Abstract
Tyrosine nitration results in altered function of selective proteins, including human smooth muscle L-type calcium channel, hCav1.2b. We report here that Cav1.2 is also subject to “denitration” . Cell lysates from activated macrophage-like cell line, RAW264.7 cells, reversed peroxynitrite-induced nitration of the carboxy terminus of Cav1.2 in a 1D gel assay. Tyrosine phosphorylation of the calcium channel by c-src kinase was blocked by nitration but reversed by pretreatment with RAW264.7 cell lysates. These findings indicate that denitration may be a physiological mechanism to restore cellular excitability during inflammation.
Keywords: nitric oxide, tyrosine, nitration, ion channel, src kinase, smooth muscle
1. Introduction
Reactive nitrogen species e.g. nitrogen monoxide, (NO·) (that can undergo interconversion to form nitrosonium (NO+), and nitroxyl anion (NO−)), peroxynitrite (ONOO−), nitrogen dioxide (NO2) and nitrylchloride (NO2Cl) act as nitrating agents that affect protein function [1,2]. Nitration of tyrosine residues, particularly by peroxynitrite, is known to occur in many disease states leading to the formation of nitrotyrosine [3]. The consequence of nitration is altered function of the target protein resulting from changes in conformation and structure of the protein, modulation of the catalytic activity of enzymes, susceptibility to proteolysis and/or affecting signal transduction by hindering tyrosine phosphorylation [4–6].
Ion channels represent a significant target for modulation by reactive nitrogen species [7]. These may be affected either directly or indirectly leading to altered cellular excitability. We have recently established the potential role of tyrosine nitration within the C-terminus of the smooth muscle L-type calcium channel [8,9]. Our studies have shown that colonic inflammation results in the nitration of Y1837 and Y2134 within the C-terminus of the human smooth muscle calcium channel, Cav1.2b, and prevents phosphorylation by the tyrosine kinase, c-src kinase. Impaired ability of c-src kinase to phosphorylate the calcium channel results in decreased calcium currents and muscle contraction during colonic inflammation [8]. Analogous to protein phosphorylation and dephosphorylation, the process of denitration has been suggested although the specific enzyme(s) in this process have not been identified [10–13]. Denitration may be of importance in the regulatory processes within cells allowing for “repair” of the affected protein without invoking de novo synthesis. Denitrase activity is preferentially observed in some tissues, e.g. spleen, lung and activated macrophages and demonstrates substrate specificity [10]. Proteins thought to be specific substrates for denitrase include the histone H1.2, albumin and calmodulin. Irie et al [11] reported the presence of denitrase activity in the mouse cell line, RAW264.7 which has similarities with macrophages. In the present study, we examined the denitrase activity from these cells towards the nitration of tyrosine residues in the carboxy-terminus of the human smooth muscle L-type calcium channels.
2. Materials and Methods
2.1 Materials
The anti-CT antibody was generated from the carboxy-terminus of human Cav1.2b (amino acids 1809–2138) by ProSci Inc (Poway, CA). All other antibodies used in this study were purchased as noted. The secondary antibodies (anti-mouse IRDye 680 and anti-rabbit IRDye 800CW) were obtained from LiCor Biosciences (Lincoln, NE). Glutathione Separose 4B bead was obtained from Amersham Biosciences (Piscataway, NJ) and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Murine macrophage-like RAW264.7 cells were obtained from Sigma-Aldrich.
2.2. Construction of GST fusion protein
Glutathione-S-transferase (GST) fusion proteins were constructed for the C-terminus of the human Cav1.2b (amino acids 1809–2138), denoted as GST-CT. The sense primer was designed to contain a BamHI site in the 5’ end and the antisense primer was designed to contain an EcoRI site at the 5’ end. The resultant PCR product was subcloned in frame with the GST open frame of the bacterial expression plasmid pGEX-6P-3 (Amersham Biosciences) between BamHI and EcoRI sites. In frame cloning was confirmed by cDNA sequencing of the resultant plasmid. GST-CT fusion protein was generated as previously described [9].
