Abstract
This paper presents a systematic study of the galvanic replacement reaction between 23.5 nm single-crystal Ag nanospheres and HAuCl4 in an aqueous medium. We have monitored both morphological and spectral changes as the molar ratio of HAuCl4 to Ag is increased. The replacement reaction on single-crystal Ag nanospheres results in the formation of a series of hollow and porous nanostructures composed of Au–Ag alloys. By varying the molar ratio of HAuCl4 to Ag, we are able to control the size and density of the pores. In addition, the localized surface plasmon resonance peaks of these nanostructures can be readily tuned from 408 to 791 nm as the product becomes increasingly more hollow and porous.
Introduction
Metal nanostructures have been extensively studied over the past decades because of their interesting catalytic, electronic, magnetic, and optical properties.1-4 The intrinsic properties of a metal nanostructure can be maneuvered by controlling the size, shape, composition, crystallinity, and structure (hollow vs solid).5-8 Recently, hollow metal nanostructures have attracted a great deal of attention because of their unique structural and optical properties such as high surface areas, low densities, and tunable localized surface plasmon resonance (LSPR) features.9 This new class of metal nanostructures are useful in a number of applications including surface-enhanced Raman scattering (SERS), optical imaging, photothermal therapy, as well as encapsulation and site specific delivery of drugs.10-15
We have developed a single-step approach based on the galvanic replacement reaction to the generation of hollow metal nanostructures. In this approach, silver nanoparticles with a variety of shapes (including cubes, plates, rods, and wires) are used as a sacrificial template to react with an aqueous HAuCl4 solution. By controlling the template and the stoichiometry of the reaction, we have obtained Au-based nanoboxes, nanocages, nanorattles, and nanotubes.13-20 In addition, it has been demonstrated that the LSPR peaks of these hollow nanostructures can be readily shifted to the near-infrared region by controlling the thickness, porosity, and elemental composition of the walls, which, in turn, can be simply achieved by adjusting the volume of HAuCl4 solution added into the suspension of Ag template. This synthetic strategy has also been successfully extended to other noble metals such as Pt and Pd.20,21
We and other groups have investigated the galvanic replacement reaction between spherical Ag nanoparticles and HAuCl4.17,22-24 In most of these studies, however, multiply twined particles (MTPs) were used as the template and/or an organic solvent was used as the reaction medium. In this article, we report a facile method for producing hollow and porous Au-based nanostructures via the galvanic replacement reaction between 23.5 nm single-crystal Ag nanospheres and HAuCl4 in an aqueous medium. A water-based system provides a more environmentally benign route because it does not involve toxic organic solvents. For biomedical applications, it is also critical to have the metal hollow nanostructures dispersed in an aqueous medium in order to be effectively applied to a biological system. For most applications, the nanostructures need to be robust enough to maintain their open structures during postchemical and/or physical treatments even though their walls are highly porous and thin (typically <5 nm in thickness). As we have demonstrated in previous studies, when the Ag template is a single crystal, the Au-based hollow nanostructure derived from it is essentially single crystalline20 and, thus, mechanically stronger than its polycrystalline counterpart. That explains why previous work on this reaction has been mainly focused on single-crystal Ag nanocubes. When the dimensions of a Ag nanocube are reduced to the regime below 30 nm, it tends to be truncated at corners (and slightly at edges) to become a cuboctahedron with a rounded profile. Because a nanocube and a cuboctahedron is enclosed by {100} and a mix of {100} and {111} facets, respectively, their reaction pathways with HAuCl4 are expected to be different. Herein, we present a detailed study of the structural, morphological, and spectral changes involved in the replacement reaction when single-crystal Ag nanospheres are employed as the sacrificial template.
Experimental Section
Preparation of Single-Crystal Ag Nanospheres
In a typical synthesis, 5 mL of ethylene glycol (EG, J. T. Baker, 9300–01) was heated with magnetic stirring in an oil bath at 153 °C for 1 h. Two 3 mL EG solutions were then simultaneously added into the hot EG at a rate of 45 mL/h through a two-channel syringe pump (KDS-200, Stoelting, Wood Dale, IL). One of the solutions contained 94 mM silver nitrate (AgNO3, Aldrich, 209139–100G), and the other one contained 147 mM of poly(vinyl pyrrolidone) (PVP, Mw ≈ 55 000, Aldrich, 856568–100G) and 0.22 mM of NaCl (Fisher, S271–500). The molar concentration was calculated in terms of the repeating unit of PVP. Upon injection of the AgNO3 solution, the reaction mixture went through a series of color changes that included yellow, light yellow, colorless, light yellow, and strong yellow. The final product exhibited a strong yellow color due to the LSPR peak of single-crystal Ag nanospheres. The final product was collected by centrifugation and washed with acetone and then with water to remove most of the EG and PVP. During the washing process, the suspension was centrifuged at 14 000 rpm for either 10 or 30 min (depending on whether acetone or water was used) to remove the EG and the excess PVP. Finally, the precipitate was redispersed in deionized water for further use.
