Abstract
Cecropin B is a cationic antimicrobial peptide originally isolated from the diapausing pupae of the giant silk moth, Hylphora cecropia. Cecropin B elicits its antimicrobial effects through disruption of the anionic cell membranes of gram-negative bacteria. Previous work by our laboratory demonstrated that a constitutively expressed cecropin B transgene conferred enhanced resistance to bacterial infection in medaka. The development of antibiotic resistance by pathogenic bacteria is a growing problem. The potential for fish bacterial pathogens to develop resistance to cecropin B was addressed in this study. Four fish bacterial pathogens were selected for the study based on their importance in aquaculture. Vibrio anguillarum, Vibrio vulnificus, and Yersinia ruckeri all exhibited inducible resistance to cecropin B. The inducible resistance of these three pathogens was correlated with reversible changes in their ultrastructures, as observed by scanning electron microscopy. V. anguillarum was demonstrated to become more adhesive to a CHSE-214 cell monolayer and to cause increased cumulative mortality in medaka following exposure to cecropin B. This work demonstrates that the resistance of fish bacterial pathogens to cecropin B is inducible and suggests that resistance to other cationic antimicrobial peptides may occur through similar means. The observed changes in ultrastructure and infectivity suggest that resistance to antimicrobial peptides is an integral part of the pathogenesis of fish gram-negative bacterial pathogens.
The demand for new and effective treatments of bacterial disease persists with the continuous emergence of antibiotic-resistant strains of bacterial pathogens in both health care and agriculture environments (22, 23). Overfishing continues to deplete natural populations of food fish, and many nations have responded with the development of land-based intensive aquaculture. The stressful conditions that exist in high-density recirculating aquaculture systems often require the use of antibiotics in order to maintain a healthy population of fish for harvest (1). The agricultural use of antibiotics that are also used to treat humans makes food products less desirable for consumers and also threatens groundwater contamination and the development of antibiotic-resistant human pathogens (1). Antimicrobial peptides (AMPs) offer an attractive alternative to conventional antibiotics for the treatment and prevention of disease in fish.
AMPs are an important component of innate immune defense in all kingdoms of life (8). The cecropins are a family of cationic α-helical AMPs that range from 35 to 39 amino acids in length. The cationic charge of the cecropins allows their attraction and subsequent binding to the relatively anionic membranes of bacteria (4, 7, 32). Cecropin B, the best studied, was originally isolated from the diapausing pupae of the giant silk moth, Hylaphora cecropia, by Boman and coworkers in 1981 (33). The secondary structure of cecropin B consists of an amphipathic amino-terminal helix joined to a largely hydrophobic carboxy-terminal helix by a hinge region (32). The multitargeted action of AMPs gives them an advantage over conventional antibiotics (34). While β-lactam and aminoglycoside antibiotics target specific bacterial enzymes for their antimicrobial effects, cationic AMPs derive their target specificity through electrostatic affinity and secondary-structure characteristics that, in concert, afford them multiple bacterial targets, including lipopolysaccharide (LPS), peptidoglycan, and membrane phospholipids, as well as intracellular targets, including those involved in nucleic acid synthesis, protein synthesis, and the activities of various enzymes (13, 15, 17, 18). It is thought that the targeting of multiple bacterial macromolecules by AMPs makes the generation of resistant bacterial strains less probable because the alteration of more than one bacterial macromolecule would be required for complete resistance. There are examples of bacteria that are constitutively resistant to cationic AMPs. The alanylation of cell envelope teichoic acids confers resistance to cationic AMPs in Staphylococcus aureus (20). Inducible resistance to cationic AMPs has been reported for a number of bacterial pathogens (12, 21).
A very well studied example of inducible resistance to cationic AMPs is the PhoQ/PhoP two-component response regulator system of Salmonella enterica serovar Typhimurium. The PhoQ membrane-bound histidine sensor kinase is directly activated by cationic AMPs and, in concert with its cognate response regulator, PhoP, controls inducible resistance to cationic AMPs by modulating the expression of genes that are essential for survival within the host macrophage (3, 24). Recent studies of the crystal structure of PhoQ have revealed that PhoQ possesses a highly negative surface in close proximity to the inner membrane that forms metal bridges with the membrane in the presence of high concentrations of Ca2+ and Mg2+ (11). Loss of the metal bridge is thought to result in the electrostatic repulsion of the anionic surface of PhoQ and the phospholipids of the inner membrane, resulting in a positional or conformational change in PhoQ and activation of PhoQ signaling (11). The activation of PhoQ/PhoP-regulated gene expression by cationic AMPs is repressed by Mg2+ (3). These findings suggest that cationic AMPs disrupt the metal bridge between PhoQ and the inner membrane in their activation of PhoQ/PhoP-regulated gene expression and inducible AMP resistance.