2.3. Nitration of GST fusion proteins and immunoblots
GST fusion proteins were incubated in 500 µM 3-morpholinosydnonimine (SIN-1) (TOCRIS Bioscience, Ellisville, MO) for 1 hr followed by peroxynitrite (150 µM, Cayman Chemical, Ann Arbor, MI) three times at 4 min intervals at 30°C. 50 µL glutathione separose beads were added and incubated for 30 min. The pellet was centrifuged at 500 × g for 5 min and washed three times with PBS buffer. SDS loading buffer was added and then subjected to SDS-PAGE. Confirmation of nitrated GST-CT was determined by anti-c-terminus (anti-CT) and anti-GST (Millipore, Billerica, MA) antibodies. Nitrated GST-CT proteins were subjected to SDS-PAGE and were transferred onto nitrocellulose membrane. After blocking with 5% (w/v) non-fat milk blocking buffer, corresponding membrane strips were cut and incubated with appropriate amount cell lysates (5 mg/mL) for 2 hours at 30°C. Membrane strips were then washed with PBS and peroxynitrite-treated GST-CT was probed for nitrotyrosine by Western blot using anti-nitrotyrosine (anti-NY, Calbiochem, La Jolla, CA) antibody. The immunoreactive bands were quantitated using an Odyssey imaging system (LiCor Biosciences). All results represent the average intensity ± standard error of the means (SE) from at least three separate experiments.
2.4. Nitration of calcium channel proteins
Human embryonic kidney (HEK) 293 cells were maintained and grown to ~80% confluence in DMEM (Invitrogen, Carlsbad, CA) medium supplemented with 10% fetal bovine serum (Invitrogen), 100units/mL penicillin/streptomycin (Invitrogen) in a humidified atmosphere of 5% CO2 and 95% O2 at 37°C. HEK293 cells were co-transfected with cDNA of human jejunal voltage-gated calcium channel Cav1.2b and β2, treated with the nitrating agent 3-morpholinosydnonimine (500 µM) and sodium peroxynitrite (150 µM) three times at 4-min intervals. The transfected cells were then treated with the cell lysates (200 µg/mL) from LPS-activated RAW264.7 cells at 37°C for 1 hour. After washing with phosphate buffer saline (PBS), cells were harvested and solubilized in RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA) supplemented with protease inhibitors (Roche Diagnostics, Indianapolis, IN) containing 0.2 mM phenylmethylsulfonyl fluoride, 10 µg/ml Calpain I, 10 µg/mL Calpain II, and 0.1 mM sodium orthovanadate. After incubation for 30 min on ice, cell debris was pelleted by centrifugation (10,000 × g, 10 min, 4°C). The supernatant was aliquoted and stored at −80°C. Protein concentration was determined by the BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL) prior to use in the experiments and standard protocols for immunoblot were performed using anti-Cav1.2 (Calbiochem) and anti-nitrotryosine antibodies.
For measuring the effect of denitration on smooth muscle calcium channel, colon were excised from mice and treated with SIN-1 and ONOO−. Calcium channel protein samples were prepared as previously described [8] and examined for nitrotyrosine as above. Denitration of colonic smooth muscle calcium channel by cell lysates from RAW264.7 cells were determined in similar fashion to the transfected HEK cells.
2.5. Preparation of cell lysate from LPS-activated RAW264.7 cells
Murine macrophage-like RAW264.7 cells were maintained and grown to ~80% confluence in DMEM medium supplemented with 10% fetal bovine serum, 100units/mL penicillin/streptomycin in a humidified atmosphere of 5% CO2 and 95% O2 at 37°C. For the source of denitrase, cells were treated with 50 ng/mL of bacterial antigen lipopolysaccharide, LPS (Sigma-Aldrich) for 24 hours, and then were harvested and resuspended in lysis buffer (20 mM Tris, pH 8.0, 1% Nonidet P-40, 0.15 M NaCl, 1 mM Na2PO4, and 1 mM EGTA). The supernatant was subjected to denitration assay.
