Abstract
The roles of flavin-containing monooxygenases (FMOs) in the oxidation of seleno-L-methionine (SeMet) to L-methionine selenoxide (MetSeO) were investigated using cDNA-expressed human FMOs, purified rat liver FMOs, and rat liver microsomes. MetSeO and the N-2,4-dinitrophenyl-derivatives of SeMet and MetSeO were synthesized and characterized by 1H-NMR and ESI/MS. These reference compounds were then used to develop a sensitive HPLC assay to monitor SeMet oxidation to MetSeO. Formation of MetSeO in rat liver microsomes was time-, protein concentration-, SeMet concentration-, and NADPH-dependent. The microsomal activity exhibited a SeMet Km value (mean ±S.D.; n=4) of 0.91 ± 0.29 mM and a Vmax value of 44 ± 8.0 nmol MetSeO/mg protein/min. Inclusion of 1-benzylimidazole, superoxide dismutase or deferoxamine caused no inhibition of the rat liver microsomal activity. Because these results suggested the involvement of FMOs in the oxidation of SeMet in rat liver microsomes, formation of MetSeO was also examined using cDNA-expressed human and purified rat FMOs. The results showed that both rat and human FMO1 and FMO3 but not FMO5 can catalyze the reaction. The SeMet kinetic constants were obtained with purified rat liver FMO3 (Km = 0.11 mM, Vmax = 280 nmol/mg protein/min) and rat liver FMO1 (Km = 7.8 mM, Vmax = 1200 nmol/mg protein/min). Because SeMet has anti-cancer, chemopreventive, and toxic properties, the kinetic results suggest FMO3 is likely to play a role in the biological activities of SeMet at low exposure conditions.
INTRODUCTION
Seleno-L-methionine (SeMet1), a naturally occurring analogue of methionine, has potent anti-cancer activities against human tumor cells (1–3) and has been suggested to have chemopreventive properties in human clinical studies where decreases in total cancer incidence were observed (4,5). The cancer chemopreventive properties of SeMet have also been demonstrated in animal models (6,7). Furthermore, SeMet has been shown to cause acute and chronic toxicity in various animal and avian models, but the mechanisms of the biological effects of SeMet remain unclear (reviewed in 8).
Metabolism of SeMet in animal and human tissues can occur by multiple enzymes. Metabolism by the methionine transsulfuration pathway yields selenocystathionine and selenocysteine (9), whereas metabolism by methionine γ-lyase and glutamine transaminase yields methylated Se and 4-methylseleno-2-oxobutanoic acid, respectively (10,11). SeMet can also be stored in the body in proteins in place of methionine, especially when methionine levels in the diet are low (8,12).
Previously, our laboratory has shown that L-methionine is a substrate for flavin-containing monooxygenases (FMOs; FMO1–4). cDNA-expressed rabbit and human FMO3 and purified rat liver FMO3 were shown to preferentially oxidize L-methionine to its corresponding sulfoxide compared to rabbit, rat and human FMO1, rabbit FMO2, and human FMO4 (13–16); human and rabbit FMO5 did not catalyze this metabolic reaction.
The primary objective of the current study was to determine if SeMet could be oxidized to its corresponding selenoxide by cDNA-expressed and purified human and rat FMOs (Scheme 1). We also examined the ability of rat liver microsomes to metabolize SeMet to its corresponding selenoxide and characterized this microsomal activity. For these experiments, the N-2,4-dinitrophenyl (DNP)-derivatives of SeMet and MetSeO were first prepared and characterized and then these reference materials were used to develop an HPLC assay to quantitate the formation of MetSeO in enzymatic incubations after derivatization with 1-fluoro-2,4-dinitrobenzene.
Scheme 1.
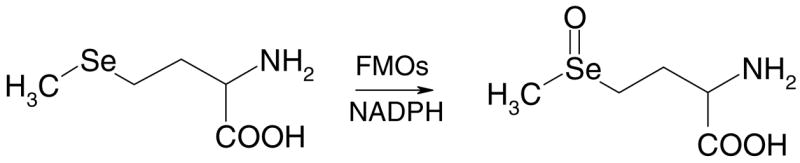
Scheme of the oxidation of seleno-L-methionine to seleno-L-methionine selenoxide by FMOs.
MATERIALS AND METHODS
Caution
The following chemicals are hazardous and should be handled carefully: SeMet, MetSeO, and 1-fluoro-2,4-dinitrobenzene.
Materials
NADPH, SeMet, L-methionine, methimazole, catalase (from bovine liver), superoxide dismutase (from bovine liver), deferoxamine and 1-fluoro-2,4-dinitrobenzene were obtained from Sigma-Aldrich. HPLC-grade acetonitrile was purchased from EM Science. SeMet and 30% hydrogen peroxide were obtained from Acros Organics (Pittsburgh, PA). Microsomes containing cDNA-expressed human FMO1, FMO3, and FMO5 were purchased from BD-Biosciences-Gentest (Woburn, MA) and the FAD content of these microsomes was provided by the vendor. Purified rat liver FMO1 and FMO3 were obtained by using dye-affinity and ADP-sepharose columns as described previously (14). FMO3 was purified nearly 72-fold over the initial microsomal Lmethionine S-oxidase activity and FMO1 was purified nearly 297-fold over the initial microsomal S-benzyl-L-cysteine S-oxidase activity. All other chemicals were of the highest grade commercially available.
