update. Clin. Microbiol. Infect. 11:256-280. [DOI] [PubMed] [Google Scholar]
- 42.Winslow, D. L., J. Damme, and E. Dieckman. 1983. Delayed bactericidal activity of beta-lactam antibiotics against Listeria monocytogenes: antagonism of chloramphenicol and rifampin. Antimicrob. Agents Chemother. 23:555-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise, R. 2003. Maximizing efficacy and reducing the emergence of resistance. J. Antimicrob. Chemother. 51(Suppl. 1):37-42. [DOI] [PubMed] [Google Scholar]
- 44.Wise, R., J. M. Andrews, G. Marshall, and G. Hartman. 1999. Pharmacokinetics and inflammatory-fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob. Agents Chemother. 43:1508-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. USA 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]