Abstract
Lung microdialysis has been used with rats to investigate antibiotic distribution after single-dose administration. However, conducting such experiments after intravenous infusion at steady state would constitute a more convenient alternative, which was evaluated here, using levofloxacin (LVX) as a test compound. Microdialysis probes were inserted in blood and muscle, used as a comparator, between 9:00 a.m. and 11:00 a.m. Intravenous LVX infusion was started 6 h later and maintained until the end of the experiment at a rate of 1.0 mg·h−1. Lung microdialysis probes were inserted on the morning of the next day. Rats were kept anesthetized during dialysate collection. In vivo probe recoveries were estimated by retrodialysis using a calibrator method, with ciprofloxacin (CIP) as the calibrator. LVX and CIP were analyzed in dialysates by high-performance liquid chromatography. The steady-state tissue-to-blood unbound-drug concentration ratios were 1.00 ± 0.15 in muscle tissues and 1.06 ± 0.40 in lungs, suggesting passive distribution of LVX in tissue. Although providing no information on rate of distribution, microdialysis investigations following drug infusion at steady state appear to be an interesting approach for characterization of antibiotic distribution in rat lungs.
Several different methodological approaches are available for assessing the distribution of antibiotics in the lungs of animals and patients (14). It is now recognized that drug concentrations in whole tissue homogenates are difficult to interpret and therefore not informative (19). In humans, the most popular experimental approach for characterizing antibiotic distribution in lungs relies on bronchoalveolar lavages (BAL) (3, 4, 5), and micro-BAL was recently proposed (2). Microdialysis is an appealing technique that has increasingly been used in recent years to investigate antibiotic distribution in the extracellular fluids (ECFs) of various tissues, including lungs, in both rats and humans (6, 9, 13, 16, 18, 23). One of the major advantages of microdialysis over BAL or micro-BAL is that microdialysis allows multiple determinations for the same subject and therefore full description of the drug concentrations versus the time profile following administration. However, for human lungs, microdialysis studies are limited to patients with elective thoracic surgery, and for rats, these studies require that the animals be kept anesthetized during open chest surgery (6, 18). As a consequence, for antibiotics with long elimination half-lives, it may be difficult to maintain the study long enough to properly characterize major pharmacokinetic parameters, such as total drug concentrations versus areas under the concentration-time curves (AUCs). Comparison of total AUCs for unbound-drug concentrations in lung ECF and plasma may provide important information, such as indirect evidence for the involvement of active efflux transport systems (7). These systems, including P glycoprotein, are present in the brain and are responsible for the lower AUCs for unbound-drug concentrations in brain ECF than in plasma, as previously demonstrated with several anti-infectious drugs by use of microdialysis (8, 10, 12, 17). The presence of P glycoprotein in lungs is suspected (11, 15, 21, 22) and therefore potentially responsible for restricted drug distribution in tissue, although this has never been documented, at least to our knowledge. It should be possible to do that by comparing the total AUCs for unbound-drug concentrations in lung tissue ECF and plasma following single-dose administration, but interestingly, comparison of unbound-drug concentrations following intravenous infusion at steady state provided exactly the same information (7). The advantage of steady-state conditions is that they require a single concentration determination for plasma and tissue, which is much simpler and probably more accurate than determination of AUCs after single-dose administration. The feasibility and optimization of such an alternative approach is now being tested, using levofloxacin (LVX) as a representative antibiotic.
MATERIALS AND METHODS
Chemicals.
Commercial solutions of LVX (5 mg·ml−1; Sanofi Aventis, Paris, France) and ciprofloxacin (CIP) (2 mg·ml−1; Panpharma, Fougères, France) were used for intravenous infusion of rats and probe recovery determinations, respectively. All chemicals used were of analytical grade, and solvents were high-performance liquid chromatography grade.
Animals.
Eight male Sprague Dawley rats from Janvier Laboratories (Le Genest-St-Isle, France), weighing 316 ± 49 g, were used for the lung distribution study. Three extra rats were used for preliminary microdialysis probe recovery assessments. All animals were acclimatized in wire cages with a 12-h light-dark cycle for a minimum of 5 days before the beginning of the experiment to allow them to adjust to the new environment. During this period, they had access to food (A03; Safe, Villemoisson-sur-Orge, France) and water. This work was done in accordance with NIH guidelines (20).