Male Sprague-Dawley rats (210–260 g) were obtained from Sasco (Omaha, NE) and kept on a 12 h light/dark schedule and allowed food and water ad libitum. “Washed” liver microsomes were prepared by subcellular fractionation as previously described and stored at −80°C until use (17).
Synthesis and characterization of MetSeO
MetSeO was synthesized from SeMet by the addition of H2O2 (18). Briefly, SeMet (0.14 mmole) was dissolved in 400 μL H2O and hydrogen peroxide (0.14 mmole) was added. The reaction was stirred on ice for 15 min after which it was lyophilized to dryness. The reaction yield was 85% and the MetSeO obtained was ≥99% pure as determined by HPLC with UV detection at 220 nm. HPLC analyses of MetSeO were carried out on a Beckman Ultrasphere 5 μcolumn (4.6 × 250 mm) using 1% acetonitrile in water adjusted to pH 2.5 with trifluoroacetic acid) at a flow rate of 1 mL/min. The retention times of SeMet and MetSeO were 8.7 and 2.7 min, respectively. Electrospray mass spectra (ESI-MS) were obtained on a Kratos DS-55 (Manchester, England) ultra high resolution mass spectrometer fitted with a Kratos DS- 55 data system. Spectra were recorded over the range of m/z 50–500. ESI-MS m/z (relative abundance) were as follows: 216 (82Se) (M++1, 6%); 214 (80Se) (M++1, 20%); 212 (78Se) (M++1, 8%); 211 (77Se) (M++1, 3%); 210 (76Se) (M++1, 3%); 198 (82Se) (M+1- H2O, 20%); 196 (80Se) (M+1- H2O, 100%); 194 (78Se) (M+1-H2O, 49%); 193 (77Se) (M+1-H2O, 18%); 192 (76Se) (M+1-H2O, 20%); 168 (80Se) (M - (H2O and CO2), 20%). These fragments are similar to what was observed by Gammelgaard, et al. (19). 1H-NMR spectra were obtained on a 500 MHz Bruker spectrometer. MetSeO NMR spectra were obtained at three different pHs (pH 2.4, 5.5, 11.5) because previous studies have shown that at pHs of 4.0–8.0 additional peaks corresponding to the methionine selenoxide hydrate (MetSe(OH)2) are detected (20, 21). Chemical shifts were reported in ppm from sodium 3-(trimethylsilyl)tetradeutero sodium propionate as internal standard. MetSeO was dissolved in D2O alone and with DCl or NaOD. At pH 11.5 (D2O/NaOD), a mixture of the two diastereomers (1:1 based on integral) of MetSeO was obtained as follows: 3.37 (1H, dd, CH(NH2)COOH); 3.10–3.13 and 2.97–3.05 (2 H, 2m, Se(O)CH2); 2.68 and 2.69 (3H, 2s, -SeCH3); 2.05–2.17 and 1.96–2.03 (2H, 2m, CH2CH(NH2)COOH). The chemical shifts observed are similar to values reported in the literature (20, 21). At pH 5.5 (D2O alone), a mixture of MetSeO (60%) and MetSe(OH)2 (40%) as based on integral was observed with the following chemical shifts: 4.34 (1H, dd, CH(NH2)COOH, MetSe(OH)2); 3.86 (1H, dd, CH(NH2)COOH, MetSeO); 3.667–3.78 (2H, m, -Se(OH)2CH2, MetSe(OH)2); 2.97–3.31 (2H, m, Se(O)CH2, MetSeO); 2.79 (3H, s, CH3Se, MetSe(OH)2); 2.70 (3H, s, -SeCH3, MetSeO); 2.53–2.69 (2H, m, CH2CH(NH2)COOH, MetSe(OH)2); 2.35–2.39 (2H, m, CH2CH(NH2)COOH, MetSeO. At pH 2.4 (D2O/DCl), a mixture of both MetSeO and MetSe(OH)2 was observed. The chemical shifts obtained were as follows: 4.20 (1H, s, CH(NH2)COOH); 3.89–3.97 and 3.65–3.79 (2H, 2m, Se(O)CH2); 3.15 and 3.09 (3H, 2s, -SeCH3); 2.55–2.80 (2H, m, CH2CH(NH2)COOH). Because the peaks were quite broad and often contained peaks from both forms, assignment to either the selenoxide or hydrate was not made.
