Abstract
Raltegravir is a novel human immunodeficiency virus type 1 (HIV-1) integrase inhibitor with potent in vitro activity (95% inhibitory concentration of 31 nM in 50% human serum). This article reports the results of an open-label, sequential, three-period study of healthy subjects. Period 1 involved raltegravir at 400 mg twice daily for 4 days, period 2 involved tenofovir disoproxil fumarate (TDF) at 300 mg once daily for 7 days, and period 3 involved raltegravir at 400 mg twice daily plus TDF at 300 mg once daily for 4 days. Pharmacokinetic profiles were also determined in HIV-1-infected patients dosed with raltegravir monotherapy versus raltegravir in combination with TDF and lamivudine. There was no clinically significant effect of TDF on raltegravir. The raltegravir area under the concentration time curve from 0 to 12 h (AUC0-12) and peak plasma drug concentration (Cmax) were modestly increased in healthy subjects (geometric mean ratios [GMRs], 1.49 and 1.64, respectively). There was no substantial effect of TDF on raltegravir concentration at 12 h postdose (C12) in healthy subjects (GMR [TDF plus raltegravir-raltegravir alone], 1.03; 90% confidence interval [CI], 0.73 to 1.45), while a modest increase (GMR, 1.42; 90% CI, 0.89 to 2.28) was seen in HIV-1-infected patients. Raltegravir had no substantial effect on tenofovir pharmacokinetics: C24, AUC, and Cmax GMRs were 0.87, 0.90, and 0.77, respectively. Coadministration of raltegravir and TDF does not change the pharmacokinetics of either drug to a clinically meaningful degree. Raltegravir and TDF may be coadministered without dose adjustments.
The worldwide incidence of human immunodeficiency virus type 1 (HIV-1) infection remains considerable. The number of individuals infected with HIV continues to grow, as does the number of deaths due to AIDS (3, 9, 23). Despite advances in the development of treatment for HIV-1 infection, new agents are still needed to address issues of viral resistance and patient nonadherence due to toxicity.
Raltegravir (MK-0518; Merck & Co., Inc.) is an agent in a new class of antiretroviral drugs, the HIV integrase inhibitors, with a novel mechanism of action (10). HIV integrase inserts viral DNA into the cellular DNA of the host cell and thus is essential for viral replication (1, 5, 10). Raltegravir has potent in vitro activity, blocking HIV replication with a 95% inhibitory concentration (IC95) of 31 nM in 50% normal human serum (Isentress [raltegravir] package insert; Merck & Co., Inc., Whitehouse Station, NJ [http://www.fda.gov/cder/foi/label/2007/022145lbl.pdf; accessed 31 October 2007]). In placebo-controlled trials of viremic patients, raltegravir administered twice daily has been shown to be efficacious in reducing HIV viral load to undetectable levels (i.e., <400 and <50 HIV RNA copies/ml) in both treatment-naïve patients (14, 15) and heavily treated, multidrug-resistant patients (6-8) at doses of 100 to 600 mg administered twice daily.
The current recommendations for treatment of HIV infection require combination therapy (3, 9, 17, 23), in part to help address the issue of drug resistance (13, 19, 20, 22). Therefore, it is expected that raltegravir will be given in combination with other anti-HIV drugs such as tenofovir disoproxil fumarate (TDF) (Viread [TDF] package insert, 2007 update; Gilead Sciences, Foster City, CA [http://www.fda.gov/cder/foi/label/2007/021356s021,021752s011lbl.pdf; accessed 31 October 2007]). The prodrug TDF is converted in vivo to tenofovir, a nucleotide reverse transcriptase inhibitor eliminated primarily by renal excretion and the molecule measured for pharmacokinetic (PK) assays. Tenofovir has not been characterized as a potent inhibitor or inducer of drug-metabolizing enzymes, but it has been shown in clinical studies to interact with didanosine, atazanavir, and lopinavir-ritonavir via mechanisms that are not entirely clear (Viread [TDF] package insert, 2007 update).
Raltegravir is metabolized predominantly by glucuronidation mediated by the isoenzyme UGT1A1; raltegravir is not a substrate for cytochrome P-450 enzymes (1, 5). Similar to tenofovir, raltegravir does not appear to be an inducer or inhibitor of enzymes involved in drug metabolism (11, 18; Isentress [raltegravir] package insert). Given the characteristics of each drug, there is no a priori reason to expect that there will be a clinically meaningful drug interaction between TDF and raltegravir. However, due to the unanticipated and unexplained drug interactions previously observed with TDF, a clinical assessment of the effects of each drug on the PK of the other is appropriate. This paper describes the results of a two-way drug-drug interaction study between raltegravir and TDF conducted in healthy subjects, as well as an assessment of the PK of raltegravir administered as monotherapy versus in combination with TDF and lamivudine in HIV-1-infected patients.