Microdialysis experiment.
The day before the experiments, between 9:00 a.m. and 11:00 a.m., rats were anesthetized by an isoflurane (Forene; Abbot, Rungis, France) air mixture equipped with a polyethylene vein femoral catheter for LVX administration and with CMA/20 microdialysis probes (polycarbonate; cutoff, 20,000 Da; membrane length, 10 mm) (CMA microdialysis, Phymep, Paris, France) in blood and muscle, as previously described (18), for drug concentration determinations. After the rats woke up and at 6 to 7 hours postsurgery, infusion of the 5-mg·ml−1 solution of LVX was started at a rate of 1.0 mg·h−1, maintained overnight to reach steady state, and continued until the end of the experiment. On the morning of day 0, the rats were anesthetized, tracheotomized, mechanically ventilated, and torachotomized for lung microdialysis probe insertion (polyether sulfone; cutoff, 6,000 Da; membrane length, 10 mm; outer diameter, 0.6 mm) (LMP 5.35.35; Microbiotech, Stockholm, Sweden), as previously described (18). Probe recoveries were estimated by retrodialysis using the calibrator method. A solution of CIP (2 μg·ml−1) was perfused into the three probes at a flow rate of 2 μl·min−1 for 15 min. A flow rate of 0.5 μl·min−1 was then maintained for 60 min before dialysate collection and continued until the end of the experiment. Dialysates were collected from blood, muscle, and lung probes every 45 min over 270 min, corresponding to six samples. The relative recovery induced by loss of the calibrator (CIP) was calculated for each collection interval according to the following equation: RLCIP = (Cin − Cout)/Cin, where Cin and Cout correspond to the CIP concentrations in the perfusate and in the dialysates collected, respectively. The actual LVX concentrations were estimated by correcting the measured concentrations in the dialysates by the recovery induced by loss of CIP as determined during the same collection interval. For each rat and medium, the ECF concentrations at steady state are presented as a means ± standard deviations (SD) derived from six consecutive determinations.
The in vitro recoveries induced by gain and loss of CIP and LVX were evaluated with three probes (two CMA/20 probes and one linear probe) for 240 min at a flow rate of 1 μl·min−1 and concentrations of 3 μg·ml−1. In vivo recoveries induced by loss of CIP and LVX were compared for three dedicated rats: A, B, and C. A mixture of the two compounds, each at a concentration of 2 μg·ml−1, was perfused in each probe at a flow rate of 0.5 μl·min−1 for 270 min, and dialysates were collected in fractions every 45 min.
Microdialysis sample analysis.
Simultaneous analysis of LVX and CIP in dialysates was performed by high-performance liquid chromatography, as previously described (17). Dialysates were injected directly after dilution in phosphate buffer (pH = 7) (1/1, vol/vol). Both compounds were analyzed at the same wavelength (excitation λ = 285 nm, emission λ = 490 nm). The between-day variabilities of the assay were characterized on each day of the analysis and were less than 20% and 10% at the two concentration levels (0.125 and 1 μg·ml−1, respectively).
RESULTS
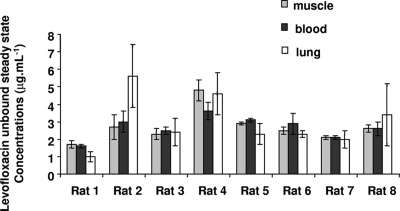
Steady-state conditions preclude retrodialysis by use of the drug method for estimation of probe recoveries, and considering that our objective was to conduct an experiment within a limited period of time, retrodialysis by use of the calibrator method was the best method. CIP had been previously validated as an appropriate calibrator for another fluoroquinolone antibiotic, norfloxacin (17). CIP was therefore tested again as a potential calibrator for LVX. The in vitro recoveries induced by gain and loss of CIP and LVX were not statistically different (data not shown). The differences between the in vivo recoveries induced by loss of LVX and CIP in blood, muscle, and lungs were lower than 7% in all cases except for rat C in the lung (Table 1). The individual values determined for the recoveries induced by CIP loss in rats receiving LVX (rats 1 to 8) are presented in Table 2. The individual steady-state concentrations of unbound LVX in each medium are presented in Fig. 1.
