Abstract
Thrombospondin-1 (TSP) induces endothelial cell (EC) actin reorganization and focal adhesion disassembly and influences multiple EC functions. To determine whether TSP might regulate EC–EC interactions, we studied the effect of exogenous TSP on the movement of albumin across postconfluent EC monolayers. TSP increased transendothelial albumin flux in a dose-dependent manner at concentrations ≥1 μg/ml (2.2 nM). Increases in albumin flux were observed as early as 1 h after exposure to 30 μg/ml (71 nM) TSP. Inhibition of tyrosine kinases with herbimycin A or genistein protected against the TSP-induced barrier dysfunction by >80% and >50%, respectively. TSP-exposed monolayers exhibited actin reorganization and intercellular gap formation, whereas pretreatment with herbimycin A protected against this effect. Increased staining of phosphotyrosine-containing proteins was observed in plaque-like structures and at the intercellular boundaries of TSP-treated cells. In the presence of protein tyrosine phosphatase inhibition, TSP induced dose- and time-dependent increments in levels of phosphotyrosine-containing proteins; these TSP dose and time requirements were compatible with those defined for EC barrier dysfunction. Phosphoproteins that were identified include the adherens junction proteins focal adhesion kinase, paxillin, γ-catenin, and p120Cas. These combined data indicate that TSP can modulate endothelial barrier function, in part, through tyrosine phosphorylation of EC proteins.
INTRODUCTION
Thrombospondin-1 (TSP)1 is an ∼420-kDa trimeric glycoprotein secreted by numerous tissues, including vascular smooth muscle and endothelial cells (ECs), and is also present in the ECM (Mosher, 1990; Lahav, 1993; Bornstein, 1995). TSP is not only expressed in tissues relevant and anatomically proximal to the vasculature, it is also present within the intravascular compartment circulating both in the plasma (Lahav, 1993) and in monocytes and the α-granules of platelets (Mosher, 1990; Lahav, 1993). Monocytes and platelets both continuously traffic through the microvasculature where they interact with the endothelial surface. Whether TSP is presented to the vascular endothelium, in vivo, through an endocrine, paracrine, and/or autocrine pathway is unknown.
TSP influences multiple EC functions, including cell attachment to and spreading on substrates (Lawler et al., 1988; Mosher, 1990; Taraboletti et al., 1990; Lahav, 1993; Bornstein, 1995), cell motility (Taraboletti et al., 1990), and angiogenesis (Taraboletti et al., 1990; Iruela-Arispe et al., 1991). TSP is a member of a small but growing number of structurally dissimilar counteradhesive proteins that have been grouped together on a functional basis (Sage and Bornstein, 1991; Murphy-Ullrich, 1995). By definition, each of these so-called counteradhesive proteins, at least under certain conditions, can alter cell-substrate interactions and actin cytoskeletal organization. This so-called counteradhesive effect includes inhibition of cell–ECM adherens junction or focal adhesion (FA) formation as well as stimulation of FA disassembly (Sage and Bornstein, 1991; Murphy-Ullrich, 1995). This emphasis on single-cell morphology and on the cell–matrix interface has encouraged studies of cells under subconfluent conditions. Only recently has it been appreciated that counteradhesive proteins may also influence EC–EC interactions (Goldblum et al., 1994a).
The vascular endothelium presents a selective barrier that actively regulates movement of circulating macromolecules and cells into extravascular tissues and compartments (Malik et al., 1989; Luscinskas et al., 1991). A structure–function relationship appears to exist between EC actin organization/shape and barrier function. Agents that disrupt actin microfilaments increase endothelial permeability, previous F-actin stabilization protects against this increase, and established mediators of permeability induce actin reorganization (Shasby et al., 1982; Bussolino et al., 1987; Goldblum et al., 1993). In EC, F-actin is arranged into both central transcytoplasmic cables and a peripheral band (Wong and Gotlieb, 1986). These microfilaments are linked to two types of adherens junctions, FAs (Clark and Brugge, 1995; Parsons and Parsons, 1997) and the zonula adherens (ZA) (Kemler, 1993; Gumbiner, 1996; Barth et al., 1997). The cytoplasmic filaments terminate within the FA, and the subcortical filaments interdigitate with the ZA. Because the ZA mechanically couples the peripheral actin cytoskeleton to the surface receptors that mediate homophilic cell–cell adhesion, it is strategically located for regulation of the paracellular pathway. The signal transduction pathways that regulate the state of assembly of these adherens junctions are incompletely understood; however, protein tyrosine phosphorylation of components within the FA (Clark and Brugge, 1995; Parsons and Parsons, 1997) and ZA (Kemler, 1993; Gumbiner, 1996; Barth et al., 1997) are associated with changes in the state of adherens junction assembly and increased vascular permeability (Abedi and Zachary, 1997; Esser et al., 1998).
Multiple domains within TSP recognize various EC surface receptors (Mosher, 1990; Lahav, 1993; Bornstein, 1995). Although the specific signaling pathways to which each of these receptors is coupled remain undefined, several EC receptors, including CD36 (Bull et al., 1994), αvβ3 (Blystone et al., 1996) and the integrin-associated protein (IAP) (Gao et al., 1996), have been coupled to tyrosine phosphorylation events. CD36 tightly associates with several src family kinases (Bull et al., 1994). Sequences within TSP that bind to the IAP receptor have been demonstrated to induce tyrosine phosphorylation of focal adhesion kinase (FAK), paxillin, and a unidentified 90-kDa protein in human melanocytes (Gao et al., 1996). TSP also interacts with other endogenous proteins including TGFβ (Murphy-Ullrich et al., 1992; Schultz-Cherry and Murphy-Ullrich, 1993), a cytokine associated with multiple biological activities, including wound healing, proliferation, and angiogenesis (Roberts and Sporn, 1993). Activation of latent TGFβ by TSP has been demonstrated both in vitro (Schultz-Cherry and Murphy-Ullrich, 1993; Schultz-Cherry et al., 1995) and in vivo (Crawford et al., 1998). That TSP activates TGFβ, induces tyrosine phosphorylation and actin reorganization, and influences angiogenesis supports the concept that TSP bioactivity is mediated through changes in EC–EC homophilic adhesion. In this study, we demonstrate that TSP regulates an endothelial paracellular pathway, in part, through a tyrosine phosphorylation-dependent pathway.
MATERIALS AND METHODS
Human TSP Preparation
TSP was purified as described previously (Murphy-Ullrich et al., 1992; Schultz-Cherry and Murphy-Ullrich, 1993). Briefly, fresh human platelets (Birmingham American Red Cross, Birmingham, AL) were thrombin-stimulated, and the platelet releasate was applied to a heparin-Sepharose CL-6B (Pharmacia, Piscataway, NJ) affinity column preequilibrated with Tris-buffered saline (TBS)-C (0.01 M Tris-HCl, 0.15 M NaCl, 0.1 mM CaCl, pH 7.4. The bound TSP was eluted and applied to an A0.5 M gel filtration column (Bio-Rad Laboratories, Richmond, CA) preequilibrated with TBS-C, pH 7.4. TSP that was depleted of bound TGFβ, i.e., stripped TSP (sTSP), was similarly prepared except that the gel filtration column was equilibrated at pH 11.0. TGFβ activity in TSP preparations was measured using the normal rat kidney soft agar colony formation assay as described previously (Murphy-Ullrich et al., 1992; Schultz-Cherry and Murphy-Ullrich, 1993).
Endothelial Cell Culture
Bovine pulmonary artery endothelial cells (ECs) (American Tissue Culture Collection, Rockville, MD) were cultured in DMEM (Sigma, St. Louis, MO) enriched with 20% heat-inactivated (56°C, 30 min) fetal bovine serum (Hyclone Laboratories, Logan, UT), l-glutamine 4 mM, nonessential amino acids, and vitamins in the presence of penicillin (50 u/ml) and streptomycin (50 μg/ml) (Sigma) as described previously (Goldblum et al., 1993). To determine whether these ECs expressed CD36 at the protein level, EC lysates were resolved by electrophoresis and electrotransferred. Milk-fat globule membranes of bovine mammary epithelial cells generously provided by Dr. I. H. Mather (University of Maryland, College Park, MD) were used as the positive control for bovine CD36 (Greenwalt and Mather, 1985). The blot was incubated with rabbit anti-human CD36 antibody (Alessio et al., 1996) kindly provided by Drs. N.N. Tandon and G.A. Jamieson (American Red Cross, Rockville, MD) followed by HRP-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma) and developed with enhanced chemiluminescence (ECL) (Amersham, Arlington Heights, IL). In the bovine ECs used in these studies, no CD36 expression was detected (Young, unpublished observations).