(Portions of the data from healthy subjects were presented at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy [24], and partial summaries of the data from patients appear in references 14 and 15.)
MATERIALS AND METHODS
Study design.
This report describes two clinical studies in which raltegravir was coadministered with TDF. The first study (protocol 008), study A, was an open-label, three-period, single-center study of healthy subjects. In period 1, raltegravir (400 mg) was administered to all subjects every 12 h for 4 days; on day 4, only the morning dose was administered. In period 2, all subjects were administered TDF (300 mg) once daily for 7 days. In period 3, all subjects received both TDF (300 mg once daily) and raltegravir (400 mg every 12 h) for 4 days. There was no washout period between periods 2 and 3. On non-PK sampling days, TDF was administered with food while raltegravir was administered without regard to food. On PK sampling days (period 1, day 4; period 2, day 7; and period 3, day 4), the study drug was administered in the fasted state.
The second study (protocol 004), study B, was an international, 29-site, double-blind, randomized, dose-ranging study in treatment-naïve HIV-1-infected patients that included intensive PK sampling in a cohort of patients. Part I of this study consisted of 10 days of twice-daily dosing with either placebo or raltegravir monotherapy at doses of 100, 200, 400, or 600 mg in a total of 35 patients. Thirty of these patients continued into part II of the study, which examined the safety, tolerability, and efficacy of raltegravir versus efavirenz, in combination with tenofovir and lamivudine, for up to 48 weeks. Patients who received raltegravir in part I received the same dosage of raltegravir in part II, and patients who received placebo in part I received efavirenz (600 mg once daily) in part II. All patients in part II also received TDF (300 mg once daily) and lamivudine (300 mg once daily). Further details of parts I and II of this study have been described previously (14, 15).
Subjects.
For study A, healthy (HIV negative), nonsmoking, male subjects, aged 18 to 45 years, weighing within ±20% of ideal body weight were eligible for enrollment. General good health was determined from physical examination, laboratory tests, and medical history; subjects with a history of metabolic, endocrine, neurologic, psychiatric, hematologic, oncologic, cardiovascular, gastrointestinal, hepatic, renal, or genitourinary disease were excluded. Two weeks prior to the study start through the poststudy visit, subjects were required not to take prescription or nonprescription medications or herbal treatments, including potential cytochrome P-450 inducers.
For study B, HIV-1-infected men and women aged 18 years or older were eligible for enrollment if they had a plasma HIV-1 RNA level of ≥5,000 copies/ml and a CD4+ T-cell count of ≥100 cells/mm3 at screening. Prohibited medications included carbamazepine, phenobarbital, phenytoin, primidone, rifabutin, rifampin, gemfibrozil, and herbal remedies (including, but not limited to St. John's wort and garlic supplements). Other inclusion and exclusion criteria were previously described (15).
Written informed consent was obtained from all subjects prior to study entry. Each protocol was reviewed and approved by the Institutional Review Board or Ethical Review Committee of each participating site and conducted in accordance with the guidelines on good clinical practice and ethical standards for human experimentation based on those of the Declaration of Helsinki.
PK sampling and assays.
For study A, in periods 1 and 3, plasma samples for the raltegravir assay were collected at predose on days 1 to 4 (period 1) or 2 to 4 (period 3) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h postdose on day 4. In periods 2 and 3, serum samples were collected for tenofovir assay at predose on days 1 and 5 to 7 (period 2) or days 2 to 4 (period 3) and at 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h postdose on day 7 (period 2) or day 4 (period 3).
For study B, plasma was collected for raltegravir assay at predose and 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 12 h following the morning dose on day 10 in part I of the study and at the same time points following the morning dose at week 2 in part II of the study.
Raltegravir samples were analyzed using reverse-phase high-pressure liquid chromatography (HPLC) with tandem mass spectrometry (MS/MS) in positive ionization mode using an atmospheric pressure chemical ionization interface (API 4000 HPLC-MS/MS; Applied Biosystems, Foster City, CA), as described in reference 16. The lower limit of quantitation was 2 ng/ml, and the assay was linear from 2 to 1,000 ng/ml. The raltegravir assays from study A were performed at Merck Research Laboratories (West Point, PA); similar methods were used at Bioanalytical Systems, Inc. (McMinnville, OR), to assay samples for study B.