TABLE 1.
In vivo recoveries induced by loss of CIP and LVXa
| Rat | Compound | Recovery (mean % ± SD) for:
|
||
|---|---|---|---|---|
| Blood | Muscle | Lung | ||
| A | LVX | 43.6 ± 5.6 | 38.9 ± 1.7 | 11.4 ± 3.8 |
| CIP | 42.9 ± 5.9 | 35.9 ± 1.5 | 11.3 ± 3.8 | |
| B | LVX | 48.0 ± 3.0 | 36.8 ± 0.7 | 12.8 ± 3.0 |
| CIP | 49.1 ± 3.1 | 36.1 ± 1.8 | 10.9 ± 4.0 | |
| C | LVX | 51.0 ± 2.1 | 36.1 ± 3.9 | 6.9 ± 2.4 |
| CIP | 56.6 ± 2.0 | 39.1 ± 4.4 | 11.9 ± 2.4 | |
CIP and LVX were perfused as a Ringer solution (CIP/LVX mixture at 2 μg·ml−1each) at a flow rate of 0.5 μl·min−1 for 270 min in the blood, muscle, and lung probes of three rats. Values shown are means ± SD for six determinations.
TABLE 2.
In vivo recoveries induced by loss of CIPa
| Rat | Recovery (mean % ± SD) for:
|
||
|---|---|---|---|
| Blood | Muscle | Lung | |
| 1 | 44.6 ± 3.1 | 45.0 ± 3.9 | 31.5 ± 7.5 |
| 2 | 41.0 ± 3.6 | 38.4 ± 4.4 | 9.4 ± 2.5 |
| 3 | 32.6 ± 2.2 | 48.4 ± 2.9 | 22.8 ± 10.0 |
| 4 | 74.6 ± 1.1 | 30.8 ± 1.4 | 18.8 ± 3.5 |
| 5 | 38.4 ± 1.1 | 45.0 ± 1.4 | 10.4 ± 3.3 |
| 6 | 46.3 ± 4.3 | 46.6 ± 2.4 | 12.1 ± 8.2 |
| 7 | 73.4 ± 1.2 | 48.5 ± 1.7 | 15.9 ± 3.9 |
| 8 | 73.4 ± 1.3 | 48.5 ± 1.8 | 15.9 ± 3.1 |
CIP was perfused as a Ringer solution (2 μg·ml−1 and 0.5 μl·min−1) in the blood, muscle, and lung probes of eight rats receiving an intravenous infusion of LVX (1.0 mg·h−1) at steady state. Values shown are means ± SD for six determinations.
FIG. 1.
Mean concentrations of unbound LVX (n = 6) in muscle ECF, blood, and lung ECF samples of eight rats receiving an intravenous infusion of LVX (1.0 mg·h−1) at steady state.
DISCUSSION
CIP recoveries induced by loss in rats treated with LVX were generally consistent with those obtained during the validation phase (rats A to C), although relatively high recoveries were occasionally observed, especially in blood. Recovery induced by loss of CIP in lungs was three- to fourfold lower on average than that in muscle or blood (Table 2), again consistent with observations made for rats A to C as well as with previous experiments (6, 18). These relatively low recoveries observed in lungs are most likely due to the different natures and membrane cutoffs compared to those of the CMA/20 probes. The between-rat variability in lung probe recoveries, with extreme values ranging between 9.2% ± 3.6% and 31.5% ± 7.5%, was also larger than that for other media (Table 2). Together, these data suggest that CIP may be considered an appropriate calibrator for LVX, although discrepancies between the estimated recovery induced by the loss of the calibrator and the actual recovery of the tested compound may occasionally occur, especially in lungs, possibly leading to outlier values for estimated LVX concentrations.