Considering the ancient evolution of AMPs, it is not surprising that bacterial pathogens have evolved systems to sense AMPs and resist their antimicrobial action. The expression and secretion of AMPs by host tissues, as well as the membrane-disrupting action of AMPs, are points where bacterial pathogens actively resist their antimicrobial effects and evade the host innate immune response (37). Mechanisms of AMP resistance among bacteria include the inhibition of AMP gene expression in host tissues, the triggering of AMP secretion by host cells and subsequent sequestering of AMPs, protease production, and changes in bacterial-membrane structure (37). Indeed, the ability of bacterial pathogens to infect and persist within the host rests heavily on the subversion of the innate immune response (26, 37). The adaptive resistance of bacteria to AMPs could reduce the selection pressure of the peptides on the bacteria, reducing the probability of the generation of mutants further.
The convenience of the relatively short coding sequences of AMP genes has promoted studies of their abilities to enhance disease resistance in various transgenic applications. The resultant transgenic organisms, expressing AMP genes, exhibit enhanced resistance to bacterial infection (5, 16, 30, 36). Our laboratory has successfully enhanced the innate immune defense of fish through the genomic integration of a cecropin B transgene (30). In order to better understand the practical application of transgenic cecropin B as a prophylactic measure for bacterial infection in fish, we investigated the abilities of gram-negative fish bacterial pathogens to adaptively resist cecropin B. Here, we report that three of the four fish bacterial pathogens tested resist cecropin B through a reversible adaptation. The observed changes in susceptibility were correlated with dramatic differences in the outer surfaces of the bacterial cells, as observed through scanning electron microscopy (SEM). Exposure to cecropin B increased the infectivity of Vibrio anguillarum based on results observed in both CHSE-214 cell adhesion assays and medaka immersion challenge studies.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
All bacterial pathogens were cultured in Bacto tryptic soy broth and on identical solid media containing 1.5% Bacto agar. Culture media for vibrios additionally contained 1% NaCl. All bacterial pathogens were cultured at 25°C. The culture tubes were rotated at 250 rpm, and 96-well plates were rotated at 200 rpm. The strains of V. anguillarum and Pseudomonas fluorescens used in this study were isolates recovered from infected fish and were provided by Frank Hetrick, Department of Microbiology, University of Maryland, College Park, MD. The strains of Vibrio vulnificus and Yersinia ruckeri used in the study were isolates recovered from infected fish and were provided by the Oregon Department of Fisheries and Wildlife, Salem, OR. The cecropin B used in the study was a synthetic, mature sequence of cecropin B with greater than 95% purity and was purchased from Genemed Synthesis Inc., San Antonio, TX. The peptide was suspended in phosphate-buffered saline (PBS), pH 7.3, and stored as aliquots at −80°C. All of the cecropin B used was frozen and thawed once and used shortly after being thawed.
Susceptibility testing.
The susceptibilities of various fish bacterial pathogens to cecropin B were determined using a modified version of the methods described by Lambert and Pearson (21a). All bacterial cultures were streaked from glycerol stocks onto the appropriate solid media. Plates were incubated overnight, and single colonies were picked and used to inoculate overnight broth cultures. The overnight broth cultures were diluted 1:1,000 and allowed to grow to exponential phase for subsequent use in susceptibility tests. The culture optical density was determined at 600 nm (OD600), and all cultures were kept on ice until they were diluted. The susceptibilities of all bacterial pathogens were determined starting from an initial density of 1 × 107 CFU/ml. Aliquots were placed in 1.5-ml tubes, and an equal volume of culture medium containing cecropin B was added. The tubes were briefly mixed by vortexing, and 200-μl aliquots were placed in 96-well polystyrene tissue culture plates. The plates were incubated in a rotary shaker at 200 rpm. The OD570s of the cultures were measured using a Bio-Rad 3700 plate reader. The growth of cultures was monitored every 4 h for a total of five readings over the course of 16 h. The definite integral of the resultant growth curves was determined using Simpson's rule. Areas under the curves of cultures containing cecropin B were divided by the area under the curve of the control culture (without cecropin B) to determine the fractional area. The fractional area was plotted versus the concentration of cecropin B, producing an inhibition profile by cecropin B for each fish bacterial pathogen. The MIC of cecropin B was determined as the x intercept of the best-fit trend line for the inhibition profile. Data are expressed as the mean and standard deviation of three separate experiments, each conducted in triplicate.
Exposure of bacterial pathogens to cecropin B and passaging.
The concentration of cecropin B used for the exposure of fish bacterial pathogens was empirically determined as the concentration that resulted in consistent growth to mid-log phase over the 16-hour incubation period (OD600, ∼0.4). At the end of 16 h, the OD600 was determined, and the cultures were diluted to 1 × 107 CFU/ml and assayed for susceptibility to cecropin B as described above.
Following the 16-hour exposure period, the cultures were diluted to 1 × 107 CFU/ml and incubated without cecropin B for 16 h. The resultant stationary-phase cultures were then diluted 1:1,000 and cultured to mid-log phase for subsequent susceptibility testing as described above. Each culture to stationary phase and subsequent culture to mid-log phase was considered one passage.
SEM.