Galvanic Replacement Reaction
In a typical synthesis, a fixed amount (0.2 nM, 300 μL) of the Ag nanospheres was dispersed in 3 mL of water containing 1 mg/mL PVP in a 50 mL flask under magnetic stirring and then heated at 100 °C for 10 min. A specific amount (as indicated in the text) of 0.03 mM HAuCl4 aqueous solution was added into the flask through a syringe pump at a rate of 45 mL/h under magnetic stirring. The solution was heated for another 10 min until the color of the system became stable. Once cooled to room temperature, the sample was centrifuged and washed with a saturated NaCl aqueous solution to remove the AgCl and with water several times to remove PVP and NaCl before characterization by scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
Instrumentation
The SEM (or TEM) sample was prepared by placing a drop of the final product (suspended in water) on a silicon wafer (or carbon-coated copper grid) and dried in the fume hood. After that, the sample was transferred into a gravity-fed flow-cell and washed for 1 h with deionized water to remove the remaining PVP. Finally, the sample was dried and stored in a vacuum. SEM images were taken using an FEI filed-emission microscope (Sirion XL) operated at an accelerating voltage of 10 kV. Energy-dispersive X-ray (EDX) measurements were done with the EDAX system attached to the same microscope. TEM imaging was performed using a Philips 420 microscope operated at 120 kV. High-resolution TEM was obtained using a JEOL JEM-2100F operated at 200 kV. The UV–vis–NIR spectra were recorded using a Cary 50 spectrophotometer (Varian, Palo Alto, CA).
Results and Discussion
In order to produce single-crystal Ag nanospheres with improved monodispersity and in high yields, we modified the polyol synthesis that used chloride and oxygen to generate single-crystal, truncated nanocubes.25 The key to high yields of single-crystal Ag nanospheres is to control the crystallinity of seeds formed in the nucleation stage. In the presence of chloride, twinned seeds can be selectively etched due to the presence of oxygen from air, leaving only single-crystal seeds in the reaction solution. The single-crystal seeds subsequently grow to generate Ag nanocubes. In order to obtain single-crystal nanospheres (i.e., cuboctahedra), we have to stop the reaction just before the seeds have evolved into faceted cubes. Figure 1 shows a TEM image of the Ag nanospheres with an average diameter of 23.5 nm and a polydispersity of <10%. The homogeneous contrast across each particle shown on the TEM image indicates that the spherical Ag nanoparticles were single crystalline. The inset shows a high-resolution TEM image of the Ag nanosphere, clearly indicating that the Ag nanosphere is a single crystal without any twin defect in the lattice.
Figure 1.
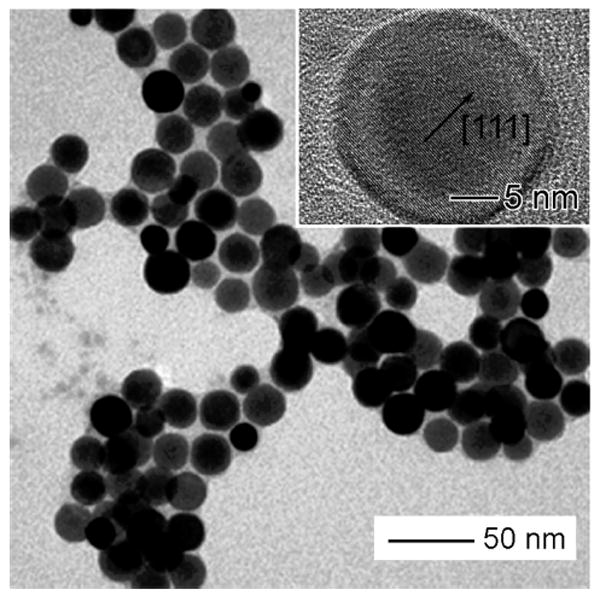
TEM image of single-crystal Ag nanospheres produced using the polyol synthesis in the presence of oxygen and chloride. The average diameter of these Ag nanospheres was 23.5 nm and a polydispersity of <10%. Inset: high-resolution TEM image of a single Ag nanosphere.