3. Results
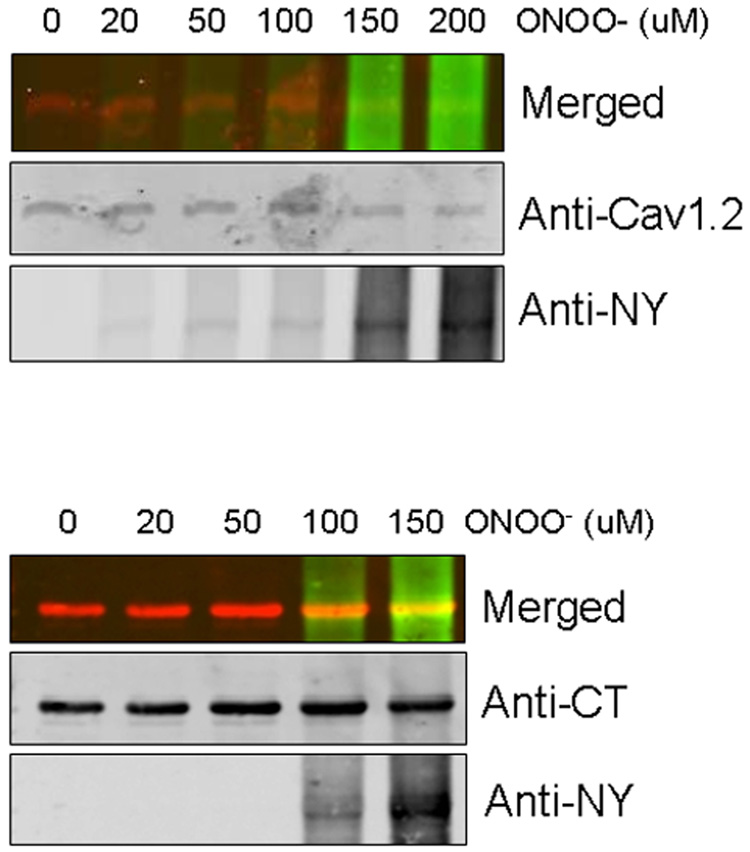
The concentration of peroxynitrite that produced consistent nitration of the calcium channel was determined by treating Cav1.2 transfected HEK293 cells with a combination of SIN-1 (500 µM) and varying peroxynitrite concentrations. Nitrotyrosine formation was confirmed by western blots using the anti-nitrotyrosine antibody. Figure 1 (top panel) shows that 150 µM peroxynitrite induced nitration of the full-length calcium channel protein. We have previously shown that nitration of tyrosine residues occurs within the C-terminus of the calcium channel. A GST-fusion protein of the C-terminus of Cav1.2 was incubated with varying concentrations of peroxynitrite, run on SDS-PAGE gel and immunoblotted with anti-CT (rabbit) and anti-nitrotyrosine (mouse) antibodies. The merged image shows that nitration of the CT fragment was evident at 100 – 150 µM. In the following experiments, 150 µM peroxynitrite concentration was chosen as the final concentration to induce nitration of tyrosine residues.
Figure 1.
Nitrotyrosine formation of the calcium channel by peroxinitrite. Nitration of Cav1.2b transfected HEK293 cells and GST-CT proteins were generated by ONOO− as described in “Materials and Methods”. Western blots demonstrating the effect of increasing ONOO− concentration on a) Cav1.2b (top panel) calcium channel and b) CT fragments (bottom right). Samples were co-immunoblotted with anti-nitrotyrosine (anti-NY) and calcium channel antibodies (anti-Cav1.2 or anti-CT). IR-conjugated secondary antibodies were used : anti-NY (green) and anti-calcium channel (red). Merged images in top panels show nitration of the calcium channel.
