Abstract
The reverse transcriptase (RT) inhibitor tenofovir (TFV) is highly effective in the simian immunodeficiency virus (SIV) macaque model of human immunodeficiency virus infection. The current report describes extended safety and efficacy data on 32 animals that received prolonged (≥1- to 13-year) daily subcutaneous TFV regimens. The likelihood of renal toxicity (proximal renal tubular dysfunction [PRTD]) correlated with plasma drug concentrations, which depended on the dosage regimen and age-related changes in drug clearance. Below a threshold area under the concentration-time curve for TFV in plasma of ∼10 μg·h/ml, an exposure severalfold higher than that observed in humans treated orally with 300 mg TFV disoproxil fumarate (TDF), prolonged TFV administration was not associated with PRTD based on urinalysis, serum chemistry analyses, bone mineral density, and clinical observations. At low-dose maintenance regimens, plasma TFV concentrations and intracellular TFV diphosphate concentrations were similar to or slightly higher than those observed in TDF-treated humans. No new toxicities were identified. The available evidence does not suggest teratogenic effects of prolonged low-dose TFV treatment; by the age of 10 years, one macaque, on TFV treatment since birth, had produced three offspring that were healthy by all criteria up to the age of 5 years. Despite the presence of viral variants with a lysine-to-arginine substitution at codon 65 (K65R) of RT in all 28 SIV-infected animals, 6 animals suppressed viremia to undetectable levels for as long as 12 years of TFV monotherapy. In conclusion, these findings illustrate the safety and sustained benefits of prolonged TFV-containing regimens throughout development from infancy to adulthood, including pregnancy.
Because at present it is not possible to cure human immunodeficiency virus (HIV) infection, prolonged treatment with combinations of anti-HIV drugs is required in order to prevent disease progression. The feasibility of giving HIV-infected persons a normal life span through chronic treatment will be determined by a number of factors, including compliance and cost, but especially the long-term safety and efficacy of the drugs.
The nucleotide reverse transcriptase (RT) inhibitor tenofovir {TFV; 9-[2-(phosphonomethoxy)propyl]adenine}, in the form of its orally bioavailable prodrug TFV disoproxil fumarate (TDF), has become one of the most commonly used anti-HIV drugs due to its favorable efficacy and safety profile, based on data collected over more than 7 years for HIV-infected adults (12, 24, 35, 37, 47, 49).
The acyclic nucleoside phosphonates cidofovir, adefovir, and TFV are renally excreted by a combination of glomerular filtration and active tubular secretion (14, 15, 18, 38). The effective uptake of acyclic nucleoside phosphonates by organic anion transporters in proximal tubules leads to accumulation in tubular cells and dose-limiting toxicity in animals (5, 63). Renal toxicity is usually manifested as renal insufficiency and proximal renal tubular dysfunction (PRTD). During clinical trials of TDF, the frequency of clinically significant renal changes was very low among populations with normal renal values at baseline (and not different from the frequencies seen with other highly active antiretroviral therapy regimens [24, 27, 37, 49, 53, 69]); furthermore, renal toxicity has been observed infrequently through continued clinical monitoring as described in case reports and cohort study reports (8, 21, 22, 27, 35, 37, 44, 47, 48, 53, 70, 71). Some reports on TDF-treated populations describe small reductions in creatinine clearance that remained within the normal range and were thus of uncertain clinical significance (22, 24, 25, 37, 40, 70). Prolonged TDF treatment of HIV-infected adults was associated with a small decrease in bone mineral density (BMD) during the first 48 weeks of treatment, but this decrease was nonprogressive (through week 288) and was not associated with any clinical symptoms (12, 24). These decreases were associated with markers of bone metabolism suggesting a slight increase in bone turnover (full prescribing information for Viread [TDF]; available from Gilead Sciences). Some but not all reports have described a similar loss in BMD in children who received TDF-containing regimens (23, 26, 30).
Because of many similarities in host physiology and disease pathogenesis, simian immunodeficiency virus (SIV) infection of macaques is a well-established and valuable animal model of HIV infection for testing of many aspects of drug treatment, including efficacy and safety (66). Studies with this animal model have demonstrated that a once-daily dosage regimen of TFV was highly efficacious for prophylaxis and therapy and still had partial benefit in the presence of viral variants with a lysine-to-arginine mutation at codon 65 (K65R) in RT, associated with approximately fivefold-reduced susceptibility to TFV in vitro (57, 59-62, 65, 67). Previously we reported that prolonged treatment of macaques with a high dose of TFV (30 mg/kg of body weight subcutaneously once daily) resulted in PRTD, a Fanconi-like syndrome characterized by glucosuria, aminoaciduria, hypophosphatemia, and bone pathology; these effects were completely or partially reversible following drug withdrawal or dosage reduction, respectively (63). In contrast, chronic administration of a low dose of TFV (10 mg/kg subcutaneously once daily) starting at birth did not seem to be associated with any adverse health effects within 3 years of treatment (63).
While some limited early data have been published previously (63), the current report summarizes extended observations on the pharmacokinetics, safety, and efficacy of prolonged TFV regimens in the macaque model, including during pregnancy. The new analysis allows us to refine the therapeutic window of chronic TFV exposure that balances antiviral efficacy with a favorable safety profile. These observations in the macaque model support the long-term treatment of HIV-infected humans with TDF-containing regimens.
MATERIALS AND METHODS
Animals, virus inoculation, and TFV administration.
All animals were rhesus macaques (Macaca mulatta) from the type D retrovirus-free and simian T-cell-lymphotropic virus type 1-free colony at the California National Primate Research Center (CNPRC). Newborn macaques were hand reared in a primate nursery in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care. We adhered strictly to the Guide for the Care and Use of Laboratory Animals, prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council (46). When necessary, animals were immobilized with ketamine HCl (Parke-Davis, Morris Plains, NJ) at 10 mg/kg injected intramuscularly. Blood samples were collected regularly for monitoring viral and immunologic parameters as described previously (65). Complete blood counts were performed on EDTA-anticoagulated blood samples. Samples were analyzed using an automated electronic cell counter (Baker 9000; Serono Baker Diagnostics) and, from November 2002 onward, a Pentra 60C+ analyzer (ABX Diagnostics); differential cell counts were determined manually. Three-color and four-color flow cytometry techniques (including CD3, CD4, CD8, and CD20) were performed as described previously (58-60, 65).
The animals described in this report included all 32 animals that were given subcutaneous TFV treatment for ≥1 year in experiments starting from 1995 onward, and their offspring. Twenty-eight of these 32 animals were infected with SIV or RT-SHIV (a chimeric SIV containing HIV type 1 [HIV-1] RT) either at birth or at a juvenile age. More-detailed descriptions of the viral inoculum stocks and the early virological and immunological data have been published previously for all of these animals (58-60, 65). Plasma viral RNA concentrations in these animals were determined by a quantitative branched-chain DNA (bDNA) assay for SIV-infected animals or by a real-time reverse transcription-PCR (RT-PCR) assay for SIV gag, as described previously (58, 65).
TFV powder (supplied by Gilead Sciences, Inc., Foster City, CA) was suspended in distilled water, dissolved by addition of NaOH to a final pH of 7.0 at a final concentration of 40 or 60 mg/ml, and filter sterilized (pore size, 0.2 μm; Nalgene, Rochester, NY). Although it is very stable at ambient temperatures, the TFV solution was stored at 4°C, and new stock solutions were prepared every few months. TFV was administered once per day subcutaneously into the back of the animal, at dosage regimens described below. Unless indicated otherwise, TFV dosages were adjusted weekly according to body weight.
As explained in Results, following the onset of renal toxicity and hypophosphatemia, some animals were administered a phosphate supplement once or twice daily; depending on the animal's weight, each dose consisted of 1 or 2 packages of Neutra-Phos (250 mg phosphorus, 164 mg sodium, and 278 mg potassium per package; Baker Norton Pharmaceuticals, Miami, FL) dissolved in a 100-ml solution of Tang (Kraft Foods). A pediatric multivitamin tablet with 200 mg calcium (CentrumKids Extra Calcium; Lederle, Madison, NJ) and/or one-quarter of an Amino Fuel tablet (Twin Laboratories Inc., Ronkonkoma, NY) with amino acids was administered to some animals once daily.
Serum chemistry panels.
Standard chemistry panels (including sodium, potassium, chloride, calcium, total CO2, anion gap, calcium, phosphorus, creatinine, blood urea nitrogen, glucose, alanine aminotransferase, alkaline phosphatase, total protein, albumin, gamma-glutamyltransferase, creatine phosphokinase, aspartate transaminase, total bilirubin, lactate dehydrogenase, cholesterol, and triglycerides) were performed with the Dacos (Coulter Electronics, Hialeah, FL) or the Hitachi 717 (Roche Biomedical, Indianapolis, IN) system. Serum samples were generally collected from animals after overnight fasting.
Urine analysis.
Urine was collected by cystocentesis. Urine was analyzed for specific gravity by a refractometer; the pH and the presence of protein, glucose, ketone, bilirubin, and occult blood were determined using Multistix strips (Bayer Corporation, Elkhart, IN). Urine sediments were examined microscopically.
Radiographs and DXA.
Radiographs were taken with a Thompson CGR X-ray unit (model SPG 515S) with the following settings: 100 mA, 0.10 s, 46 to 54 kVp (kilovoltpeak). Dual-energy X-ray absorptiometry (DXA) scans were performed with a Norland Eclipse compact DXA system. Animals were placed in standardized positions for scans. The bone mineral content (BMC), bone mineral area (BMA), and BMD (calculated as BMC/BMA) were determined for each site. Machine software calculates BMC based on the X-ray absorption characteristics of the tissue and determines BMA by calculating the projected bone area using an edge detection algorithm to find the bone margins within the DXA image. Measurement sites included lumbar vertebrae 2 to 4 (L2 to L4), distal radius and ulna (DR+U), femoral neck, and global proximal femur.
TFV pharmacokinetics.
Blood was collected by venipuncture at various times (0, 0.5, 1, 2, 4, 8, and 24 h; in some experiments, blood was also collected at 6 h) after subcutaneous TFV administration and was spun for 10 min at 900 × g to separate the plasma or serum from the cells. Plasma and serum were immediately stored at −70°C. At selected time points, peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient separation (lymphocyte separation medium; MP Biomedicals, Aurora, OH) and washed in a cold (+4°C) 0.9% NaCl solution. To lyse contaminating red blood cells, previously published techniques were used (20). Briefly, the cells were incubated for 2 min with 2 ml of ammonium salt solution (3.5 g ammonium chloride and 36 mg ammonium carbonate per 500 ml water) and then washed twice with cold 0.9% NaCl, and the cell pellets were stored at −70°C until analysis.
Concentrations of TFV in plasma or serum were determined either by a validated high-performance liquid chromatography (HPLC) method with fluorescence derivatization with a limit of quantitation (LOQ) of 25 ng/ml (54) or by a validated HPLC coupled to a positive-ion electrospray tandem mass spectrometry (LC-MS-MS) detection method with an LOQ of 3.7 ng/ml (63).
Plasma was prepared by protein precipitation by the addition of 2 equivalents of acetonitrile containing 0.2% formic acid and 150 nM adefovir (Gilead Sciences), which was chosen as an internal standard. Following filtration, samples were dried and reconstituted in mobile phase A (0.2% formate in water) before analysis using LC-MS-MS. Chromatography was performed on a Synergi Hydro RP-80 Å 4-μm, 75- by 2.0-mm column (Phenomenex, Torrance, CA). Analytical separation was accomplished using 0.2% formate as a buffer and a multistage gradient between 1% and 95% acetonitrile over 3.5 min by maintaining a flow rate of 250 μl/min with a Shimadzu LC-20AD tertiary pump system (Shimadzu Scientific Instruments, Columbia, MD). The column was reequilibrated for 1 min before injection of the next sample. Parent/daughter mass-to-charge (m/z) transitions of 288.2/176.1 and 274.1/162.0 (resolution in atomic mass units) for TFV and adefovir (internal standard) were monitored on an API-4000 triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA). Concentrations were determined based on calibration curves (1/x, weighted) with linearity in excess of an r2 of 0.99 and with a typical LOQ of 3.7 ng/ml.