When these Ag nanospheres are mixed with aqueous HAuCl4, galvanic replacement reaction occurs immediately. Figure 2 summarizes all of the major structural changes involved in the galvanic replacement reaction after different volumes of HAuCl4 have been added. The most striking feature shown in these electron micrographs is the structural reconstruction of the silver nanosphere in the course of the galvanic replacement reaction. In contrast to other silver nanostructures (e.g., nanocubes) that often resulted in hollow nanostructures with morphology more or less similar to that of the silver template, the single-crystal Ag nanosphere is transformed into hollow and porous nanostructures with enlarged {111} facets during the replacement reaction. More specifically, the faceted hollow nanostructures derived from the single-crystal Ag nanosphere have an octahedral shape, as shown in the illustrations. Other groups have also observed the formation of faceted hollow nanostructures when single-crystal Ag nanospheres are utilized as a template for the galvanic replacement reaction. For instance, Yin and Alivisatos found that single-crystal hollow octahedra could be obtained through the galvanic replacement reaction between 11.0 nm single-crystal Ag nanospheres and AuCl3 in an organic medium (o-dichlorobenzene) in the presence of oleylamine as a surfactant.24 In the present work, we use 23.5 nm single-crystal Ag nanospheres dispersed in an aqueous solution and some PVP is added as a capping agent before the replacement reaction. The results of these studies clearly indicate that the structural evolution is insensitive to parameters such as the size of the Ag nanospheres, the capping agent, and the dispersion medium. It seems to be mainly caused and determined by the rounded curvature on the surface of the nanosphere.
Figure 2.
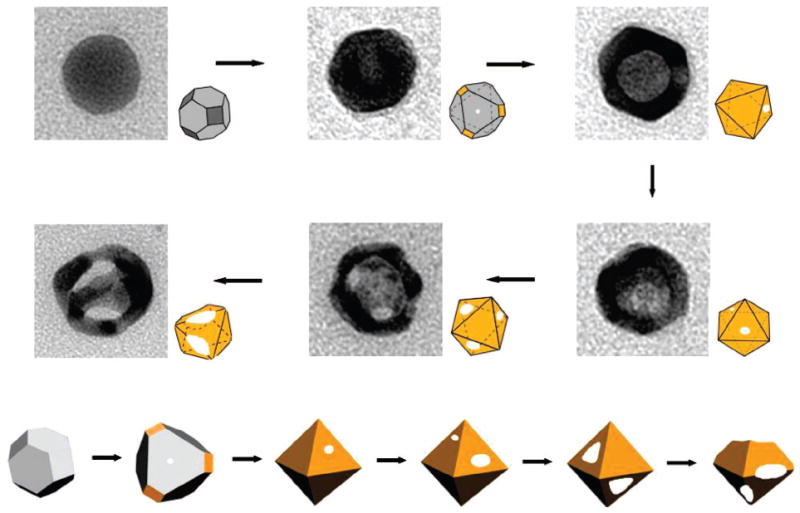
TEM images detailing all major morphological and structural changes involved in the galvanic replacement reaction between Ag nanosphere (23.5 nm in diameter) and an aqueous HAuCl4 solution.
The mechanism of structural transformation in the replacement reaction can be explained as the following. The structure of a nanocrystal highly depends on the ratio between the areas of crystallographic facets that enclose the nanocrystal. The single-crystal Ag nanosphere is actually a cuboctahedron enclosed by a mix of {111} and {100} facets. As demonstrated in our previous work, PVP tends to preferentially cover the {100} rather than {111} facets.26,27 Any of {111} regions unprotected by PVP will serve as primary site for the dissolution of Ag and eventually become pores when aqueous HAuCl4 solution is added. The Ag atoms in the interior will diffuse to the reaction sites and react with HAuCl4 to generate Au atoms, which will be deposited preferentially onto {100} facets.28,29 The cuboctahedron evolves into an octahedron because of a relatively higher rate of atomic addition to the {100} facets. This mechanism is consistent with the structural evolution shown in Figure 2.