Assay of Transendothelial Albumin Flux
Transendothelial 14C-BSA flux was assayed as described previously (Goldblum et al., 1993). Briefly, gelatin-impregnated polycarbonate filters (Nucleopore, Pleasanton, CA) mounted in chemotactic chambers (ADAPS,, Dedham, MA) were inserted into wells of 24-well plates. Each upper compartment was seeded with 2 × 105 ECs and cultured for 72 h. The baseline barrier function of each monolayer was determined by applying 14C-BSA to each upper compartment (0.5 ml) for 1 h at 37°C, after which the lower compartment (1.5 ml) was counted for 14C activity. Only monolayers retaining ≥97% of the 14C-BSA were studied. The monolayers were then exposed to increasing TSP concentrations for 6 h in media containing 10% fetal bovine serum. On the basis of the established dose–response relationship, other monolayers were exposed to TSP-enriched medium at a fixed TSP concentration (30 or 20 μg/ml) for increasing exposure times. Simultaneous controls with medium alone were performed for each time point. Transfer of 14C-BSA across EC monolayers was again assayed and expressed as picomoles per hour.
To ensure that the increases in 14C-BSA flux across EC monolayers ascribed to our TSP preparations were due to TSP and not a contaminant(s), a murine monoclonal anti-TSP IgG antibody (Ab 133) (Schultz-Cherry and Murphy-Ullrich, 1993) was used. EC monolayers were exposed for 6 h to TSP (30 μg/ml), TSP preincubated with Ab 133 (90 μg/ml, 1 h, 25°C) or Ab 133 alone. Transfer of 14C-BSA across EC monolayers was then assayed and expressed as picomoles per hour as described above. To exclude the contribution of endotoxin or bacterial lipopolysaccharide (LPS), transendothelial 14C-BSA flux was assayed after 6 h exposures to 30 μg TSP/ml, TSP preincubated with polymixin B (PMB) immobilized onto agarose beads (Sigma), LPS derived from Escherichia coli:0111:B4 (Sigma) 100 ng/ml, and LPS preincubated with PMB in the presence of 10% fetal bovine serum. Because LPS-induced barrier dysfunction is profoundly serum dependent, the TSP-induced changes were also studied under strict serum-starvation conditions as described previously (Goldblum et al., 1994).
To determine whether TGFβ might contribute to TSP-induced barrier dysfunction, human recombinant TGFβ1, an anti-TGFβ antibody (R&D Systems, Minneapolis, MN), as well as a peptide demonstrated to block activation of latent TGFβ (GGWSHW) (Schultz-Cherry et al., 1995), each were tested in the barrier function assay. To further exclude TGFβ bioactivity, TSP that was depleted of bound TGFβ, i.e. sTSP, was compared with equivalent concentrations of native TSP in the same barrier function assay. To determine whether ECM proteins other than TSP could augment transendothelial 14C-BSA flux, equimolar concentrations to TSP at 30 μg/ml (i.e., 71 nM for trimeric TSP) of human fibronectin, human vitronectin, and bovine type I collagen (all purchased from Collaborative Biomedical Products, Bedford, MA) each were simultaneously tested in the barrier function assay.
In other experiments, EC monolayers were pretreated with the protein synthesis inhibitor cycloheximide (Sigma) 50 μg/ml, 0.5 h before and throughout the TSP or media exposures. This concentration of cycloheximide inhibited >95% of EC protein synthesis as measured by [35S] methionine (New England Nuclear, DuPont, Boston, MA) incorporation into trichloracetic acid–precipitable protein as we have described previously (Goldblum et al., 1993). Simultaneous cycloheximide controls were also included.
Effect of Protein Tyrosine Kinase/Phosphatase Inhibition
To determine whether the state of EC protein tyrosine phosphorylation mediated TSP-induced changes in barrier function, experiments were performed in the presence of either protein tyrosine kinase (PTK) or protein tyrosine phosphatase (PTP) inhibitors (Young et al., 1998). The concentration of each inhibitor was chosen on the basis of its activity in the barrier function assay; for each agent the maximal concentration that did not alter albumin flux was used. EC monolayers were pretreated with genistein (50 μg/ml or 185 μM), sodium orthovanadate (vanadate) (2.5 μM), or phenylarsine oxide (PAO) (0.1 μM) (Sigma), 0.5 h before and throughout the exposure to TSP or media. Herbimycin A (1.0 μM) (Sigma) was introduced ∼16 h before and throughout the TSP or media exposure. The presence of dimethylsulfoxide 0.1% was controlled for in simultaneous media controls.
Immunoblotting for EC Phosphotyrosine
Phosphotyrosine immunoblotting was performed as described previously (Young et al., 1998). Postconfluent EC monolayers were exposed to TSP or to media alone, in the presence or absence of PTK or PTP inhibitors for increasing exposure times. The cells were then lysed with ice-cold lysis buffer containing 50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM ethylene glycol tetraacetic acid, 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml leupeptin, 1 mg/ml pepstatin, 1 mg/ml aprotinin, 100 mg/ml type-1 DNAse, 1 mM Na3VO4, 1 mM NaF, 10 mM pyrophosphate, 500 μM paranitrophenol, and 1 mM PAO (all purchased from Sigma). The lysates were centrifuged, and the supernatants were collected. All samples were assayed for protein concentration with a standard Bio-Rad DC Protein assay kit (Bio-Rad Chemical Division). The samples were resolved by electrophoresis on an 8–16% gradient SDS-polyacrylamide gel (Novex, San Diego, CA) and were transferred onto polyvinylidene difluoride membranes (ESA, Chelmsford, MA). To insure equal protein loading and transfer, each blot was stained with Fast Green concentrate (Sigma). The blot was blocked in 5% nonfat dry milk and incubated with biotinylated antiphosphotyrosine mAb (0.8 μg/ml) (4G10, Upstate Biotechnology, Lake Placid, NY) followed by HRP-conjugated streptavidin (Upstate Biotechnology) (0.5 μg/ml). The blot was developed with ECL and exposed to x-ray film (DuPont, Newark, DE) for increasing times. To confirm equivalent protein loading, blots were stripped with 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl, pH 6.7, and incubated with 0.5 μg/ml murine antiphysarum β-tubulin IgG 2b (Boehringer Mannheim, Indianapolis, IN) followed by HRP-conjugated anti-mouse IgG (Transduction Laboratories, Lexington, KY), and developed with ECL. Autoradiographs were scanned by laser densitometry (Molecular Dynamics, Sunnyvale, CA). In selected experiments, ECs exposed to human fibronectin, human vitronectin, and bovine type I collagen were similarly processed for phosphotyrosine immunoblotting.
F-Actin Epifluorescence Microscopy and Immunolocalization of Phosphotyrosines
To maintain EC monolayers under experimental conditions identical to our permeability assay, we stained monolayers directly on polycarbonate filters as described previously (Goldblum et al., 1993; Young et al., 1998). ECs grown to confluence on filters were exposed for 6 h to TSP (20 μg/ml) or to media alone. Selected cultures were incubated with herbimycin A (1.0 μM) for 16 h before and throughout the 6 h interval. The monolayers were fixed in 3.7% formaldehyde for 20 min, rendered permeable in 0.5% Triton X-100 in HEPES buffer for 5 min, and stained with the F-actin probe fluorescein–phalloidin (1.65 × 10−7M) (Molecular Probes, Eugene, OR) for 20 min. In other experiments, ECs cultured on filters were probed for phosphotyrosine-containing proteins as described previously (Young et al., 1998). The monolayers were washed with PBS containing 1 mM vanadate, fixed in 4% paraformaldehyde for 20 min followed by absolute methanol (10 min, −20°C), washed, and incubated for 1 h with FITC-conjugated antiphosphotyrosine antibody (5 μg/ml) (UBI). The filters and their attached monolayers were mounted cell-side up on microscope slides and photographed through a Zeiss Axioskop 20 Microscope (Carl Zeiss, Thornwood, NY) equipped for epifluorescence.
Assay of EC Injury
To determine whether TSP-induced changes in endothelial barrier function could be explained by EC injury, TSP-exposed and medium control monolayers were studied for 51Cr release as we have described previously (Goldblum et al., 1994). Briefly, ECs were labeled with [51Cr]-sodium chromate (Amersham), and the labeled monolayers were incubated for 6 h with either TSP (30 μg/ml) or medium alone. The supernatants were centrifuged and counted. All washed monolayers were solubilized with 1% Triton X-100 (Sigma) to induce maximum release. The lysates were centrifuged, and the supernatants were counted for 51Cr activity. EC injury was expressed as [51Cr supernatant)/(51Cr supernatant + 51Cr cell lysate)] × 100%.
Identification of Phosphotyrosine-containing Proteins
EC lysates were precleared by incubation with antimurine IgG cross-linked to agarose (Sigma) for 1 h at 4°C and then incubated overnight at 4°C with specific murine mAbs raised against paxillin, β-catenin, γ-catenin, p120Cas, (Transduction Laboratories), or FAK (UBI). The resultant immune complexes were immobilized by incubation with antimurine IgG cross-linked to agarose, centrifuged, washed, boiled for 5 min in sample buffer, and again centrifuged. The supernatants were processed for immunoblotting with antiphosphotyrosine (4G10) antibody as described above. To control for discrepancies in the amount of immunoprecipitated protein, blots were stripped and reprobed with the immunoprecipitating antibody. The blots were subsequently incubated with HRP-conjugated antimurine IgG (Transduction Laboratories) and developed with ECL. Autoradiographs were scanned by laser densitometry, and the phosphotyrosine-containing bands were normalized to the precipitated protein of interest.
Statistical Methods
ANOVA was used to compare the mean responses among experimental and control groups for all experiments. The Dunnett and Scheffe F-test was used to determine between which groups significant differences existed. A p value of < 0.05 was considered significant.