Pharmacokinetic parameters were derived by noncompartmental analysis using WinNonlin, version 2.1, 3.1, or 5.0.1 (Pharsight Corporation, Mountain View, CA). While the TFV bioavailability (F) after subcutaneous administration is expected to be ∼100%, all clearance values in this report refer to apparent clearance (CL/F) rather than absolute clearance (CL).
Quantitation of intracellular TFV-DP.
PBMC samples were lysed in 0.5 ml of cold 70% methanol. Following centrifugation, cell debris was separated from the PBMC extract and used to evaluate the exact number of cells by using a validated biochemical assay as previously described (6). A 225-μl aliquot of lysed PBMC was used for direct quantitation of TFV diphosphate (TFV-DP). The PBMC extract was dried and resuspended before analysis in 1 mM phosphate buffer containing 225 nM 2-chloro-ATP (Sigma-Aldrich, St. Louis, MO), chosen as an internal standard. Intracellular TFV-DP concentrations were determined by LC-MS-MS using a method derived from previously described ion-pairing and low-flow nucleotide detection methods (50, 68). Chromatography was performed on an Acquity BEH-C18 1.7-μm, 2.1- by 5.0-mm column with additional use of a VanGuard precolumn (Waters, Wexford, Ireland). Analytical separation was accomplished using 3 mM formate and 10 mM 1,5-dimethylhexylamine (Sigma-Aldrich, St. Louis, MO) as an ion-pairing buffer and a multistage gradient between 5% and 75% acetonitrile over 6.0 min by maintaining a flow rate of 100 μl/min with a Shimadzu LC-20AD tertiary pump system. The column was washed for 1 min, followed by reequilibration for 2.5 min before injection of the next sample. Parent/daughter m/z transitions of 448.1/176.1 and 542.0/169.9 (resolution in atomic mass units) for TFV-DP and 2-chloro-ATP (internal standard) were monitored on an API-4000 triple-quadrupole mass spectrometer. Concentrations were determined based on calibration curves (1/x, weighted) with linearity in excess of an r2 of 0.99 and with a typical lower limit of quantification of 39.1 fmol/sample. This value was then divided by the number of cells per sample (typically ∼1 million) to obtain the final concentration in femtomoles per million cells.
Measurement of SIV-specific cell-mediated immune responses.
Flow cytometry assays with intracellular cytokine staining were performed according to standard protocols using 1 × 106 cells per stimulus in RPMI with 10% heat-inactivated fetal bovine serum. A peptide pool of SIVmac239gag p27 was used at 5 μg/ml; aldrithiol-2 (AT-2)-inactivated SIVmac239 was used at 300 ng/ml. Cultures were stimulated with Staphylococcus enterotoxin B (200 ng/ml) as a positive control. Negative-control cultures consisted of medium only. Costimulatory antibodies to CD49d and CD28 were added, and cultures were incubated at 37°C under 5% CO2 for 6 h (10). Brefeldin A (10 μg) was added 1 h after the start of the incubation. Monensin was used instead of brefeldin A to assess the cytotoxic function of T cells by measuring the surface expression of CD107a/b (7). Data were acquired (300,000 lymphocyte events) on a FACS ARIA instrument (Becton Dickinson) and analyzed using FlowJo software, version 8.1 (TreeStar, Ashland, OR). Data were reported as frequencies of positive CD4+ or CD8+ T cells and were considered positive if the value was ≥2-fold the medium-only value.
Statistical analysis.
Statistical analyses were performed with Prism 4 for Mac and Instat 3 (GraphPad Software Inc., San Diego, CA). Statistical analysis of disease-free survival was done using a log rank test. A P value of <0.05 was considered statistically significant.
RESULTS
Physiologic age-related changes in systemic TFV clearance.
To explore normal age-related changes in systemic TFV CL/F, a single dose of TFV (10 or 30 mg/kg) was administered subcutaneously to 23 uninfected animals of different ages, and plasma samples were collected over a 24-h period; 2 animals were given two doses of TFV (10 mg/kg subcutaneously) 19 months apart. TFV clearance was low at birth but then increased rapidly over the first year of life to maximum levels (∼1,000 ml/h/kg). When juvenile animals matured into adulthood as assessed by both age and weight, TFV clearance decreased to reach normal levels of approximately 400 to 750 ml/h/kg (Fig. 1A and B). For comparison, the renal blood flow and glomerular filtration rate in rhesus macaques of 5 kg have been reported to be 1,650 ml/h/kg and 125 ml/h/kg, respectively (17).
FIG. 1.
Changes in TFV clearance: effects of age and dosage regimens. Twenty-four-hour pharmacokinetic studies with subcutaneous administration of TFV were performed. (A and B) Weight- and age-related changes in TFV CL/F, respectively, following a single-dose administration of 10 or 30 mg/kg (subcutaneously) to 23 healthy, uninfected macaques of different ages; 2 of these 23 animals (male 31007 and female 31456) were given two doses of TFV (10 mg/kg,= subcutaneously) 19 months apart. (C) TFV CL/F in animals that received prolonged TFV regimens (once daily subcutaneously; regimens are outlined in Table 1). Pharmacokinetic data were collected either from animals that never showed PRTD (blue) or from animals prior to (red) or after (black) the development of PRTD. Animal 33091 had glucosuria at a single time point when the TFV clearance was 400 ml/h/kg, but this disappeared when clearance increased again after dosage reduction. (D) An overlay of graphs B and C with animals grouped according to the presence of PRTD demonstrates that animals that received chronic TFV treatment and never showed signs of PRTD throughout their observation periods had apparent TFV clearances indistinguishable from those of animals that received a single dose. Based on the available data, the horizontal dotted line indicates the arbitrary cutoff value of reduced TFV clearance (<400 ml/h/kg) that was associated with PRTD in juvenile and adult animals.
Pharmacokinetics and toxicity during prolonged TFV treatment. (i) Animal population.
Thirty-two animals that were part of several different studies had in common that they received uninterrupted daily administration of TFV for at least 1 year. While early data on 11 animals have been reported previously (63), the summary in Table 1 includes additional animals and extended data. While 4 of the 32 animals were uninfected and were used to study only the safety of prolonged TFV administration, the remaining 28 animals were from studies that investigated the long-term therapeutic efficacy of TFV monotherapy against SIV or RT-SHIV infection.
TABLE 1.
Summary of regimens of prolonged subcutaneous TFV administration to macaques
| Animal informationa
|
TFV dosage regimenb
|
PKc
|
Clinical outcome
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen and animal no. | Sex | SIV infection status | Age at start or change of treatment | Wt at start or change of treatment (kg) | TFV dose | Age at time of PK (mo) | Wt at time of PK (kg) | Cmax (μg/ml) | AUC (μg·h/ml) | TFV CL/F (ml/h/kg) | Onset (age) and patternd of:
|
Health status | |
| Glucosuria | Hypophosphatemia | ||||||||||||
| Regimens of only ≤10 mg/kg | |||||||||||||
| 31121* | M | − | 2 days | 0.6 | 10 mg/kg | 10 | 2.1 | 16.2 | 17.3 | 578 | Neg | Neg | |
| 30 | 4.3 | 17.4 | 18.0 | 556 | Neg | Neg | |||||||
| 40 | 6.0 | 26.8 | 48.4 | 206 | 40 mo | Neg | |||||||
| 42 mo | 5.8 | 5 mg/kg | 46 | 6.4 | 10.3 | 16.7 | 300 | Persistent | Neg | ||||
| 62 mo | 8.5 | 2.5 mg/kg | 63 | 8.5 | 5.2 | 11.6 | 216 | Persistent | Neg | ||||
| 63 mo | 8.4 | 1.25 mg/kg | Persistent | 75 mo | |||||||||
| 75 mo | 9.0 | Stopped | Intermittent | Intermittent | |||||||||
| 99 mo | 11.3 | 1.3 mg/kg (single dose) | 100 | 11.3 | 3.1 | 11.0 | 120 | Intermittent trace | Intermittent | Healthy at 10 yr | |||
| 31122* | F | − | 1 day | 0.5 | 10 mg/kg | 10 | 1.8 | 10.2 | 9.6 | 1,045 | Neg | Neg | |
| 30 | 3.8 | 10.0 | 9.9 | 1,015 | Neg | Neg | |||||||
| 40 | 4.8 | 16.0 | 12.2 | 821 | Neg | Neg | |||||||
| 46 | 5.4 | 14.9 | 15.7 | 635 | Neg | Neg | |||||||
| 64 | 8.4 | 8.2 | 15.0 | 667 | Neg | Neg | |||||||
| 83 mo | 7.70 | Constant 75 mg | 100 | 7.80 | 8.77 | 18.9 | 520 | Neg | Neg | ||||
| 109 mo | 7.20 | Constant 19 mg | 111 | 6.70 | 4.13 | 6.0 | 470 | Neg | Neg | Healthy at 10 yr | |||
| 31042* | F | + | 3 wk | 0.5 | 10 mg/kg | 28 | 2.6 | 10.4 | 13.1 | 765 | Neg | AIDS at 42 mo | |
| Regimens of >10 mg/kg; no PRTD | |||||||||||||
| 30576 | M | + | 24 mo | 3.2 | 10 mg/kg | Neg | |||||||
| 29 mo | 3.4 | 20 mg/kg | 34 | 3.7 | 18.3 | 18.8 | 1,064 | Neg | Neg | ||||
| 44 mo | 4.9 | 10 mg/kg | Neg | Neg | AIDS at 60 mo | ||||||||
| 33093 | M | + | 3 mo | 0.8 | 30 mg/kg | Neg | Neg | ||||||
| 7 mo | 0.9 | 20 mg/kg | Neg | Neg/NE | |||||||||
| 14 mo | 1.4 | 10 mg/kg | Neg | NE | AIDS at 17 mo | ||||||||
| 32989 | M | + | 4 mo | 1.4 | 30 mg/kg | 7 mo (single trace) | Neg | ||||||
| 7 mo | 2.1 | 20 mg/kg | Neg | Neg | |||||||||
| 12 mo | 2.1 | 10 mg/kg | Neg | Neg/NE | AIDS at 21 mo | ||||||||