In order to determine whether nitration of the C-terminus of the calcium channel was subject to denitration by activated macrophages, 1D membrane assay of GST-CT was performed. GST-CT was incubated with peroxynitrite, separated by electrophoresis, transferred to PVDF membranes and treated with cell lysates from activated RAW264.7 cells. Immunoblots were performed with anti-CT and anti-nitrotyrosine antibodies. Treatment with macrophage cell lysates resulted in marked down-regulation of nitrotyrosine (Figure 2). Heat inactivation of the cell lysates or incubation with lysis buffer alone prevented denitration. We further examined the specificity of the denitrase activity in macrophages by determining the effect of cell lysates prepared from CHO cells. Figure 3 shows that unlike RAW264.7 cells, the lysates from CHO cells did not result in denitration of GST-CT.
Figure 2.
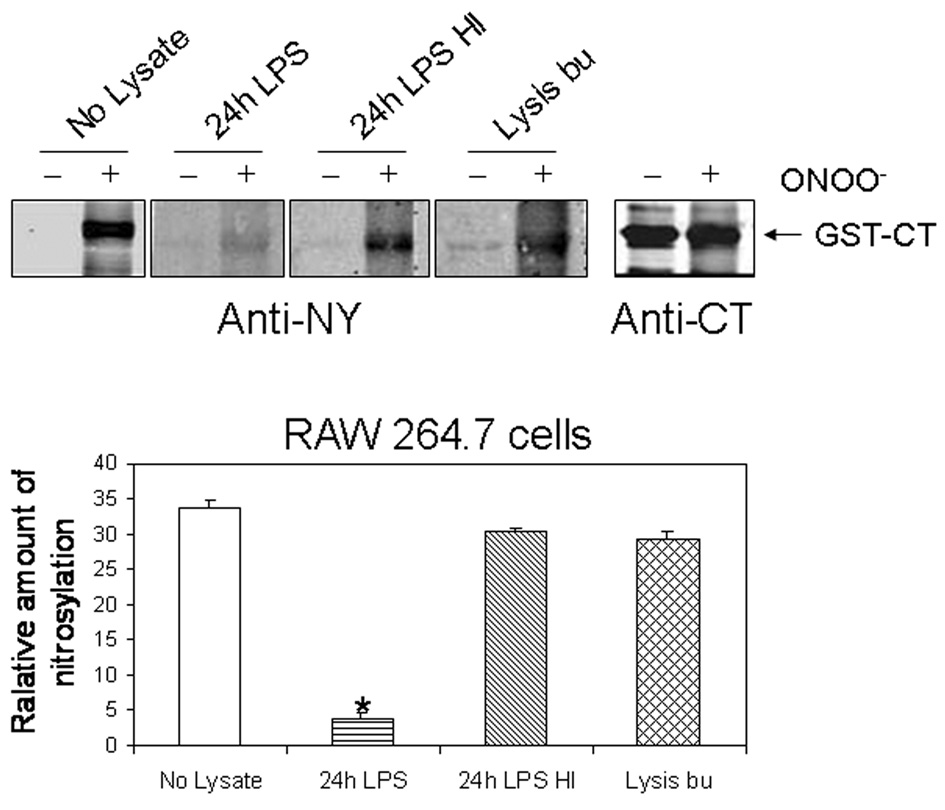
The effect of cell lysates from LPS-activated macrophage RAW264.7 on nitrated GST-CT (150 ug). Nitrotyrosine immunoreactivity was detected with anti-NY antibody. The intensity of nitrated GST-CT was reduced by 88% following treatment with cell lysates from 24h activated RAW264.7 cells but not when incubated with heat inactivated cell lysates or lysis buffer only. The presence of GST-CT proteins was confirmed by anti-CT antibody. Relative amounts of nitration were calculated as means ± SE of three independent experiments. * p < 0.005 vs. No lysate
Figure 3.