RESULTS
Effect of TSP on Transendothelial 14C-BSA Flux
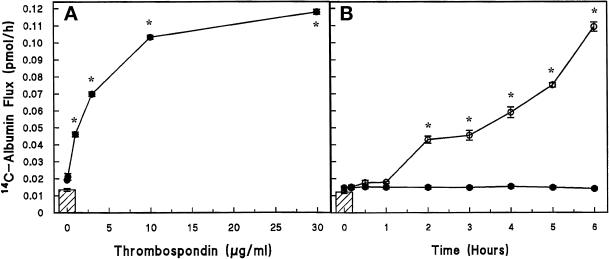
TSP increased transendothelial 14C-BSA flux in a concentration-dependent manner (Figure 1A). The mean (±SE) pretreatment transendothelial 14C-BSA flux was 0.008 ± 0.002 pmol/h (n = 30) and the mean (±SE) 14C-BSA transfer across naked filters without EC monolayers was 0.189 ± 0.003 pmol/h (n = 6). The lowest TSP concentration that induced a significant increment in 14C-BSA flux compared with the media control was 1.0 μg/ml. The maximum mean (±SE) 14C-BSA flux of 0.118 ± 0.002 pmol/h was seen with TSP 30 μg/ml, at which point the TSP-induced effect had begun to plateau or saturate (Figure 1A). The effect of TSP on endothelial barrier function was also time dependent (Figure 1B). Transendothelial 14C-BSA flux was assayed after exposure to TSP (20 μg/ml) or media alone over a time period from 10 min to 6 h. 14C-BSA flux across media control monolayers did not change throughout the 6-h study period. TSP (20 μg/ml) induced significant increments in 14C-BSA flux compared with the simultaneous media control at ≥2 h (Figure 1B). In other studies, 30 μg/ml TSP significantly increased 14C-BSA flux across EC monolayers as early as 1 h compared with the simultaneous media control (0.033 ± 0.001 pmol/h, n = 9 vs. 0.015 ± 0.002 pmol/h, n = 10, respectively) with further time-dependent increments (Young, unpublished observations).
Figure 1.
Dose- and time-dependent effect of TSP on transendothelial 14C-BSA flux. Mean (±SE) pretreatment baseline transendothelial 14C-BSA flux is shown by the cross-hatched bars. *Significantly increased compared with the simultaneous media control at p < 0.05. (A) Closed circles represent mean (±SE) 14C-BSA flux in picomoles per hour immediately after 6-h exposures to increasing concentrations of TSP (0, 0.1, 1, 3, 10, and 30 μg/ml) in the presence of 10% serum (n = 5). (B) Circles represent mean (±SE) 14C-BSA flux in picomoles per hour immediately after increasing exposure times (0, 0.17, 0.5, 1, 2, 3, 4, 5, and 6 h) to TSP (20 μg/ml) (○) (n = 4) and simultaneous medium controls (●) (n = 4).
Effect of TSP on EC Injury
A 51Cr release assay was used to determine whether a TSP exposure (30 μg/ml, 6 h) that compromises endothelial barrier function also might induce EC injury or death over the same time period. This assay detects defects in the plasma membrane that permit passage of molecules ≤1000 Da. EC monolayers preloaded with 51Cr were exposed for 6 h to TSP (30 μg/ml) or media alone. Mean (±SE) 51Cr release from TSP-exposed EC was not significantly different than release from the simultaneous media controls (12.68 ± 0.35%, n = 8, vs. 11.97 ± 0.51%, n = 8, respectively), indicating that TSP-induced changes in barrier function were not the result of cytotoxicity.
Neutralization of TSP and Exclusion of Endotoxin
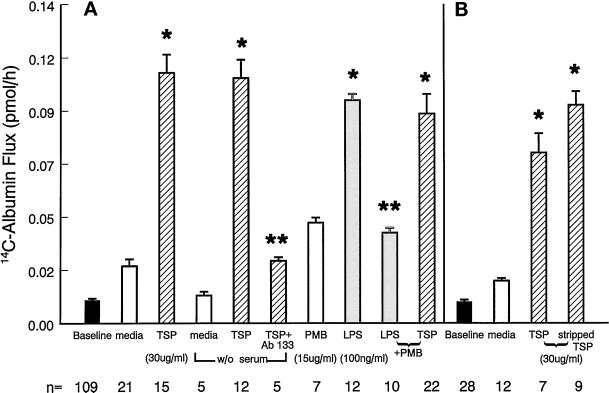
To ensure that the TSP-induced increases in 14C-BSA flux across EC monolayers could be ascribed to TSP bioactivity, TSP was tested both in the absence and presence of anti-TSP 133 IgG (Figure 2A). Preincubation of TSP (30 μg/ml) with Ab 133 (Schultz-Cherry and Murphy-Ullrich, 1993) reduced the TSP effect by >80%. Endotoxin, in the presence of serum accessory molecules, is known to influence endothelial barrier function (Goldblum et al., 1994). One TSP preparation analyzed for endotoxin activity by a chromogenic limulus amebocyte lysate assay (Associates of Cape Cod, Inc., Woods Hole, MA) was found to contain ∼0.36 ng endotoxin/μg TSP. To determine whether these levels of endotoxin contributed to the TSP effect in the presence of serum, the ability of either serum deprivation or of PMB adsorption to decrease TSP-induced barrier dysfunction was examined (Figure 2A). The TSP effect in the presence or absence of serum was not significantly different. The effect of TSP preincubated with a concentration of PMB that neutralizes 100 ng/ml LPS was not different from the effect of TSP alone. It is therefore unlikely that TSP-induced changes in endothelial barrier function can be ascribed to endotoxin contamination; however, these findings do not exclude the possibility of synergistic action between TSP and trace amounts of endotoxin.
Figure 2.
Neutralization of TSP and exclusion of endotoxin and TGFβ. Vertical bars represent mean (±SE) transendothelial 14C-BSA flux in picomoles per hour. Mean (±SE) pretreatment baseline is shown by the closed bars, and n indicates the number of monolayers studied. (A) 14C-BSA flux immediately after 6-h exposures to TSP (30 μg/ml) and the simultaneous media control both in the presence of 10% serum, TSP and the media control under serum-free conditions, TSP + Ab 133 (90 μg/ml), PMB (15 μg/ml), LPS (100 ng/ml), LPS + PMB, and TSP + PMB. *Significantly increased compared with the appropriate media control at p < 0.05. **Significantly decreased compared with simultaneous control at p < 0.05. (B) 14C-BSA flux immediately after 6-h exposures to TSP (30 μg/ml) or stripped TSP (30 μg/ml) (cross-hatched bars) or the simultaneous media control (open bars). *Significantly increased compared with simultaneous media control at p < 0.05.
Exclusion of TGFβ
TSP is associated with variable amounts of active TGFβ, usually ranging from 30 to 70 pg TGFβ/μg TSP. We therefore tested up to >500-fold higher concentrations of TGFβ (0.1–500 ng/ml) in the barrier function assay (Table 1). Six-hour exposures to TGFβ at concentrations of ≥1.0 ng/ml increased albumin flux compared with the simultaneous media control; however, the level of flux obtained with 1.0 ng/ml TGFβ could not account for the level of flux observed with 30 μg/ml TSP. Furthermore, significant increments in flux were observed with ≥1.0 μg/ml TSP. At these concentrations, the estimated amount of associated TGFβ (≥0.035 ng/ml) activity would be insufficient to significantly increase flux compared with media controls (Table 1). To further assess whether the TSP-associated TGFβ activity contributed to the TSP effect, native TSP (30 μg/ml) was preincubated with anti-TGFβ antibody and then tested in the permeability assay. Although the anti-TGFβ antibody at either 100 or 250 μg/ml diminished the TSP effect (Table 2), it only partially blocked it (30–35% inhibition), suggesting that associated TGFβ activity or TSP-stimulated TGFβ activity accounts for only a fraction of the observed TSP activity. To determine whether the TSP-associated TGFβ activity was a requirement for the TSP effect, TSP that was depleted of bound TGFβ, i.e., sTSP, was compared with equivalent concentrations of native TSP with its associated TGFβ activity for the ability to augment transendothelial 14C-BSA flux (Figure 2B). TSP depleted of TGFβ retained its ability to induce changes in barrier function. In fact, on a molar basis, sTSP induced significantly greater barrier dysfunction than did TSP complexed with TGFβ. Although TGFβ can exert its own intrinsic activity in the barrier function assay, it also might mask a site on the TSP molecule that mediates increased vascular permeability. To determine the contribution of latent TGFβ activation, the peptide GGWSHW known to block sTSP activation of latent TGFβ (Schultz-Cherry et al., 1995) and an anti-TGFβ antibody each were tested for their ability to diminish sTSP-induced barrier dysfunction (Table 2). The anti-TGFβ antibody and the GGWSHW peptide each decreased the sTSP effect by ∼15%, whereas the control peptide (GGYSHW) did not. These data suggest that at higher TSP concentrations, TGFβ activity can explain up to 30% of the TSP effect and that approximately one-half of this TGFβ contribution (∼15%) is due to TSP activation of latent TGFβ.