Tenofovir quantitation was conducted at Bioanalytical Systems, Inc. (West Lafayette, IN). Tenofovir was removed from heparinized serum by solid-phase extraction. Tenofovir was then separated and detected by a liquid chromatography-MS/MS system using a 100-by-4.6-mm Ultra IBD column (Restek Corp., Bellefonte, PA) with a mobile phase of 20% acetonitrile-0.1% formic acid. The internal standard was 2′-deoxyadenosine-5′-monophosphoric acid monohydrate. The lower limit of quantitation was 5 ng/ml, and the assay was linear from 5 to 500 ng/ml. The mass transitions used were 288.2 to 175.9 (m/z) for tenofovir and 332.0 to 135.9 (m/z) for the internal standard. Two sets of low-, medium-, and high-quality control samples were evaluated with each run. For these quality control samples, interday accuracy was 99.3 to 104.2% and interday precision was 6.7 to 13.2% (coefficient of variation).
PK methods.
For plasma raltegravir concentrations and serum tenofovir concentrations, the area under the concentration-versus-time curve (AUC) was calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations. Actual recorded sampling times were used for these analyses. Peak plasma drug concentration (Cmax), time to Cmax (Tmax), and concentrations at 12 h postdose (C12 [for raltegravir]) and 24 h postdose (C24 [for tenofovir]) were obtained by inspection of the concentration data.
Safety and tolerability.
For study A, the safety and tolerability of raltegravir and TDF were assessed by clinical evaluation of vital signs, physical examinations, electrocardiograms, and laboratory safety evaluations, including hematology, chemistry, and urinalysis. Adverse experiences (AEs) were monitored throughout the study. The investigator assessed AEs with respect to intensity (mild, moderate, or severe), duration, seriousness (serious or not serious), outcome, and relationship to study drug.
Clinical and laboratory safety evaluations were also included in study B (14, 15).
Statistical methods.
The PK parameters Cmax, C12, and AUC from 0 to 12 h (AUC0-12) for raltegravir and C24 and AUC0-24 for tenofovir were natural log transformed, and confidence intervals (CIs) for the means (and for the difference of two means) were constructed on the natural log scale. Exponentiation was performed on the means (and mean differences) and lower and upper limits of the CIs prior to reporting. With the exception of Tmax, all CIs were based on an analysis of variance (ANOVA) model with treatment as a fixed effect and with the subject as a random effect (with compound symmetric covariance structure assumed). For C12 and C24, only those values arising from the final dosing interval of each period were included in the ANOVA model. All PK parameters were analyzed in separate models.
Ninety-five percent CIs were constructed for the geometric means (GMs) of raltegravir C12, Cmax, and AUC0-12 for each treatment regimen, and 90% CIs were constructed for the respective GM ratios (GMRs [raltegravir plus TDF versus raltegravir alone]). For Tmax, the Hodges-Lehman estimate of the true median difference ([raltegravir plus TDF] − [raltegravir alone]) was computed, as was a 90% CI for the true median difference. Similar methods were used for analysis of the tenofovir PK parameters.
RESULTS
Subject demographics and baseline characteristics.
In study A, 10 men with a mean age of 31.4 years (range, 18 to 43 years) and a mean weight of 81.4 kg (range, 61.4 to 90.0 kg) were enrolled. The racial composition of this group was Hispanic (n = 7), white (n = 2), and black (n = 1). One subject discontinued due to a laboratory AE. Nine subjects completed the study and were included in the PK analysis. All 10 subjects were included in the safety evaluation.
In study B, 30 patients completed the PK substudy; of these, 25 were in treatment arms containing raltegravir and were included in the PK analyses described here. These 25 patients (24 men and 1 woman) had a mean age of 40.6 years (range, 22 to 68 years), a mean weight of 75.7 kg (range, 56.8 to 101.6 kg), and a racial composition of white (n = 16), Hispanic (n = 7), and black (n = 2).
PK of raltegravir.
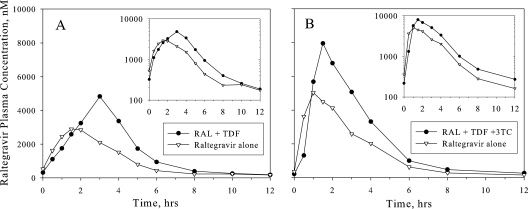
Figure 1 shows the arithmetic mean plasma raltegravir concentration-versus-time profile after multiple-dose administration of raltegravir alone and in combination with multiple-dose TDF in both healthy subjects and treatment-naïve HIV-1-infected patients. Table 1 displays the raltegravir PK parameters in healthy subjects from study A, and Table 2 shows them in patients from study B. In healthy subjects, raltegravir C12 and Tmax were essentially unchanged by coadministration with TDF, while AUC0-12 and Cmax were increased by approximately 49% and 64%, respectively. In patients, raltegravir C12, AUC0-12, and Cmax were all increased by approximately 30 to 40% for coadministration of raltegravir plus TDF plus lamivudine compared to raltegravir alone.
