Abstract
Malaria and trypanosomiasis are diseases which afflict millions and for which novel therapies are urgently required. We have tested two well-characterized cell-penetrating peptides (CPPs) for antiparasitic activity. One CPP, designated TP10, has broad-spectrum antiparasitic activity against Plasmodium falciparum, both blood and mosquito stages, and against blood-stage Trypanosoma brucei brucei.
Vector-borne protozoan diseases are responsible for high levels of human morbidity and mortality (13), with malaria in particular resulting in 1 to 3 million deaths a year (30). There is an urgent need to identify and develop novel compounds to treat infection or prevent transmission from the mosquito vector. Antimicrobial peptides (AMPs) have been put forward as one potential class of novel antimalarials (1, 7, 8). The mosquito innate immune response includes the production of several AMPs (21), although the effectiveness of such effector molecules in vivo is unclear (4). Cell-penetrating peptides (CPPs), on the other hand, are molecules which can translocate into cells without causing membrane damage, leading to their proposed use as vectors for delivering therapeutic cargo to treat various conditions and diseases. Unlike AMPs, CPPs are either derived from protein transduction domains or designed to mimic the structure and sequence of such domains (36). However, AMPs and CPPs show significant similarities in charge, structure, and initial membrane interactions (12). Two of the best-characterized CPPs are pVEC (9) and TP10 (32), which can successfully translocate into various cell types (22, 26, 27) and demonstrate antimicrobial activity (24, 26). Our aim was to investigate whether pVEC and TP10 are capable of exerting antiparasitic effects against Plasmodium falciparum, the most deadly of the species that cause human malaria. In addition, we tested Trypanosoma brucei brucei, of which other subspecies of the T. brucei genus (gambiense and rhodesiense) are the causative agents of human African trypanosomiasis (2).
P. falciparum assays.
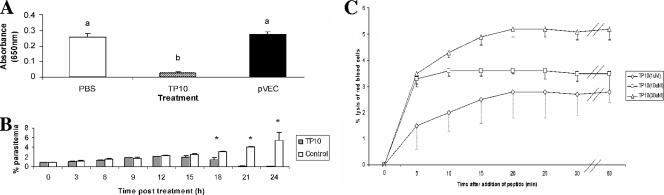
pVEC and TP10 (Table 1) were synthesized as described previously (18) and dissolved in NANOpure water prior to use. Using P. falciparum clone HB3 cultured from 1% parasitemia with malaria culture medium and 10% serum (34), we used the Malstat assay to measure parasite lactate dehydrogenase as an indirect measure of growth (14). Following a screen of peptide concentration (1, 10, and 30 μM) and incubation period (3, 6, and 24 h), only TP10 was found to reduce parasitemia, at 30 μM after 24-h incubation. TP10 treatment led to a significant reduction of parasitemia by ∼99% (one-way analysis of variance; P < 0.0001) (Fig. 1A). In contrast, pVEC had no effect against the parasite. This activity was confirmed microscopically for each experiment using acridine orange fluorescence (data not shown). Monitoring TP10 activity every 3 h up to 24 h posttreatment (Fig. 1B) revealed an arrest in growth at 15 h (Student's t test; P = 0.071). By 18 h, the parasitemia began to fall (Student's t test; P = 0.007), and by 21 h, most of the parasites had died (Student's t test; P < 0.0001 and P = 0.03, respectively). Since the cultures were asynchronous, this indicates that TP10 is active against all blood-stage forms. One unusual observation in the TP10-treated cultures was that the medium changed to a rust color 18 h after treatment. If this is due to TP10 disrupting hemozoin formation, leading to a buildup of free oxidized heme, then that in turn could kill the parasite (20). It is also possible that TP10 acts in an indirect manner. A recent study demonstrated that phospholipase A2 hydrolysis of human serum lipoproteins in parasite culture medium generates toxic antiplasmodial lipid by-products (11). While the main route of TP10 uptake is believed to be via the endocytic pathway (25), it has recently been suggested that perturbation of the lipid bilayer is required to maximize TP10 uptake (35). However, uptake does not necessarily translate into toxicity, as two newly designed CPPs are reported to accumulate in P. falciparum-infected red blood cells (RBCs) without killing the parasite but never enter noninfected RBCs (10). This may be related