Table 1.
Concentration-dependent effect of TGFβ on endothelial barrier function
| TGFβ (ng/ml) | Barrier function (pmol/h)a
|
||
|---|---|---|---|
| n | Mean | SEM | |
| 0 | 14 | 0.026 | 0.003 |
| 0.1 | 3 | 0.034 | 0.005 |
| 1.0 | 11 | 0.042 | 0.003b |
| 10.0 | 11 | 0.051 | 0.001b |
| 100.0 | 11 | 0.052 | 0.003b |
| 500.0 | 3 | 0.071 | 0.005b |
Transendothelial 14C-BSA flux was determined after 6-h exposure to TGFβ.
Significantly increased compared with simultaneous media control at p < 0.05.
Table 2.
Contribution of TGFβ to TSP-induced endothelial barrier dysfunction
| Treatment | Barrier function (pmol/h)a
|
||
|---|---|---|---|
| n | Mean | SEM | |
| Media control | 6 | 0.021 | 0.001 |
| TSP (30 μg/ml) | 9 | 0.136 | 0.004b |
| TSP + anti-TGFβ (100 μg/ml) | 4 | 0.089 | 0.003b,c |
| TSP + anti-TGFβ (250 μg/ml) | 3 | 0.095 | 0.004b,c |
| Media control | 6 | 0.022 | 0.001 |
| sTSP (15 μg/ml) | 6 | 0.111 | 0.001b |
| sTSP + anti-TGFβ (100 μg/ml) | 6 | 0.093 | 0.003b,d |
| sTSP + GGWSHW (10 μM) | 6 | 0.095 | 0.004b,d |
| sTSP + GGYSHWe (10 μM) | 6 | 0.110 | 0.007b |
Transendothelial 14C-BSA flux was determined after 6-h exposure to TSP, sTSP, or media alone.
Significantly increased compared with simultaneous media control at p < 0.05.
Significantly decreased compared with TSP (30 μg/ml) alone at p < 0.05.
Significantly decreased compared with sTSP (15 μg/ml) alone at p < 0.05.
Control peptide for GGWSHW.
Effect of ECM Proteins on Endothelial Barrier Function
To determine whether ECM proteins other than TSP might similarly influence endothelial barrier function, monolayers were exposed for 6 h to equimolar concentrations of TSP, fibronectin, vitronectin, type I collagen, or media alone (Table 3). Of the ECM proteins tested, only TSP increased transendothelial 14C-BSA flux compared with the simultaneous media controls.
Table 3.
Effect of ECM proteins on endothelial barrier function
| Treatment | Barrier function (pmol/h)a
|
||
|---|---|---|---|
| n | Mean | SEM | |
| Media control | 3 | 0.017 | 0.001 |
| TSP | 3 | 0.119 | 0.006b |
| Fibronectin | 4 | 0.017 | 0.001 |
| Vitronectin | 4 | 0.016 | 0.001 |
| Collagen I | 3 | 0.020 | 0.001 |
Transendothelial 14C-BSA flux was determined after 6-h exposures to media or equimolar concentrations (71 nM) of TSP, fibronectin, vitronectin, and collagen I.
Significantly increased compared with the media control at p < 0.05.
Effect of Protein Synthesis Inhibition on TSP-induced Changes in Endothelial Barrier Function
The increase in monolayer permeability was evident after 2–6 h of TSP exposure. It is possible that during this prolonged stimulus-to-response lag time, TSP could induce EC synthesis of a second protein mediator(s) that could alter barrier function in an autocrine/paracrine manner. To investigate this possibility, the ability of TSP to stimulate this response was examined in the presence of a protein synthesis inhibitor. Pretreatment of monolayers with cycloheximide (50 μg/ml, 6 h) failed to block the TSP-induced increment in albumin flux (0.062 ± 0.001 pmol/h, n = 7 vs. 0.073 ± 0.008 pmol/h, n = 5). Cycloheximide alone failed to increase mean (±SE) 14C-BSA flux compared with the media control (0.016 ± 0.001 pmol/h, n = 5 vs. 0.019 ± 0.001 pmol/h, n = 12, respectively). Thus, de novo protein synthesis does not appear to be necessary for the response to TSP.
Effect of PTK Inhibition on TSP-induced Changes in Endothelial Barrier Function
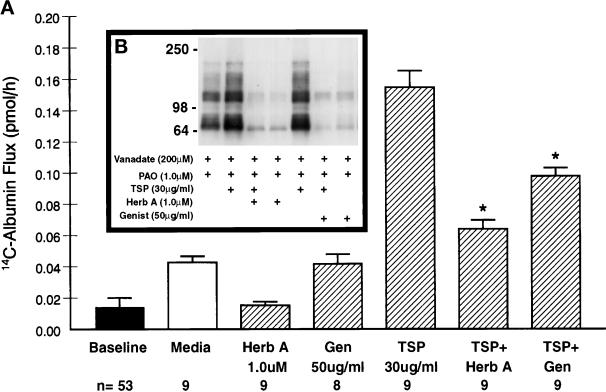
To determine whether protein tyrosine phosphorylation mediated TSP-induced changes in barrier function, TSP was presented to monolayers in the presence of one of two PTK inhibitors (Figure 3A). The mean (±SE) pretreatment baseline barrier function was 0.014 ± 0.006 pmol/h (n = 53). 14C-BSA flux across EC monolayers treated with either herbimycin A (1.0 μM) or genistein (185 μM) alone was not increased compared with the media controls. A 6-h exposure to TSP (30 μg/ml) significantly increased transendothelial 14C-BSA flux compared with the media control. Pretreatment of monolayers with either herbimycin A or genistein protected against the TSP-induced increment by >80% and >50%, respectively. Therefore, two structurally and functionally dissimilar PTK inhibitors each significantly diminished the effect of TSP in this permeability assay.
Figure 3.
Effect of PTK inhibition on TSP-induced changes in endothelial barrier function and tyrosine phosphorylation of EC proteins. (A) Vertical bars represent mean (±SE) transendothelial 14C-BSA flux in picomoles per hour immediately after 6-h exposures to TSP (30 μg/ml), genistein (50 μg/ml), herbimycin A (1.0 μM), TSP + genistein, TSP + herbimycin A, and the simultaneous media control. Herbimycin A was introduced 16 h before the standard 6-h study period. The presence of dimethylsulfoxide 0.1% was controlled for in the media controls. The pretreatment baseline is shown by the closed bar. n indicates the number of monolayers studied. *Significantly decreased compared with treatment with TSP alone at p < 0.05. (B) ECs were exposed for 1 h to vanadate (200 μM) and PAO (1.0 μM) in the absence or presence of TSP (30 μg/ml) and in the absence or presence of either herbimycin A (1.0 μM) or genistein (50 μg/ml). Herbimycin A was introduced 16 h before the 1-h study period. The ECs were lysed and processed for immunoblotting for phosphotyrosine-containing proteins. Molecular weights in kilodaltons are indicated on the left.
Effect of PTP Inhibition on TSP-induced Changes in Endothelial Barrier Function
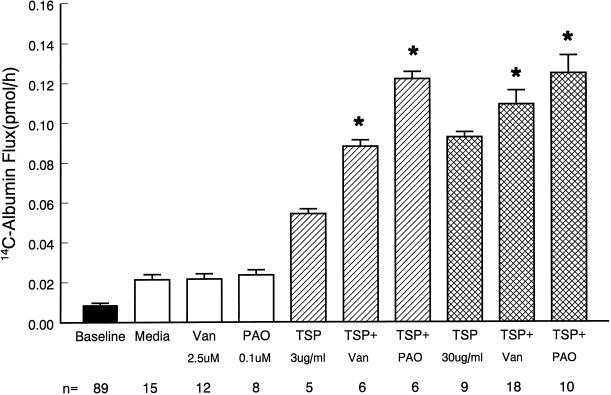
Consistent with the suggested involvement of PTK activity in TSP-induced loss of barrier function (Figure 3), inhibition of PTP activity enhanced the TSP effect (Figure 4). The mean (±SE) pretreatment baseline barrier function was 0.012 ± 0.001 pmol/h (n = 89). Pretreatment of monolayers with either vanadate (2.5 μM) or PAO (0.1 μM) alone for 6 h did not significantly increase 14C-BSA flux compared with media controls. In the presence of TSP 3 μg/ml, vanadate and PAO each enhanced the TSP effect by 62 and 120%, respectively (Figure 4). At a higher TSP concentration of 30 μg/ml, these same two PTP inhibitors enhanced the TSP effect by 17 and 33%, respectively. The ability of these two structurally and functionally dissimilar PTP inhibitors to enhance the TSP effect offers further evidence that TSP influences EC barrier function through protein tyrosine phosphorylation.
Figure 4.
Effect of PTP inhibition on TSP-induced changes in endothelial barrier function. Vertical bars represent mean (±SE) transendothelial flux in picomoles per hour immediately after 6-h exposures to TSP (3 or 30 μg/ml), vanadate (Van) (2.5 μM), PAO (0.1 μM), TSP + Van, TSP + PAO, and the simultaneous media control (open bar). The mean (±SE) pretreatment baseline is shown by the closed bar. n indicates the number of monolayers studied. *Significantly increased compared with treatment with TSP alone at p < 0.05.