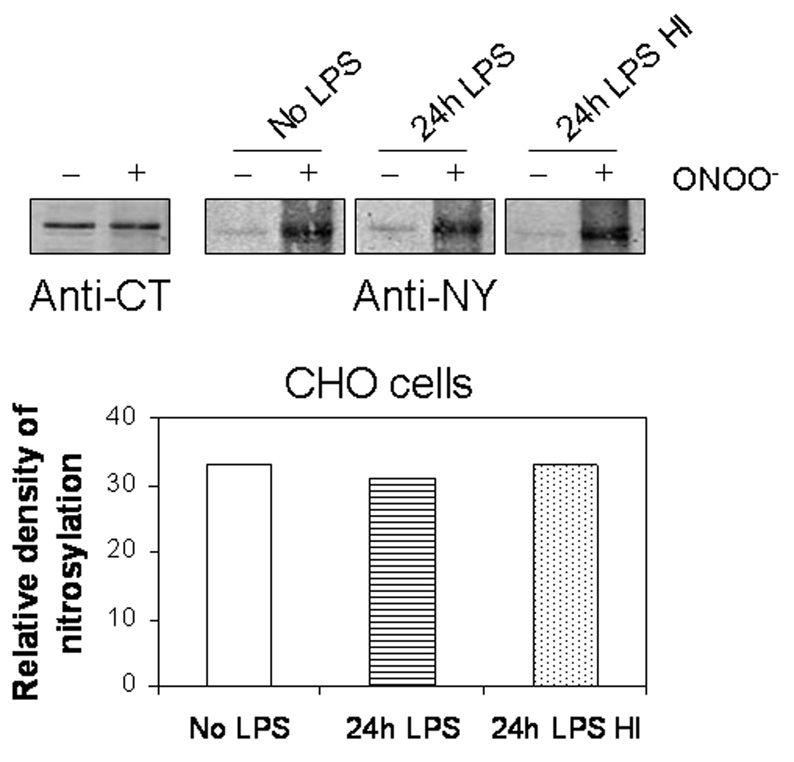
Lack of denitrase activity in cell lysates of CHO cells. Treatment of nitrated CT with cell lysates from CHO cells did not reduce tyrosine nitration. Nitration of GST-CT proteins was analyzed by Western blot after peroxynitrite treated proteins were incubated with cell lysates from LPS-treated CHO cells. Similar results were obtained from 3 separate experiments.
We have previously demonstrated that nitration of tyrosine residues prevents src-kinase mediated tyrosine phosphorylation resulting in reduced calcium currents [9]. To determine whether denitration restores the ability for src-kinase to induce phosphorylation, GST-CT was incubated with peroxynitrite, run on a SDS-PAGE gel, transferred to PVDF membrane followed by treatment with RAW264.7 cell lysates. The membrane strips were then incubated with src kinase (in kinase buffer) to induce tyrosine phosphorylation and immunoblotted with anti-phosphotyrosine antibody (anti-PY20, Santa Cruz Biosciences) (Figure 4). GST-CT could be phosphorylated after treatment with cell lysates from RAW cells but not in its absence.
Figure 4.
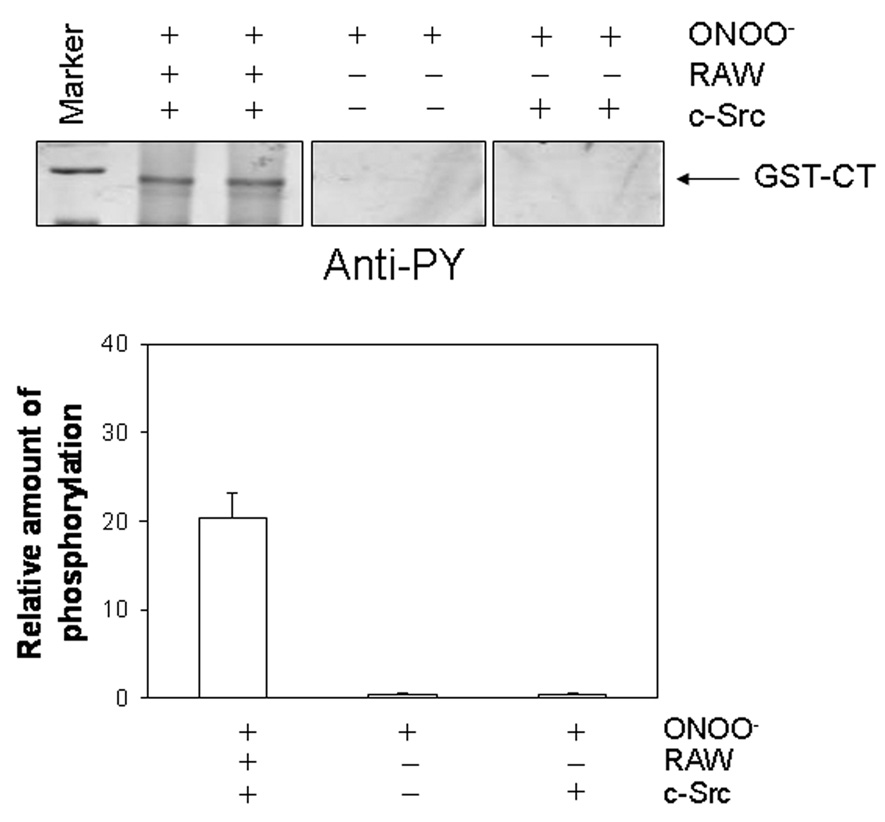
Denitration restores src kinase induced tyrosine phosphorylation. GST-CT proteins were nitrated by ONOO− and either treated with cell lysates from LPS-activated RAW264.7 cells or not prior to phosphorylation by c-src kinase (9 