FIG. 1.
Arithmetic mean plasma raltegravir concentrations following multiple doses of raltegravir (RAL) at 400 mg twice daily with and without coadministration of multiple doses of TDF alone at 300 mg once daily or TDF at 300 mg once daily plus lamivudine (3TC) at 300 mg once daily. (A) Healthy young men (n = 9 [inset, semilog scale]). (B) Treatment-naïve HIV-positive patients (n = 6 [inset, semilog scale]).
TABLE 1.
Comparison of plasma raltegravir PK for healthy young men administered multiple doses with and without coadministration of multiple doses of TDFa
| Treatment | GM (CI) for parameter (n = 9):
|
|||
|---|---|---|---|---|
| C12 (nM)b | AUC0-12 (μM h−1)b | Cmax (μM)b | Tmax (h) | |
| Raltegravir alone (95% CI) | 142.3 (86.0-35.5) | 10.29 (6.56-16.13) | 2.87 (1.75-4.71) | 1.5c |
| Raltegravir + tenofovir (95% CI) | 146.4 (88.5-242.4) | 15.35 (9.79-24.07) | 4.71 (2.87-7.72) | 3.0c |
| Raltegravir + tenofovir-raltegravir ratio (90% CI) | 1.03 (0.73-1.45) | 1.49 (1.15-1.94) | 1.64 (1.16-2.32) | 1.0 (−0.3-1.8)d |
| P | 0.879 | 0.023 | 0.029 | 0.281 |
Subjects were administered multiple doses of raltegravir (400 mg twice daily) with and without coadministration of multiple doses of TDF (300 mg once daily).
The GM was computed from the least-squares estimate from an ANOVA performed on the natural-log-transformed values.
Median reported for Tmax.
Hodges-Lehman estimate of median treatment difference and 90% CI for true median treatment difference.
TABLE 2.
Comparison of raltegravir plasma PK for HIV-1-infected patients administered multiple doses of raltegravir with and without coadministration of multiple doses of TDF and lamivudinea
| Parameter and raltegravir treatment | nb | GM (90% CI)
|
||
|---|---|---|---|---|
| Wk 2 of part II | Day 10 of part I | GMR of wk 2 of part II to day 10 of part Ic | ||
| AUC0-12 (μM h−1) | ||||
| All doses | 25 | 13.9 (10.8-18.0) | 9.9 (7.7-12.6) | 1.41 (1.11-1.79) |
| 100 mg b.i.d. | 6 | 5.4 (2.9-9.9) | 5.5 (4.2-7.2) | 0.98 (0.57-1.67) |
| 200 mg b.i.d. | 7 | 14.9 (12.2-18.2) | 9.4 (6.8-13.1) | 1.58 (1.16-2.15) |
| 400 mg b.i.d. | 6 | 25.3 (20.9-30.8) | 14.3 (7.6-26.7) | 1.78 (0.86-3.66) |
| 600 mg b.i.d. | 6 | 18.3 (10.6-31.6) | 12.9 (5.8-28.4) | 1.42 (0.73-2.78) |
| Cmax (μM) | ||||
| All doses | 25 | 4.2 (3.0-5.9) | 3.2 (2.4-4.3) | 1.33 (0.96-1.85) |
| 100 mg b.i.d. | 6 | 1.2 (0.5-3.0) | 2.0 (1.3-3.1) | 0.63 (0.28-1.40) |
| 200 mg b.i.d. | 7 | 4.6 (3.6-6.0) | 3.3 (1.9-5.9) | 1.39 (0.89-2.17) |
| 400 mg b.i.d. | 6 | 8.6 (6.5-11.3) | 4.5 (2.0-10.2) | 1.90 (0.76-4.77) |
| 600 mg b.i.d. | 6 | 6.3 (3.2-12.4) | 3.3 (1.4-8.1) | 1.89 (0.94-3.80) |
| C12 (nM) | ||||
| All doses | 25 | 147.7 (100.5-217.0) | 103.7 (77.6-138.6) | 1.42 (0.89-2.28) |
| 100 mg b.i.d. | 6 | 155.1 (72.5-331.5) | 45.0 (22.8-89.1) | 3.44 (1.40-8.46) |
| 200 mg b.i.d. | 7 | 182.6 (98.5-338.6) | 112.4 (78.4-161.1) | 1.62 (1.01-2.61) |
| 400 mg b.i.d. | 6 | 239.2 (150.2-381.0) | 141.7 (87.6-229.1) | 1.69 (1.12-2.54) |
| 600 mg b.i.d. | 6 | 67.7 (16.3-280.6) | 159.3 (71.8-353.4) | 0.43 (0.07-2.41) |
Subjects were administered multiple doses of raltegravir twice daily (b.i.d.) with and without coadministration of multiple doses of TDF (300 mg once daily) and lamivudine (300 mg once daily).