Effect of PTK Inhibition on TSP-induced Intercellular Gap Formation
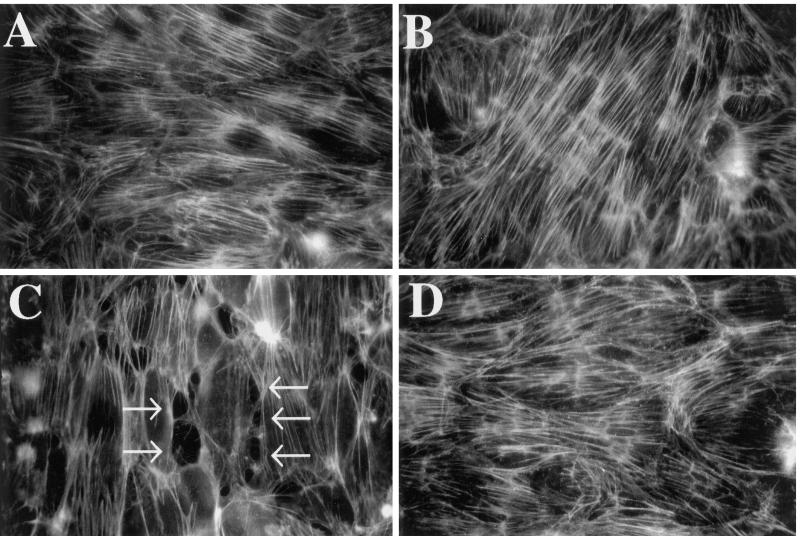
On the basis of data that PTK activity is involved in the TSP-induced loss in barrier function, the role of protein tyrosine phosphorylation in opening of the paracellular pathway was assessed. Accordingly, EC monolayers exposed to TSP (30 μg/ml) ± herbimycin A (1.0 μM) were stained with fluorescein–phalloidin, an F-actin–specific reagent. By fluorescence microscopy, monolayers incubated with herbimycin A or media alone exhibited continuous transcytoplasmic actin filaments and cell-to-cell apposition without intercellular gaps (Figure 5, A and B). After exposure to TSP for 6 h, isolated ellipsoid disruptions within the F-actin lattice occurred exclusively at the cell–cell interface (Figure 5C). In EC monolayers preincubated with herbimycin A for 16 h before and throughout the 6-h exposure to TSP, no intercellular gaps could be demonstrated (Figure 5D). Therefore, PTK inhibition blocked the TSP-induced formation of intercellular gaps in these tightly confluent EC monolayers.
Figure 5.
Effect of PTK inhibition on TSP-induced intercellular gap formation. EC monolayers grown on filters were exposed for 6 h to media or TSP (30 μg/ml) in the presence or absence of herbimycin A (1.0 μM), the latter introduced 16 h before the addition of TSP. The monolayers were fixed, rendered permeable, stained with fluorescein–phalloidin, and examined by epifluorescence microscopy. (A) Medium control; (B) herbimycin A control; (C) TSP; (D) TSP + herbimycin A. Arrows in C indicate intercellular gaps. Magnification, 750×.
Immunolocalization of Phosphotyrosines in TSP-exposed EC
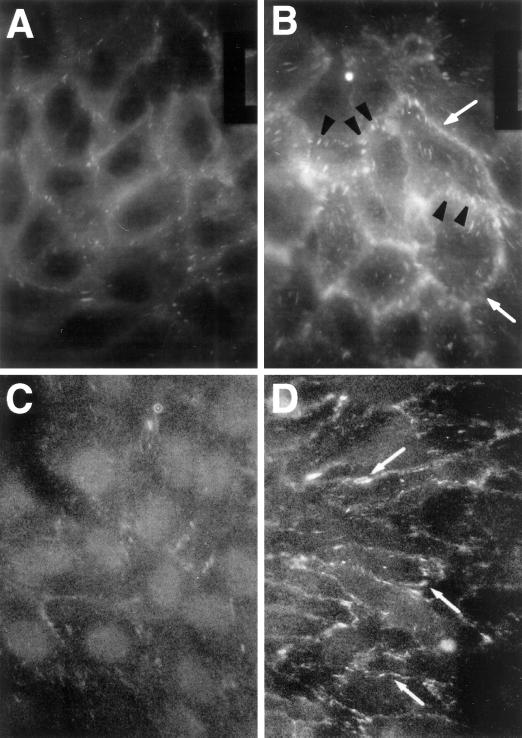
Because TSP-stimulated protein tyrosine phosphorylation appears necessary for intercellular gap formation and increases in albumin flux, we asked whether phosphoproteins localized to cell–cell junctions might be involved. When EC monolayers were exposed to TSP (20 μg/ml) for either 10 min (Figure 6B) or 1 h (Figure 6D), and then probed with FITC-conjugated antiphosphotyrosine antibody, TSP-exposed EC displayed a fluorescence signal predominately restricted to intercellular boundaries. In addition, plaque-like structures were evident both at intercellular boundaries and, to a lesser degree, throughout the cell bodies. In contrast, the control cells provided with media alone showed only relatively weak immunostaining for phosphotyrosine-containing proteins (Figure 6, A and C). These data suggest that TSP, after exposure times as brief as 10 min, preferentially stimulates tyrosine phosphorylation of proteins that are either enriched to or upon phosphorylation translocate to cell–cell junctions in confluent EC monolayers.
Figure 6.
Immunolocalization of phosphotyrosine-containing proteins in TSP-exposed ECs. ECs on filters were exposed for 10 min or 1 h to media or TSP (20 μg/ml), fixed, incubated with FITC-conjugated antiphosphotyrosine antibody, and analyzed by epifluorescence microscopy. (A) Medium control, 10 min; (B) TSP, 10 min; (C) medium control, 1 h; (D) TSP, 1 h. Arrowheads in B point to phosphotyrosine signal restricted to FA-like structures, and arrows in B and D point to phosphotyrosine signal restricted to intercellular boundaries of ECs exposed to TSP. Magnification, 750×.
TSP-induced Tyrosine Phosphorylation of EC Proteins
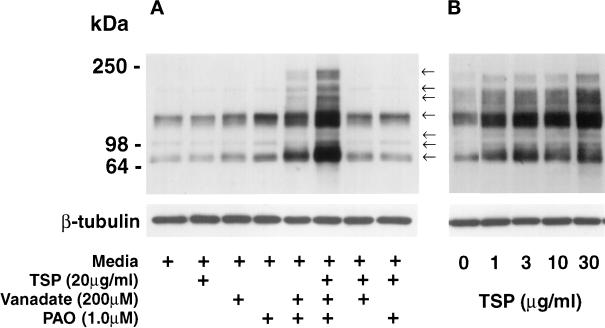
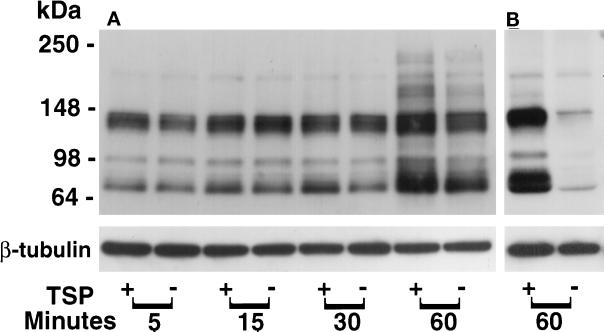
As a first step to determine which EC proteins might be tyrosine-phosphorylated in response to TSP, extracts obtained from EC were processed for phosphotyrosine immunoblotting. Exposure of EC to a range of TSP concentrations for varying time intervals demonstrated no consistent increases in protein tyrosine phosphorylation in the absence of PTP inhibition; however, in the presence of both PTP inhibitors, vanadate (200 μM) and PAO (1.0 μM), exogenous TSP was clearly associated with increased tyrosine phosphorylation of EC proteins (Figure 7A). This was not as evident when only one PTP inhibitor was present. After an exposure of 1 h in the presence of both vanadate (200 μM) and PAO (1.0 μM), TSP concentrations as low as 1.0 μg/ml increased the phosphotyrosine signal as compared with the effect seen with vanadate and PAO alone; over a TSP range of 1–30 μg/ml, this increase in signal appears to be concentration dependent (Figure 7B). TSP (20 μg/ml) in the presence of both vanadate (200 μM) and PAO (1.0 μM) increased the phosphotyrosine signal compared with the simultaneous vanadate/PAO control after exposure times of 1 h [Figure 8A, compare (+) and (−) lanes]. After normalization to β-tubulin, one of the phosphotyrosine-containing bands (∼66 kDa) was consistently increased as early as 0.5 h. Although the bands identified by the antiphosphotyrosine antibody increased over time in both the TSP-exposed and vanadate/PAO control EC, at 1 h there was a greater signal in the cells that were incubated with TSP (Figure 8A). When ECs were exposed to equimolar concentrations of 3 ECM proteins other than TSP (i.e., fibronectin, vitronectin, and type I collagen), protein tyrosine phosphorylation was not increased compared with simultaneous controls (Young, unpublished observations). To determine whether PTP inhibition was required throughout the TSP stimulus or only before cell lysis, ECs were exposed to TSP (30 μg/ml) or media alone in the presence of PTP inhibition with vanadate and PAO only during the final 0.25 h of the incubation (Figure 8B). Under these conditions, the fold increase of protein tyrosine phosphorylation (∼10-fold) was greater than the TSP-induced fold increase detected with PTP inhibition present throughout the TSP exposure ∼2-fold (Figure 8, A and B).