units/mL) incubation. Immunoblots with anti-phosphotyrosine (anti-PY) antibody showed phosphorylation of nitrated GST-CT after treatment of RAW264.7 cell lysates whereas nitration of GST-CT prevented phosphorylation by c-src kinase (right panel). Bottom graph shows relative amount of signal intensity for phosphorylation (n=3)
Denitrase activity of macrophage cells was also examined against nitration of the full-length Cav1.2b transfected in HEK293 cells (Figure 5A) and native colonic smooth muscle calcium channels (Figure 5B). The transfected cells or the smooth muscle tissues were treated with peroxynitrite (150 uM), lysed and 1D membrane assay performed. The PVDF membrane strips were blotted with anti-Cav1.2 and anti-nitrotyrosine antibodies. Figure 5 shows that treatment with RAW cells also induces denitration of the full-length heterologously expressed and native smooth muscle calcium channels.
Figure 5.
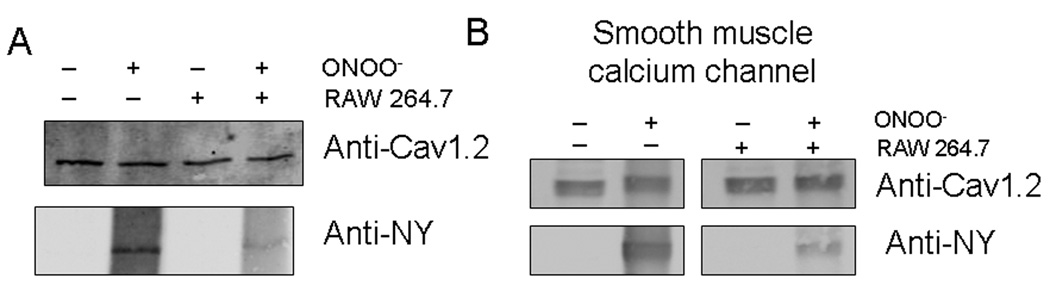
Denitration of heterologously expressed Cav1.2b (A) and colonic smooth muscle calcium channel (B). Treatment of HEK293 transfected cells and mouse colonic smooth muscle with ONOO− resulted in nitration of calcium channels. Nitrotyrosine was significantly reduced by treatment with cell lysates from RAW264.7 cells. The presence of calcium channels were confirmed with anti-Cav1.2 antibodies. Figure is representative of 3 separate experiments.