n represents the number of patients who had intensive PK data at both week 2 of part II and day 10 of part I in the treatment group.
The GMR is the ratio of the GM of PK parameters from cohort I subjects at week 2 of the combination therapy phase (part II) to those on day 10 of the monotherapy phase (part I). The CI was calculated based on the paired t distribution. (Note that raltegravir was administered with tenofovir and lamivudine in part II; raltegravir was administered alone in part I.)
PK of tenofovir.
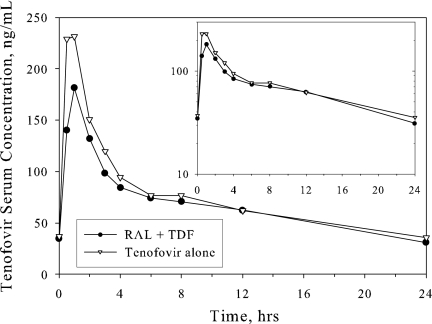
Figure 2 displays the mean serum tenofovir concentration-versus-time profile, and Table 3 displays the PK parameters of tenofovir after multiple-dose administration of TDF alone and in combination with multiple-dose raltegravir in healthy subjects. Relative to administration of TDF alone, the coadministration of raltegravir with TDF resulted in little change in tenofovir PK with C24, AUC0-24, and Cmax decreasing by averages of approximately 13%, 10%, and 23%, respectively. There was no substantial effect on Tmax.
FIG. 2.
Arithmetic mean serum tenofovir concentrations following multiple doses of TDF at 300 mg once daily with and without coadministration of multiple doses of raltegravir at 400 mg twice daily in healthy young men. Inset, semilog scale.
TABLE 3.
Comparison of serum tenofovir PK for healthy young men administered multiple doses of TDF with and without raltegravira
| Treatment | GM (CI) for parameter (n = 9):
|
|||
|---|---|---|---|---|
| C24 (ng/ml)b | AUC0-24 (ng h−1/ml)b | Cmax (ng/ml)b | Tmax (h) | |
| Tenofovir alone (95% CI) | 34.4 (28.0-42.3) | 1,737 (1,485-2,032) | 263 (223-311) | 0.5c |
| Tenofovir + raltegravir (95% CI) | 29.9 (24.3-36.8) | 1,563 (1,336-1,828) | 202 (171-239) | 1.0c |
| Tenofovir + raltegravir-tenofovir ratio (90% CI) | 0.87 (0.74-1.02) | 0.90 (0.82-0.99) | 0.77 (0.69-0.85) | 0.3 (0.0-0.8)d |
| P | 0.137 | 0.067 | 0.001 | 0.883 |
Subjects were administered multiple doses of TDF at 300 mg once daily with and without raltegravir at 400 mg twice daily.
The GM was computed from the least-squares estimate from an ANOVA performed on the natural-log-transformed values.
Median reported for Tmax.
Hodges-Lehman estimate of median treatment difference and 90% CI for true median treatment difference.
Safety and tolerability.
In study A, coadministration of multiple doses of raltegravir with multiple doses of TDF was generally well tolerated. No serious clinical AEs were reported. Of the eight clinical and laboratory AEs reported by four subjects, all were considered by the investigator to be nonserious and possibly related to the study drug. The most common AE was headache. All clinical AEs were mild in intensity and transient in nature. One subject was discontinued from the study due to the laboratory AEs of increased levels of both alanine aminotransferase and aspartate aminotransferase. The abnormal lab values, which were recorded after administration of TDF alone in period 2, returned to baseline levels after discontinuation.
In study B, all doses of raltegravir in combination with TDF and lamivudine were well tolerated in patients, and further details have been published previously (14, 15).