Synthesis and characterization of dinitrophenyl-seleno-L-methionine and dinitrophenyl- L-methionine selenoxide
1-Fluoro-2,4-dinitrobenzene (0.095 mmol) was added to a stirred suspension of SeMet (0.10 mmol) and anhydrous sodium carbonate (0.3 mmol) in anhydrous tetrahydrofuran (1mL). The reaction was kept in the dark and was stirred for 1 h at 40°C followed by 8 h at room temp. Water (15 mL) was added and the pH adjusted to 12 with 1 M Na2CO3. The mixture was washed twice with 15 mL CH2Cl2. The pH was then adjusted to 2 with 1 N HCl and extracted twice with CH2Cl2 (15 mL). The combined organic phases were dried over magnesium sulfate and the solvent was evaporated at 10°C under reduced pressure. The yellow solid obtained was 96% pure as determined by HPLC at 220 nm and 365 nm. The yield was 90%. The compound was characterized by NMR and EI-MS. The 1H-NMR was carried out in D2O/NaOD and the signals were as follows: 2.00 (3H, s, CH3Se-); 2.28–2.37 (2H, m, -CH2CH(NH-)COOH); 2.61–2.73 (2H, m, -SeCH2-); 4.38 (1H, dd, -CH (NH-)COOH); 7.00 (1H, d, HorthoAr); 8.29 (1H, dd, HmetaAr); 9.10 (1H, d, HmetaAr). EI-MS fragmentation, m/z (relative abundance) is as follows: 363 (80Se) (M+, 35%); 222 (CH3SeSeCH3+ O2), 50%); 190 (CH3SeSeCH3, 80%); 109 (CH3SeCH2, 100%).
Dinitrophenyl-L-methionine selenoxide was synthesized by adding hydrogen peroxide (30%, 0.025 mmol) to a solution of dinitrophenyl-seleno-L-methionine (0.017 mmol) dissolved in NaOD (0.017 mmol) in D2O (0.5 mL). The solution was stirred 10 min at 0°C in the dark. Reaction yield was 100% and purity was 96%. 1H-NMR (D2O/NaOD) mixture of two diastereomers (1:1 ratio as based on integral) was as follows: 2.40–2.52 (2H, m, -CH2CH(NH-)COOH; 2.65 and 2.67 (3H, 2s, CH3Se(O)-); 2.97–3.07 and 3.14–3.21(2H, 2 m, -Se(O)CH2); 4.43–4.47 (1H, m, -CH(NH-)COOH); 7.03 and 7.04 (1H, 2d, HorthoAr); 8.32 (1H, dd, HmetaAr); 9.12 (1H, s, HmetaAr). EI-MS fragmentation m/z (relative abundance) was as follows: 379 (80Se) (M+, 3.5%); 316 (80Se) (M-(CO2 + H2O), 3%); 267 (M-CH3SeOH, 100%).
Microsomal enzymatic assays
The buffer used to prepare solutions for the enzymatic reactions contained 0.1 M KH2PO4, 0.1 M KCl, 5 mM EDTA, pH 7.4. Enzymatic reactions were carried out in a total volume of 0.5 mL in a Dubnoff metabolic incubator with continuous shaking by incubating microsomal protein (0.3–1.0 mg) at 37°C for 5 min in the presence or absence of NADPH (2 mM) and catalase (2800 units) before SeMet was added to start the enzymatic reaction. Catalase was included in the incubations to remove any H2O2 resulting from uncoupled oxidation of NADPH. The SeMet concentration was 5 mM in the time course and protein dependence experiments and in the experiments to determine the effect of superoxide dismutase (537 units), deferoxamine (10 μM), or 1-benzylimadazole (1 mM) on the microsomal activity. Kinetic constants were determined using SeMet concentrations ranging from 0.05–10 mM using an incubation time of 20 min. Data were analyzed using nonlinear regression (SigmaPlot, SPSS Inc., Chicago, IL). For all metabolic incubations, the reaction was terminated with the addition of 0.5 mL ethanol and centrifuged for 15 min at 3000 rpm in a Beckman TJ-6 centrifuge to precipitate the protein. An aliquot of the supernatant (0.5 mL) was derivatized with 15 μL 2,4-dinitrofluorobenzene (10% v/v in ethanol) followed by addition of 50 μL KOH/KHCO3 then allowed to react for 24 h in the dark at room temperature.
MetSeO recovery from incubation mixtures was determined by incubating 0.5 mM MetSeO with 2 mM NADPH and catalase (2800 units) for 20 min at 37°C in the presence of 0.53 mg rat liver microsomal protein. A concurrent control of 0.5 mM MetSeO in buffer only was also carried out for 20 min at 37°C. MetSeO (104.9 ± 8.4 % of control value; mean ±S.D., n=5) was detected in the incubations containing microsomes and NADPH.
Experiments to examine the effect of SeMet on L-methionine oxidation in rat liver microsomes were carried out by incubating10 mM methionine in the presence of 10 mM SeMet for 20 min as described above. After these metabolic reactions were terminated using an equal volume of ice-cold ethanol, samples were derivatized with 1- fluoro-2,4-dinitrobenzene and analyzed for methionine sulfoxide formation as previously described (14).
Protein concentrations for the microsomal samples were determined by the method of Lowry et al (22) using bovine albumin as standard. Protein concentrations of the purified rat liver FMOs were carried out by the bicinchoninic acid method (Pierce Chemical Co., Rockford, IL).