to the changes in erythrocyte membrane composition due to the formation of new permeability pathways (19). In order to determine whether hemolysis played a role in TP10 toxicity against the parasites, the CPP was incubated with uninfected RBCs and the lytic activity measured at 650 nm. RBC lysis peaked at 5% within 20 min at 30 μM (Fig. 1C). Hemolysis is unlikely to contribute significantly to TP10's antiparasitic effects, since a drop in parasitemia was not detected until 15 h posttreatment. Gametocytes of P. falciparum clone 3D7 were cultured as described elsewhere (6) and used in membrane feeding experiments to infect Anopheles gambiae Keele strain mosquitoes (17) as described previously (6). Fifty to 100 female mosquitoes (5 to 7 days old) were offered infectious blood meals supplemented with 30 μM TP10, pVEC, or phosphate-buffered saline (control). Ten days after feeding, midguts were dissected, and the prevalence (percent infection) and intensity (number of oocysts seen on a whole mosquito midgut) were noted. TP10 consistently reduced the prevalence of oocyst infection compared to that for the control by 35 to 45%, with statistical significance in experiment 1 (P < 0.0001) and experiment 3 (P = 0.014) (Table 2) as determined using Fisher's exact test. Experiment 2 just failed to reach significance, which could be due to the low infectivity in the control feed (P = 0.07). In terms of infection intensity, TP10 reduced the number of oocysts in infected mosquitoes in all feeds, although this only reached statistical significance in experiment 1 (P < 0.0001) as determined using the Genmod procedure comparing oocyst distribution fitted to negative binomials (3). It is worth noting that infection prevalence and intensity in experimental feeds are much higher than those observed in the field with P. falciparum. Therefore, a 50% reduction in prevalence could be enough to push the oocyst numbers to zero in an applied field study. As exflagellation was observed for all infective blood meals, TP10 is likely to act against the zygotes or ookinetes. Our conclusion is that TP10 is highly active against both blood-stage and vector-stage malaria parasites.
TABLE 1.
Amino acid sequences and predicted peptide propertiesa
FIG. 1.
CPP activity against asynchronous blood-stage P. falciparum cultures. (A-C) Following 24-h incubation with 30 μM pVEC or TP10 at 2% hematocrit, antiparasitic activity was measured using the lactate dehydrogenase assay (A). Background absorbance obtained with uninfected RBCs was subtracted from the other readings. Bars with different letters are significantly different (one-way analysis of variance; P < 0.0001). (B) The time course of TP10 activity was monitored every 3 h over the 24-h incubation period and compared to control cultures (i.e., phosphate-buffered saline [PBS] added instead of a CPP) to determine the time course of its antimalarial activity. *, indicates a significant difference (P < 0.05) using Student's t test (pairwise comparison). (C) The hemolytic activity of TP10 against human RBCs was analyzed over three different concentrations (1, 10, and 30 μM) and compared to a Triton X-100 positive control, where lysis was assumed to be 100%. Error bars in all figures represent the standard errors of the means (n = 3).
TABLE 2.
Effects of CPPs on the establishment of P. falciparum (3D7 clone) sporogonic development in the mosquito vector A. gambiae following a gametocyte membrane feeding
| Expt no. | CPP treatment | No. of oocyst-positive mosquitoes/total no. dissected (% prevalence of oocyst infection) | Median no. of oocysts/infected mosquito (range) |
|---|---|---|---|
| 1 | TP10 | 21/42 (50)a | 2 (1-6)b |
| pVEC | 25/40 (63)a | 2 (1-23) | |
| Control (no CPP) | 37/40 (93) | 4 (1-11) | |
| 2 | TP10 | 13/43 (30) | 1 (1-3) |
| pVEC | 19/43 (44) | 2 (1-5) | |
| Control (no CPP) | 23/47 (49) | 1 (1-5) | |
| 3 | TP10 | 17/34 (50)a | 4 (1-43) |
| pVEC | 24/35 (69) | 4 (1-24) | |
| Control (no CPP) | 31/40 (78) | 4 (1-29) |
Significantly different (P of <0.0001, P equals 0.0013, and P equals 0.01 for TP10 [experiment 1], pVEC [experiment 1], and TP10 [experiment 2], respectively) from the value of control mosquitoes infected, as calculated using chi-squared or Fisher's exact tests.