Figure 7.
Concentration-dependent effect of TSP on tyrosine phosphorylation of EC proteins in the presence of PTP inhibition. ECs were exposed for 1 h to TSP or media alone with or without vanadate (200 μM), PAO (1.0 μM), or both. Cell lysates were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes, and the blots were probed with antiphosphotyrosine IgG. (A) Phosphotyrosine-containing proteins in extracts of ECs exposed to TSP (20 μg/ml, 1 h) or media alone in the presence and absence of vanadate, PAO, or both. Arrows indicate bands with apparent Mr of 240,000, 205,000, 185,000, 135,000, 110,000, 95,000, and 66,000. (B) Phosphotyrosine-containing proteins in EC extracts obtained from monolayers exposed for 1 h to increasing concentrations of TSP in the presence of fixed concentrations of both vanadate (200 μM) and PAO (1.0 μM). To confirm equivalent protein loading, each blot was stripped and reprobed with anti-β-tubulin antibody. Molecular weights in kilodaltons are indicated on the left. Each is a representative blot of three experiments.
Figure 8.
Temporal effect of TSP on protein tyrosine phosphorylation in ECs. (A) ECs were exposed to TSP (20 μg/ml) (+) or media alone (−) for increasing exposure times in the presence of fixed concentrations of both vanadate (200 μM) and PAO (1.0 μM) and were processed for detection of phosphotyrosine-containing proteins by immunoblotting as described in the legend to Figure 7. (B) ECs were exposed for 1 h to TSP (30 μg/ml) (+) or media alone (−) in the presence of vanadate and PAO only during the last 0.25 h of the 1-h incubation and were processed for phosphotyrosine immunoblotting. To indicate protein loading, each blot was stripped and reprobed with anti–β-tubulin antibody. Molecular weights in kilodaltons are indicated on the left. These are representative blots of three experiments.
Effect of PTK Inhibitors on TSP-induced Protein Tyrosine Phosphorylation
ECs exposed to TSP (30 μg/ml) in the presence of vanadate and PAO with or without either herbimycin A or genistein were processed for phosphotyrosine immunoblotting (Figure 3B). PTK inhibition with either herbimycin A or genistein decreased both basal and TSP-induced tyrosine phosphorylation of EC proteins. Herbimycin A decreased both the ∼135 and ∼66 kDa bands by >57%, whereas genistein decreased each band by >60%. In addition, both herbimycin A and genistein decreased the ∼95 kDa band by >97%. Thus, these two PTK inhibitors that protected against intercellular gap formation and loss of barrier function gained entry into ECs and diminished TSP-induced protein tyrosine phosphorylation.
Identification of Tyrosine-phosphorylated Proteins in ECs Exposed to TSP
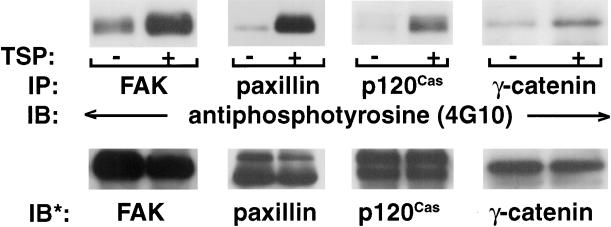
To further assess the response of ECs to TSP, we immunoscreened for proteins that comigrated with the phosphotyrosine-containing bands with apparent Mr of 135,000, 95,000, and 66,000 (Figure 8B). FAK and p120Cas comigrated with the ∼135 kDa band, β- and γ-catenin with the ∼95 kDa band, and paxillin with the ∼66 kDa band (Young, unpublished observations). Lysates from EC exposed for 1 h to TSP (30 μg/ml) or media alone in the presence of vanadate and PAO for the final 0.25 h of the incubation were each immunoprecipitated with anti-FAK, anti-paxillin, anti-β-catenin, anti-γ-catenin, or anti-p120Cas antibodies. The immunoprecipitates were processed for immunoblotting with biotinylated antiphosphotyrosine (4G10) antibody (Figure 9). As a control for any discrepancy in immunoprecipitation efficiency and/or loading of the immunoprecipitated protein, blots were stripped and reprobed with the same immunoprecipitating antibody. Under these conditions, TSP increased tyrosine phosphorylation of FAK approximately fivefold, paxillin approximately eightfold, γ-catenin approximately twofold, and p120Cas approximately threefold. The studies of β-catenin were inconclusive. Other phosphoproteins that comigrate with the ∼135, ∼95, and ∼66 kDa bands have not been excluded. In addition, other phosphotyrosine-containing proteins seen in EC exposed to TSP that migrated with an apparent Mr of 240,000, 205,000, 185,000, and 110,000 have not yet been identified (Figures 7 and 8).
Figure 9.
Identification of tyrosine-phosphorylated proteins in ECs exposed to TSP. ECs were incubated for 1 h with TSP (30 μg/ml) (+) or media alone (−) in the presence of vanadate (200 μM) and PAO (1.0 μM) only during the last 0.25 h of incubation. ECs were lysed and immunoprecipitated with antibodies raised against FAK, paxillin, p120Cas, or γ-catenin. The immunoprecipitates were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The blots were probed with bio-tinylated-antiphosphotyrosine (4G10) antibody, incubated with HRP-conjugated streptavidin, developed with ECL, and subjected to laser densitometry. For normalization of phosphotyrosine signal to loading of the immunoprecipitated protein, blots were stripped and reprobed with the immunoprecipitating antibodies, i.e., anti-FAK, anti-paxillin, anti-p120Cas, or anti-γ-catenin. IP, Immunoprecipitate; IB, immunoblot; IB*, immunoblot after stripping. These blots are representative of three experiments.
DISCUSSION
In this report, we have demonstrated that TSP influences transendothelial flux of macromolecules through a paracellular pathway, the functional state of which appears to be regulated, in part, through protein tyrosine phosphorylation. The TSP-induced increments in transendothelial albumin flux were dose and time dependent and could not be ascribed solely to endotoxin contamination, TGFβ activation, or EC injury. TSP was effective at concentrations as low as 1.0 μg/ml (2.2 nM). The EC response to TSP (20 μg/ml) was demonstrable at ≥2 h, with further time-dependent increments that were not dependent on protein synthesis. PTK inhibitors, in concentrations that blocked TSP-induced EC protein tyrosine phosphorylation, protected against TSP-induced intercellular gap formation and increments in transendothelial 14C-BSA flux. In contrast, PTP inhibition enhanced TSP-induced barrier dysfunction. The increase in phosphotyrosine immunostaining in TSP-exposed EC was initially localized to FA-like structures and intercellular boundaries and, later, almost exclusively to areas of EC–EC contact. Finally, TSP-induced protein tyrosine phosphorylation was clearly demonstrable in the presence of PTP inhibition. Several phosphoproteins were identified as the adherens junction components FAK, paxillin, γ-catenin, and p120Cas. The concentration and time requirements for TSP-induced increases in protein tyrosine phosphorylation were compatible with the dose and time requirements for TSP-induced changes in barrier function. Collectively these data suggest that TSP perturbs the tight regulation of EC protein tyrosine phosphorylation/dephosphorylation, which in turn directly/indirectly opens the paracellular pathway.
That TSP induces EC protein tyrosine phosphorylation and that PTK inhibition protects against the TSP-induced responses implicates activation of one or more EC PTKs. TSP is a multidomain molecule that recognizes several EC surface receptors that have been coupled to tyrosine phosphorylation events, including CD36 (Bull et al., 1994), αvβ3 (Blystone et al., 1996), and IAP (CD47) (Gao et al., 1996). When EC lysates were immunoblotted with an anti-CD36 antibody that recognizes bovine CD36, no CD36 expression was detected. Furthermore, the synthetic peptide CSVTCG that interacts with CD36 (Bull et al., 1994), failed to either simulate or block the TSP effect (Young, unpublished observations). That these EC do not express CD36, nor do they respond to the CSVTCG motif in TSP, implicates a CD36-independent, tyrosine phosphorylation-dependent TSP response. In other preliminary experiments, an RGD-containing sequence that binds to the αvβ3 integrin, and a binding motif in the COOH terminus that activates IAP, KRFVVMWKK (kindly provided by Dr. W. Frazier, Washington University, St. Louis, MO), each also failed to simulate or block the TSP effect (Young, unpublished observations). It is conceivable that for certain TSP-induced EC responses, simultaneous or serial costimulation of multiple receptors is required. In addition, TSP may mediate its biological effects on EC barrier function and protein tyrosine phosphorylation through an as yet undefined sequence(s) and/or receptor(s).