4. Discussion
Recent studies have suggested that nitration of tyrosine residues may be a reversible process [10–12,14]. Nitration of the tyrosine residues involves a covalent modification, resulting in the addition of a nitro group onto the ortho carbon of the tyrosine residue [4] and alters the functional properties of the target protein. Nitrotyrosine formation has been established in several pathophysiological states. Denitration is a possible mechanism by which proteins may regain their function without the need for resynthesis. In the present study we used a similar 1D assay as that reported by Irie et al. [11] to determine whether the c-terminus of the calcium channel was subject to denitrase activity of activated RAW 264.7 cells. We have previously shown that the α subunit of the human smooth muscle calcium channel is nitrated at tyrosine residues within the carboxy terminus by peroxynitrite [8,9]. This impairs tyrosine phosphorylation by src kinase [15] resulting in the reduced calcium currents. The physiological implication of the decreased calcium currents is the attenuation of muscle contraction during inflammation [16,17]. In an experimental model of colitis, we reported decreased contractions of the colonic smooth muscle due to altered regulation of calcium channel by src kinase following nitration [8].
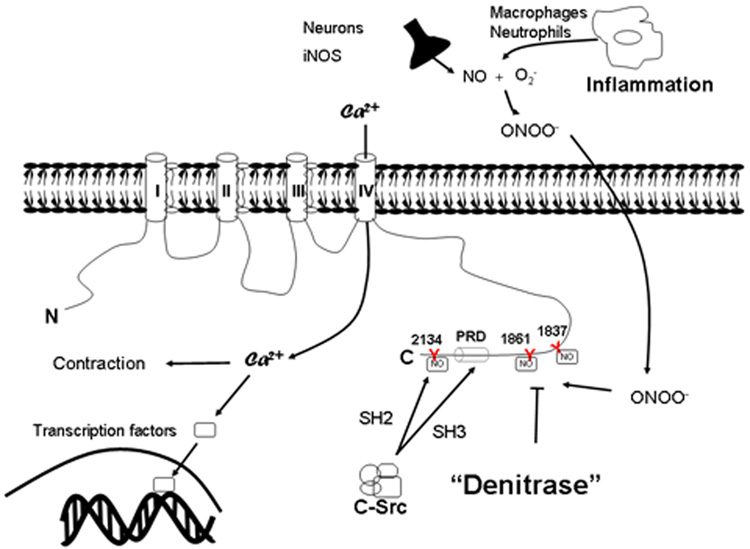
The denitrase activity of RAW264.7 cells, that was initially identified by Murad and colleagues, was shown to be selective towards histone H1.2 [11] and calmodulin [13]. Our findings suggest that the carboxy terminus of the L-type calcium channel may also be a potential substrate for the denitrase. In the GST fusion protein consisting of the terminal 229 amino acids of the calcium channel, tyrosine residues at positions 1837 and 2134 are potential phosphorylation sites with Y2134 nitration preventing anchoring of the SH2 domain of c-src with the calcium channel [9] and thus affecting c-src regulation of the calcium channel (Figure 6). Pretreatment with the cell lysates from RAW264.7 cells, however, was sufficient to allow for recovery of tyrosine phosphorylation by c-src kinase (Figure 4). In addition to the heterologously expressed channel, native smooth muscle calcium channel is also a substrate for regulation by denitrase activity. It is noteworthy that in many tissues, the levels of nitrotyrosine formation are low and that one in five 3-nitrotyrosine residues per 10,000 may be detected [4]. This is likely due to the requirement of consensus sequences in the target protein. In colonic smooth muscle tissues, pretreatment with peroxynitrite reduces calcium-influx mediated contractions but not that due to intracellular calcium release suggesting that tyrosine nitration did not affect contractile proteins at least to the same extent as the smooth muscle calcium channel [8]. Inflammation induced increases in reactive nitrogen species occurs as a result of increased neutrophils and macrophages. In this study, we examined the denitrase activity from macrophage-like cells on colonic smooth muscle. Further studies to establish whether smooth muscle cells can also synthesize “denitrase(s)” to regulate endogenous calcium channel function during inflammation will be necessary. Nevertheless, our studies establish a potential role for nitration/denitration of the calcium channel as a “switch” to regulate calcium influx during inflammation.
Figure 6.
Schematic presentation of putative sites for nitration of tyrosine residues with the carboxy-terminus of Cav1.2. Peroxynitrite formation during colonic inflammation induces nitration of tyrosine residues in the terminal segment of Cav1.2, preventing tyrosine phosphorylation and binding of SH2 and SH3 domains of c-src kinase. Regulation by c-src kinase is importantly involved in calcium –influx mediated contraction and gene transcription.