Figure 3 shows the detailed morphological evolution of single-crystal Ag spheres before and after adding different volumes of the aqueous HAuCl4 solution. The morphological evolution can be divided into two steps: formation of hollow octahedra and growth of multiple pores in surface of these structures. After adding 0.5 mL of the 0.03 mM HAuCl4 solution, most of the particles exhibit a small pinhole, and the nanospheres start to evolve into facetted particles. After 0.75 mL of the HAuCl4 aqueous solution has been added, the pinholes are enlarged, and the spherical particles completely evolve into octahedra with sharp edges and corners. After the addition of more than 1.25 mL of the HAuCl4 solution, multiple holes are observed along with further enlargement of the pinholes. Up till this point, the average size of the particles has increased to 28.6 ± 2.9 nm. After addition of 3.0 mL of the HAuCl4 solution, the nanoparticles become highly porous, and some of them have a ring-like structure. At this point, the multiple openings on the surface would coalesce via Ostwald ripening to compensate the increase in total surface energy. EDX measurements on the highly porous structures indicate that they contained approximately 65% Au and 35% Ag.
Figure 3.
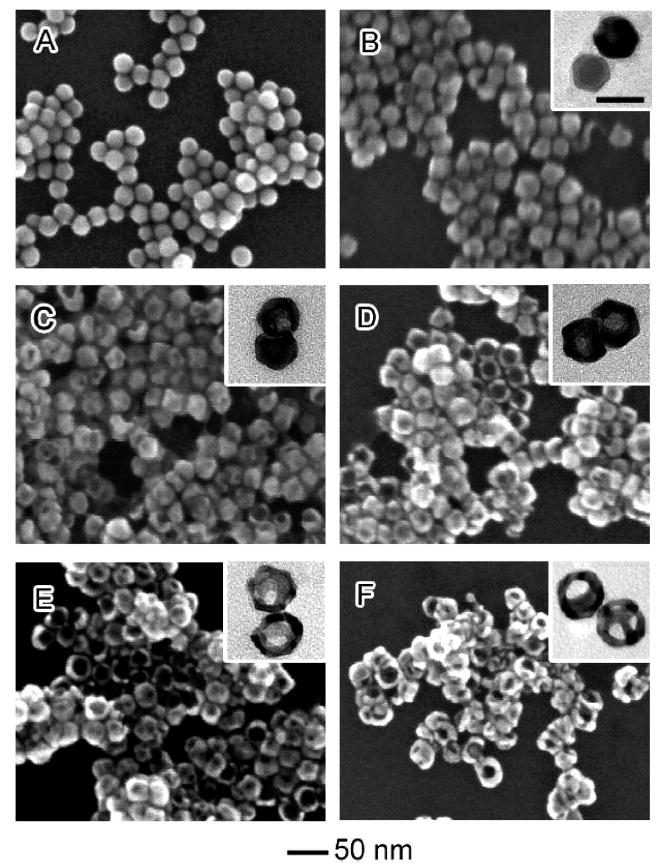
SEM images showing six different stages of the galvanic replacement reaction that involves the use of Ag nanospheres as a sacrificial template. (A–F) Ag nanospheres titrated with 0, 0.5, 0.75, 1.25, 1.75, and 3.0 mL of 0.03 mM aqueous HAuCl4, respectively. The insets show TEM images of each respective sample. Scale bar = 30 nm for all TEM images.
It is worth noting that the process of the replacement reaction shows a unique characteristic in the case of 23.5 nm single-crystal Ag spheres. For the galvanic replacement reactions involving Ag nanocubes, nanowires, and MTPs lager than 50 nm, our results indicate that the replacement proceeds through three major steps: (i) initiation of galvanic replacement by pitting at a specific site on the surface of a Ag template; (ii) formation of a pinhole-free nanobox consisting of thin, uniform walls (made of a Au–Ag alloy); and (iii) generation of pores in the wall through a dealloying process, in which Ag atoms are selectively oxidized.17 For the present system, we did not observe the formation of hollow structures with complete shells (i.e., nanoboxes). Instead, pinholes formed at the very early stage of the reaction and continuously grew into larger sizes. In addition, multiple pores could be generated in the walls, leading to the formation of nanocages at the very beginning of the reaction. This difference can be attributed to the presence of a mix of {100} and {111} facets on the surface of a nanosphere. These two facets react with HAuCl4 at different rates as the {100} facets are more heavily covered by PVP than the {111} facets.15 For the same reason, Au deposition does not occur uniformly across the entire surface of the silver template, leading to the formation of a porous Au shell from the very beginning.