The state of tyrosine phosphorylation of adherens junction proteins is central to regulating cell–cell adhesion and endothelial barrier integrity. Growth factor activation of receptor PTKs induces tyrosine phosphorylation of adherens junction proteins and cell–cell adherens junction disassembly (Hoschuetzky et al., 1994; Shibamoto et al., 1994; Esser et al., 1998; Hazan and Norton, 1998). The phosphotyrosine-containing proteins that we have now demonstrated in ECs exposed to TSP could be immunolocalized to the intercellular boundaries. Several nonreceptor PTKs are preferentially associated with the plasma membrane, including members of the src family (Tsukita et al., 1991; Matsuyoshi et al., 1992; Behrens et al., 1993; Hamaguchi et al., 1993). Src transformation increases tyrosine phosphorylation of cell–cell adherens junction proteins and diminishes cadherin-dependent, homophilic adhesion (Matsuyoshi et al., 1992; Volberg et al., 1992; Behrens et al., 1993; Hamaguchi et al., 1993). Interestingly, PTK inhibition with herbimycin A also protects against this loss of intercellular adhesion (Matsuyoshi et al., 1992; Hamaguchi et al., 1993). Therefore, nonreceptor PTKs may independently or in concert with a receptor PTK mediate the EC response to the TSP stimulus.
That TSP-induced tyrosine phosphorylation of EC proteins was consistently observed only in the presence of PTP inhibition, and that PTP inhibition enhanced TSP-induced barrier dysfunction, implicates the participation of one or more PTPs. PTPs play a crucial role in regulating protein tyrosine phosphorylation of adherens junctions (Volberg et al., 1992; Brady-Kalnay et al., 1995; Fuchs et al., 1996). Receptor PTP activity increases during contact inhibition of cultured cells in vitro (Gaits et al., 1995; Southey et al., 1995; Fuchs et al., 1996). In ECs, the activation of the receptor PTP, HPTPβ, increases 12-fold as cells progress to confluence (Gaits et al., 1995). Two receptor PTPs that belong to the immunoglobulin superfamily, PTPμ (Brady-Kalnay et al., 1995) and PTPk (Fuchs et al., 1996), each localize to cell–cell adherens junctions, directly bind ZA component proteins, and participate in homophilic cell–cell adhesion. That PTP inhibition unmasks TSP-induced tyrosine phosphorylation and enhances loss of barrier function is compatible with these findings. In fact, others have shown that previous PTP inhibition with vanadate is necessary to observe tyrosine phosphorylation of EC–EC adherens junction proteins (Lampugnani et al., 1997). In another study, microspike formation induced by TSP became more prominent in pervanadate-treated cells (Adams, 1995). This requirement for PTP inhibition may explain why tyrosine phosphorylation events in ECs have not been easily recognized in response to the TSP stimulus.
TSP induces tyrosine phosphorylation of multiple EC proteins with an apparent Mr of 240,000–66,000. We now have identified two substrates for TSP-induced tyrosine phosphorylation in ECs as the FA component proteins FAK and paxillin (Clark and Brugge, 1995; Parsons and Parsons, 1997). These proteins participate in signaling pathways that regulate the state of FA assembly and cell-substrate adhesion. Tyrosine phosphorylation of both FAK and paxillin can occur through either ECM protein–integrin interactions (Burridge et al., 1992) and/or through Rho-A–dependent stress fiber formation and FA assembly (Chrzanowska-Wodnicka et al., 1996). Interestingly, several other established mediators of increased vascular permeability, including bradykinin (Leeb-Lundberg et al., 1994), platelet-activating factor (Soldi et al., 1996), α-thrombin (Schaphorst et al., 1997), and vascular endothelial growth factor (Abedi and Zachary, 1997), also stimulate tyrosine phosphorylation of FAK and paxillin. In addition, others have demonstrated that enhanced spreading of human melanocytes in response to the TSP stimulus correlates with tyrosine phosphorylation of FAK, paxillin, and a 90-kDa protein (Gao et al., 1996). Tyrosine phosphorylation of FAK and paxillin facilitates their interactions with src homolgy-2–bearing proteins and promotes downstream signaling events. Whether TSP-induced changes in barrier function are mediated through these protein–protein interactions and signaling pathways remains to be determined.
Two other identified substrates for TSP-induced tyrosine phosphorylation are γ-catenin (or plakoglobin) and p120Cas. γ-Catenin, which is homologous to β-catenin, and p120Cas are both part of the cell–cell adherens junction (ZA) complex (Kemler, 1993; Gumbiner, 1996; Barth et al., 1997). The ZA is responsible for mediating actin-dependent, homophilic cell–cell adhesion through its transmembrane receptors, the cadherins. In ECs, γ-catenin and β-catenin each independently couple the EC-specific, vascular endothelial-cadherin to the actin cytoskeleton through their interactions with α-catenin (Lampugnani et al., 1995). While β-catenin associates with vascular endothelial-cadherin in newly formed and loosely adherent EC junctions, γ-catenin predominates in tightly confluent EC–EC junctions (Lampugnani et al., 1997). Tyrosine phosphorylation of ZA protein components reduces intercellular EC–EC adhesion (Esser et al., 1998) and disrupts the linkage between cadherin and the actin cytoskeleton (Balsamo et al., 1995; Hazan and Norton, 1998). Tyrosine phosphorylation of γ-catenin, β-catenin, and p120Cas induced by ligand occupancy of the epidermal growth factor receptor diminishes cell–cell adhesion through disruption of the cadherin-actin cytoskeletal linkage (Hazan and Norton, 1998). Thus, tyrosine phosphorylation of γ-catenin and p120Cas may directly contribute to TSP-induced opening of the paracellular pathway.
The relationship between TSP-induced opening of the endothelial paracellular pathway and tyrosine phosphorylation of both FA and ZA component proteins remains to be determined. That TSP induces tyrosine phosphorylation of proteins associated with both adherens junctions is suggestive of intracellular cooperativity between both the FA and ZA. To what extent the state of assembly of either adherens junction is influenced by the other is unclear. Recently, we have demonstrated that another counteradhesive protein, SPARC (secreted protein acidic and rich in cysteine), also induces tyrosine phosphorylation of adherens junction and possibly other EC proteins, some of which migrate with similar molecular weights (Young et al., 1998). One could speculate that these two structurally dissimilar but functionally related counteradhesive proteins both induce tyrosine phosphorylation of common substrates. Whether these two counteradhesive proteins regulate EC–EC homophilic adhesion through a common tyrosine phosphorylation-dependent signaling pathway is unclear.
TSP is operative over a wide range of complex intercellular interactions. Understanding the mechanism(s) through which TSP influences homotypic EC–EC adhesion as well as EC shape changes and migration has implications for vasculogenesis, tissue morphogenesis, and fetal development (O’Shea et al., 1990; O’Shea and Dixit, 1998), as well as angiogenesis (Taraboletti et al., 1990; Iruela-Arispe et al., 1991), within the context of wound healing (Munjal et al., 1990; DiPietro et al., 1996), tissue remodeling (Botney et al., 1992; Kuhn and Mason, 1995), and tumor cell survival (Roberts, 1996). TSP also promotes leukocyte and tumor cell motility in vitro (Taraboletti et al., 1987; Mansfield et al., 1990) and modulates tumor cell adhesion to ECs (Incardona et al., 1995). Therefore, this same TSP-responsive paracellular pathway might also accommodate migrating leukocytes and/or metastatic tumor cells. TSP has been identified as a counteradhesive protein that disrupts cell–ECM interactions. In this study, we have presented evidence for the modulation of EC–EC adhesion by TSP through a pathway dependent on protein tyrosine phosphorylation.
ACKNOWLEDGMENTS
We thank Mr. Antonio Pallero for excellent technical assistance and Ms. Shirley Taylor for excellent secretarial support. This work was supported in part by the Office of Research and Development, Department of Veterans Affairs (S.E.G.), the U.S. Army Medical Research and Development Command (grant DAMD17-94-J-4117) (S.E.G.), and grants DK-48373 (S.E.G.), HL-44575 (J.E.M.-U.), and HL-50061 (J.E.M.-U.) from National Institutes of Health. J.E.M.-U. is an Established Investigator of the American Heart Association (AHA-Genentech Special Awardie in Thrombosis) (grant 9640228N). B.A.Y. is a recipient of a Department of Defense Augmentation Award for Science and Engineering Research Training (AASERT).