| 32993 | M | + | 13 mo | 2.3 | 30 mg/kg | Neg | Neg | ||||||
| 15 mo | 2.6 | 20 mg/kg | Neg | Neg | |||||||||
| 16 mo | 2.7 | 10 mg/kg | Neg | Neg/NE | AIDS at 32 mo | ||||||||
| 32137 | M | + | 17 mo | 2.9 | 30 mg/kg | Neg | |||||||
| 19 mo | 3.1 | 20 mg/kg | 20 mo (single trace) | Neg | |||||||||
| 20 mo | 3.3 | 10 mg/kg | 28 | 3.8 | 12.0 | 11.9 | 840 | Neg | Neg | AIDS at 51 mo | |||
| 33088 | M | + | 12 mo | 2.1 | 30 mg/kg | Neg | Neg | ||||||
| 14 mo | 2.5 | 20 mg/kg | Neg | Neg | |||||||||
| 15 mo | 2.7 | 10 mg/kg | Neg | Neg | |||||||||
| 19 mo | 3.0 | Stoppede | Neg | Neg | |||||||||
| 20 mo | 3.1 | Restart, 10 mg/kg | Neg | Neg | |||||||||
| 49 mo | 6.8 | Constant 70 mg | 77 | 7.9 | 8.4 | 15.3 | 590 | Neg | Neg | Healthy at 7 yr | |||
| 33091 | M | + | 12 mo | 2.3 | 30 mg/kg | Neg | Neg | ||||||
| 14 mo | 2.6 | 20 mg/kg | Neg | Neg | |||||||||
| 15 mo | 2.8 | 10 mg/kg | Neg | Neg | |||||||||
| 19 mo | 3.1 | Stoppede | Neg | Neg | |||||||||
| 20 mo | 3.6 | Restart, 10 mg/kg | Neg | Neg | |||||||||
| 49 mo | 7.5 | Constant 75 mg | 65 | 9.0 | 8.8 | 19.4 | 400 | 75 mo (single low) | Neg | ||||
| 75 mo | 9.2 | Constant 19 mg | 77 | 9.2 | 2.21 | 4.4 | 460 | Neg | Neg | Healthy at 7 yr | |||
| Regimens of >10 mg/kg; PRTD | |||||||||||||
| 29003* | M | + | 3 wk | 0.5 | 30 mg/kg | 22 mo | 22 mo | Severe bone lesions | |||||
| 22 mo | 1.8 | Stopped | Neg | Neg | Resolution of bone lesions but AIDS at 41 mo | ||||||||
| 29049* | M | − | 3 days | 0.6 | 30 mg/kg | 16 mo | 7 mo | Severe bone lesions at 16 mo | |||||
| 29055* | M | + | 3 wk | 0.7 | 30 mg/kg | 20 mo | 12 mo | AIDS at 20 mo; moderate bone lesions | |||||
| 29278* | M | + | 3 wk | 0.5 | 30 mg/kg | 13 mo | 13 mo | Severe bone lesions | |||||
| 13.5 mo | 1.1 | 30 mg/kg 2 times per wk | Persistent | Persistent | AIDS at 21 mo | ||||||||
| 29279* | M | + | 3 wk | 0.6 | 30 mg/kg | 13 mo | 13 mo | Moderate bone lesions | |||||
| 16 mo | 1.8 | 10 mg/kg | Intermittent | Intermittent | AIDS at 22 mo | ||||||||
| 28006* | M | + | 22 mo | 3.0 | 30 mg/kg | 39 mo | 38 mo | AIDS at 39 mo; moderate bone lesions | |||||
| 27999* | M | + | 28 mo | 2.7 | 30 mg/kg | 39 | 2.8 | 61.0 | 222.0 | 135 | 39 mo | 39 mo | Euthanized at 41 mo |
| 29008* | M | + | 3 wk | 0.6 | 30 mg/kg | 25 | 2.1 | 59.0 | 101.0 | 297 | 24 mo | 23 mo | |
| 26 mo | 2.3 | 10 mg/kg | Persistent | Persistent | AIDS at 41 mo | ||||||||
| 29045* | M | + | 3 wk | 0.7 | 30 mg/kg | 24 | 2.5 | 66.0 | 124.0 | 241 | 25 mo | 22 mo | |
| 25 mo | 2.8 | 10 mg/kg | 63 | 5.7 | 29.0 | 94.0 | 106 | Persistent | Persistent/PS | ||||
| 67 mo | 6.6 | 5 mg/kg | Persistent | Intermittent/PS | |||||||||
| 74 mo | 9.2 | 2.5 mg/kg | 83 | 9.5 | 9.1 | 31.0 | 80 | Persistent | Intermittent/PS | Euthanasia at 7 yr (unrelated cause) | |||
| 33109 | M | + | 3 mo | 1.2 | 30 mg/kg | 8 mo | Neg | ||||||
| 7 mo | 1.5 | 20 mg/kg | Intermittent | Neg | |||||||||
| 14 mo | 1.8 | 10 mg/kg | Persistent | NE | AIDS at 26 mo | ||||||||
| 30162 | M | + | 33 mo | 4.8 | 10 mg/kg | 35 mo | 38 mo | ||||||
| 38 mo | 4.7 | 5 mg/kg | Persistent | Persistent | |||||||||
| 39 mo | 4.6 | 2.5 mg/kg | 44 | 5.3 | 6.1 | 11.0 | 230 | Persistent | Intermittent/PS | ||||
| 59 | 8.0 | 7.3 | 18.2 | 137 | Persistent | Intermittent/PS | AIDS at 76 mo | ||||||
| 30007 | F | + | 33 mo | 4.7 | 10 mg/kg | Neg | |||||||
| 38 mo | 4.6 | 20 mg/kg | 42 mo | 41 mo | |||||||||
| 44 mo | 4.6 | 10 mg/kg | 45 | 4.5 | 21.1 | 44.9 | 223 | Persistent | Persistent/PS | AIDS at 53 wks | |||
| 30338 | M | + | 32 mo | 4.5 | 10 mg/kg | Neg | |||||||
| 37 mo | 5.2 | 20 mg/kg | 41 | 6.0 | 24.4 | 33.2 | 603 | 53 mo | 51 mo/NE | ||||
| 53 mo | 6.4 | 10 mg/kg | Neg | NE | |||||||||
| 57 mo | 6.0 | 5 mg/kg | Neg | NE | AIDS at 61 mo | ||||||||
| 30339 | M | + | 32 mo | 3.9 | 10 mg/kg | Neg | |||||||
| 37 mo | 4.0 | 20 mg/kg | 41 | 4.6 | 19.8 | 26.5 | 755 | 51 mo | 53 mo | ||||
| 53 mo | 6.0 | 10 mg/kg | Persistent | NE | |||||||||
| 57 mo | 6.2 | 5 mg/kg | Neg | NE | AIDS at 62 mo | ||||||||
| 30340 | M | + | 32 mo | 4.3 | 10 mg/kg | Neg | |||||||
| 37 mo | 4.8 | 20 mg/kg | 41 | 5.6 | 21.7 | 31.5 | 634 | Neg | 48 mo | ||||
| 52 mo | 6.5 | 10 mg/kg | Neg | Intermittent | AIDS at 64 mo | ||||||||
| 30343 | F | + | 32 mo | 4.5 | 10 mg/kg | Neg | |||||||
| 37 mo | 4.5 | 20 mg/kg | 41 | 4.9 | 21.0 | 24.0 | 834 | 53 mo | 48 mo | ||||
| 53 mo | 5.4 | 10 mg/kg | Neg | Neg | AIDS at 62 mo | ||||||||
| 30581 | M | + | 24 mo | 3.3 | 10 mg/kg | Neg | |||||||
| 29 mo | 3.4 | 20 mg/kg | 32 | 3.6 | 18.2 | 21.2 | 945 | 36 mo | 42 | ||||
| 42 mo | 4.6 | 10 mg/kg | Persistent | Persistent | |||||||||
| 48 mo | 5.3 | 5 mg/kg | Persistent | Persistent | AIDS at 68 mo | ||||||||
| 30845 | M | + | 23 mo | 3.3 | 10 mg/kg | Neg | |||||||
| 28 mo | 3.6 | 20 mg/kg | 32 | 4.0 | 20.7 | 26.3 | 760 | 35 mo | 38 mo | ||||
| 38 mo | 4.7 | 10 mg/kg | Persistent | Persistent | |||||||||
| 43 mo | 5.3 | 5 mg/kg | Neg | Neg/PS | AIDS at 53 mo | ||||||||
| 29046* | M | − | 3 days | 0.5 | 30 mg/kg | 24 | 2.4 | 66.0 | 114.0 | 262 | 25 mo | 7 mo | |
| 25 mo | 2.4 | 10 mg/kg | 34 | 3.1 | 22.0 | 50.0 | 201 | Persistent | Persistent | ||||
| 66 | 6.2 | 24.0 | 58.0 | 174 | Persistent | Intermittent | |||||||
| 77 | 7.3 | 36.0 | 82.0 | 122 | Persistent | Intermittent | |||||||
| 78 mo | 7.4 | 5 mg/kg | 83 | 6.8 | 13.0 | 31.0 | 161 | Persistent | Intermittent | ||||
| 99 | 7.8 | 18.9 | 47.0 | 106 | Persistent | Intermittent | |||||||
| 99 mo | 7.8 | 2.5 mg/kg | Persistent | Intermittent/PS | |||||||||
| 119 mo | 7.2 | Constant 18 mg | 135 | 7.2 | 8.1 | 17.4 | 150 | Persistent | Neg/PS | ||||
| 136 mo | 7.2 | Constant 9 mg | 137 | 7.6 | 2.6 | 9.0 | 130 | Persistent | Neg/PS | ||||
| 145 mo | 7.6 | Constant 4.8 mg | 147 | 7.6 | 1.2 | 4.5 | 140 | Persistent | Neg/PS | Healthy at 13 yr | |||
| 29276* | F | + | 3 wk | 0.6 | 30 mg/kg | 15 mo | 13 mo | ||||||
| 16 mo | 1.3 | 10 mg/kg | Persistent | Persistent/ PS | |||||||||
| 24 mo | 1.7 | 5 mg/kg | 55 | 3.7 | 14.0 | 52.0 | 97 | Persistent | Intermittent/PS | ||||
| 66 mo | 4.0 | 2.5 mg/kg | 73 | 4.0 | 11.0 | 26.0 | 98 | Persistent | Intermittent/PS | ||||
| 90 mo | 4.5 | 1.25 mg/kg | Persistent | Intermittent/PS | |||||||||
| 103 mo | 4.3 | 0.8 mg/kg | Persistent | Intermittent/PS | |||||||||
| 110 mo | 4.5 | Constant 3.6 mg | Persistent | Intermittent/PS | |||||||||
| 126 mo | 4.7 | Constant 1.8 mg | 128 | 4.4 | 1.4 | 6.2 | 60 | Intermittent | Intermittent/PS | ||||
| 136 mo | 4.8 | 1.8 mg 3 times per wk | 138 | 4.6 | 1.5 | 13.5 | 30 | Intermittent | Intermittent/PS | Healthy at 12 yr | |||
| 32186 | M | + | 17 mo | 2.9 | 30 mg/kg | Neg | 19 mo | ||||||
| 19 mo | 2.9 | 20 mg/kg | 20 mo | Persistent | |||||||||
| 20 mo | 2.9 | 10 mg/kg | Persistent | Intermittent | |||||||||
| 23 mo | 3.1 | 5 mg/kg | 28 | 3.3 | 17.6 | 85.5 | 59 | Persistent | Intermittent | ||||
| 28 mo | 3.3 | 2.5 mg/kg | Persistent | Intermittent/PS | |||||||||
| 31 mo | 3.9 | Stoppede | Persistent | Neg | |||||||||
| 33 mo | 4.1 | 2.5 mg/kg | Persistent | Intermittent | |||||||||
| 40 mo | 4.6 | 1.25 mg/kg | Persistent | Neg | |||||||||
| 56 mo | 7.4 | 0.65 mg/kg | Persistent | Intermittent | |||||||||
| 61 mo | 7.9 | Constant 4.2 mg | 77 | 10.1 | 1.2 | 4.1 | 100 | Neg | Neg/PS | ||||
| 89 | 9.8 | 1.2 | 4.5 | 90 | Trace/neg | Neg/PS | Healthy at 8 yr | ||||||
| 30577 | F | + | 24 mo | 2.6 | 10 mg/kg | Neg | |||||||
| 29 mo | 2.7 | 20 mg/kg | 34 | 3.2 | 27.4 | 39.2 | 510 | 36 mo | 36 mo | ||||
| 39 mo | 3.5 | 10 mg/kg | Persistent | Persistent | |||||||||
| 48 mo | 4.4 | 5 mg/kg | Persistent | Neg | |||||||||
| 71 mo | 5.6 | 2.5 mg/kg | 86 | 5.3 | 25.5 | 35.3 | 71 | Persistent | Neg | ||||
| 86 mo | 5.3 | Stoppede | Neg | Neg | |||||||||
| 88 mo | 5.3 | Constant 14 mg | 103 | 5.5 | 4.5 | 8.4 | 290 | Intermittent trace | Neg/PS | ||||
| 104 mo | 5.5 | Constant 7 mg | 106 | 5.4 | 1.9 | 4.3 | 300 | Intermittent trace | Neg/PS | ||||
| 114 | 6.9 | 1.5 | 4.3 | 240 | Neg | Neg/PS | Healthy at 10 yr | ||||||
F, female; M, male; +, positive; −, negative. Asterisks indicate animals for which some early data were published previously (63).
In all regimens, TFV was given subcutaneously once daily unless indicated otherwise.
PK, pharmacokinetics; Cmax, maximum concentration of drug in serum or plasma.
The onset of glucosuria or hyphophosphatemia is the age at first detection; “single trace” (100 mg/dl) or “single low” (250 mg/dl) indicates glucosuria at a single time point followed by persistently negative samples. Patterns: persistent, glucosuria or hypophosphatemia at ≥75% of time points at which samples were analyzed; intermittent, <75% of time points; neg, negative; NE, not able to evaluate for renal phosphate wasting due to severe opportunistic infections affecting liver and intestinal phosphate absorption; PS, phosphate supplement (Neutra-Phos).
TFV treatment was stopped not for toxicity reasons but to monitor for virologic rebound.
(ii) General overview of dose-related toxicity and pharmacokinetics.