Significantly different (P of <0.0001 for TP10 [experiment 1]) from the value of oocyst densities, as calculated using the Genmod procedure.
T. b. brucei assays.
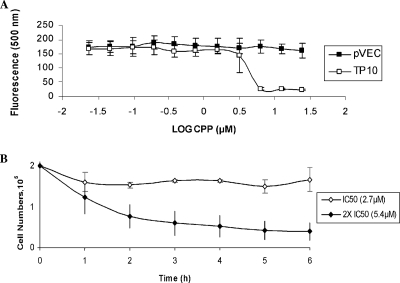
CPP activity was investigated against another protozoan parasite, bloodstream forms (BSFs) of T. b. brucei strain 427 (15). Trypanocidal activity was measured using a modification of the Alamar Blue assay (29), adapted as described previously (33), with no drug as a control. TP10 showed strong activity, with a mean IC50 (the concentration inhibiting growth by 50%) of 2.7 μM, as calculated using the GraFit 5.0 data analysis software (Erithacus). In contrast, pVEC was ineffective (Fig. 2A). In order to determine whether TP10 was lysing the parasites, BSFs were incubated at a starting density of 2 × 105 cells in HMI-9 medium in the presence of TP10 at its IC50 (2.7 μM) and 2× IC50 (5.4 μM). Treatments were monitored for the presence/absence of parasites by microscopy every hour for 6 h (Fig. 2B). The lysis half-time at 5.4 μM against BSFs was around 1.5 h. These data show that TP10 is also active against blood-stage trypanosomes.
FIG. 2.
CPP activity against blood-stage T. b. brucei. (A) The Alamar Blue assay was carried out using pVEC and TP10 to monitor parasite metabolic function over double dilution concentrations of CPP, starting from 25 μM, to determine the IC50 for each peptide. (B) The lysis assay was used to determine whether TP10 treatment at IC50 or 2× IC50 leads to morphological changes and/or lysis of the blood-stage trypanosomes. Error bars represent the standard errors of the means (n = 3).
The predicted structure of TP10 (according to the Advanced Micro Devices predictor program) is one with very high hydrophobicity and potential to form helices in membranes, suggesting that the mechanism of activity is similar to that utilized by AMPs (5; the literature is abundant with data on AMPs active against individual parasitic protozoa [16, 23, 31]). It will be interesting to see whether TP10 is active against a broader range of parasitic protozoa, such as Leishmania, Toxoplasma, and Giardia. Finally, in a practical use of CPPs, one must also consider the possible development of parasite resistance. In bacteria, mechanisms to destroy or prevent AMPs from crossing the lipid bilayer have been described previously (28), although there is no evidence to suggest that any bacteria have developed complete resistance to attack by multiple AMPs. This may be due to variability in size and amino acid composition. It is feasible that parasites would share a similar property of evolution against CPPs. Therefore, there is reason to believe that TP10, and any additional CPPs which demonstrate antiprotozoan activity, could be utilized in long-term parasite control strategies including transgenic or paratransgenic mosquitoes in the field or as human therapeutics.
Acknowledgments
We thank Klavs Berzins and the members of his group for help in initiating the parasite cultures in the Department of Immunology, Stockholm University; and we thank Liz Peat, Institute of Biomedical and Life Sciences, Glasgow University, for technical support.
Ingrid Faye acknowledges The Swedish Research Council (VR-NT 621-2004-3913), and Ülo Langel acknowledges The Swedish Research Council (VR-NT, VR-Med) and the Center for Biomembrane Research at Stockholm University. R.B.G.A. was supported by The Swedish Research Council grant VR-NT 621-2004-3913 to I.F.