In the present system, the pore size and the density of pores in the Au–Ag nanostructures could be adjusted by varying the molar ratio of HAuCl4 to Ag. As such, it is expected that the optical properties (e.g., LSPR features) of the resultant nanostructures could be readily tuned. Figure 4 shows how the LSPR spectra changed as the single-crystal Ag nanospheres were titrated with the 0.03 mM HAuCl4 aqueous solution. The 23.5 nm single-crystal Ag spheres showed a sharp SPR peak at 408 nm, similar to the value reported for single-crystal Ag nanoparticles in this size range.25 As more HAuCl4 was added, the extinction peak was continuously shifted from 408 to 791 nm. Specifically, the peak was red-shifted to 433, 474, 557, 689, and 791 nm in correlation to a volume of 0.5, 0.75, 1.25, 1.75, and 3.0 mL for the HAuCl4 solution. Note that the extinction spectrum for the sample formed with 1.25 mL of the HAuCl4 solution exhibit a broad peak, which can be attributed to the nonuniform void size and wall thickness of the nanocages as small holes may also start to develop in the walls through a dealloying process at this stage. Figure 5 shows a photograph of 11 aqueous dispersions of such nanostructures, indicating that the color could be tuned from yellow to red and light blue. Similar to other types of Ag templates, the tuning in optical properties was mainly due to a structural transformation from solid Ag nanospheres into Au–Ag alloy nanocages with pores of different sizes on their surfaces, as well as different densities of pores.
Figure 4.
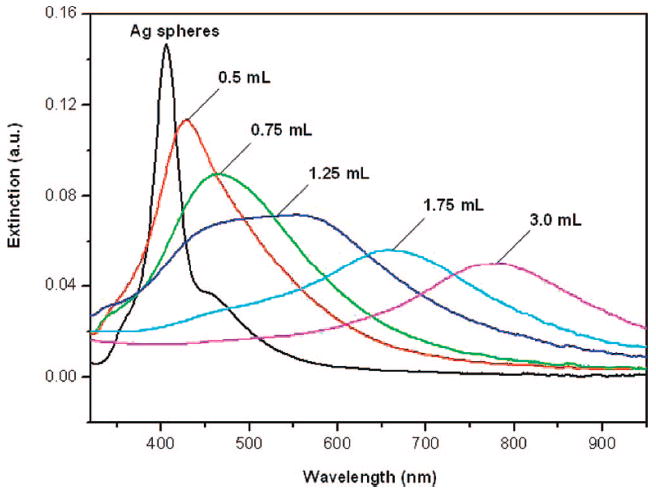
UV–vis–NIR extinction spectra of Ag nanospheres before and after they have been titrated with different volumes of 0.03 mM HAuCl4 aqueous solution.
Figure 5.

Photographs of aqueous dispersion of Ag nanospheres before and after they have been titrated with different volumes of 0.03 mM HAuCl4 solution (from left to right): 0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.5, and 3.0 mL. The major absorption peak was positioned at 408, 425, 433, 474, 493, 557, 672, 689, 712, 756, and 791 nm, respectively.
Conclusions
In summary, we have investigated the structural and morphological changes involved in the galvanic replacement reaction between 23.5 nm single-crystal Ag nanospheres and HAuCl4 in an aqueous medium. The details of this reaction are quite different from previous observations on Ag nanocubes, nanowires, and MTPs. The replacement reaction on single-crystal Ag spheres generates hollow, porous Au–Ag nanostructures with an octahedral shape at the very beginning of the reaction. In particular, the Ag nanospheres directly evolve into Au–Ag nanocages without going through the stage where a complete shell is formed. By varying the molar ratio of HAuCl4 to Ag, we are able to adjust the pore size and the density of pores. In the same way, the LSPR peaks of these nanostructures can be readily tuned to the near-infrared region, the so-called transparent window for blood and soft tissue. We believe that these hollow and porous metal nanostructures hold a great potential for biomedical applications as both contrast enhancement and therapeutic agents.
Acknowledgments
This work was supported in part by a Director’s Pioneer Award from the NIH (1DPOD000798) and a research grant from the NSF (DMR-0451788). M.H.K. was also partially supported by the Korea Research Foundation Grant funded through the Korean Government (KRF-2006-352-D00062).
Footnotes
Part of the “Larry Dalton Festschrift”.