In the current follow-up studies, the primary dose-related toxicity of prolonged TFV treatment continued to be the previously described renal toxicity, namely, PRTD, characterized by glucosuria and changes in the levels of certain serum markers (especially hypophosphatemia and elevated total alkaline phosphatase levels). In severe cases, PRTD led to bone pathology (63). Serum creatinine and potassium concentrations were less-sensitive markers of renal toxicity, since for some animals, these levels were within the range of those of untreated animals (creatinine, ≤1.4 mg/dl [within or equivalent to the mean + 2 standard deviations]; potassium, ≥2.8 mmol/liter) despite significant glucosuria and hypophosphatemia. Other serum parameters such as liver enzymes and fasting levels of glucose, triglycerides, and cholesterol were indistinguishable from those of age-matched untreated animals (data not shown); any aberrations of these serum markers in individual animals could be explained by the presence of SIV-associated pathology in animals that progressed to AIDS (e.g., opportunistic infections resulting in diarrhea, septicemia, etc.).
For the purpose of further analyses, the onset of detectable renal toxicity (PRTD) was defined as the first observation of glucosuria (in the absence of hyperglycemia) and/or hypophosphatemia. Based on the range of values seen in age-matched control animals, renally induced hypophosphatemia for animals up to the age of ∼5 years was defined as serum phosphorus concentrations of <4 mg/dl at two consecutive time points or <3 mg/dl at one time point in the absence of major gastroenteric problems (e.g., due to AIDS) that could suggest reduced intestinal absorption of phosphate; for adult animals from ∼5 years onwards, only serum phosphorus concentrations of <2.5 mg/dl were considered significant indicators of hypophosphatemia.
All available longitudinal and cross-sectional pharmacokinetic data from long-term TFV-treated animals were analyzed in order to determine whether the TFV clearance and/or the area under the plasma concentration-versus-time curve (AUC) were predictive of the presence or future development of PRTD (glucosuria and/or hypophosphatemia). For newborn and infant macaques, low TFV clearance and high plasma AUCs were not associated with detectable PRTD. For adult and juvenile macaques (all ≥10 months old), the presence of PRTD at the time of a pharmacokinetic study was associated with reduced TFV clearance (<400 ml/h/kg) in comparison to that of age-matched control animals (Fig. 1C and D). The combined analysis of TFV clearance and AUCs provided useful insights into the pathogenesis of PRTD during the different TFV regimens. As indicated in Fig. 2, the combination of low AUCs (<20 μg·h/ml) and normal TFV clearance (>400 ml/h/kg) was not associated with detectable PRTD, even for animals that were treated for more than 9 years (e.g., animal 31122 [Fig. 2, quadrant A]). Animals that had initial TFV clearances within the normal range (>400 ml/h/kg) but higher plasma AUCs (∼20 μg·h/ml or higher), either due to higher dosage regimens (20 or 30 mg/kg subcutaneously once daily) or due to a gradual age-related decrease in clearance (as explained above), were in an intermediate stage (Fig. 2, quadrant B), since the prolonged exposure eventually led to the occurrence of PRTD concomitantly with the observation of reduced TFV clearance (<400 ml/h/kg [Fig. 2, quadrant C]). Following the detection of PRTD, empirical TFV dosage reductions were implemented to bring AUCs to 10 to 20 μg·h/ml; although this led to improvement, glucosuria and hypophosphatemia did not always resolve completely, and prolonged treatment with these reduced regimens generally led to a further decrease in TFV clearance with relapses of the glucosuria and hypophosphatemia, which then necessitated further dosage reductions. As our knowledge improved over time, we learned that (i) lower AUC target values of ∼5 to 10 μg·h/ml were more appropriate for minimizing the risk of toxicity while maintaining antiviral activity (see discussions below, e.g., of animals 32186, 30577, 33088, and 33091), and (ii) for TFV dosage regimens that were adjusted weekly based on weight, the weight- and age-related physiologic decrease in clearance (Fig. 1A and B) automatically predisposed growing animals to higher plasma AUCs, which then promoted or aggravated renal toxicity. Accordingly, it was then decided to maintain all remaining TFV-treated animals (including those without any evidence of PRTD) on a fixed absolute dose (in mg of TFV) instead of a weight-adjusted dose (Table 1).
FIG. 2.
Correlation between apparent TFV clearance, plasma AUCs, and the development of PRTD. Animals had been on stable subcutaneous TFV dosage regimens for at least 4 months at the time of the pharmacokinetic studies. Symbols indicating whether PRTD (glucosuria and/or hypophosphatemia) occurred refer to the time frame after the pharmacokinetics study was performed. While some animals were previously on higher-dosage regimens (see Table 1), the dosages indicated refer to the time of the pharmacokinetic studies. Because clearance is calculated as dose/AUC, hyperbolas are predicted at a specific dose. The quadrants define where differences and changes in TFV CL/F are physiologic (i.e., due to individual variation and the effect of aging) versus pathological (i.e., nephrotoxicity). The available data suggest that the cutoff values for the different quadrants are approximately AUCs of 20 μg·h/ml and clearance values of 400 ml/h/kg. Quadrant A is the zone where no PRTD was observed; quadrant B is a pre-PRTD stage; quadrants C and D area are associated with clinical PRTD. Glucosuria was detected once for animal 33091 after the pharmacokinetic values were at the intersection of these quadrants; the glucosuria resolved following dosage reduction (which was associated with an increase in TFV clearance [quadrant A]). (Inset) Empirical model for the pathogenesis of PRTD during high-dose TFV regimens. At high TFV exposures (quadrant B), the gradual development of PRTD reduces TFV clearance; this reduced clearance, by further increasing drug exposure (i.e., AUCs), aggravates renal toxicity and drives the animals' pharmacokinetic parameters further and further into quadrant C; only following drastic dosage reductions can values move from quadrant C toward quadrant D.
For clarity of further presentation, animals are stratified according to their initial dosage regimen and the occurrence of PRTD; selected animals are described in more detail to document the model presented in Fig. 2.
(iii) Prolonged low-dose TFV regimens (always ≤10 mg/kg; n = 3).
Three infant macaques were started on a 10-mg/kg (once daily subcutaneously) TFV regimen (Table 1). SIV-infected animal 31042 started receiving TFV at the age of 3 weeks, developed K65R RT mutants, had persistent viremia, and eventually had to be euthanized with AIDS at the age of 3.5 years (without any evidence of TFV-induced toxicity [63]). The other two animals (male 31121 and female 31122) were uninfected and were started on a 10-mg/kg regimen within the first 2 days after birth. At the age of 3.5 years, when plasma TFV AUCs increased to >20 μg·h/ml, animal 31121 developed glucosuria. Although gradual dosage reductions (to obtain AUCs of 10 to 20 μg·h/ml) produced an initial improvement in the markers, eventually the glucosuria became more frequent and severe (>1,000 mg/dl), and there was a gradual increase in serum creatinine concentrations (peak, 2.2 mg/dl) and a gradual decrease in serum phosphorus concentrations (nadir, 1.7 g/dl). Accordingly, at the age of 75 months, TFV treatment was completely withdrawn, which then led to a slow improvement and stabilization of serum phosphorus (2.1 to 4.3 mg/dl) and creatinine (2.0 to 2.2 mg/dl) levels, and the glucosuria became low (≤100 mg/dl) and less frequent. Animal 31121 continued to gain weight (currently 11 kg at 10 years), and except for the laboratory parameters indicative of reduced renal function (i.e., increased creatinine levels), is clinically healthy. Throughout this whole observation period, animal 31121 received the routine CNPRC primate diet (without any extra mineral, vitamin, or amino acid dietary supplements).
Female animal 31122 never had detectable glucosuria during her 10 years of daily TFV treatment. Creatinine concentrations remained low (≤0.8 mg/dl), and serum phosphorus levels and all other serum chemistry parameters remained within the expected levels for age-matched animals. Serum phosphorus concentrations decreased transiently (to nadirs of 2.8 to 2.9 mg/dl) during consecutive pregnancies (see below), but these changes were within the range of values observed for untreated pregnant animals. Animal 31122 remains healthy at the age of 10 years.
(iv) Prolonged TFV treatment with regimens including >10 mg/kg (n = 29).
Twenty-nine animals had treatment periods ranging from 7 weeks to 25 months where the daily subcutaneous dose of TFV was 20 or 30 mg/kg. Based on the occurrence of PRTD, these 29 animals can be separated into two groups.
(a) Animals without persistent PRTD (n = 7).
Seven animals receiving a regimen of >10 mg/kg (Table 1) either never developed PRTD (n = 4) or had only a single time point at which a trace or small amount of glucose (100 to 250 mg/dl) was detected in the urine (n = 3). Following dosage reduction, glucose was absent in the urine at later time points for these three animals. Six of the seven animals without persistent PRTD were started on a daily 30-mg/kg regimen that was reduced within 2 months to 20 mg/kg and then further reduced to 10 mg/kg. The seventh animal (30576) had been on a 20-mg/kg regimen for 15 months without developing detectable PRTD; this absence of renal toxicity is probably due to a relatively high TFV clearance in this animal (1,080 ml/h/kg), leading to plasma AUCs (18.8 μg·h/ml) lower than those for eight other animals that developed PRTD within 3 to 14 months of treatment with a 20-mg/kg regimen (Table 1; Fig. 2). All serum chemistry values (including phosphorus, creatinine, and alkaline phosphatase) of these seven animals were within the range of age-matched untreated control animals. They received a regular diet (i.e., without any special mineral, vitamin, or amino acid dietary supplements) throughout their observation period.
(b) Animals with PRTD (n = 22).
The other 22 animals that had been on 20- to 30-mg/kg TFV regimens for extended periods developed PRTD (glucosuria and/or hypophosphatemia) (Table 1).
Of these 22 animals, 6 had evidence of possible PRTD-related moderate hypokalemia (i.e., at least one time point when serum potassium concentrations were <2.8 mmol/liter in the absence of other obvious explanations such as diarrhea); severe hypokalemia (serum potassium concentrations of <2 mmol/liter) was never observed. The serum creatinine concentration was an unreliable marker for renal toxicity. For 18 of these 22 animals with PRTD, serum creatinine levels remained within the normal range (≤1.4 mg/dl) throughout their observation periods; this included animals that had persistent hypophosphatemia and/or glucosuria for as long as 5 years. This finding suggests that for these animals, the renal pathology was mostly limited to the proximal renal tubuli. For four animals, serum creatinine concentrations increased to >1.4 mg/dl, but the onset of the creatinine increase was unpredictable (7 months to 4 years after the onset of hypophosphatemia or glucosuria). Peak creatinine levels ranged from 2.9 to 3.6 mg/dl; increases in creatinine levels were generally gradual and slow. An exception was animal 32186, for which creatinine levels increased suddenly (from 1.4 to 2.1 mg/dl within 6 weeks) when this chronically infected animal was depleted of CD8+ cells by the administration of a monoclonal antibody (which was associated with a transient increase in plasma viremia) (65); it is possible that immune complexes that were formed during this CD8+ cell depletion experiment may have suddenly aggravated the development of glomerulonephritis.
Following the occurrence of glucosuria, hypophosphatemia, or increased creatinine levels, sequential dosage reductions (to eventually obtain AUCs of <10 μg·h/ml) and a change to a constant, absolute dose (irrespective of body weight, as explained above) helped stabilize and/or improve the serum phosphorus and creatinine concentrations. In some animals, such low-dose maintenance regimens (AUCs, <10 μg·h/ml) led to improvement (i.e., an increase in TFV clearance and the disappearance of glucosuria [Fig. 1C; Table 1, animals 29046, 32186, and 30577]). None of these animals with elevated creatinine levels had outward symptoms of renal insufficiency.
Based on clinical observations described previously (63), eight of the animals (29045, 29046, 29276, 30007, 30162, 30577, 32186, and 33109) for which serum phosphorus concentrations did not increase sufficiently shortly after the initial TFV dosage reductions benefited from receiving dietary phosphate supplements (Neutra-Phos). Four long-term-treated animals (29045, 29046, 29276, and 30577) with poor fur quality (alopecia) were also given daily amino acid supplements (Amino Fuel) to offset the urinary loss of amino acids during PRTD; the initiation of these amino acid supplements was sometimes associated with rapid improvement of these animals' fur.
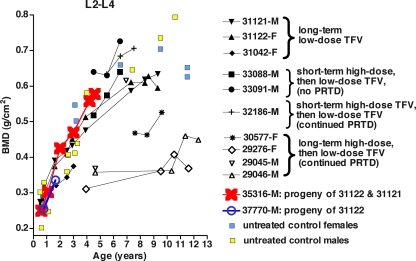
Effect of prolonged TFV administration on BMD.