HPLC Analyses
HPLC analyses of the N-2,4-DNP-derivatives of MetSeO (20 μL injection volume) were carried out with UV detection at 360 nm on a Waters Nova-Pak 5 μ reverse-phase C-18 column (3.9 × 75 mm) using a Gilson gradient controlled HPLC system. The mobile phases used were 1% acetonitrile/99% H2O containing 0.1 % trifluoroacetic acid adjusted to pH 4.5 on pump A and 75% acetonitrile/25% H2O containing 0.1 % trifluoroacetic acid adjusted to pH 4.5 on pump B. The initial mobile phase composition was 1% B which increased to 5% B over 3 min. The percentage of B then increased to 50% over the next 3 min. It further increased to 75% B over 3 min where it was held for 2.5 min. It then decreased to 1%B over 5 min where it was held for a total run time of 20.5 min. Typical retention times of the DNP-derivatives of MetSeO and SeMet were 4.8 and 7.6 min, respectively. Separation of the diastereomers of MetSeO was not achieved with this method. Quantitation of MetSeO was done by comparing peak areas after derivatizatin with 1-fluoro-2,4-dinitrofluorobenzene (corrected for any non-enzymatic activity in the absence of NADPH) to standard curves (r > 0.99) prepared using the reference compound.
Assays using cDNA-expressed human FMOs
Experiments were carried out using 0.5 and 5 mM SeMet in the presence of the pyridoxalphosphate-dependent enzyme inhibitor, aminooxyacetic acid (1 mM - final; 23), catalase (2800 units) and cDNA-expressed FMO1, FMO3 or FMO5 (0.5–0.75 nmol FAD/mL) in the presence and absence of 2 mM NADPH. Concurrent controls using the same volume of vector control microsomes (which contain no FMOs) were also included. Reactions were carried out for 15–45 min.
Assays using purified rat FMO1 and FMO3
Assays to determine the kinetic constants were carried out using 0.05–10 mM SeMet for 20 min and derivatized as described above. Data were analyzed using nonlinear regression (SigmaPlot, SPSS Inc., Chicago, IL).
Statistical analyses
Comparisons of the control activity to the activity observed in the presence of inhibitors was done using SigmaStat (SPSS Inc., Chicago, IL) with a paired t-test, with p<0.05 as the criterion for significance.
RESULTS
Characterization of MetSeO
MetSeO was synthesized by the addition of H2O2 to SeMet. The ESI-mass spectrum of the selenoxide exhibited a pseudomolecular ion of m/z 214 consistent with the expected molecular weight of SeMetO + 1. The major peak (m/z 196) corresponds to the loss of water. These results are consistent with results previously reported in the literature (19). The proton NMR spectrum obtained in the presence of NaOD (pH 11.5) was consistent with the presence of nearly equal concentrations of the two possible diastereomers of MetSeO, as indicated by the presence of two singlets (δ = 2.69 and 2.68 ppm) for the methyl protons of the two diastereomers. The splittings of the β- and γ-methylene protons were also consistent with the presence of the two diastereomers. Chiral selenoxides are known to readily undergo facile racemization in aqueous media, mostly via formation of achiral hydrates (24, 25). The spectra obtained in only D2O (pH 5.5) or in the presence of DCl (pH 2.4) show peaks corresponding to MetSeO along with additional peaks that likely are due to the hydrate form MetSe(OH)2 with two singlets present for the methyl protons (δ= 3.15 and 3.09 ppm). The presence of MetSeO diastereomers and MetSe(OH)2 formation at the various pHs is consistent with what was observed previously with other selenoxides at similar conditions (21, 25). Further oxidation of MetSeO to methionine selenone was not observed in our studies, consistent with previous reports that oxidation of selenoxides to selenones required use of oxidants (e.g. ozone and KMNO4) much more vigorous than those needed for conversion of selenides to selenoxides (26).
Characterization of the 2,4-DNP-derivatives of SeMet and MetSeO
As we have previously used 1-fluoro-2,4-dinitrobenzene to determine L-methionine sulfoxide formation from L-methionine (1–4) and this derivatization technique also worked for SeMet and MetSeO, we synthesized and characterized the DNP-derivatives of SeMet and MetSeO. The EI-MS spectrum of reference dinitrophenyl-seleno-L-methionine (DNPSeMet) exhibited the expected pseudomolecular ion (m/z 363). The relative abundances of the selenium isotopes were also comparable with the theoretical spectrum. The 1H NMR spectrum of the synthetic product was carried out in D2O with one equivalent of NaOD and was consistent with the expected chemical shifts of the DNP- and SeMet moieties. Chemical oxidation of DNP-SeMet with H2O2 resulted in the formation of dinitrophenyl-L-methionine selenoxide (DNP-MetSeO). Analysis of reference DNPMetSeO by EI-MS exhibited a pseudomolecular ion of m/z 379 consistent with the expected molecular weight (80Se) and the known relative abundance of the isotopes of selenium. The major peak at m/z 267 corresponded to loss of CH3SeOH and the formation of CH2=CHCH(NH-Ar)COOH. Similar selenoxide fragmentations have been reported previously (27). This compound was also characterized by NMR in D2O/NaOD. The integration and the splitting of the chemical shifts of the spectrum were consistent with the presence of two diastereomers of DNP-MetSeO. The peaks corresponding to the two meta protons (δ - 9.12 and 8.32 ppm) and the ortho protons (δ - 7.04 and 7.03) of the dinitrophenyl group had the expected 1:1:1 integration ratios. The peaks corresponding to the α-proton (δ - 4.47-4.46 ppm), the β-protons (δ - 2.52-2.40 pm), γ-protons (δ- 3.21-3.14 and 3.07-2.97) and the methyl group (δ- 2.67 and 2.65 ppm) had the expected 1:2:2:3 integration ratios and exhibited greater downfield chemical shifts compared to the corresponding protons of DNP-SeMet.