Cultures of various fish bacterial pathogens under various conditions were observed using a scanning electron microscope at the University of Connecticut Electron Microscopy Laboratory. Briefly, gold-palladium-covered silicon chips, 2.5 mm by 2.5 mm, were coated with 15 μl of 0.1% poly-l-lysine and allowed to air dry. Fish bacterial pathogens were cultured in broth to exponential phase, and 30 μl of culture was placed on the coated chips and incubated for 10 min at room temperature. The chips were briefly rinsed in PBS, pH 7.3, in 24-well plates and subsequently fixed in 2% glutaraldehyde/1× sodium cacodylate buffer (100 mM cacodylate, 40 mM NaCl, 0.15 mM CaCl2, 0.15 mM MgCl2) for 30 min. Following the initial fixation, all chips were rinsed in 1× sodium cacodylate buffer twice for 10 min each time before being placed in 1-dram glass shell vials containing 1% osmium tetroxide/1× sodium cacodylate buffer for overnight fixation. All of the volumes used throughout the fixation for each chip were 1 ml. The next day, the chips were rinsed twice in 1× sodium cacodylate buffer for 10 min each and dehydrated in ethyl alcohol (EtOH)/1× sodium cacodylate buffer at the following EtOH percentages: 30, 50, 70, 90, and 100%, with a final overnight incubation in 100% EtOH. It is important to note that at no point after the cultures had been applied to the chips were the chips allowed to dry. Following EtOH dehydration, the chips were incubated in a critical-point dryer for 1 h. The dried chips were then attached to SEM stubs using silver paint and were sputter coated with gold for 2 min at 1,800 kV.
CHSE-214 cell adhesion assay.
Chinook salmon embryo (CHSE-214) cells were routinely cultured in CO2-independent medium supplemented with 10% fetal bovine serum at 20°C without antibiotics. The CHSE-214 cells were seeded in 24-well tissue culture plates and grown to partial confluence (80%). Representative wells were trypsinized and enumerated by the trypan blue exclusion method. Mid-log-phase cultures of V. anguillarum were diluted to a multiplicity of infection of 100 and incubated with the cell monolayers in 0.2 ml of cell culture medium for 0, 30, 60, and 90 min at room temperature (∼22°C). Following incubation, the cell monolayers were washed twice with 0.5 ml of PBS (pH 7.2) for 2 min on a rotary shaker to remove nonadherent bacteria. The adhered cells were lysed in a PBS buffer, pH 7.3, containing 1% (vol/vol) Triton X-100, and the resulting lysates were plated on appropriate solid medium. The resultant colonies were counted, and the data were expressed as the number of infecting bacteria per CHSE-214 cell.
Medaka immersion challenge assay.
Groups of 20 medaka, 7 to 8 months of age, were immersed in a PBS solution (pH 7.3, 1.0% NaCl) containing various concentrations of V. anguillarum for 90 min. Following exposure, the fish were rinsed three times with aquarium water and returned to their original rearing tanks. The experimental fish were monitored for 14 days prior to euthanasia with MS222. Dead or moribund fish were removed daily for counting. For determination of the number of bacteria to use for infection trials, a standard curve was created using 0, 104, 105, and 106 CFU/ml for immersion challenges. The concentration that caused 30% cumulative mortality, 105 CFU/ml, was used for the immersion challenge of medaka with V. anguillarum that had previous exposure to cecropin B and V. anguillarum that did not.
RESULTS
Susceptibility testing of inducible resistance.
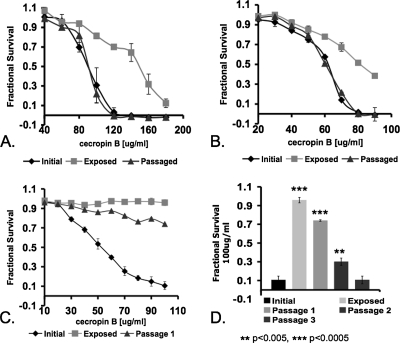
The susceptibilities of fish bacterial pathogens (P. fluorescens, V. anguillarum, V. vulnificus, and Y. ruckeri) to cecropin B were determined by the broth culture growth inhibition assay. In this assay, the inhibition profiles of bacterial pathogens resistant to cecropin B moved the inhibition profile toward the positive end of the x axis (Fig. 1). This change in the position and shape of the inhibition profiles was due to an increase in the fractional survival of the cultures with increasing concentrations of cecropin. Figure 1 depicts the dose-dependent inhibition profiles of bacterial pathogens to cecropin B. Following prior exposure to cecropin B, V. anguillarum, V. vulnificus, and Y. ruckeri, but not P. fluorescens (data not shown), exhibited increased fractional survival in susceptibility tests (Fig. 1A to C), suggesting these pathogens gained resistance to cecropin B. Those bacterial pathogens that exhibited an increase in fractional survival in culture with cecropin B displayed differences in their respective levels of resistance to cecropin B (Fig. 1A to D). V. anguillarum and V. vulnificus exhibited increased resistance to cecropin B, but the increased level of resistance was abolished following a single passage of cells in the growth medium without the presence of cecropin B (Fig. 1A and B). However, the increased resistance to cecropin B exhibited by Y. ruckeri was not abolished completely following one passage of culture in the growth medium without cecropin B (Fig. 1C). It took an additional two continuous passages of culture in the growth medium without cecropin B for Y. ruckeri to return to the original level of susceptibility (Fig. 1D). The MIC of cecropin B for each of the pathogens was determined in three discrete states: without exposure to cecropin B, after exposure to 80% to 90% of the MIC of cecropin B, and following consecutive culture without cecropin B. The MICs obtained for V. anguillarum and V. vulnificus after exposure to cecropin B were significantly greater than those without exposure to cecropin B (Table 1). P. fluorescens did not exhibit a change in the MIC for cecropin B following exposure (Table 1). Y. ruckeri exhibited such a great increase in resistance to cecropin B following exposure that the MIC was not determinable from the data obtained (Table 1 and Fig. 1C and D).