DXA scans were available for six animals with clinical PRTD (glucosuria and/or hypophosphatemia) (Fig. 3). As described previously (63), three of these animals (29045, 29046, 29276) had received a prolonged high-dose TFV regimen (30 mg/kg subcutaneously once daily) since birth and showed growth retardation with significant radiographic evidence of bone mineralization defects before dosage reductions and subsequent dietary supplements were implemented. Despite improvement, the BMDs and BMCs of these three animals remained below the range of those of normal adult animals but were not associated with any overt symptoms; no fractures have been observed. Two animals with persistent PRTD for which dosage reductions and dietary supplementation had been initiated earlier to prevent major adverse effects displayed either normal BMD (animal 32186) or reduced BMD (animal 30577) after 73 or 91 months of TFV administration, respectively. Animal 31121 (for which TFV treatment was stopped after ∼6 years due to PRTD) had BMD within the normal range throughout the observation period of 9 years (Fig. 3).
FIG. 3.
DXA scan evaluation of bones in long-term TFV-treated animals. Details on the TFV dosage regimens are provided in Table 1. DXA scans were performed on the femoral neck, global proximal femur, DR+U, and lumbar vertebrae (L2 to L4). Although all these locations gave similar results, the BMDs of lumbar vertebrae are shown because they had less variability (among both treated and untreated animals) and were therefore more useful for comparison of the TFV-treated animals. The untreated female macaques either were nulliparous or were tested at least 14 months after their most recent infant had been weaned. Except for those animals that had been on prolonged high-dose TFV regimens and had bone mineralization defects before the dosage was reduced (29045, 29046, 29276, and 30577), the TFV-treated animals had BMDs similar to those of age- and sex-matched control animals. M and F indicate male and female, respectively.
Three animals (31122, 33088, and 33091) (Table 1) that received low-dose maintenance regimens of TFV and had no PRTD or only a single observation of glucosuria throughout 6 to 10 years of TFV treatment had total body weights, as well as BMDs and BMCs for L2 to L4 and DR+U, within the range of those of age- and sex-matched control animals (Fig. 3). For TFV-treated female animal 31122, transient decreases in BMD and BMC were observed on DXA scans performed shortly after pregnancies; small decreases in BMD in the forearms and lumbar spines of uninfected and untreated pregnant humans have also been described (43).
Effect of prolonged low-dose TFV treatment during pregnancy.
Uninfected female macaque 31122 was started on prolonged TFV treatment (10 mg/kg subcutaneously once daily) at the age of 1 day (Table 1). She was housed with uninfected, TFV-treated male animal 31121. At the age of approximately 4 years, she delivered an infant, which died of neonatal septicemia. At the age of 5 years and 4 months, she delivered a healthy male infant (animal 35316). Starting at the age of 7 months, animal 35316 has been living in our outdoor colony with other animals to promote physical activity and social interactions; he has developed normally by all criteria (including behavior, weight, serum chemistry analyses, urinalysis [no glucosuria], radiographs, and BMD [Fig. 3]) throughout his observation period, currently 5 years. When animal 31122 was almost 6 years old, a third pregnancy was lost due to a placental hemorrhage on gestational day 72, necessitating a cesarean section; the dead fetus was macroscopically normal, and the cause of death was therefore most likely the placental hemorrhage. At the age of approximately 8 years, animal 31122 became pregnant by a different, uninfected, untreated male. Regular ultrasound monitoring revealed normal fetal development but a partial placenta previa; to prevent any complications, a scheduled cesarean section was performed on gestational day 156, with delivery of a healthy male infant (animal 37770; birth weight, 505 g). This infant, which was raised by a foster mother, is currently 22 months old and shows normal development by all criteria (as described for animal 35316 above). A fifth pregnancy at the age of 9 years resulted in fetal loss at approximately gestational day 53. At the age of 10 years, a sixth pregnancy, fathered again by animal 31121, resulted in a healthy male infant that was delivered by cesarean section (due to a partial placenta previa) on estimated gestational day 152.
As a comparison, the rate of prenatal mortality in rhesus macaques at the CNPRC is 17% (with the highest rate of loss during early gestation [gestational days 18 to 70]) (31), and therefore the loss of pregnancy in this animal was not deemed to be TFV related. Four pharmacokinetic studies that were performed on animal 31122 between the ages of 3 and 9 years indicate that during these consecutive pregnancies, maternal exposure to TFV (plasma AUCs) ranged from 6 to 19 μg·h/ml (Table 1; Fig. 2).
Sustained antiviral efficacy of prolonged TFV treatment.
The 28 SIV-infected, TFV-treated animals were from several studies where untreated animals had persistently high viremia levels and all developed AIDS (generally within 4 months for animals infected shortly after birth; within 1 to 2 years for animals infected as juveniles) (58-60, 64, 65). TFV therapy initiated early during the infection course generally induced rapid suppression of viremia. Although prolonged TFV therapy always resulted in the emergence of viral mutants with a K65R mutation in RT, the TFV-treated animals had significantly improved disease-free survival compared to untreated animals in all these studies (59, 60, 64, 65) (Fig. 4). Of the 28 long-term TFV-treated animals infected with K65R viral mutants, 20 had persistent viremia and eventually developed AIDS while on treatment; 1 animal (29003 [Table 1]) became viremic and developed AIDS after TFV treatment was discontinued to reduce toxicity; 1 viremic animal (27999) was AIDS free but was euthanized because of its skeletal lesions, as described previously (63). The remaining six animals have been able to suppress plasma viremia to undetectable levels for 6 to 12 years without progression toward AIDS. Whether an animal had persistent or undetectable viremia did not correlate with the TFV dosage regimen, the plasma TFV exposure levels, the timing of the first detection of K65R viral mutants, or the presence of other mutations in RT. While one animal with undetectable viremia (29045) was eventually lost from the study after 7 years of TFV treatment due to an unrelated cause (self-injurious behavior), five infected animals remained healthy and had very low or undetectable viremia (less than the cutoff value of 125 copies/ml by a bDNA assay) and normal CD4+ and CD8+ T-lymphocyte counts and CD4/CD8 lymphocyte ratios after their respective TFV treatment periods (12 years for animal 29276, 8 years for animal 30577, 7 years for animal 32186, and 6 years for animals 33088 and 33091). In four of these five animals with undetectable viremia by conventional diagnostics (29276, 30577, 32186, and 33088), no virus could be detected in plasma even by the more sensitive real-time RT-PCR assay (cutoff, 10 RNA copies per ml of plasma). The fifth animal (33091) had undetectable viremia as measured by the bDNA assay (<125 copies per ml) in 8 of the 10 samples collected from 10 months of treatment onward but had 2 intermittent samples with detectable low-level viremia by the SIV bDNA assay (128 and 678 SIV RNA copies/ml at 20 and 47 months of treatment, respectively); plasma collected after 66 months of TFV treatment had a copy number of 290/ml according to the real-time RT-PCR assay.
FIG. 4.
Effect of long-term TFV treatment on disease progression in SIV-infected macaques. Disease-free survival curves are presented for all studies that allowed evaluation of the effect of prolonged TFV treatment on SIV disease progression. Data from separately performed studies were pooled for the analysis if all input parameters (age of animals, virus isolate, dose and route of virus inoculation) were very similar. On each graph, the survival curves of TFV-treated and untreated animals were compared using the log rank test. Long-term survivors that are discussed in Results are indicated by arrows. (A) Newborn macaques were inoculated orally with a high dose of SIVmac251, and TFV treatment was started on five animals (29003, 29008, 29045, 29055, and 31042) 3 weeks later (59, 64). Three of these TVF-treated animals developed AIDS while on treatment; one animal, 29003, had TFV interrupted at the age of 95 weeks and developed AIDS at 177 weeks; animal 29045 was lost from the study at the age of 7 years due to an unrelated cause (self-injurious behavior). (B) Twelve newborn macaques were inoculated intravenously with a high dose of K65R mutants of SIV, and 3 weeks later, six of them were started on TFV as described previously (60). TFV-treated animal 29276 is currently AIDS free at the age of 12 years. (C) Four-week-old infant macaques were inoculated orally with SIVmac251, and three animals (32990, 33093, and 33109) were started on TFV treatment approximately 11 weeks later, when animals were already immunosuppressed (64). (D) Eleven juvenile macaques (ages, 12 to 17 months) were inoculated orally with SIVmac251, and five animals were started on TFV treatment 2 weeks later, as described previously (66). Two animals (32137 and 32993) developed AIDS, while the other three animals (32186, 33088, and 33091) are currently AIDS free after more than 6 years of SIV infection.
In a subset of these six animals with undetectable viremia, transient CD8+ cell depletion (via injection of the anti-CD8 monoclonal antibody cM-T807) (n = 4) or short-term interruption of TFV treatment (7 to 9 weeks) (n = 4), described previously, had led to a transient increase in viremia (58, 65). Thus, the drug concentrations in these animals, even after the dosage reductions (Table 1), were associated with antiviral activity.
PBMC of the five surviving SIV-infected animals with very low or undetectable viremia were tested for the presence of SIV-specific cell-mediated immune responses using flow cytometry assays for intracellular staining of three cytokines: interleukin 2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α). All five animals had detectable SIV-specific CD4+ T lymphocytes; four of the five animals had SIV-specific cytokine-secreting CD8+ T lymphocytes in peripheral blood (Table 2). There was no obvious correlation between the patterns of cytokine secretion and the duration of TFV treatment.
TABLE 2.
SIV-specific cell-mediated immune responses in long-term TFV-treated animals with low or undetectable levels of viremiaa
| Animal no. | SIV stimulusb | CD4+ CD3+ T lymphocytes
|
CD8+ CD3+ T lymphocytes
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As % of gated lymphocytes | % Positive for:
|
As % of gated lymphocytes | % Positive for:
|
|||||||||||
| IL-2 | IFN-γ | TNF-α | IFN-γ+ and TNF-α | IL-2 | IFN-γ | TNF-α | CD107a/b | IFN-γ+and CD107a/b | TNF-α and CD107a/b (% IFN-γ+c) | IFN-γ and TNF-α (% CD107a/b+d) | ||||
| 29276 | p27 | 37.2 | 0.014 | 0.103 | 0.177 | 0.110 | 23.4 | 0.014 | 0.350 | 0.764 | 0.510 | 0.140 | 0.630 (12.2) | 0.110 (100.0) |
| AT-2 | 36.6 | 0.014 | 0.103 | 0.197 | 0.110 | 23.2 | 0.028 | 0.000 | 0.000 | 0.030 | 0.000 | 0.000 (0.000) | 0.000 (0.000) | |
| 30577 | p27 | 48.6 | 0.002 | 0.073 | 0.177 | 0.066 | 14.1 | 0.025 | 1.333 | 1.860 | 2.080 | 1.400 | 1.570 (92.9) | 1.210 (94.7) |
| AT-2 | 49.0 | 0.003 | 0.233 | 0.397 | 0.230 | 14.5 | 0.000 | 0.113 | 0.420 | 0.000 | 0.130 | 0.310 (45.4) | 0.140 (92.3) | |
| 33088 | p27 | 38.7 | 0.000 | 0.008 | 0.012 | 0.000 | 27.2 | 0.011 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 (0.000) | 0.000 (0.000) |
| AT-2 | 37.8 | 0.002 | 0.130 | 0.273 | 0.150 | 26.5 | 0.008 | 0.000 | 0.000 | 0.030 | 0.000 | 0.000 (0.000) | 0.000 (0.000) | |
| 33091 | p27 | 32.1 | 0.008 | 0.217 | 0.400 | 0.210 | 15.9 | 0.028 | 0.100 | 0.160 | 0.170 | 0.000 | 0.000 (0.000) | 0.000 (0.000) |
| AT-2 | 31.7 | 0.008 | 0.307 | 0.610 | 0.310 | 15.6 | 0.005 | 0.000 | 0.070 | 0.220 | 0.000 | 0.000 (0.000) | 0.000 (0.000) | |
| 32186 | p27 | 54.8 | 0.000 | 0.023 | 0.077 | 0.042 | 29.0 | 0.000 | 0.039 | 0.040 | 0.000 | 0.000 | 0.000 (0.000) | 0.000 (0.000) |
| AT-2 | 55.0 | 0.000 | 0.121 | 0.283 | 0.140 | 29.1 | 0.027 | 0.004 | 0.015 | 0.000 | 0.000 | 0.000 (0.000) | 0.000 (0.000) | |
PBMC were collected after prolonged TFV treatment(12 years for animal 29276, 8 years for animal 30577, and 6 years for animals 32186, 33088, and 33091) and tested using flow cytometry assays with intracellular cytokine staining. Values are given after subtraction of the readings for medium-only control wells; negative values were assigned an arbitrary value of zero. Boldfaced data indicate positive immune responses(i.e., ≥2-fold above medium-only levels). Staphylococcus enterotoxin B was used as a positive control to ensure cell viability and the ability of cells to secrete cytokines(data not shown).