The between-rat variability, like the between-medium variability, was rather limited, and the average steady-state unbound-drug concentrations in the various rats and media compared favorably in all cases except the lung data for rat 2 (Fig. 1). Error bars corresponding to SD associated with these estimated average concentrations (n = 6) were most often higher in lung ECFs than in other media, illustrating the greater uncertainty of concentration estimates for this tissue than for plasma or muscle. This observation, which again is consistent with previous findings (6, 18), is probably a consequence of the decreased lung probe recoveries. Because the flow rate was already quite low (0.5 μl·min−1), it does not seem possible to substantially increase lung probe recovery by further reducing the flow. Using different probes with increased recovery may potentially be of greater benefit.
The ECF steady-state tissue-to-blood unbound-drug concentration ratios were 1.00 ± 0.15 in muscle and 1.06 ± 0.40 in lungs, suggesting passive distribution of LVX in tissue (7). These values are in partial agreement with data previously obtained from humans and published mostly by researchers from the same group (1, 13, 24, 25). The first LVX microdialysis distribution study with humans was conducted on the skeletal muscle tissues of patients with sepsis (24). Unbound-drug concentrations in plasma were derived from measured total plasma concentrations, assuming an average bound fraction of 35%. The tissue-to-plasma unbound-drug AUC ratio from time 0 to 8 h was used to characterize LVX distribution and was estimated at 0.85. The second study was conducted with subcutaneous adipose tissue, using a similar procedure, except that AUCs were estimated from time 0 to 10 h and plasma protein binding was assumed to be 25% (1). Average tissue-to-plasma unbound-drug AUC ratios were again close to unity (1.1 ± 0.6 for healthy and 1.2 ± 1.0 for inflamed subcutaneous adipose tissue). The third article described the first microdialysis study of distribution in lungs (13) and led to the conclusion that LVX penetration in this tissue was less than that in muscle (24) or subcutaneous adipose tissue (1). However, in this study, the AUCs of unbound LVX in lungs were compared to total plasma AUCs, with a mean ratio of 0.6. Therefore, after correcting the plasma AUC for protein binding as previously done by these authors, and considering that 30% of LVX is bound in plasma, one obtains a mean tissue-to-plasma unbound-drug AUC ratio of 1.9, which is actually higher than the previously reported values close to unity (1, 24). The last study was then conducted to clarify the apparently conflicting data between muscle or subcutaneous tissue and lungs (25). Unfortunately, distinct subjects were enrolled in the study, with muscle and subcutaneous-tissue distribution being investigated in healthy volunteers and lung distribution in patients undergoing elective lung surgery. Furthermore, concentrations of unbound drug in tissue as determined by microdialysis were again compared with total plasma concentrations, making interpretation of AUC ratios difficult, especially since LVX plasma protein binding may differ between healthy volunteers and patients. But interestingly, in this last study total AUCs were used to characterize LVX distribution in tissue. Unfortunately, microdialysate samples were collected over an 8-h period of time, which, considering the delayed peak in tissue (between 1 and 3 h) and the relatively long elimination half-life of LVX (estimated at 5 h in this study), does not allow precise estimation of total AUCs. This study led to the conclusion that determining LVX levels in the ECFs of soft tissues cannot serve as a surrogate for predicting its pharmacokinetics in lungs (25), whereas the results of our study, which was conducted on rats but measured LVX concentrations in both tissues of each animal, suggest the opposite.
Microdialysis tissue distribution studies following single-dose administrations should rely on comparison between total AUCs for unbound drug in tissue and plasma (7), but as exemplified by this series of studies conducted with LVX, this is not always done, and it may be difficult to accurately estimate these total AUCs. On many occasions, the AUCs for two consecutive administrations at steady state should be equal to the total AUCs observed after single doses, but these AUCs may not be more practical to estimate. Therefore, comparison of unbound-drug concentrations in tissue ECF and plasma following intravenous infusion at steady state appears to be an interesting alternative for characterizing drug distribution in tissue, particularly in looking for an active efflux transport phenomenon. Although a single concentration measurement in tissue and plasma would be enough from a theoretical standpoint, six consecutive series of determinations were conducted for this initial study. Yet, this was without major benefit since these individual values were always consistent, and the number of determinations should be reduced in future studies. Yet, as opposed to microdialysis studies conducted with multiple drug determinations after single-dose administrations, the major limitation of this proposed approach, based on single-point determination at steady state, is that it does not provide any information on the rate of distribution in tissue, which can be modified in the presence of various pathophysiological conditions, such as altered blood flow.