FIG. 1.
Inducible resistance of fish bacterial pathogens to cecropin B. V. anguillarum (A), V. vulnificus (B), and Y. ruckeri (C and D) susceptibilities to 100 μg/ml cecropin B following multiple passages without cecropin B. Each data point represents three repeat experiments with three replicates each. The concentrations of cecropin B used for the exposure of V. anguillarum, V. vulnificus, and Y. ruckeri were 120 μg/ml, 70 μg/ml, and 90 μg/ml, respectively. The error bars represent the standard deviation. **, P < 0.005; ***, P < 0.0005.
TABLE 1.
MICs of cecropin B for bacterial pathogens at discrete points of cecropin B resistance
| Bacterium | MIC (μg/ml)a | Exp [CecB] (μg/ml)b | AE MIC (μg/ml)c | AP MIC (μg/ml)d |
|---|---|---|---|---|
| V. anguillarum | 147 ± 4 | 120 | 209 ± 12 | 142 ± 2 |
| V. vulnificus | 85 | 70 | 136 ± 9 | 85 ± 1 |
| Y. ruckeri | 100 ± 4 | 90 | NDe | ND |
| P. fluorescens | 25 ± 1 | 20 | 24 ± 1 | ND |
MIC without prior exposure to cecropin B; mean and standard deviation of three experimental repeats with three replicates each.
Concentration of cecropin B (CecB) used for exposure; mean of three experimental repeats with three replicates each.
MIC after exposure; mean and standard deviation of three experimental repeats with three replicates each.
MIC after passage; mean and standard deviation of three experimental repeats with three replicates each.
ND, not determined.
Cecropin B resistance affects the infectivity of V. anguillarum.
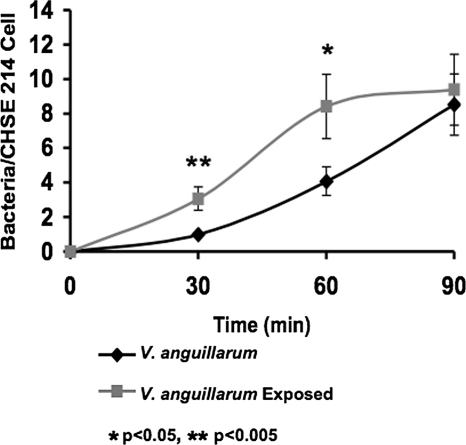
An important consideration when using any antimicrobial agent in a recirculating aquaculture system is the potential for the selection of new strains of bacterial pathogens with enhanced resistance to the antibiotic. Although the susceptibility testing of V. anguillarum, V. vulnificus, and Y. ruckeri against cecropin B demonstrated that they responded with a reversible, inducible resistance, the effects of such resistance on the infectivities of the pathogens required assessment. For this purpose, a cell monolayer adhesion assay was adopted using CHSE-214 cells as the host and V. anguillarum as the bacterial pathogen. Following 30 min and 60 min of incubation, cultures of cecropin B-exposed V. anguillarum exhibited a significant increase in adhesion to CHSE-214 cells compared to that of unexposed cultures (Fig. 2). At 90 min, the numbers of adhering bacteria per CHSE-214 cell were indistinguishable between exposed and unexposed cultures (Fig. 2). These results indicate that exposure of V. anguillarum to cecropin B results in an increase in the rate of adhesion of V. anguillarum to CHSE-214 cells.
FIG. 2.
Fish cell adhesion assay. CHSE-214 cell monolayers were incubated with unexposed V. anguillarum and V. anguillarum that had been exposed to cecropin B. The concentration of cecropin B used for the exposure of V. anguillarum was 120 μg/ml. The data represent three separate repeat experiments, each with three replicates. The error bars represent the standard deviation. *, P < 0.05; **, P < 0.005.