A peptide pool of SIVmac239gag p27 was used at 5 μg/ml; AT-2-inactivated SIVmac239 was used at 300 ng/ml.
Frequency of IFN-γ-secreting cells as a percentage of the gated TNF-α+ CD107a/b+ CD8+ CD3+ lymphocyte population.
Frequency of CD107a/b+ cells as a percentage of the gated IFN-γ+ TNF-α+ CD8+ CD3+ lymphocyte population.
These TFV-treated animals continue to be monitored indefinitely.
Intracellular TFV-DP concentrations during single-dose or prolonged TFV treatment.
During a 24-h pharmacokinetics study, multiple PBMC samples were collected from the seven surviving long-term TFV-treated animals (doses, 0.4 to 9.1 mg/kg subcutaneously) and from four single-dosed juvenile macaques (4 mg/kg subcutaneously) for the measurement of intracellular concentrations of the active metabolite, TFV-DP. For the animals on prolonged dosing with TFV, intracellular TFV-DP concentrations varied little over the 24-h sampling period (coefficient of variation, <20%) (data not shown), indicating the long half-life of TFV-DP in PBMC; TFV-DP concentrations measured 24 h after the daily drug dose ranged from 45 to 319 fmol per million PBMC (Table 3). Four juvenile macaques that were given a single subcutaneous dose of TFV (4 mg/kg) had TFV-DP concentrations of 24 to 76 fmol per million PBMC 24 h after dosing. The average ratio of the intracellular TFV-DP concentration at 24 h postdose to the mean extracellular TFV concentration in plasma over the 24-h period was 0.47 for the chronically dosed animals, which was higher than the ratio of 0.27 for the single-dosed animals (P = 0.027 by a two-tailed t test on log-transformed ratios) (Table 3). This significant difference is indicative of the effective intracellular accumulation of TFV-DP over multiple doses of TFV.
TABLE 3.
Intracellular levels of TFV-DP during single-dose or prolonged TFV treatmenta
| Dose and animal no. | TFV dose (mg/kg) | Plasma TFV AUC0-24 (μg·h/ml) | Intracellular TFV-DP concn (fmol/106 PBMC) 24 h after dosing | Ratio of [intracellular TFV-DP] at 24 h to [mean plasma TFV] during dosing intervalb |
|---|---|---|---|---|
| Chronic dose | ||||
| 29046 | 0.6 | 4.5 | 52 | 0.40 |
| 29276 | 0.4 | 13.5 | 107 | 0.57 |
| 30577 | 1.0 | 4.3 | 48 | 0.38 |
| 31122 | 2.8 | 6.0 | 75 | 0.43 |
| 32186 | 0.4 | 4.5 | 53 | 0.41 |
| 33088 | 9.1 | 15.3 | 319 | 0.72 |
| 33091 | 2.1 | 4.4 | 45 | 0.35 |
| Single dose | ||||
| 35391 | 4 | 6.0 | 76 | 0.44 |
| 35410 | 4 | 4.1 | 29 | 0.32 |
| 35434 | 4 | 5.8 | 31 | 0.18 |
| 35942 | 4 | 4.2 | 24 | 0.19 |
TFV was administered subcutaneously. Chronically dosed animals were on stable TFV regimens (administered once daily) for at least 7 weeks when the 24-h pharmacokinetics study was performed (see Table 1); an exception was animal 29276, which was dosed three times per week. Blood collected at specific time points after dosing was also used to isolate PBMC in order to measure intracellular TFV-DP levels. AUC0-24, AUC from 0 to 24 h.
The ratio of the intracellular TFV-DP concentration (24 h after dosing) to the mean plasma TFV concentration was calculated by assuming an approximate cell volume of 0.2 pl per cell (e.g., 100 fmol/106 PBMC means 500 nM); the mean plasma TFV concentration was calculated by dividing the AUC by the dose interval and converting it also to nanomolar concentrations in order to calculate the unitless ratio. The geometric mean of the ratios for chronically dosed animals (0.47) was significantly higher than that for single-dosed animals (0.27) (P = 0.027 by a two-tailed t test on log-transformed ratios).
DISCUSSION
Data on the long-term safety and efficacy of TFV are important, considering (i) the increasing use of TDF in regimens to treat HIV-infected persons, including in developing countries, (ii) the ongoing preexposure prophylaxis trials investigating whether daily administration of TDF can reduce the rate of HIV infection among high-risk populations (1), and (iii) the anticipated regulatory approval of TDF for the treatment of hepatitis B virus-infected persons. Because these groups include women of reproductive age, available data on any long-term effects of in utero exposure to TFV are also very useful. While only long-term studies of humans can provide definitive answers, safety and efficacy data obtained from nonhuman primate models of HIV infection are highly relevant, due to the many similarities in physiology (including drug metabolism, infant development, kidney and bone physiology) and disease pathogenesis (28, 36, 52, 66, 72). An advantage of the macaque model is that the relative durability of TFV's efficacy and safety within a species can be evaluated in a more timely manner, because (i) without treatment, the disease course in macaques infected with virulent SIV isolates is accelerated compared to that in HIV-infected humans (41), and (ii) the whole development from infancy to adulthood and parenthood takes place in approximately 4 to 6 years. So far, studies in macaque models have been quite predictive of the effects of TFV in humans and have played an important role in guiding its clinical development. The data in the current report further support the feasibility of prolonged TFV treatment regimens.
All animals described in this report have in common that they have been on prolonged TFV regimens (administered once daily subcutaneously) for at least 1 year. At the initiation of these studies (from 1995 onward, i.e., prior to the clinical development of the oral TDF regimen for humans), our understanding of the interactions between pharmacologic, virologic, and host factors during TFV therapy was very limited. Accordingly, relatively high-dose subcutaneous TFV regimens were used in an attempt to maximize antiviral efficacy. Following observations of toxicity, the dosage regimens were adjusted empirically to define the optimal window of drug exposure that would balance a minimal risk for toxicity with sufficient antiviral activity (with the emergence of K65R viral mutants as a confounding variable) to maintain overall health and delay the progression of SIV disease. Over time, the ongoing collection of safety and efficacy data from multiple macaque studies and the ability to measure intracellular TFV-DP concentrations have gradually refined our knowledge.
In the current follow-up studies, the primary dose-dependent toxicity continued to be renal toxicity, namely, the development of PRTD, in which chronic hypophosphatemia can secondarily lead to bone disease, as described previously (63). No novel toxicities were observed in any other organ systems. Although the relatively small number of animals limits the ability to detect small differences, serum parameters such as fasting concentrations of glucose, triglycerides, and cholesterol were indistinguishable from those of age-matched untreated animals; this is consistent with the observations of favorable lipid profiles in HIV-infected people treated with TDF-containing regimens (24, 45).
The renal toxicity of high-dose TFV regimens in macaques is related to the renal drug clearance, which occurs through a combination of glomerular filtration and active tubular secretion (14, 16, 18). The degree of toxicity in tubular cells is likely determined by the local, intracellular concentration of TFV (or its phosphorylated metabolites), which is determined by the balance between drug uptake from the plasma at the basolateral membrane (mediated by organic anion transporters) and the active secretion of TFV at the apical brush-border membrane into the tubular lumen (mediated by ATP-binding cassette transporters such as MRP4) (14, 34, 51).
The current report provides additional insights into the correlation between age-dependent changes in pharmacokinetics and the likelihood of the development and resolution of PRTD. As reported previously (63), no PRTD was detected with short-term high-dose TFV regimens in infant macaques, despite relatively high plasma drug concentrations (AUCs, >45 μg·h/ml). This is likely because the organic anion transporter mechanisms in the proximal renal tubuli are poorly developed at birth (11, 39); thus, even with high plasma TFV concentrations, the low uptake of TFV from the plasma by the renal tubular cells prevented accumulation to toxic intracellular concentrations. When infant macaques mature into juvenile animals, these uptake mechanisms develop and result in a higher renal clearance of TFV. However, the available data suggest that for juvenile and adult macaques, if plasma TFV concentrations are persistently high (AUCs, >20 μg·h/ml), the uptake of the drug from plasma (at the basolateral membrane) exceeds the secretion into the tubular lumen (at the apical brush-border membrane); in other words, the secretory transport mechanisms at the apical membrane are the rate-limiting step in the renal secretion of TFV. In this situation, the gradual accumulation of TFV in these renal tubular cells leads, perhaps once a threshold is reached, to a dysfunction of the tubular cells that severely limits the transport of molecules in both directions, resulting in significantly reduced TFV secretion (TFV clearance, <400 ml/h/kg), which coincides with the detection of glucosuria and hypophosphatemia due to reduced reabsorption from the urinary filtrate. Once this process of renal toxicity is triggered, a vicious cycle is initiated in which, unless dosage reductions are implemented, increased exposure to TFV promotes further renal toxicity, the loss of proximal tubular cells, and eventually tubular atrophy (63).
In our studies with juvenile and adult macaques, our initial attempts to define a target window for plasma drug exposure were complicated because a normal, physiologic decrease in renal TFV clearance occurs when juvenile animals mature into adulthood (Fig. 1). This is probably because for adult macaques, the kidney volume is independent of the animal's sex, total body size, and weight (32, 33). Accordingly, for a TFV dosage regimen that is based on body weight (as is common practice in veterinary medicine), a heavy (due to muscle, fat, or pregnancy) adult macaque will experience higher plasma TFV AUCs than a lighter adult animal with kidneys of a similar size. In other words, as animals mature into adulthood, an increase in AUCs (and in the likelihood of PRTD development) can be avoided only by reducing the dosage regimen per kilogram of body weight, or dosing with a constant absolute amount of drug (irrespective of body weight fluctuations, but more reflective of the kidney capacity). The use of a constant dose is similar to routine practice with most drugs for human adults, including TDF, for which a fixed dose (one 300-mg tablet per day) is used within a relatively wide range of body weights (38).
The current data on several of the animals demonstrated that during prolonged subcutaneous monotherapy with TFV, plasma concentrations lower (AUC, 4 to 10 μg·h/ml) than our previously set target levels (AUC, 10 to 20 μg·h/ml [63]) still effectively suppress virus replication. Such plasma AUCs are within a range two- to threefold higher than the steady-state AUCs (∼3 μg·h/ml) obtained with the clinical dose of TDF (one 300-mg tablet per day) for humans (4). In addition, intracellular TFV-DP concentrations in animals with plasma AUCs of 4 to 15 μg·h/ml were 45 to 319 fmol per million PBMC, values similar to those seen in humans chronically treated with oral TDF (∼50 to 300 fmol/million PBMC [29, 50]). Such similarities in drug concentrations underscore the growing relevance of these chronic low-dose TFV studies of macaques to model prolonged treatment regimens of humans with TDF. The fact that the relatively low plasma TFV concentrations in these chronically treated macaques provided intracellular concentrations that were sufficient to control viremia can be explained by the long intracellular half-life of TFV-DP in humans (median, 150 h; range, 60 to >175 h [29]), which is predicted to lead to intracellular accumulation. Consistent with this hypothesis, an increase of approximately twofold in intracellular TFV-DP concentrations was observed following multiple doses (Table 3). These pharmacokinetic data also suggest that in future macaque studies with prolonged subcutaneous TFV regimens, the most rapid antiviral response can probably be achieved by a relatively high induction dose (aimed at quickly inducing sufficient intracellular TFV-DP concentrations), followed by a lower-dose maintenance regimen to avoid toxicity.