DISCUSSION
Drug-drug interactions are particularly important considerations in treating HIV-infected populations and can often be problematic (3, 9, 23). TDF is commonly used in anti-HIV regimens and is likely to be coadministered with raltegravir. No interaction between raltegravir and TDF was anticipated based on in vitro, preclinical, or clinical data; however, TDF is associated with other drug interactions of unknown mechanism: for example, with atazanavir (21), didanosine (12), and saquinavir-ritonavir (2). A study of healthy subjects (study A) was thus conducted to examine the safety and tolerability of raltegravir at 400 mg twice daily alone and in combination with TDF at 300 mg once daily as well as to examine the effects of coadministration on the PK of both raltegravir and tenofovir. In addition, the PK of raltegravir were examined in a small cohort of patients in a study in treatment-naïve HIV-1-infected patients (study B) when administered as monotherapy versus in combination with TDF at 300 mg once daily and lamivudine at 300 mg once daily.
In both healthy subjects and HIV-1-infected patients, TDF modestly increased the steady-state raltegravir AUC (increased by 49% in study A) and Cmax (increased by 64% in study A). The mean effect of TDF on steady-state raltegravir C12 differed between the two studies, with essentially no effect in healthy subjects and an approximately 40% increase in patients. In study B, raltegravir was dosed in combination with both TDF and lamivudine. Lamivudine is predominantly renally eliminated via active organic cationic secretion and has not been reported to be an inhibitor of UDP glucuronosyltransferases (Epivir [lamivudine] package insert, 2006 update; GlaxoSmithKline, Philadelphia, PA [http://www.fda.gov/cder/foi/label/2006/020564s026lbl.pdf; accessed 31 October 2007]). Given this, and the similarity of the results in patients to those in volunteers administered raltegravir and TDF without lamivudine, the modest increase in plasma raltegravir levels observed in patients with the combination therapy is likely attributable to an effect of tenofovir and not lamivudine. The mechanism behind the observed increase in plasma raltegravir levels in the presence of tenofovir is unknown. Renal clearance plays only a relatively minor role in elimination of raltegravir (11), and so an interaction at the level of renal excretion seems unlikely to explain the observed results.
Raltegravir is an agent in a new class of antiretroviral agents, and there are insufficient clinical data at this time to definitively say which PK parameters are most important in determining efficacy and safety. For other classes of antiretroviral agents, however, there is a reasonable but imperfect association of efficacy with trough drug concentration (Ctrough) values that exceed the protein-adjusted IC95 in the HIV spread assay. Since the observed effects of tenofovir on raltegravir C12 range from virtually no change (as observed in healthy subjects in study A) to a modest increase (as observed in patients in study B), the interaction is unlikely to have a significant impact on the efficacy of raltegravir. The modest increases seen in raltegravir AUC0-12 and Cmax values observed in the presence of tenofovir are also unlikely to be of concern given the lack of safety issues associated with exposure and maximum plasma drug concentrations seen to date (6-8, 11, 14, 15, 18 Isentress [raltegravir] package insert). Of particular note, the coadministration of raltegravir (400 mg twice daily) with TDF (300 mg once daily) and lamivudine (300 mg once daily) has shown good safety and efficacy in HIV-1-infected patients on long-term dosing (14). These collective data support that the observed effect of tenofovir on raltegravir PK is not clinically significant and that no dose adjustment is needed for raltegravir when it is coadministered with TDF.
As anticipated based on preclinical and in vitro data, raltegravir had no substantial effect on tenofovir PK. Multiple-dose coadministration of TDF and raltegravir led to a slight decrease in mean tenofovir Cmax with less of an effect on tenofovir AUC0-24. There was no meaningful effect on tenofovir C24. The effect of raltegravir on tenofovir is similar in magnitude to the reported slight decrease in tenofovir PK parameter values observed on codosing of rifampin with TDF (4). No dose adjustment is recommended for TDF when it is coadministered with rifampin (Viread [tenofovir disoproxil fumarate] package insert, 2007 update), which implies that the observed effect of raltegravir on TDF is also of no clinical significance.
The administration of raltegravir in combination with TDF was generally well tolerated, with no particular safety issue of concern. Present anti-HIV therapies, including nucleoside and nonnucleoside reverse transcriptase inhibitors and protease inhibitors, have toxicity and tolerability issues (3, 9, 23). Raltegravir, an HIV integrase inhibitor, is a member of a new class of antiretroviral agents. Prior clinical experience with raltegravir (6-8, 11, 14, 15, 18; Isentress [raltegravir] package insert), in conjunction with safety data from this study, demonstrates a favorable safety profile and indicates that raltegravir may not have the same toxicity and tolerability issues associated with currently marketed agents.
In summary, the results of this study indicate that coadministration of raltegravir and TDF is generally safe and well tolerated and does not alter the PK of either raltegravir or tenofovir to a clinically meaningful extent, indicating that raltegravir and TDF may be coadministered without dose adjustment.