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Arrighi, R. B., C. Nakamura, J. Miyake, H. Hurd, and J. G. Burgess. 2002. Design and activity of antimicrobial peptides against sporogonic-stage parasites causing murine malarias. Antimicrob. Agents Chemother. 46:2104-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett, M. P., R. J. Burchmore, A. Stich, J. O. Lazzari, A. C. Frasch, J. J. Cazzulo, and S. Krishna. 2003. The trypanosomiases. Lancet 362:1469-1480. [DOI] [PubMed] [Google Scholar]
- 3.Bell, A. S., and L. C. Ranford-Cartwright. 2004. A real-time PCR assay for quantifying Plasmodium falciparum infections in the mosquito vector. Int. J. Parasitol. 34:795-802. [DOI] [PubMed] [Google Scholar]
- 4.Blandin, S., L. F. Moita, T. Kocher, M. Wilm, F. C. Kafatos, and E. A. Levashina. 2002. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep. 3:852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 6.Carter, R., L. C. Ranford-Cartwright, and P. Alano. 1993. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies, p. 67-89. In J. E. Hyde (ed.), Methods in molecular biology, vol. 21. Protocols in molecular parasitology. Humana Press Inc., Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 7.Conde, R., F. Z. Zamudio, M. H. Rodriguez, and L. D. Possani. 2000. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 471:165-168. [DOI] [PubMed] [Google Scholar]
- 8.Dagan, A., L. Efron, L. Gaidukov, A. Mor, and H. Ginsburg. 2002. In vitro antiplasmodium effects of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 46:1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmquist, A., M. Lindgren, T. Bartfai, and Ü. Langel. 2001. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp. Cell Res. 269:237-244. [DOI] [PubMed] [Google Scholar]
- 10.Guergnon, J., F. Dessauge, V. Dominguez, J. Viallet, S. Bonnefoy, V. J. Yuste, O. Mercereau-Puijalon, X. Cayla, A. Rebollo, S. A. Susin, P. E. Bost, and A. Garcia. 2006. Use of penetrating peptides interacting with PP1/PP2A proteins as a general approach for a drug phosphatase technology. Mol. Pharmacol. 69:1115-1124. [DOI] [PubMed] [Google Scholar]
- 11.Guillaume, C., C. Deregnaucourt, V. Clavey, and J. Schrevel. 2004. Anti-Plasmodium properties of group IA, IB, IIA and III secreted phospholipases A2 are serum-dependent. Toxicon 43:311-318. [DOI] [PubMed] [Google Scholar]
- 12.Henriques, S. T., M. N. Melo, and M. A. Castanho. 2006. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem. J. 399:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill, C. A., F. C. Kafatos, S. K. Stansfield, and F. H. Collins. 2005. Arthropod-borne diseases: vector control in the genomics era. Nat. Rev. Microbiol. 3:262-268. [DOI] [PubMed] [Google Scholar]
- 14.Hirai, M., and I. Faye. 2004. Evaluation of parasitemia by lactate dehydrogenase assay, p. 21-22. In I. Ljungstrom, H. Perlmann, M. Schlichtherle, A. Scherf, and M. Wahlgren (ed.), Methods in malaria research, 4th ed. MR4/ATCC, Manassas, VA.
- 15.Hirumi, H., and K. Hirumi. 1994. Axenic culture of African trypanosome bloodstream forms. Parasitol. Today 10:80-84. [DOI] [PubMed] [Google Scholar]
- 16.Hu, Y., and S. Aksoy. 2005. An antimicrobial peptide with trypanocidal activity characterized from Glossina morsitans morsitans. Insect Biochem. Mol. Biol. 35:105-115. [DOI] [PubMed] [Google Scholar]
- 17.Hurd, H., P. J. Taylor, D. Adams, A. Underhill, and P. Eggleston. 2005. Evaluating the costs of mosquito resistance to malaria parasites. Evolution 59:2560-2572. [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, Y., U. Soomets, and Ü. Langel. 2006. Design and synthesis of cell-penetrating peptides, p. 537-552. In Ü. Langel (ed.), Handbook of cell-penetrating peptides, 2nd ed. Taylor & Francis, Boca Raton, FL.