ACKNOWLEDGEMENTS
This study was supported by NIH DK46367. We thank Dr Gianrico Farrugia for providing human jejunal hCav1.2b construct.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- 1.Bian K, Murad F. Nitric oxide (NO)--biogeneration, regulation, and relevance to human diseases. Front Biosci. 2003;8:d264–d278. doi: 10.2741/997. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 4.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 6.Kong SK, Yim MB, Stadtman ER, Chock PB. Peroxynitrite disables the tyrosine phosphorylation regulatory mechanism: Lymphocyte-specific tyrosine kinase fails to phosphorylate nitrated cdc2(6–20)NH2 peptide. Proc Natl Acad Sci U S A. 1996;93:3377–3382. doi: 10.1073/pnas.93.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matalon S, et al. Regulation of ion channel structure and function by reactive oxygen-nitrogen species. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1184–L1189. doi: 10.1152/ajplung.00281.2003. [DOI] [PubMed] [Google Scholar]
- 8.Ross GR, Kang M, Shirwany N, Malykhina AP, Drozd M, Akbarali HI. Nitrotyrosylation of Ca2+ Channels Prevents c-Src Kinase Regulation of Colonic Smooth Muscle Contractility in Experimental Colitis. J Pharmacol Exp Ther. 2007;322:948–956. doi: 10.1124/jpet.107.123075. [DOI] [PubMed] [Google Scholar]
- 9.Kang M, Ross GR, Akbarali HI. COOH-terminal association of human smooth muscle calcium channel Ca(v)1.2b with Src kinase protein binding domains: effect of nitrotyrosylation. Am J Physiol Cell Physiol. 2007;293:C1983–C1990. doi: 10.1152/ajpcell.00308.2007. [DOI] [PubMed] [Google Scholar]
- 10.Kamisaki Y, et al. An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc Natl Acad Sci U S A. 1998;95:11584–11589. doi: 10.1073/pnas.95.20.11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irie Y, Saeki M, Kamisaki Y, Martin E, Murad F. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in proteins. Proc Natl Acad Sci U S A. 2003;100:5634–5639. doi: 10.1073/pnas.1131756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorg B, Qvartskhava N, Voss P, Grune T, Haussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 13.Smallwood HS, Lourette NM, Boschek CB, Bigelow DJ, Smith RD, Pasa-Tolic L, Squier TC. Identification of a denitrase activity against calmodulin in activated macrophages using high-field liquid chromatography--FTICR mass spectrometry. Biochemistry. 2007;46:10498–10505. doi: 10.1021/bi7009713. [DOI] [PubMed] [Google Scholar]
- 14.Kuo WN, Kanadia RN, Shanbhag VP, Toro R. Denitration of peroxynitrite-treated proteins by 'protein nitratases' from rat brain and heart. Mol Cell Biochem. 1999;201:11–16. doi: 10.1023/a:1007024126947. [DOI] [PubMed] [Google Scholar]
- 15.Hu XQ, Singh N, Mukhopadhyay D, Akbarali HI. Modulation of voltage-dependent Ca2+ channels in rabbit colonic smooth muscle cells by c-Src and focal adhesion kinase. J Biol Chem. 1998;273:5337–5342. doi: 10.1074/jbc.273.9.5337. [DOI] [PubMed] [Google Scholar]
- 16.Reddy SN, Bazzocchi G, Chan S, Akashi K, Villanueva-Meyer J, Yanni G, Mena I, Snape WJ., Jr. Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289–1297. doi: 10.1016/0016-5085(91)90079-z. [DOI] [PubMed] [Google Scholar]
- 17.Annese V, Bassotti G, Napolitano G, Usai P, Andriulli A, Vantrappen G. Gastrointestinal motility disorders in patients with inactive Crohn's disease. Scand J Gastroenterol. 1997;32:1107–1117. doi: 10.3109/00365529709002989. [DOI] [PubMed] [Google Scholar]