Acknowledgments
We thank all of the subjects who participated in this study and Tuli Ahmed and Neal Azrolan of Merck & Co., Inc., for assistance with the preparation of the manuscript. We also thank Bach-Yen Nguyen of Merck & Co., Inc., for her contribution and role to the POOY data.
This study was funded by Merck & Co., Inc., which reviewed and approved the manuscript.
Other than employees of Merck & Co., Inc., all authors have been investigators for the sponsor. Employees may hold stock and/or stock options in the company.
K. Lasseter performed the enrollment of subjects and/or data collection, analysis and interpretation of data, and preparation of the manuscript. S. Breidinger, E. Freidman, and J. Stek performed analysis and interpretation of data and preparation of the manuscript. K. Gottesdiener, J. Chen, M. Iwamoto, J. Kost, J. Stone, H. Teppler, J. Wagner, and L. Wenning contributed to the study concept and design, analysis and interpretation of data, and preparation of the manuscript.
Footnotes
Published ahead of print on 14 July 2008.
REFERENCES
- 1.Asante-Appiah, E., and A. M. Skalka. 1999. HIV-1 integrase: structural organization, conformational changes, and catalysis. Adv. Virus Res. 52:351-369. [DOI] [PubMed] [Google Scholar]
- 2.Chittick, G. E., J. Zong, M. R. Blum, J. J. Sorbel, J. A. Begley, N. Adda, and B. P. Kearney. 2006. Pharmacokinetics of tenofovir disoproxil fumarate and ritonavir-boosted saquinavir mesylate administered alone or in combination at steady state. Antimicrob. Agents Chemother. 50:1304-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. 2006. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC.
- 4.Droste, J. A. H., C. P. W. G. M. Verweij-van Wissen, B. P. Kearney, R. Buffels, P. J. vanHorssen, Y. A. Hekster, and D. M. Burger. 2005. Pharmacokinetic study of tenofovir disoproxil fumarate combined with rifampin in healthy volunteers. Antimicrob. Agents Chemother. 49:680-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito, D., and R. Craigie. 1999. HIV integrase structure and function. Adv. Virus Res. 52:319-333. [DOI] [PubMed] [Google Scholar]
- 6.Grinsztejn, B., B. Y. Nguyen, C. Katlama, J. M. Gatell, A. Lazzarin, D. Vittecoq, C. J. Gonzalez, J. Chen, and R. D. Isaacs. 2006. Potent antiretroviral effect of MK-0518, a novel HIV-1 integrase inhibitor, in patients with triple-class resistant virus, abstr. 159LB. 13th Conf. Retrovir. Opportunistic Infect., Denver, CO.
- 7.Grinsztejn, B., B. Y. Nguyen, C. Katlama, J. M. Gatell, A. Lazzarin, D. Vittecoq, C. J. Gonzalez, J. Chen, C. M. Harvey, and R. D. Isaacs. 2006. Potent efficacy of Mk-0518, a novel HIV-1 integrase inhibitor, in patients with triple-class resistant virus: 24-week data, abstr. H-1670b, p. 195. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. (Final program.)
- 8.Grinsztejn, B., B. Y. Nguyen, C. Katlama, J. M. Gatell, A. Lazzarin, D. Vittecoq, C. J. Gonzalez, J. Chen, C. M. Harvey, and R. D. Isaacs. 2007. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369:1261-1269. [DOI] [PubMed] [Google Scholar]
- 9.Hammer, S. M., M. S. Saag, M. Schechter, J. S. Montaner, R. T. Schooley, D. M. Jacobsen, M. A. Thompson, C. C. Carpenter, M. A. Fischl, B. G. Gazzard, J. M. Gatell, M. S. Hirsch, D. A. Katzenstein, D. D. Richman, S. Vella, P. G. Yeni, and P. A. Volberding. 2006. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA Panel. JAMA 296:827-843. [DOI] [PubMed] [Google Scholar]
- 10.Hazuda, D. J., P. Felock, M. Witmer, A. Wolfe, K. Stillmock, J. A. Grobler, A. Espeseth, L. Gabryelski, W. Schleif, C. Blau, and M. D. Miller. 2000. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 287:646-650. [DOI] [PubMed] [Google Scholar]
- 11.Kassahun, K., I. McIntosh, D. Cui, D. Hreniuk, S. Merschman, K. Lasseter, N. Azrolan, M. Iwamoto, J. A. Wagner, and L. A. Wenning. 2007. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the HIV-1 integrase enzyme. Drug Metab. Dispos. 35:1657-1663. [DOI] [PubMed] [Google Scholar]