Effects of SeMet on L-methionine sulfoxidation in rat liver microsomes and characterization of the SeMet selenoxidation activity of rat liver microsomes
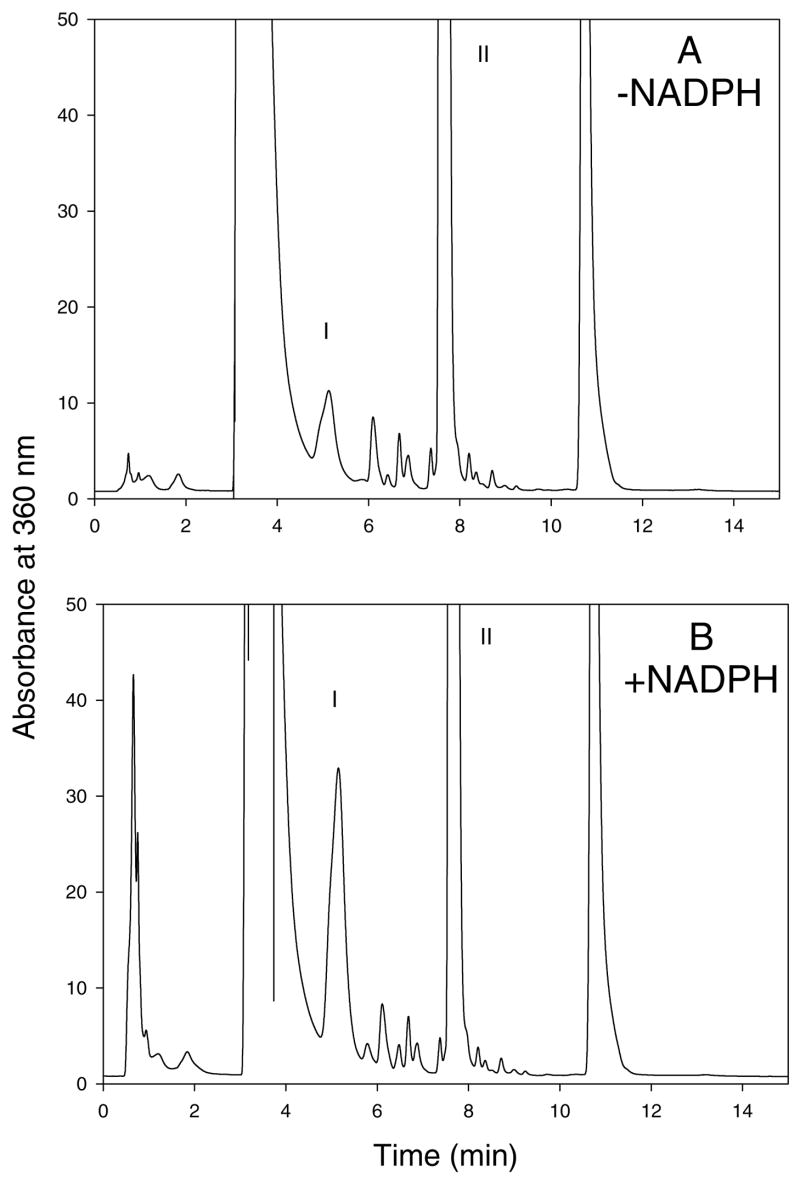
Inclusion of 10 mM SeMet in incubations of 10 mM L-methionine with rat liver microsomes resulted in almost complete inhibition of L-methionine sulfoxide formation. The amount of methionine sulfoxide detected in incubations containing SeMet was only 1 ± 1% (mean ±S.D., n=3) of the amount of methionine sulfoxide detected in control incubations containing SeMet (data not shown). Because of this nearly complete inhibition of methionine sulfoxide formation, we investigated the ability of rat liver microsomes to oxidize SeMet. Incubations of SeMet with rat liver microsomes in the presence of NADPH following derivatization with Sanger’s reagent resulted in the detection of a peak on the chromatogram that co-eluted with that of chemically synthesized DNP-MetSeO (Figure 1B). The formation of this peak in rat liver microsomes was dependent on incubation time (Figure 2A), protein concentration (Figure 2B) and was substantially reduced in the absence of NADPH (Figure 1A). Analysis of the rat liver microsomal kinetic data by nonlinear regression (see Figure 2C for a representative experiment) resulted in an apparent SeMet Km value (mean ± S.D., n=4) of 0.91 ± 0.29 mM and a Vmax value of 44 ± 8 nmol MetSeO/mg protein/min; therefore, the Vmax/Km ratio was 51 ± 10 (Table 1).