- 19.Kirk, K., H. M. Staines, R. E. Martin, and K. J. Saliba. 1999. Transport properties of the host cell membrane. Novartis Found. Symp. 226:55-66. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, S., M. Guha, V. Choubey, P. Maity, and U. Bandyopadhyay. 2007. Antimalarial drugs inhibiting hemozoin (beta-hematin) formation: a mechanistic update. Life Sci. 80:813-828. [DOI] [PubMed] [Google Scholar]
- 21.Levashina, E. A. 2004. Immune responses in Anopheles gambiae. Insect Biochem. Mol. Biol. 34:673-678. [DOI] [PubMed] [Google Scholar]
- 22.Mae, M., H. Myrberg, Y. Jiang, H. Paves, A. Valkna, and Ü. Langel. 2005. Internalisation of cell-penetrating peptides into tobacco protoplasts. Biochim. Biophys. Acta 1669:101-107. [DOI] [PubMed] [Google Scholar]
- 23.McGwire, B. S., C. L. Olson, B. F. Tack, and D. M. Engman. 2003. Killing of African trypanosomes by antimicrobial peptides. Infect. Dis. 188:146-152. [DOI] [PubMed] [Google Scholar]
- 24.Nekhotiaeva, N., A. Elmquist, G. K. Rajarao, M. Hallbrink, Ü. Langel, and L. Good. 2004. Cell entry and antimicrobial properties of eukaryotic cell-penetrating peptides. FASEB J. 18:394-396. [DOI] [PubMed] [Google Scholar]
- 25.Padari, K., P. Saalik, M. Hansen, K. Koppel, R. Raid, Ü. Langel, and M. Pooga. 2005. Cell transduction pathways of transportans. Bioconjug. Chem. 16:1399-1410. [DOI] [PubMed] [Google Scholar]
- 26.Palm, C., S. Netzereab, and M. Hällbrink. 2006. Quantitatively determined uptake of cell-penetrating peptides in non-mammalian cells with an evaluation of degradation and antimicrobial effects. Peptides 27:1710-1716. [DOI] [PubMed] [Google Scholar]
- 27.Parenteau, J., R. Klinck, L. Good, Ü. Langel, R. J. Wellinger, and S. A. Elela. 2005. Free uptake of cell-penetrating peptides by fission yeast. FEBS Lett. 579:4873-4878. [DOI] [PubMed] [Google Scholar]
- 28.Peschel, A., and H. G. Sahl. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4:529-536. [DOI] [PubMed] [Google Scholar]
- 29.Raz, B., M. Iten, Y. Grether-Buhler, R. Kaminsky, and R. Brun. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 68:139-147. [DOI] [PubMed] [Google Scholar]
- 30.RBM Partnership. 2005. World malaria report 2005. http://rollbackmalaria.org/wmr2005/.
- 31.Savoia, D., S. Scutera, S. Raimondo, S. Conti, W. Magliani, and L. Polonelli. 2006. Activity of an engineered synthetic killer peptide on Leishmania major and Leishmania infantum promastigotes. Exp. Parasitol. 113:186-192. [DOI] [PubMed] [Google Scholar]
- 32.Soomets, U., M. Lindgren, X. Gallet, M. Hällbrink, A. Elmquist, L. Balaspiri, M. Zorko, M. Pooga, R. Brasseur, and Ü. Langel. 2000. Deletion analogues of transportan. Biochim. Biophys. Acta 1467:165-176. [DOI] [PubMed] [Google Scholar]
- 33.Stewart, M. L., G. J. Bueno, A. Baliani, B. Klenke, R. Brun, J. M. Brock, I. H. Gilbert, and M. P. Barrett. 2004. Trypanocidal activity of melamine-based nitroheterocycles. Antimicrob. Agents Chemother. 48:1733-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 35.Yandek, L. E., A. Pokorny, A. Floren, K. Knoelke, Ü. Langel, and P. F. Almeida. 2007. Mechanism of the cell-penetrating peptide transportan 10 permeation of lipid bilayers. Biophys. J. 92:2434-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zorko, M., and Ü. Langel. 2005. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv. Drug. Deliv. Rev. 57:529-545. [DOI] [PubMed] [Google Scholar]