In order to determine if the observed difference in the adhesion of V. anguillarum to CHSE-214 cells translated into a difference in the capacity of the bacteria to infect fish, a medaka immersion challenge assay was developed. Y. ruckeri, V. anguillarum, and V. vulnificus were tested for the ability to infect medaka via immersion. Of the three bacterial pathogens studied, only V. anguillarum caused mortality over the 14-day observation period (data not shown). For this reason, V. anguillarum was the only bacterial pathogen tested in the immersion challenge assay.
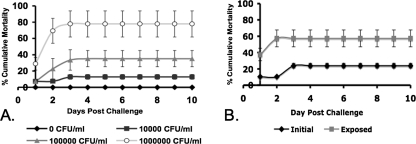
The effects of immersion in different concentrations (CFU/ml) of V. anguillarum on the cumulative mortality of medaka were determined (Fig. 3A). A concentration of V. anguillarum 775 (1 × 105 CFU/ml) that resulted in a cumulative mortality of ∼30% was selected for immersion challenge assay with medaka because it would better accommodate an increase in cumulative mortality. As shown in Fig. 3, immersion challenge with V. anguillarum exposed to cecropin B resulted in cumulative mortality comparable to that caused by unexposed V. anguillarum. These results are in good agreement with those shown in Fig. 2.
FIG. 3.
Immersion challenge assay. (A) Groups of 20 medaka were challenged with various concentrations of V. anguillarum by immersion. (B) Groups of 20 medaka were challenged with 1 × 105 CFU/ml of V. anguillarum with or without prior exposure to cecropin B. The concentration of cecropin B used for the exposure of V. anguillarum was 120 μg/ml. The data represent three separate experiments. The error bars represent the standard deviation.
Ultrastructural changes in bacterial pathogens after treatment with cecropin B.
A number of mechanisms of AMP resistance have been described for gram-negative bacterial pathogens (26). Cationic AMPs target the anionic outer layer of gram-negative bacterial pathogens, where the initial binding of the peptide to the bacterium occurs. The changes in susceptibility to cecropin B described in this work could be correlated with ultrastructural changes in the outer surfaces of the bacterial cells. SEM was used to observe any changes in the ultrastructure of the outer surfaces of V. anguillarum, V. vulnificus, and Y. ruckeri associated with resistance to cecropin B.
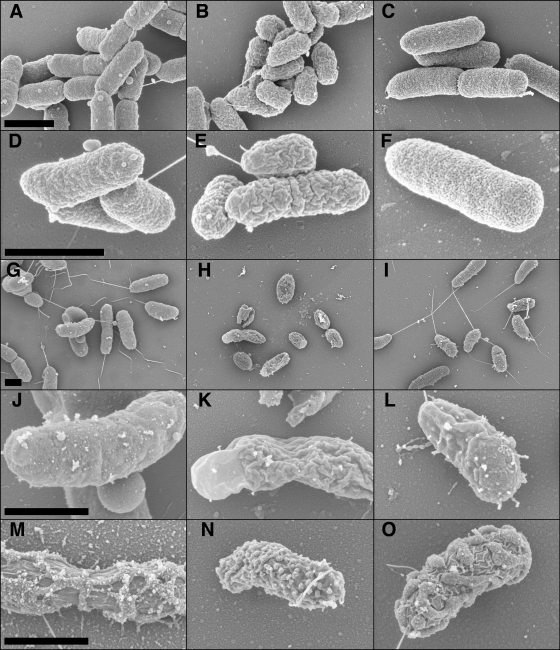
Prior to culturing Y. ruckeri with cecropin B, the outer surface the cell was smooth (Fig. 4A and D), but following exposure to cecropin B, prominent ridges formed on the outer surface of the cell and the surface appeared smoother (Fig. 4B and E). Also, individual cells appeared smaller (compare Fig. 4B to A). Following three consecutive passages of the cecropin B-resistant cells in the medium without cecropin B, the outer layer of Y. ruckeri returned to the state observed prior to exposure to cecropin B, concurrent with an increase in susceptibility to killing by cecropin B (Fig. 4C and F).
FIG. 4.
SEM of ultrastructural changes that were correlated with cecropin B resistance. Y. ruckeri (A to F), V. vulnificus (G to L), and V. anguillarum (M to O) were cultured to three distinct points in respect to cecropin B resistance The ultrastructures of cultures were observed via SEM. (A, D, G, J, and M) Cells without exposure to cecropin B. (B, E, H, K, and N) Cells exposed to cecropin B and resistant to cecropin B. (C, F, I, L, and O) Resistant cells cultured to regain the nonresistant state. The concentrations of cecropin B used for the exposure of V. anguillarum, V. vulnificus, and Y. ruckeri were 120 μg/ml, 70 μg/ml, and 90 μg/ml, respectively. Bar, 1 μm.
V. vulnificus displayed profound changes correlated with cecropin B resistance (Fig. 4G to L). Cells in a state of increased resistance to cecropin B exhibited reduced cell size and dramatic loss of flagella, as well as a rugose membrane shape similar to that observed for Y. ruckeri (compare Fig. 4H and K to G and J). After V. vulnificus was cultured in the growth medium without cecropin B for one passage, the cells regained their flagella and the outer membrane returned to its condition prior to exposure to cecropin B (Fig. 4I and L).