- 12.Kearney, B. P., J. R. Sayre, J. F. Flaherty, S. S. Chen, S. Kaul, and A. K. Cheng. 2005. Drug-drug and drug-food interactions between tenofovir disoproxil fumarate and didanosine. J. Clin. Pharmacol. 45:1360-1367. [DOI] [PubMed] [Google Scholar]
- 13.Little, S. J., S. Holte, J. P. Routy, E. S. Daar, M. Markowitz, A. C. Collier, R. A. Koup, J. W. Mellors, E. Connick, B. Conway, M. Kilby, L. Wang, J. M. Whitcomb, N. S. Hellmann, and D. D. Richman. 2002. Antiretroviral-drug resistance among patients recently infected with HIV. N. Engl. J. Med. 347:385-394. [DOI] [PubMed] [Google Scholar]
- 14.Markowitz, M., B. Y. Nguyen, E. Gotuzzo, F. Mendo, W. Ratanasuwan, C. Kovacs, G. Prada, J. O. Morales-Ramirez, C. S. Crumpacker, R. D. Isaacs, L. R. Gilde, H.Wan, M. D. Miller, L. A. Wenning, and H. Teppler. 2007. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naïve patients with HIV-1 infection. Results of a 48-week controlled study. J. Acquir. Immune Defic. Syndr. 46:125-133. [DOI] [PubMed] [Google Scholar]
- 15.Markowitz, M., J. O. Morales-Ramirez, B. Y. Nguyen, C. M. Kovacs, R. T. Steigbigel, D. A. Cooper, R. Liporace, R. Schwartz, R. Isaacs, L. R. Gilde, L. Wenning, J. Zhao, and H. Teppler. 2006. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 43:509-515. [DOI] [PubMed] [Google Scholar]
- 16.Merschman, S. A., P. T. Vallano, L. A. Wenning, B. K. Matuszewski, and E. J. Woolf. 2007. Determination of the HIV integrase inhibitor, MK-0518 (raltegravir), in human plasma using 96-well liquid-liquid extraction and HPLC-MS/MS. J. Chromatogr. B 857:15-19. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien, M. E., R. A. Clark, C. L. Besch, L. Myers, and P. Kissinger. 2003. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J. Acquir. Immune Defic. Syndr. 34:407-414. [DOI] [PubMed] [Google Scholar]
- 18.Petry, A. S., L. A. Wenning, M. Laethem, M. De Smet, J. T. Kost, S. Merschman, K. Strohmaier, S. Ramael, K. Lasseter, K. M. Gottesdiener, J. A. Wagner, and M. Iwamoto. 2006. Safety, tolerability, and pharmacokinetics after single and multiple doses of MK-0518 in healthy subjects, abstr. A-376, p. 8. Abstr. Intersci. Conf. Antimicrob. Agents Chemother. [DOI] [PubMed]
- 19.Richman, D. D., S. C. Morton, T. Wrin, N. Hellmann, S. Berry, M. F. Shapiro, and S. A. Bozzette. 2004. The prevalence of antiretroviral drug resistance in the United States. AIDS 18:1393-1401. [DOI] [PubMed] [Google Scholar]
- 20.Shet, A., L. Berry, H. Mohri, S. Mehandru, C. Chung, A. Kim, P. Jean-Pierre, C. Hogan, V. Simon, D. Boden, and M. Markowitz. 2006. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1: a decade of experience. J. Acquir. Immune Defic. Syndr. 41:439-446. [DOI] [PubMed] [Google Scholar]
- 21.Taburet, A. M., C. Piketty, C. Chazallon, I. Vincent, L. Gérard, V. Calvez, F. Clavel, J. P. Aboulker, and P. M. Girard. 2004. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 48:2091-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UK Group on Transmitted HIV Drug Resistance. 2005. Time trends in primary resistance to HIV drugs in the United Kingdom: multicentre observational study. BMJ 331:1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UNAIDS—Joint United Nations Programme on HIV/AIDS. 2006. Report on the global AIDS epidemic. http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp. Accessed 31 October 2007.
- 24.Wenning, L. A., E. Friedman, J. T. Kost, S. Merschman, K. Lasseter, N. Azrolan, K. M. Gottesdiener, J. A. Wagner, J. Stone, and M. Iwamoto. 2006. Lack of a significant drug interaction between MK-0518 and tenofovir disoproxil fumarate (TDF), abstr. A-375, p. 8. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemotherapy. American Society for Microbiology, Washington, DC.