In conclusion, lung microdialysis investigations following drug infusion at steady state appear to be an interesting alternative to the same type of experiments conducted after single-dose administration for characterization of distribution of antibiotics in lungs.
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Bellmann, R., G. Kuchling, P. Dehghanyar, M. Zeitlinger, E. Minar, B. X. Mayer, M. Müller, and C. Joukhadar. 2004. Tissue pharmacokinetics of levofloxacin in human soft tissue infections. Br. J. Clin. Pharmacol. 57:563-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boselli, E., D. Breilh, S. Djabarouti, C. Guillaume, T. Rimmelé, J. B. Gordien, F. Xuereb, M. C. Saux, and B. Allaouchiche. 2007. Reliability of mini-bronchoalveolar lavage for the measurement of epithelial lining fluid concentrations of tobramycin in critically ill patients. Intensive Care Med. 33:1519-1523. [DOI] [PubMed] [Google Scholar]
- 3.Conte, J. E., Jr., J. A. Golden, M. G. Kelley, and E. Zurlinden. 2005. Intrapulmonary pharmacokinetics and pharmacodynamics of meropenem. Int. J. Antimicrob. Agents 26:449-456. [DOI] [PubMed] [Google Scholar]
- 4.Conte, J. E., Jr., J. A. Golden, M. McIver, and E. Zurlinden. 2006. Intrapulmonary pharmacokinetics and pharmacodynamics of high-dose levofloxacin in healthy volunteer subjects. Int. J. Antimicrob. Agents 28:114-121. [DOI] [PubMed] [Google Scholar]
- 5.Conte, J. E., Jr., J. A. Golden, M. McIver, E. Little, and E. Zurlinden. 2007. Intrapulmonary pharmacodynamics of high-dose levofloxacin in subjects with chronic bronchitis or chronic obstructive pulmonary disease. Int. J. Antimicrob. Agents 30:422-427. [DOI] [PubMed] [Google Scholar]
- 6.Dahyot, C., S. Marchand, G. L. Pessini, C. Pariat, B. Debaene, W. Couet, and O. Mimoz. 2006. Microdialysis study of imipenem distribution in skeletal muscle and lung extracellular fluids of Acinetobacter baumannii-infected rats. Antimicrob. Agents Chemother. 50:2265-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahyot, C., S. Marchand, M. Bodin, B. Debaene, O. Mimoz, and W. Couet. 2008. Application of basic pharmacokinetic concepts to analysis of microdialysis data: illustration with imipenem muscle distribution. Clin. Pharmacokinet. 47:181-189. [DOI] [PubMed] [Google Scholar]
- 8.de Lange, E. C., S. Marchand, D. van den Berg, I. C. van der Sandt, A. G. de Boer, A. Delon, S. Bouquet, and W. Couet. 2000. In vitro and in vivo investigations on fluoroquinolones; effects of the P-glycoprotein efflux transporter on brain distribution of sparfloxacin. Eur. J. Pharm. Sci. 12:85-93. [DOI] [PubMed] [Google Scholar]
- 9.de la Peña, A., T. Dalla Costa, J. D. Talton, E. Rehak, J. Gross, U. Thyroff-Friesinger, A. I. Webb, M. Müller, and H. Derendorf. 2001. Penetration of cefaclor into the interstitial space fluid of skeletal muscle and lung tissue in rats. Pharm. Res. 18:1310-1314. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, J. E., K. R. Brouwer, and P. J. McNamara. 2002. GF120918, A P-glycoprotein modulator, increases the concentration of unbound amprenavir in the central nervous system in rats. Antimicrob. Agents Chemother. 46:2284-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endter, S., U. Becker, N. Daum, H. Huwer, C. M. Lehr, M. Gumbleton, and C. Ehrhadt. 2007. P-glycoprotein (MDR1) functional activity in human alveolar epithelial cell monolayers. Cell Tissue Res. 328:77-84. [DOI] [PubMed] [Google Scholar]