Although prolonged TFV monotherapy led to the selection of K65R viral mutants in all animals, the virological responses were variable, and this variability could not be explained by drug concentrations or the presence of other RT mutations. Following the emergence of K65R mutants, some TFV-treated animals maintained persistent viremia and, although more slowly than untreated animals, eventually progressed to AIDS. However, six TFV-treated animals were able to suppress K65R viremia to undetectable or very low levels and to maintain this suppression throughout the observation period (presently as long as 12 years of infection). Although several of these animals had PRTD (due to early high-dose regimens), their prolonged AIDS-free survival is remarkable and, to our knowledge, unprecedented considering that (i) these animals were inoculated with virus stocks that were very virulent (i.e., all untreated animals had persistent viremia and developed disease within several months or years), (ii) TFV monotherapy was used, and (iii) we and others have demonstrated that in the absence of TFV treatment, K65R viral mutants are as virulent as wild-type virus (42, 60, 61). By either withdrawing TFV treatment or depleting CD8+ cells, we demonstrated previously that both continued TFV therapy and CD8+ cell-mediated immune responses were required to maximally suppress K65R viremia in such animals (58, 65). Accordingly, a combination of factors (including antiviral immune responses and drug-mediated effects) is responsible for the successful and sustained suppression of viremia in TFV-treated animals (58, 65, 66). In other words, the variable virologic outcome among animals with K65R viral mutants but similar drug exposure likely reflects the individual variability in antiviral immune responses that are needed to suppress these K65R mutants. While the TFV-treated animals with low or undetectable viremia showed measurable SIV-specific cell-mediated immune responses in peripheral blood, more research is needed to further elucidate the exact nature of these immune responses (e.g., epitope recognition, effector frequency in lymphoid tissues and at mucosal sites); information from such studies may be useful for the development of better immunotherapeutic strategies that can complement drug therapy. These findings of reduced viremia of K65R SIV mutants associated with strong antiviral immune responses in TFV-treated macaques may explain observations that viremia in persons with detectable K65R HIV-1 mutants can be suppressed by TFV-containing drug regimens (13) and are consistent with clinical observations of strong antiviral immune responses in HIV-1-infected people receiving highly active antiretroviral therapy who have low-level viremia with drug-resistant virus (2, 19).
An important concern about long-term HIV therapy is the safety of a drug regimen during pregnancy (9). Growth restriction had previously been reported for some newborn macaques born to adult female macaques treated during pregnancy with a high dose of TFV (30 mg/kg subcutaneously) (55, 56). However, as explained previously (63), this was probably because this high-dose TFV regimen induced PRTD with phosphate depletion in the mothers (and the reduced transplacental transfer of phosphate led to fetal deprivation), rather than having a direct transplacental effect on the growing fetus. In our studies, female macaque 31122 had been started at birth on continuous low-dose TFV treatment; although her plasma TFV AUCs were four- to sixfold higher than those with the oral TDF regimen in humans, this animal has not demonstrated any signs of toxicity by the age of 10 years and has delivered three healthy offspring. These offspring showed normal pre- and postnatal development (including renal parameters and bone development) throughout their observation periods (as long as 5 years after birth). Although the number of these animals is small and therefore further investigation is warranted, these preliminary observations with TFV during pregnancy are consistent with the available data from the Antiretroviral Pregnancy Registry, which show no indications of an increased risk of birth defects after in utero exposure to TFV (3).
In conclusion, the current follow-up studies provide further information on the pharmacokinetics, safety, and efficacy of long-term TFV regimens in macaques. While high-dose subcutaneous TFV regimens in macaques caused PRTD (which was managed with dosage reduction and, if needed, dietary supplements), prolonged treatment with reduced doses that maintained plasma exposure levels below a certain threshold were safe and were associated with persistent virologic, immunologic, and clinical benefits in multiple animals. These observations support the long-term treatment of HIV-infected humans with TFV-containing regimens. Continued monitoring of these animals as they progress toward geriatric age will provide further valuable information on prolonged treatment with TFV-containing regimens, with the ultimate goal of giving HIV-infected persons a normal life span.
Acknowledgments
We thank P. Allen, V. Bakula, I. Cazares-Shaw, T. Dearman, L. Hirst, A. Ignatov, B. Rodello, W. von Morgenland, and the staff of the Clinical Laboratories and Veterinary and Colony Services of the California National Primate Research Center for expert technical assistance. The SIVmac239gag p27 peptides were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. AT-2-inactivated SIVmac239 was provided by J. Lifson (SAIC Frederick, Inc., National Cancer Institute, Frederick, MD).
This research was supported by Gilead Sciences; E. Glaser Pediatric AIDS Foundation grants PG-50609, PG-50757, and PG-50853 to K.K.A.V.R.; and grant RR-00169 from the National Center for Research Resources (NCRR; a component of NIH) to the California National Primate Research Center.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
Footnotes
Published ahead of print on 23 June 2008.
REFERENCES
- 1.AIDS Vaccine Advocacy Coalition. March 2005, posting date. Will a pill a day prevent HIV? Anticipating the results of the tenofovir “PREP” trials. http://avac.org/pdf/tenofovir.pdf.
- 2.Alatrakchi, N., C. Duvivier, D. Costagliola, A. Samri, A. Marcelin, G. Kamkamidze, M. Astriti, R. Agher, V. Calvez, B. Autran, and C. Katlama. 2005. Persistent low viral load on antiretroviral therapy is associated with T cell-mediated control of HIV replication. AIDS 19:25-33. [DOI] [PubMed] [Google Scholar]
- 3.Antiretroviral Pregnancy Registry Steering Committee. 2007. Antiretroviral Pregnancy Registry interim report for 1 January 1989 through 31 July 2007. Registry Coordinating Center, Wilmington, NC.
- 4.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedard, J., S. May, M. Lis, L. Tryphonas, J. Drach, J. Huffman, R. Sidwell, L. Chan, T. Bowlin, and R. Rando. 1999. Comparative study of the anti-human cytomegalovirus activities and toxicities of a tetrahydrofuran phosphonate analogue of guanosine and cidofovir. Antimicrob. Agents Chemother. 43:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benech, H., F. Theodoro, A. Herbet, N. Page, D. Schlemmer, A. Pruvost, J. Grassi, and J. R. Deverre. 2004. Peripheral blood mononuclear cell counting using a DNA-detection-based method. Anal. Biochem. 330:172-174. [DOI] [PubMed] [Google Scholar]
- 7.Betts, M. R., and R. A. Koup. 2004. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 75:497-512. [DOI] [PubMed] [Google Scholar]
- 8.Blaas, S., A. Schneidewind, T. Gluck, and B. Salzberger. 2006. Acute renal failure in HIV patients with liver cirrhosis receiving tenofovir: a report of two cases. AIDS 20:1786-1787. [DOI] [PubMed] [Google Scholar]
- 9.Brogly, S. B., N. Ylitalo, L. M. Mofenson, J. Oleske, R. Van Dyke, M. J. Crain, M. J. Abzug, M. Brady, P. Jean-Philippe, M. D. Hughes, and G. R. Seage III. 2007. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS 21:929-938. [DOI] [PubMed] [Google Scholar]
- 10.Buxbaum, S., F. B. Kraus, A. Hahn, O. Beck, B. Kabartas, H. W. Doerr, and B. Ludwig. 2006. Flow cytometric analysis of virus-specific T lymphocytes: practicability of detection of HCMV-specific T lymphocytes in whole blood in patients after stem cell transplantation. J. Immunol. Methods 311:164-173. [DOI] [PubMed] [Google Scholar]
- 11.Calcagno, P. L., and M. I. Rubin. 1963. Renal extraction of para-aminohippurate in infants and children. J. Clin. Investig. 42:1632-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassetti, I., J. V. Madruga, J. M. Suleiman, A. Etzel, L. Zhong, A. K. Cheng, and J. Enejosa. 2007. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin. Trials 8:164-172. [DOI] [PubMed] [Google Scholar]
- 13.Chappell, B. J., N. A. Margot, and M. D. Miller. 2007. Long-term follow-up of patients taking tenofovir DF with low-level HIV-1 viremia and the K65R substitution in HIV-1 RT. AIDS 21:761-763. [DOI] [PubMed] [Google Scholar]
- 14.Cihlar, T., E. S. Ho, D. C. Lin, and A. S. Mulato. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20:641-648. [DOI] [PubMed] [Google Scholar]
- 15.Cundy, K. 1999. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin. Pharmacokinet. 36:127-143. [DOI] [PubMed] [Google Scholar]
- 16.Cundy, K. C., C. Sueoka, G. R. Lynch, L. Griffin, W. A. Lee, and J.-P. Shaw. 1998. Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Antimicrob. Agents Chemother. 42:687-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 18.Deeks, S. G., P. Barditch-Crovo, P. S. Lietman, F. Hwang, K. C. Cundy, J. F. Rooney, N. S. Hellmann, S. Safrin, and J. Kahn. 1998. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob. Agents Chemother. 42:2380-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeks, S. G., J. N. Martin, E. Sinclair, J. Harris, T. B. Neilands, H. T. Maecker, E. Hagos, T. Wrin, C. J. Petropoulos, B. Bredt, and J. M. McCune. 2004. Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral-treated individuals with drug-resistant human immunodeficiency virus type 1. J. Infect. Dis. 189:312-321. [DOI] [PubMed] [Google Scholar]
- 20.Durand-Gasselin, L., D. Da Silva, H. Benech, A. Pruvost, and J. Grassi. 2007. Evidence and possible consequences of the phosphorylation of nucleoside reverse transcriptase inhibitors in human red blood cells. Antimicrob. Agents Chemother. 51:2105-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Sahly, H. M., L. Teeter, T. Zerai, R. A. Andrade, C. Munoz, C. Nnabuife, and R. Hunter. 2006. Serum creatinine changes in HIV-seropositive patients receiving tenofovir. AIDS 20:786-787. [DOI] [PubMed] [Google Scholar]
- 22.Fux, C. A., A. Christen, S. Zgraggen, M. G. Mohaupt, and H. Furrer. 2007. Effect of tenofovir on renal glomerular and tubular function. AIDS 21:1483-1485. [DOI] [PubMed] [Google Scholar]
- 23.Gafni, R. I., R. Hazra, J. C. Reynolds, F. Maldarelli, A. N. Tullio, E. DeCarlo, C. J. Worrell, J. F. Flaherty, K. Yale, B. P. Kearney, and S. L. Zeichner. 2006. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy: impact on bone mineral density in HIV-infected children. Pediatrics 118:e711-e718. [DOI] [PubMed] [Google Scholar]
- 24.Gallant, J. E., S. Staszewski, A. L. Pozniak, E. DeJesus, J. M. Suleiman, M. D. Miller, D. F. Coakley, B. Lu, J. J. Toole, and A. K. Cheng. 2004. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 292:191-201. [DOI] [PubMed] [Google Scholar]
- 25.Gallant, J. E., M. A. Parish, J. C. Keruly, and R. D. Moore. 2005. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin. Infect. Dis. 40:1194-1198. [DOI] [PubMed] [Google Scholar]
- 26.Giacomet, V., S. Mora, L. Martelli, M. Merlo, M. Sciannamblo, and A. Vigano. 2005. A 12-month treatment with tenofovir does not impair bone mineral accrual in HIV-infected children. J. Acquir. Immune Defic. Syndr. 40:448-450. [DOI] [PubMed] [Google Scholar]
- 27.Gitman, M. D., D. Hirschwerk, C. H. Baskin, and P. C. Singhal. 2007. Tenofovir-induced kidney injury. Expert Opin. Drug Saf. 6:155-164. [DOI] [PubMed] [Google Scholar]
- 28.Gribnau, A. A. M., and L. G. M. Geijsberts. 1981. Developmental stages in the rhesus monkey (Macaca mulatta). Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 29.Hawkins, T., W. Veikley, R. L. St Claire III, B. Guyer, N. Clark, and B. P. Kearney. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406-411. [DOI] [PubMed] [Google Scholar]
- 30.Hazra, R., R. I. Gafni, F. Maldarelli, F. M. Balis, A. N. Tullio, E. DeCarlo, C. J. Worrell, S. M. Steinberg, J. Flaherty, K. Yale, B. P. Kearney, and S. L. Zeichner. 2005. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy for pediatric HIV infection. Pediatrics 116:e846-e854. [DOI] [PubMed] [Google Scholar]
- 31.Hendrie, T. A., P. E. Peterson, J. J. Short, A. F. Tarantal, E. Rothgam, M. I. Hendrie, and A. G. Hendrickx. 1996. Frequency of prenatal loss in a macaque breeding colony. Am. J. Primatol. 40:41-53. [DOI] [PubMed] [Google Scholar]
- 32.Hill, L. R., K. R. Hess, L. C. Stephens, P. T. Tinkey, and R. E. Price. 1999. Comparison of kidney weight and volume to selected anatomical parameters in the adult female rhesus monkey (Macaca mulatta). J. Med. Primatol. 28:67-72. [DOI] [PubMed] [Google Scholar]
- 33.Hill, L. R., K. R. Hess, L. C. Stephens, R. E. Price, and K. N. Gray. 2001. Correlation of kidney weight and volume and selected skeletal parameters to sex in the adult rhesus monkey (Macaca mulatta). J. Med. Primatol. 30:56-60. [DOI] [PubMed] [Google Scholar]
- 34.Imaoka, T., H. Kusuhara, M. Adachi, J. D. Schuetz, K. Takeuchi, and Y. Sugiyama. 2007. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol. Pharmacol. 71:619-627. [DOI] [PubMed] [Google Scholar]
- 35.Izzedine, H., J. S. Hulot, D. Vittecoq, J. E. Gallant, S. Staszewski, V. Launay-Vacher, A. Cheng, and G. Deray. 2005. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol. Dial. Transplant. 20:743-746. [DOI] [PubMed] [Google Scholar]
- 36.Jerome, C. P., and P. E. Peterson. 2001. Nonhuman primate models in skeletal research. Bone 29:1-6. [DOI] [PubMed] [Google Scholar]
- 37.Jones, R., J. Stebbing, M. Nelson, G. Moyle, M. Bower, S. Mandalia, and B. Gazzard. 2004. Renal dysfunction with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy regimens is not observed more frequently. A cohort and case-control study. J. Acquir. Immune Defic. Syndr. 37:1489-1495. [DOI] [PubMed] [Google Scholar]
- 38.Kearney, B. P., J. F. Flaherty, and J. Shah. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 43:595-612. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Anaya, A., J. D. Unadkat, L. A. Schumann, and A. L. Smith. 1990. Pharmacokinetics of zidovudine (azidothymidine). II. Development of metabolic and renal clearance pathways in the neonate. J. Acquir. Immune Defic. Syndr. 3:1052-1058. [PubMed] [Google Scholar]
- 40.Mauss, S., F. Berger, and G. Schmutz. 2005. Antiretroviral therapy with tenofovir is associated with mild renal dysfunction. AIDS 19:93-95. [DOI] [PubMed] [Google Scholar]
- 41.McChesney, M. B., E. T. Sawai, and C. J. Miller. 1998. Simian immunodeficiency virus, p. 322-345. In R. Ahmed and I. Chen (ed.), Persistent viral infections. John Wiley & Sons, Ltd., New York, NY.