V. anguillarum 775 cells also exhibited outer-layer changes following exposure to cecropin B. Before cecropin B exposure, V. anguillarum cells produced extracellular products that covered the outer layer and diffused into the medium (Fig. 4M). The amount of extracellular product produced and secreted by the cells was greatly reduced following exposure to cecropin B, as was apparent from the small spherical structures on the outer surfaces of the cells (Fig. 4N). Once the previously exposed cells of V. anguillarum 775 were passaged without cecropin B in the medium, the small spherical structures began to increase in size and covered the outer layers of the cells (Fig. 4O). This structural change was correlated with a return of susceptibility to cecropin B to that exhibited by unexposed cultures.
DISCUSSION
This work has demonstrated that fish bacterial pathogens exhibit an inducible resistance to the AMP cecropin B. The ability of human bacterial pathogens to sense AMPs and to respond by resistance to their toxic effects is well documented, in contrast to the relative absence of information regarding this capacity for fish bacterial pathogens (37). Immunity to bacterial infection depends greatly on the innate immune response in ectothermic animals, such as fish, largely due to the dependence of the development of adaptive immunity on temperature (25, 31). Many bacterial infections cause mortality less than 4 weeks after inoculation (29). This places a great demand on the innate immune response of the fish in fighting bacterial infections. AMPs present a rapid defense against bacterial infection. The results of the studies presented in this paper demonstrate that fish bacterial pathogens have the capacity to resist cecropin B killing with an inducible and reversible response. The induction of resistance to cecropin B suggests that AMP resistance is a sensitive response to a change in the environment. The resultant physiological differences could aid the bacterial pathogens in surviving within the host. The extended period during which increased resistance to cecropin was maintained by Y. ruckeri following multiple culture passages without cecropin B suggests that a phase variation event may be responsible for the increased resistance to cecropin B. Yersinia enterocolitica has been shown to employ a DNA adenine methyltransferase (Dam) for the phase-variable regulation of virulence gene expression (14). Future studies are required to determine the involvement of Dam and/or DNA mismatch repair mechanisms in the resistance of Y. ruckeri to killing by AMPs.
Ultrastructural changes in the bacterial membrane have been linked to AMP toxicity in killed bacteria (2, 19). This work presents a unique look at the ultrastructural changes related to AMP resistance in growing mid-exponential cultures of bacteria. Outer membrane remodeling has been shown to be partly responsible for the adaptive resistance of S. enterica serovar Typhimurium to polymyxin B (12). The findings presented here further support the involvement of structural modifications of the outer membranes of gram-negative bacteria in AMP resistance by providing SEM evidence of broad ultrastructural changes that are correlated with cecropin B resistance. The observed ultrastructural changes included the loss of flagella and changes in the shape and texture of the outer membrane.
Bacterial flagellin has been shown to be a ligand for host Toll-like receptors (TLRs) (9, 35). A recent study demonstrated that fish soluble TLR5 amplifies the human TLR5-mediated response through the direct binding of flagellin (35). This finding indicates a role for bacterial flagellin as a molecular pattern that stimulates the immune response in fish and that the absence of flagella could provide a benefit to pathogens in evading detection by host molecular pattern recognition receptors. The loss of flagella by V. vulnificus following exposure to cecropin B supports this possibility and suggests that cecropin B may play an equivalent role as a molecular pattern recognized by bacteria that stimulates responses that are advantageous for the survival of the bacteria within the host.
All three pathogens displayed dramatic differences in the shapes and textures of their outer membranes. Consequently, the question arises as to whether the changes resulted from the release of LPS from the outer membrane of the bacteria. If the exposure of bacteria to cecropin B results in the release of LPS, it would certainly explain such a consistent ultrastructural change being observed in three different species of fish bacterial pathogens. Chelating agents, such as EDTA, are known to cause the release of LPS from bacteria by removing the divalent cations that stabilize the arrangement of neighboring LPS molecules in the outer membrane (35). The possibility that cecropin B causes the release of LPS from the outer membrane by displacing the stabilizing divalent cations requires further study.
The observed relationship between the inducible resistance to cecropin B and infectivity in V. anguillarum suggests that AMP resistance is an integral part of successful host colonization. Both increased adhesion to CHSE-214 cell monolayers and increased cumulative mortality in medaka were correlated with resistance to cecropin B in V. anguillarum. V. anguillarum is known to enter its fish host through the portals of the skin, the gut, and the gills and has been shown to move by chemotaxis toward mucus secretions from multiple epithelial tissues, including the skin and the intestine (27). Fish epithelial tissues secrete innate immune effectors, including AMPs (10, 28). The increase in the cumulative mortality of medaka following challenge with V. anguillarum that was previously exposed to cecropin B suggests that successful colonization of the fish by V. anguillarum depends on its ability to resist the actions of AMPs. The observed increase in infectivity suggests that AMPs could serve as signals for the physiological changes related to host colonization by V. anguillarum. The ultrastructural changes in the outer membrane of V. anguillarum following exposure to cecropin B could be the cause of its increased infectivity, its increased resistance to cecropin B, or both. This relationship requires further confirmation.