Abbreviations used:
- EC
endothelial cell
- FA
focal adhesion
- FAK
focal adhesion kinase
- IAP
integrin-associated protein
- LPS
lipopolysaccharide
- PAO
phenylarsine oxide
- PMB
polymixin B
- PTK
protein tyrosine kinase
- PTP
protein tyrosine phosphatase
- sTSP
stripped thrombospondin-1
- TSP
thrombospondin-1
- ZA
zonula adherens
REFERENCES
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- Adams JC. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the antiadhesive activities of thrombospondin-1. J Cell Sci. 1995;108:1977–1990. doi: 10.1242/jcs.108.5.1977. [DOI] [PubMed] [Google Scholar]
- Alessio M, DeMonte L, Scirea A, Gruarin P, Tandon NN, Sitia R. Synthesis, processing and intracellular transport of CD36 during monocytic differentiation. J Biol Chem. 1996;271:1770–1776. doi: 10.1074/jbc.271.3.1770. [DOI] [PubMed] [Google Scholar]
- Balsamo J, Ernst H, Zanin MKB, Hoffman S, Lilien J. The interaction of the retina cell surface N-acetylgalactosaminylphosphotransferase with an endogenous proteoglycan ligand results in inhibition of cadherin-mediated adhesion. J Cell Biol. 1995;129:1391–1401. doi: 10.1083/jcb.129.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AIM, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Roy FV, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-src gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystone SD, Lindberg FP, Williams MP, McHugh KP, Brown EJ. Inducible tyrosine phosphorylation of the β3 integrin requires the αV integrin cytoplasmic tail. J Biol Chem. 1996;271:31458–31462. doi: 10.1074/jbc.271.49.31458. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botney MD, Kaiser LR, Cooper JD, Mecham RP, Parghi D, Rody J, Parks WC. Extracellular matrix protein gene expression in atherosclerotic hypertensive pulmonary arteries. Am J Pathol. 1992;140:357–364. [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Rimm DL, Tonks NK. Receptor protein tyrosine phosphatase PTPμ associates with cadherins and catenins in vivo. J Cell Biol. 1995;130:977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull HA, Brickell PM, Dowd PM. Src-related protein tyrosine kinases are physically associated with the surface antigen CD36 in human dermal microvascular endothelial cells. FEBS Lett. 1994;351:41–44. doi: 10.1016/0014-5793(94)00814-0. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F, Camussi G, Aglietta M, Braquet P, Bosia A, Pescarmona G, Sanavio F, D’Urso N, Marchisio PC. Human endothelial cells are target for platelet-activating factor. I. Platelet-activating factor induces changes in cytoskeleton structures. J Immunol. 1987;139:2439–2446. [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–238. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SMF, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111:1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Muller T, Lerch MM, Ullrich A. Association of the human protein-tyrosine phosphatase κ with members of the armadillo family. J Biol Chem. 1996;271:16712–16719. doi: 10.1074/jbc.271.28.16712. [DOI] [PubMed] [Google Scholar]
- Gaits F, Li RY, Ragab A, Ragab-Thomas JMF, Chap H. Increase in receptor-like protein tyrosine phosphatase activity and expression level on density-dependent growth arrest of endothelial cells. Biochem J. 1995;311:97–103. doi: 10.1042/bj3110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates alpha v beta 3 function through integrin-associated proteins. J Cell Biol. 1996;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum SE, Brann TW, Ding X, Pugin J, Tobias PS. Lipopolysaccharide (LPS)-binding protein and soluble CD14 function as accessory molecules for LPS-induced changes in endothelial barrier function in vitro. J Clin Invest. 1994;93:692–702. doi: 10.1172/JCI117022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum SE, Ding X, Brann TW, Campbell-Washington J. Bacterial lipopolysaccharide induces actin reorganization, intercellular gap formation, and endothelial barrier dysfunction in pulmonary vascular endothelial cells: concurrent F-actin depolymerization and new actin synthesis. J Cell Physiol. 1993;157:13–23. doi: 10.1002/jcp.1041570103. [DOI] [PubMed] [Google Scholar]
- Goldblum SE, Ding X, Funk SE, Sage EH. SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc Natl Acad Sci USA. 1994a;91:3448–3452. doi: 10.1073/pnas.91.8.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwalt DE, Mather IH. Characterization of an apically derived epithelial membrane glycoprotein from bovine milk, which is expressed in capillary endothelia in diverse tissues. J Cell Biol. 1985;100:397–408. doi: 10.1083/jcb.100.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 1993;12:307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan RB, Norton L. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J Biol Chem. 1998;273:9078–9084. doi: 10.1074/jbc.273.15.9078. [DOI] [PubMed] [Google Scholar]
- Hoschuetzky H, Aberle H, Kemler R. β-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona F, Lewalle JM, Morandi V, Lambert S, Legrand Y, Foidart JM, Legrand C. Thrombospondin modulates human breast adenocarcinoma cell adhesion to human endothelial cells. Cancer Res. 1995;55:166–173. [PubMed] [Google Scholar]
- Iruela-Arispe ML, Bornstein P, Sage H. Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci USA. 1991;88:5026–5030. doi: 10.1073/pnas.88.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Mason RJ. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol. 1995;147:1759–1769. [PMC free article] [PubMed] [Google Scholar]
- Lahav J. The functions of thrombospondin and its involvement in physiology and pathophysiology. Biochim Biophys Acta. 1993;1182:1–14. doi: 10.1016/0925-4439(93)90146-r. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J Cell Sci. 1997;110:2065–2077. doi: 10.1242/jcs.110.17.2065. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Weinstein R, Hynes RO. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988;107:2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LMF, Song X-H, Mathis SA. Focal adhesion-associated proteins p125FAK and paxillin are substrates for bradykinin-stimulated tyrosine phosphorylation in swiss 3T3 cells. J Biol Chem. 1994;269:24328–24334. [PubMed] [Google Scholar]
- Luscinskas FW, Cybulsky MI, Kiely JM, Peckins CS, Davis VM, Gimbrone MA., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991;146:1617–1625. [PubMed] [Google Scholar]
- Malik AB, Lynch JJ, Cooper JA. Endothelial barrier function. J Invest Dermatol. 1989;93:62S–67S. doi: 10.1111/1523-1747.ep12581072. [DOI] [PubMed] [Google Scholar]
- Mansfield PJ, Boxer LA, Suchard SJ. Thrombospondin stimulates motility of human neutrophils. J Cell Biol. 1990;111:3077–3086. doi: 10.1083/jcb.111.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF. Physiology of thrombospondin. Annu Rev Med. 1990;41:85–97. doi: 10.1146/annurev.me.41.020190.000505. [DOI] [PubMed] [Google Scholar]
- Munjal ID, Blake DA, Sabet MD, Gordon SR. Thrombospondin: biosynthesis, distribution, and changes associated with wound repair in corneal endothelium. Eur J Cell Biol. 1990;52:252–263. [PubMed] [Google Scholar]
- Murphy-Ullrich JE. Antiadhesive proteins of the extracellular matrix: thrombospondin, tenascin, and SPARC. Trends Glycosci Glycotechnol. 1995;7:89–100. [Google Scholar]
- Murphy-Ullrich JE, Schultz-Cherry S, Höök M. Transforming growth factor-β complexes with thrombospondin. Mol Biol Cell. 1992;3:181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea KS, Dixit VM. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol. 1988;107:2737–2748. doi: 10.1083/jcb.107.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea KS, Rheinheimer JST, Dixit VM. Deposition and role of thrombospondin in the histogenesis of the cerebellar cortex. J Cell Biol. 1990;110:1275–1283. doi: 10.1083/jcb.110.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Parsons SJ. Src family tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- Roberts A, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Roberts DD. Regulation of tumor growth and metastasis by thrombospondin-1. FASEB J. 1996;10:1183–1191. [PubMed] [Google Scholar]
- Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- Schaphorst KL, Pavalko FM, Patterson CE, Garcia JG. Thrombin-mediated focal adhesion plaque reorganization in endothelium: role of protein phosphorylation. Am J Respir Cell Mol Biol. 1997;17:443–455. doi: 10.1165/ajrcmb.17.4.2502. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-β secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Shasby DM, Shasby SS, Sullivan JM, Peach MJ. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982;51:657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura M, Takeichi N, Ito F. Tyrosine phosphorylation of β-catenin and plakoglobin enhanced by hepatocyte growth factor and the epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- Soldi R, Sanavio F, Aglietta M, Primo L, Defilippi P, Marchisio PC, Bussolino F. Platelet-activating factor (PAF) induces the early tyrosine phosphorylation of focal adhesion kinase (p125FAK) in human endothelial cells. Oncogene. 1996;13:515–525. [PubMed] [Google Scholar]
- Southey MC, Findlay DM, Kemp BE. Regulation of membrane-associated tyrosine phosphatases in UMR 106.06 osteoblast-like cells. Biochem J. 1995;305:485–490. doi: 10.1042/bj3050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G, Roberts DD, Liotta LA. Thrombospondin-induced tumor cell migration: haptotaxis and chemotaxis are mediated by different molecular domains. J Cell Biol. 1987;105:2409–2415. doi: 10.1083/jcb.105.5.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111:765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Akiyama T, Yamanashi Y, Yamamoto T, Tsukita S. Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J Cell Biol. 1991;113:867–879. doi: 10.1083/jcb.113.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg T, Zick Y, Dror R, Sabanay I, Gilon C, Levitzki A, Geiger B. The effect of tyrosine-specific protein phosphorylation on the assembly of adherens-type junctions. EMBO J. 1992;11:1733–1742. doi: 10.1002/j.1460-2075.1992.tb05225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MKK, Gotlieb AI. Endothelial cell monolayer integrity. I. Characterization of dense peripheral band of microfilaments. Arteriosclerosis. 1986;6:212–219. doi: 10.1161/01.atv.6.2.212. [DOI] [PubMed] [Google Scholar]
- Young BA, Wang P, Goldblum SE. The counteradhesive protein SPARC regulates an endothelial paracellular pathway protein tyrosine. Biochem Biophys Res Commun. 1998;251:320–327. doi: 10.1006/bbrc.1998.9475. [DOI] [PubMed] [Google Scholar]