- 42.Metzner, K. J., J. M. Binley, A. Gettie, P. Marx, D. F. Nixon, and R. I. Connor. 2006. Tenofovir treatment augments anti-viral immunity against drug-resistant SIV challenge in chronically infected rhesus macaques. Retrovirology 3:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.More, C., P. Bettembuk, H. P. Bhattoa, and A. Balogh. 2001. The effects of pregnancy and lactation on bone mineral density. Osteoporos. Int. 12:732-737. [DOI] [PubMed] [Google Scholar]
- 44.Moreno, S., P. Domingo, R. Palacios, J. Santos, V. Falco, J. Murillas, V. Estrada, J. Ena, and M. L. Alvarez. 2006. Renal safety of tenofovir disoproxil fumarate in HIV-1 treatment-experienced patients with adverse events related to prior NRTI use: data from a prospective, observational, multicenter study. J. Acquir. Immune Defic. Syndr. 42:385-387. [DOI] [PubMed] [Google Scholar]
- 45.Moyle, G. J., C. A. Sabin, J. Cartledge, M. Johnson, E. Wilkins, D. Churchill, P. Hay, A. Fakoya, M. Murphy, G. Scullard, C. Leen, and G. Reilly. 2006. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS 20:2043-2050. [DOI] [PubMed] [Google Scholar]
- 46.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 47.Nelson, M. R., C. Katlama, J. S. Montaner, D. A. Cooper, B. Gazzard, B. Clotet, A. Lazzarin, K. Schewe, J. Lange, C. Wyatt, S. Curtis, S. S. Chen, S. Smith, N. Bischofberger, and J. F. Rooney. 2007. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS 21:1273-1281. [DOI] [PubMed] [Google Scholar]
- 48.Padilla, S., F. Gutierrez, M. Masia, V. Canovas, and C. Orozco. 2005. Low frequency of renal function impairment during one-year of therapy with tenofovir-containing regimens in the real-world: a case-control study. AIDS Patient Care STDS 19:421-424. [DOI] [PubMed] [Google Scholar]
- 49.Pozniak, A. L., J. E. Gallant, E. Dejesus, J. R. Arribas, B. Gazzard, R. E. Campo, S. S. Chen, D. McColl, J. Enejosa, J. J. Toole, and A. K. Cheng. 2006. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes—a 96-week analysis. J. Acquir. Immune Defic. Syndr. 43:535-540. [DOI] [PubMed] [Google Scholar]
- 50.Pruvost, A., E. Negredo, H. Benech, F. Theodoro, J. Puig, E. Grau, E. Garcia, J. Molto, J. Grassi, and B. Clotet. 2005. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 49:1907-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ray, A. S., T. Cihlar, K. L. Robinson, L. Tong, J. E. Vela, M. D. Fuller, L. M. Wieman, E. J. Eisenberg, and G. R. Rhodes. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 50:3297-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts, J., E. W. Ford, and J. L. Southers. 1998. Urinary system, p. 312-323. In B. T. Bennett, C. R. Abee, and R. Henrickson (ed.), Nonhuman primates in biomedical research diseases. Academic Press, San Diego, CA.
- 53.Sax, P. E., J. E. Gallant, and P. E. Klotman. 2007. Renal safety of tenofovir disoproxil fumarate. AIDS Read. 17:90-92, 99-104, C3. [PubMed] [Google Scholar]
- 54.Shaw, J. P., C. M. Sueoka, R. Oliyai, W. A. Lee, M. N. Arimilli, C. Kim, and K. C. Cundy. 1997. Metabolism and pharmacokinetics of a novel oral prodrug of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm. Res. 14:1824-1829. [DOI] [PubMed] [Google Scholar]
- 55.Tarantal, A. F., M. L. Marthas, J.-P. Shaw, K. Cundy, and N. Bischofberger. 1999. Administration of 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) to gravid and infant rhesus macaques (Macaca mulatta): safety and efficacy studies. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:323-333. [DOI] [PubMed] [Google Scholar]
- 56.Tarantal, A. F., A. Castillo, J. E. Ekert, N. Bischofberger, and R. B. Martin. 2002. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta). J. Acquir. Immune Defic. Syndr. 29:207-220. [DOI] [PubMed] [Google Scholar]
- 57.Tsai, C.-C., K. E. Follis, T. W. Beck, A. Sabo, R. F. Grant, N. Bischofberger, and R. E. Benveniste. 1995. Prevention of simian immunodeficiency virus infection in macaques by 9-(2-phosphonylmethoxypropyl)adenine (PMPA). Science 270:1197-1199. [DOI] [PubMed] [Google Scholar]
- 58.Van Rompay, K. K., J. A. Johnson, E. J. Blackwood, R. P. Singh, J. Lipscomb, T. B. Matthews, M. L. Marthas, N. C. Pedersen, N. Bischofberger, W. Heneine, and T. W. North. 2007. Sequential emergence and clinical implications of viral mutants with K70E and K65R mutation in reverse transcriptase during prolonged tenofovir monotherapy in rhesus macaques with chronic RT-SHIV infection. Retrovirology 4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, C. J. Berardi, A. S. Mulato, A. Spinner, R. P. Tarara, D. R. Canfield, S. Telm, N. Bischofberger, and N. C. Pedersen. 1996. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob. Agents Chemother. 40:2586-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, P. D. Lamy, P. J. Dailey, D. R. Canfield, R. P. Tarara, N. Bischofberger, and N. C. Pedersen. 1999. 9-[2-(Phosphonomethoxy)propyl]adenine (PMPA) therapy prolongs survival of infant macaques inoculated with simian immunodeficiency virus with reduced susceptibility to PMPA. Antimicrob. Agents Chemother. 43:802-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Rompay, K. K. A., M. D. Miller, M. L. Marthas, N. A. Margot, P. J. Dailey, R. P. Tarara, D. R. Canfield, J. M. Cherrington, N. L. Aguirre, N. Bischofberger, and N. C. Pedersen. 2000. Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J. Virol. 74:1767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Rompay, K. K. A., M. B. McChesney, N. L. Aguirre, K. A. Schmidt, N. Bischofberger, and M. L. Marthas. 2001. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J. Infect. Dis. 184:429-438. [DOI] [PubMed] [Google Scholar]
- 63.Van Rompay, K. K. A., L. L. Brignolo, D. J. Meyer, C. Jerome, R. Tarara, A. Spinner, M. Hamilton, L. L. Hirst, D. R. Bennett, D. R. Canfield, T. G. Dearman, W. Von Morgenland, P. C. Allen, C. Valverde, A. B. Castillo, R. B. Martin, V. F. Samii, R. Bendele, J. Desjardins, M. L. Marthas, N. C. Pedersen, and N. Bischofberger. 2004. Biological effects of short-term and prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob. Agents Chemother. 48:1469-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Rompay, K. K. A., R. Singh, L. Brignolo, J. R. Lawson, K. A. Schmidt, B. Pahar, D. R. Canfield, R. P. Tarara, N. Bischofberger, and M. Marthas. 2004. The clinical benefits of tenofovir for simian immunodeficiency virus-infected macaques are larger than predicted by its effects on standard viral and immunological parameters. J. Acquir. Immune Defic. Syndr. 36:900-914. [DOI] [PubMed] [Google Scholar]
- 65.Van Rompay, K. K. A., R. P. Singh, B. Pahar, D. L. Sodora, C. Wingfield, J. R. Lawson, M. L. Marthas, and N. Bischofberger. 2004. CD8+ cell-mediated suppression of virulent simian immunodeficiency virus during tenofovir treatment. J. Virol. 78:5324-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Rompay, K. K. A. 2005. Antiretroviral drug studies in non-human primates: a valid animal model for innovative drug efficacy and pathogenesis studies. AIDS Rev. 7:67-83. [PubMed] [Google Scholar]
- 67.Van Rompay, K. K. A., B. P. Kearney, J. J. Sexton, R. Colón, J. R. Lawson, E. J. Blackwood, W. A. Lee, N. Bischofberger, and M. L. Marthas. 2006. Evaluation of oral tenofovir disoproxil fumarate and topical tenofovir GS-7340 to protect infant macaques against repeated oral challenges with virulent simian immunodeficiency virus. J. Acquir. Immune Defic. Syndr. 43:6-14. [DOI] [PubMed] [Google Scholar]
- 68.Vela, J. E., L. Y. Olson, A. Huang, A. Fridland, and A. S. Ray. 2007. Simultaneous quantitation of the nucleotide analog adefovir, its phosphorylated anabolites and 2′-deoxyadenosine triphosphate by ion-pairing LC/MS/MS. J. Chromatogr. B 848:335-343. [DOI] [PubMed] [Google Scholar]
- 69.Vigano, A., G. V. Zuccotti, L. Martelli, V. Giacomet, L. Cafarelli, S. Borgonovo, S. Beretta, G. Rombola, and S. Mora. 2007. Renal safety of tenofovir in HIV-infected children: a prospective, 96-week longitudinal study. Clin. Drug Investig. 27:573-581. [DOI] [PubMed] [Google Scholar]
- 70.Winston, A., J. Amin, P. Mallon, D. Marriott, A. Carr, D. A. Cooper, and S. Emery. 2006. Minor changes in calculated creatinine clearance and anion-gap are associated with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy. HIV Med. 7:105-111. [DOI] [PubMed] [Google Scholar]
- 71.Zimmermann, A. E., T. Pizzoferrato, J. Bedford, A. Morris, R. Hoffman, and G. Braden. 2006. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin. Infect. Dis. 42:283-290. [DOI] [PubMed] [Google Scholar]
- 72.Zoetis, T., and M. Hurtt. 2003. Species comparison of anatomical and functional renal development. Birth Defects Res. B 68:111-120. [DOI] [PubMed] [Google Scholar]