Figure 1.
A typical reverse-phase HPLC chromatogram of the reaction mixture of SeMet (5 mM) with rat liver microsomes (1 mg/mL) in the absence (A) and presence (B) of NADPH following derivatization with 2,4-dinitrofluorobenzene, showing the N-2,4- dinitrophenyl derivatives of MetSeO (I) and SeMet (II). The incubation time was 20 min.
Figure 2.
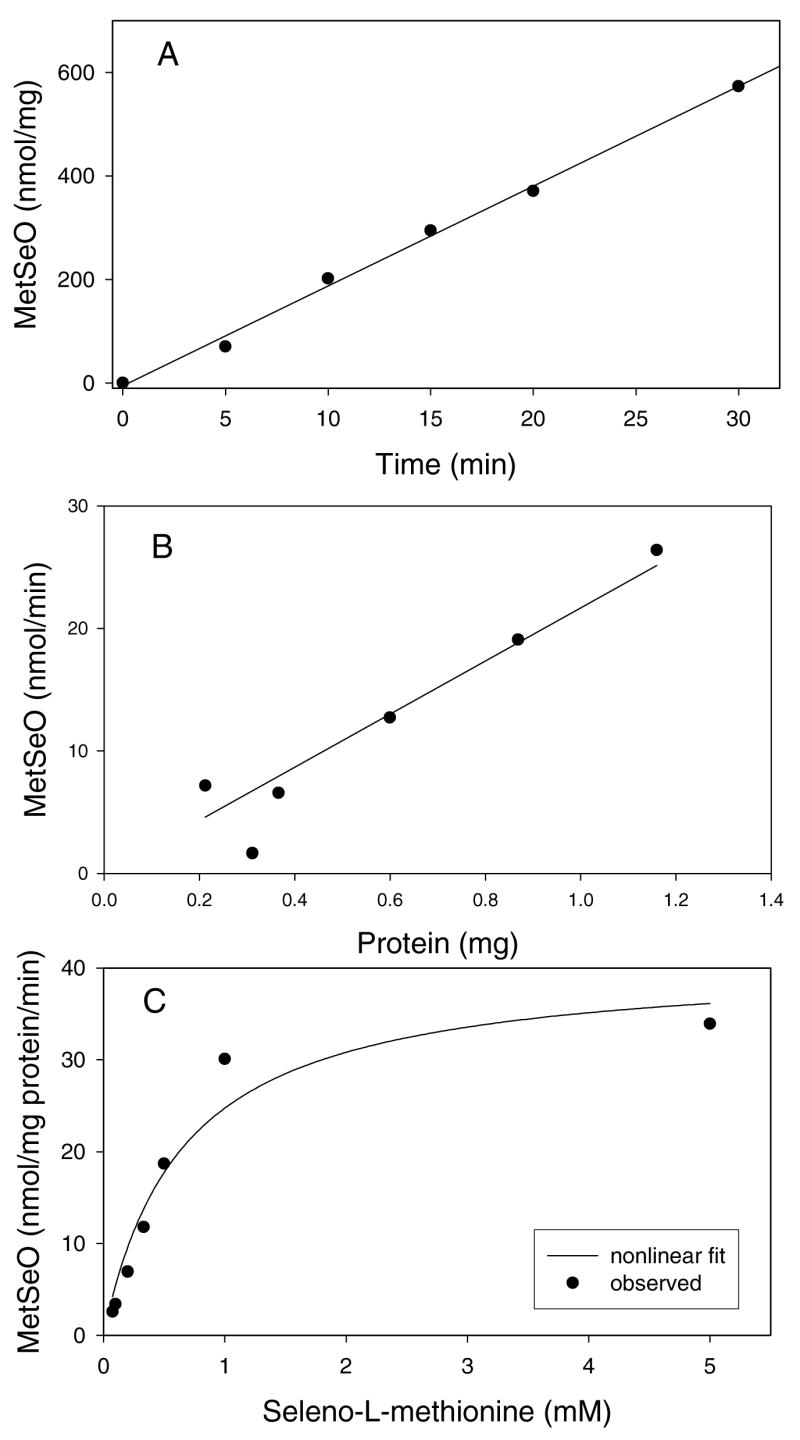
Representative time course (A), protein dependence (B) and nonlinear curve fitting plot (C) of MetSeO formation by rat liver microsomes. Details on experimental conditions can be found in the Materials and Methods.
Table 1.