The relationship between bacterial pathogens and their hosts is dictated by their mutual recognition (6). The function of AMPs in inducing physiological changes in the bacteria, resulting in reduced susceptibility to AMPs, the loss of pathogen-associated molecular patterns, and enhanced infectivity, indicates that AMPs are host-associated molecular patterns for bacterial pathogens. Further study of this relationship will lead to a greater understanding of the dynamics of host-pathogen interactions and aid in the development of novel therapeutic modalities for treatment of bacterial infection that limit the development of antibiotic-resistant mutant strains of bacterial pathogens.
Acknowledgments
This research was supported by a grant from the U.S. Department of Agriculture (CONTR 58-1930-0-009) to T.T.C.
Footnotes
Published ahead of print on 12 May 2008.
REFERENCES
- 1.Anonymous. 2002. The use of antimicrobials outside human medicine: information from the World Health Organization on the health consequences. J. Environ. Health 64:66, 62. [PubMed] [Google Scholar]
- 2.Arzese, A., B. Skerlavaj, L. Tomasinsig, R. Gennaro, and M. Zanetti. 2003. Antimicrobial activity of SMAP-29 against the Bacteroides fragilis group and clostridia. J. Antimicrob. Chemother. 52:375-381. [DOI] [PubMed] [Google Scholar]
- 3.Bader, M. W., S. Sanowar, M. E. Daley, A. R. Schneider, U. Cho, W. Xu, R. E. Klevit, H. Le Moual, and S. I. Miller. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122:461-472. [DOI] [PubMed] [Google Scholar]
- 4.Bechinger, B. 1997. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 156:197-211. [DOI] [PubMed] [Google Scholar]
- 5.Boudreaux, C. M., R. E. Corstvet, R. K. Cooper, and F. M. Enright. 2005. Effects of cecropin B transgene expression on Mannheimia haemolytica serotype 1 colonization of the nasal mucosa of calves. Am. J. Vet. Res. 66:1922-1930. [DOI] [PubMed] [Google Scholar]
- 6.Bowdish, D. M., and R. E. Hancock. 2005. Anti-endotoxin properties of cationic host defence peptides and proteins. J. Endotoxin Res. 11:230-236. [DOI] [PubMed] [Google Scholar]
- 7.Bulet, P., and R. Stocklin. 2005. Insect antimicrobial peptides: structures, properties and gene regulation. Protein Pept. Lett. 12:3-11. [DOI] [PubMed] [Google Scholar]
- 8.Bulet, P., R. Stocklin, and L. Menin. 2004. Anti-microbial peptides: from invertebrates to vertebrates. Immunol. Rev. 198:169-184. [DOI] [PubMed] [Google Scholar]
- 9.Caron, G., D. Duluc, I. Fremaux, P. Jeannin, C. David, H. Gascan, and Y. Delneste. 2005. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J. Immunol. 175:1551-1557. [DOI] [PubMed] [Google Scholar]
- 10.Chen, S. L., W. Li, L. Meng, Z. X. Sha, Z. J. Wang, and G. C. Ren. 2007. Molecular cloning and expression analysis of a hepcidin antimicrobial peptide gene from turbot (Scophthalmus maximus). Fish Shellfish Immunol. 22:172-181. [DOI] [PubMed] [Google Scholar]
- 11.Cho, U. S., M. W. Bader, M. F. Amaya, M. E. Daley, R. E. Klevit, S. I. Miller, and W. Xu. 2006. Metal bridges between the PhoQ sensor domain and the membrane regulate transmembrane signaling. J. Mol. Biol. 356:1193-1206. [DOI] [PubMed] [Google Scholar]
- 12.Ernst, R. K., T. Guina, and S. I. Miller. 2001. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 3:1327-1334. [DOI] [PubMed] [Google Scholar]
- 13.Evans, E. W., and B. G. Harmon. 1995. A review of antimicrobial peptides: defensins and related cationic peptides. Vet. Clin. Pathol. 24:109-116. [DOI] [PubMed] [Google Scholar]
- 14.Falker, S., M. A. Schmidt, and G. Heusipp. 2005. DNA methylation in Yersinia enterocolitica: role of the DNA adenine methyltransferase in mismatch repair and regulation of virulence factors. Microbiology 151:2291-2299. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich, C. L., D. Moyles, T. J. Beveridge, and R. E. Hancock. 2000. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao, A. G., S. M. Hakimi, C. A. Mittanck, Y. Wu, B. M. Woerner, D. M. Stark, D. M. Shah, J. Liang, and C. M. Rommens. 2000. Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat. Biotechnol. 18:1307-1310. [DOI] [PubMed] [Google Scholar]