References and Notes
- 1.Teng XW, Black D, Watkins NJ, Gas YL, Yang H. Nano Lett. 2003;3:261. [Google Scholar]
- 2.Sanders AW, Routenberg DA, Wiley B, Xia Y, Dufresne ER, Reed MA. Nano Lett. 2006;6:1822. doi: 10.1021/nl052471v. [DOI] [PubMed] [Google Scholar]
- 3.Tao A, Sinsermsuksakul P, Yang P. Angew Chem Int Ed. 2006;45:4597. doi: 10.1002/anie.200601277. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Yang Y. J Am Chem Soc. 2002;124:5290. [Google Scholar]
- 5.Krebig U, Vollmer M. Optical Properties of metal Clusters. Spriger; New York: 1995. [Google Scholar]
- 6.El-Sayed MA. Acc Chem Res. 2001;34:257. doi: 10.1021/ar960016n. [DOI] [PubMed] [Google Scholar]
- 7.Jackson JB, Halas NJ. J Phys Chem B. 2001;105:2473. [Google Scholar]
- 8.Westcott SL, Oldburg SJ, Lee TR, Halas FJ. Chem Phys Lett. 1999;300:651. [Google Scholar]
- 9.Selvakannan PR, Sastry M. Chem Commun. 2005:1684. doi: 10.1039/b418566h. [DOI] [PubMed] [Google Scholar]
- 10.Schwartzber AM, Oshiro TY, Zhang JZ, Huser T, Talley CE. Anal Chem. 2006;78:4732. doi: 10.1021/ac060220g. [DOI] [PubMed] [Google Scholar]
- 11.Loo C, Lin A, Hirsch L, Lee MH, Barton J, Halas N, West J, Drezek R. Technol Cancer Res Treat. 2004;3:22. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 12.Shukla S, Priscilla A, Banerjee M, Bhonde RR, Ghatak J, Satyam PV, Sastry M. Chem Mater. 2005;17:5000. [Google Scholar]
- 13.Chen J, Saeki F, Wiley B, Cang H, Cobb MJ, Li ZY, Au L, Zhang H, Kimmey MBX, Li D, Xia Y. Nano Lett. 2005;5:473. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Wiley B, Li Z-Y, Campbell D, Saeki F, Cang H, Au L, Lee J, Li XD, Xia Y. Adv Mater. 2005;17:2255. [Google Scholar]
- 15.Chen J, McLellan JM, Siekkinen A, Xiong Y, Li ZY, Xia Y. J Am Chem Soc. 2006;128:14776. doi: 10.1021/ja066023g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Mayers B, Xia Y. Adv Mater. 2003;1:5–641. [Google Scholar]
- 17.Sun Y, Xia Y. J Am Chem Soc. 2004;12:6–3892. doi: 10.1021/ja039734c. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Wiley B, Li Z-Y, Xia Y. J Am Chem Soc. 2004;12:6–9399. doi: 10.1021/ja048789r. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Xia Y. Adv Mater. 2004;15:264. [Google Scholar]
- 20.Sun Y, Mayers B, Xia Y. Nano Lett. 2002;2:481. [Google Scholar]
- 21.Chen J, Wiley B, McLellan J, Xiong Y, Li Z-Y, Xia Y. Nano Lett. 2005;5:2058. doi: 10.1021/nl051652u. [DOI] [PubMed] [Google Scholar]
- 22.Lu X, Tuan H-Y, Chen J, Li Z-Y, Korgel BA, Xia Y. J Am Chem Soc. 2007;129:1733. doi: 10.1021/ja067800f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Lee JY, Too HP. J Phys Chem B. 2005;109:19208. doi: 10.1021/jp052242x. [DOI] [PubMed] [Google Scholar]
- 24.Yin Y, Erdonmez C, Aloni S, Alivisatos AP. J Am Chem Soc. 2006;128:12671. doi: 10.1021/ja0646038. [DOI] [PubMed] [Google Scholar]
- 25.Wiley B, Herricks T, Sun Y, Xia Y. Nano Lett. 2004;4:1733. [Google Scholar]
- 26.Sun Y, Mayers B, Herricks T, Xia Y. Nano Lett. 2003;3:955. [Google Scholar]
- 27.Xiong Y, Chen J, Wiley B, Xia Y, Yin Y, Li Z-Y. Nano Lett. 2005;5:1237. doi: 10.1021/nl0508826. [DOI] [PubMed] [Google Scholar]
- 28.Wang ZL. J Phys Chem B. 2000;104:1153. [Google Scholar]
- 29.Zhang JM, Ma F, Xu W. Appl Surf Sci. 2004;229:34. [Google Scholar]