- 12.Granero, L., M. Santiago, J. Cano, A. Machado, and J. E. Peris. 1995. Analysis of ceftriaxone and ceftazidime distribution in cerebrospinal fluid of and cerebral extracellular space in awake rats by in vivo microdialysis. Antimicrob. Agents Chemother. 39:2728-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutschala, D., K. Skhirtladze, A. Zuckermann, W. Wisser, P. Jaksch, B. X. Mayer-Helm, H. Burgmann, E. Wolner, M. Müller, and E. M. Tschernko. 2005. In vivo measurement of levofloxacin penetration into lung tissue after cardiac surgery. Antimicrob. Agents Chemother. 49:5107-5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiem, S., and J. J. Schentag. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie, E. M., R. G. Deeley, and S. P. Cole. 2005. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 204:216-237. [DOI] [PubMed] [Google Scholar]
- 16.Liu, P., M. Muller, M. Grant, A. I. Webb, B. Obermann, and H. Derendorf. 2002. Interstitial tissue concentrations of cefpodoxime. J. Antimicrob. Chemother. 50(Suppl.):19-22. [DOI] [PubMed] [Google Scholar]
- 17.Marchand, S., M. Chenel, I. Lamarche, C. Pariat, and W. Couet. 2003. Dose ranging pharmacokinetics and brain distribution of norfloxacin using microdialysis in rats. J. Pharm. Sci. 92:2458-2465. [DOI] [PubMed] [Google Scholar]
- 18.Marchand, S., C. Dahyot, I. Lamarche, O. Mimoz, and W. Couet. 2005. Microdialysis study of imipenem distribution in skeletal muscle and lung extracellular fluids of noninfected rats. Antimicrob. Agents Chemother. 49:2356-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouton, J. W., U. Theuretzbacher, W. A. Craig, P. M. Tulkens, H. Derendorf, and O. Cars. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235-237. [DOI] [PubMed] [Google Scholar]
- 20.National Institutes of Health. 1985. Principles of laboratory animal care. NIH publication 85-23. National Institutes of Health, Bethesda, MD.
- 21.Scheffer, G. L., A. C. Pijnenborg, E. F. Smit, M. Müller, D. S. Postma, W. Timens, P. van der Valk, E. G. de Vries, and R. J. Scheper. 2002. Multidrug resistance related molecules in human and murine lung. J. Clin. Pathol. 55:332-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steimer, A., H. Franke, E. Haltner-Ukomado, M. Laue, C. Ehrhardt, and C. M. Lehr. 2007. Monolayers of porcine alveolar epithelial cells in primary culture as an in vitro model for drug absorption studies. Eur. J. Pharm. Biopharm. 66:372-382. [DOI] [PubMed] [Google Scholar]
- 23.Zeitlinger, M., M. Müller, and C. Joukhadar. 2005. Lung microdialysis—a powerful tool for the determination of exogenous and endogenous compounds in the lower respiratory tract. AAPS J. 7:E600-E608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeitlinger, M. A., P. Dehghanyar, B. X. Mayer, B. S. Schenk, U. Neckel, G. Heinz, A. Georgopoulos, M. Müller, and C. Joukhadar. 2003. Relevance of soft-tissue penetration by levofloxacin for target site bacterial killing in patients with sepsis. Antimicrob. Agents Chemother. 47:3548-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeitlinger, M. A., F. Traunmüller, A. Abrahim, M. R. Müller, Z. Erdogan, M. Müller, and C. Joukhadar. 2007. A pilot study testing whether concentrations of levofloxacin in interstitial space fluid of soft tissues may serve as a surrogate for predicting its pharmacokinetics in lung. Int. J. Antimicrob. Agents 29:44-50. [DOI] [PubMed] [Google Scholar]