Apparent Kinetic Constants for Seleno-L-Methionine Selenoxide Formation by Purified Rat Liver FMOs and Rat Liver Microsomesa.
| Enzyme | Vmaxb (nmol/mg protein/min) | Kmb (mM) | Vmax/Km | R |
|---|---|---|---|---|
| Rat Liver FMO1 | 1200c | 7.8 | 150 | 0.98 |
| Rat Liver FMO3 | 280 | 0.11 | 2500 | 0.96 |
| Rat Liver Microsomes | 44 ± 8.0 | 0.91 ± 0.29 | 51 ± 10 | 0.97 |
The enzyme was incubated with varying concentrations of seleno-L-methionine (0.05- 10 mM) and NADPH (2 mM) for 20 min as described in the Materials and Methods.
Apparent kinetic constants were determined using nonlinear curve fitting as described in the Materials and Methods. Experiments with purified rat liver FMOs were carried out only once because of the limited amounts of purified FMOs available for these experiments and therefore no statistics were provided. The rat liver microsomal values (means ± S.D.) were determined from four experiments.
Values were rounded to two significant figures.
To help determine which enzymes were responsible for SeMet oxidation in the microsomal system, the effects of various enzyme inhibitors and reactive oxygen scavengers were examined. Inclusion of 1-benzylimidazole, a general cytochrome P450 inhibitor, had no effect on the enzymatic activity (94.9 ± 12.3% of the control activity, mean ± S.D., n=4) in rat liver microsomes. No inhibition of the selenoxide formation was observed when superoxide dismutase (92.3 ± 9.1% of the control activity, mean ± S.D., n=4) or deferoxamine (89.4 ± 11.1% of the control activity, mean ± S.D., n=4), an iron chelator that inhibits hydroxy radical production by iron-catalyzed reactions, was included in the incubations. Thus, these results provide evidence against the involvement of cytochrome P450s or reactive oxygen species in this metabolic reaction.
Ability of cDNA-expressed FMO1, FMO3, and FMO5 to oxidize SeMet to MetSeO
Because cytochrome P450s and reactive oxygen species were not responsible for this metabolic reaction, the ability of cDNA-expressed human FMOs to oxidize SeMet was examined. Activity was observed with FMO1 and FMO3 using both 0.5 and 5 mM SeMet (Figure 3). The activity observed with FMO1 was slightly higher than that observed with FMO3 at both concentrations (Figure 3). With FMO1, there was a nearly 3-fold increase in activity when the SeMet concentration was increased from 0.5 mM to 5 mM. With FMO3, there was a nearly 3.2-fold increase activity in activity from 0.5 to 5 mM SeMet. FMO5 did not increase the amount of MetSeO over that observed with insect microsomes that contain no FMOs (data not shown).
Figure 3.
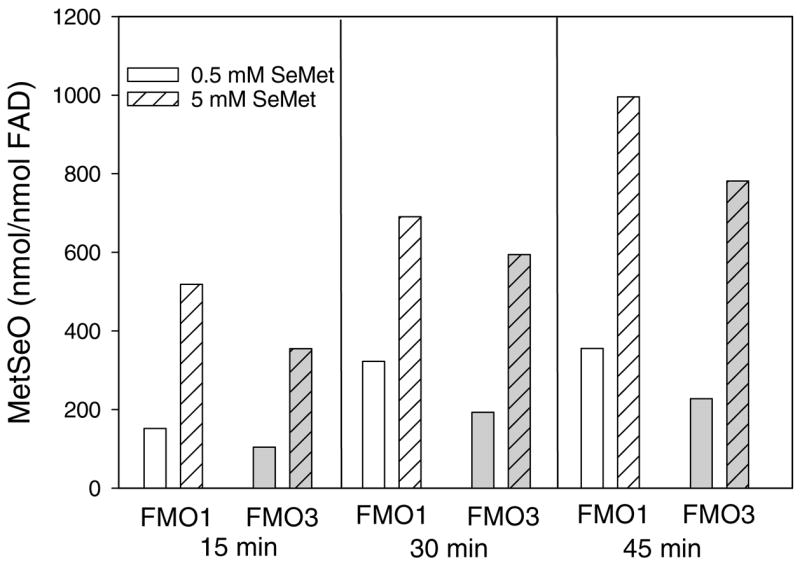
Time-dependent formation of MetSeO by cDNA-expressed human FMO1 and FMO3.
MetSeO formation in incubations of SeMet with purified rat FMO1 and FMO3
The kinetic constants for purified rat liver FMOs were determined using nonlinear curve fitting (Figure 4A and 4B). With purified rat liver FMO1, the Km value was determined to be 7.8 mM and the Vmax value was 1200 nmol/mg protein/min, resulting in a V/K ratio of 150 (Table 1). With the purified rat liver FMO3, the SeMet Km value was determined to be 0.11 mM and the Vmax value was 280 nmol/mg protein/min, resulting in a V/K ratio of 2500 (Table 1).
Figure 4.