- 17.Hale, J. D., and R. E. Hancock. 2007. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Exp. Rev. Anti-Infect. Ther. 5:951-959. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, R. E., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 19.Henk, W. G., W. J. Todd, F. M. Enright, and P. S. Mitchell. 1995. The morphological effects of two antimicrobial peptides, hecate-1 and melittin, on Escherichia coli. Scanning Microsc. 9:501-507. [PubMed] [Google Scholar]
- 20.Kristian, S. A., X. Lauth, V. Nizet, F. Goetz, B. Neumeister, A. Peschel, and R. Landmann. 2003. Alanylation of teichoic acids protects Staphylococcus aureus against Toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J. Infect. Dis. 188:414-423. [DOI] [PubMed] [Google Scholar]
- 21.Kwon, D. H., and C. D. Lu. 2006. Polyamines induce resistance to cationic peptide, aminoglycoside, and quinolone antibiotics in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 50:1615-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Lambert, R. J., and J. Pearson. 2000. Susceptibility testing: accurate and reproducible minimum inhibitory concentration (MIC) and noninhibitory concentration (NIC) values. J. Appl. Microbiol. 88:784-790. [DOI] [PubMed] [Google Scholar]
- 22.Li, X. Z., M. Mehrotra, S. Ghimire, and L. Adewoye. 2007. β-Lactam resistance and β-lactamases in bacteria of animal origin. Vet. Microbiol. 121:197-214. [DOI] [PubMed] [Google Scholar]
- 23.McGowan, J. E., Jr. 2006. Resistance in nonfermenting gram-negative bacteria: multidrug resistance to the maximum. Am. J. Infect. Control 34:S29-S37, S64-S73. [DOI] [PubMed] [Google Scholar]
- 24.Miller, S. I., W. S. Pulkkinen, M. E. Selsted, and J. J. Mekalanos. 1990. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect. Immun. 58:3706-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nath, S., S. Kales, K. Fujiki, and B. Dixon. 2006. Major histocompatibility class II genes in rainbow trout (Oncorhynchus mykiss) exhibit temperature dependent downregulation. Immunogenetics 58:443-453. [DOI] [PubMed] [Google Scholar]
- 26.Nizet, V. 2006. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr. Issues Mol. Biol. 8:11-26. [PubMed] [Google Scholar]
- 27.O'Toole, R., S. Lundberg, S. A. Fredriksson, A. Jansson, B. Nilsson, and H. Wolf-Watz. 1999. The chemotactic response of Vibrio anguillarum to fish intestinal mucus is mediated by a combination of multiple mucus components. J. Bacteriol. 181:4308-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan, C. Y., J. Y. Chen, Y. S. Cheng, C. Y. Chen, I. H. Ni, J. F. Sheen, Y. L. Pan, and C. M. Kuo. 2007. Gene expression and localization of the epinecidin-1 antimicrobial peptide in the grouper (Epinephelus coioides), and its role in protecting fish against pathogenic infection. DNA Cell Biol. 26:403-413. [DOI] [PubMed] [Google Scholar]
- 29.Powell, M., J. Carson, and R. van Gelderen. 2004. Experimental induction of gill disease in Atlantic salmon Salmo salar smolts with Tenacibaculum maritimum. Dis. Aquat. Organ. 61:179-185. [DOI] [PubMed] [Google Scholar]
- 30.Sarmasik, A., G. Warr, and T. T. Chen. 2002. Production of transgenic medaka with increased resistance to bacterial pathogens. Mar. Biotechnol. 4:310-322. [DOI] [PubMed] [Google Scholar]
- 31.Sharp, G. J., A. W. Pike, and C. J. Secombes. 1992. Sequential development of the immune response in rainbow trout [Oncorhynchus mykiss (Walbaum, 1792)] to experimental plerocercoid infections of Diphyllobothrium dendriticum (Nitzsch, 1824). Parasitology 104:169-178. [DOI] [PubMed] [Google Scholar]
- 32.Steiner, H., D. Andreu, and R. B. Merrifield. 1988. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim. Biophys. Acta 939:260-266. [DOI] [PubMed] [Google Scholar]
- 33.Steiner, H., D. Hultmark, A. Engstrom, H. Bennich, and H. G. Boman. 1981. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292:246-248.7019715 [Google Scholar]
- 34.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 35.Tsujita, T., A. Ishii, H. Tsukada, M. Matsumoto, F. S. Che, and T. Seya. 2006. Fish soluble Toll-like receptor (TLR)5 amplifies human TLR5 response via physical binding to flagellin. Vaccine 24:2193-2199. [DOI] [PubMed] [Google Scholar]
- 36.Tzou, P., J. M. Reichhart, and B. Lemaitre. 2002. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proc. Natl. Acad. Sci. USA 99:2152-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yount, N. Y., and M. R. Yeaman. 2005. Immunocontinuum: perspectives in antimicrobial peptide mechanisms of action and resistance. Protein Pept. Lett. 12:49-67. [DOI] [PubMed] [Google Scholar]