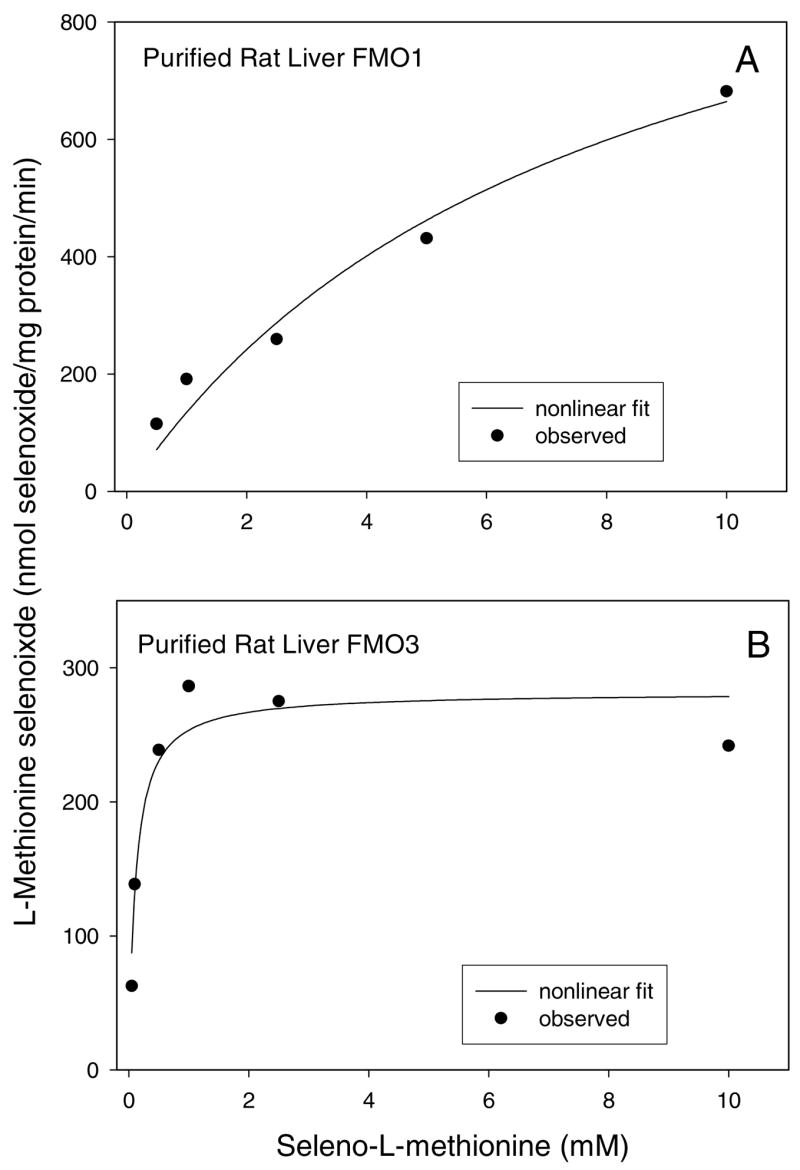
Nonlinear curve fitting plot of L-methionine selenoxide formation by purified rat liver FMO1 (A) and FMO3 (B).
DISCUSSION
In this study, the oxidation of SeMet by FMOs and the characterization of this metabolic reaction in rat liver microsomes have been studied after development of a specific HPLC assay to detect the DNP-derivative of MetSeO. An advantage of this method is that it allows monitoring of product formation rather than monitoring NADPH oxidation that is not specific and can be associated with multiple reactions in the microsomal system (28).
The results clearly show that SeMet is a substrate for FMOs. The purified rat liver and cDNA-expressed human FMO data show that SeMet can be oxidized by both FMO1 and FMO3. Similar to L-methionine, no activity was observed with cDNAexpressed human FMO5. Although we did not determine kinetic values with cDNA-expressed human FMOs, we previously have shown that for many substrates such as Lmethionine, S-allyl-L-cysteine, and S-benzyl-L-cysteine, kinetic values obtained with either purified rat liver FMOs or cDNA-expressed rabbit FMOs were similar to the corresponding values obtained with cDNA-expressed human FMOs (13,15, 29). With SeMet, the Vmax values obtained with purified rat liver FMO1 and FMO3 (1200 and 280 nmol/mg/min, respectively) are similar to the corresponding values (1266 and 261 nmol/mg/min, respectively) with L-methionine (14). Interestingly, with both SeMet and L-methionine, the Vmax/Km ratio is much higher with rat liver FMO3 than with rat liver FMO1. The Km values observed with SeMet for FMO1 and FMO3 (7.8 and 0.1 mM, respectively) are much lower than the Km values observed previously with L-methionine (48.0 and 3.4 mM, respectively; 14). A similar trend was observed by Chen and Ziegler (30) for the metabolism of dialkyl- and alkylaryl-selenides compared to their sulfur and nitrogen analogues by pig liver FMO1 (31). Interestingly, SeMet is oxidized chemically to its corresponding selenoxide by peroxynitrite with second-order rate constants that were 10- to 100-fold higher than the rate constants obtained for the same reaction with methionine (32).
MetSeO formation in rat liver microsomes was time-, protein-, substrate concentration-, and NADPH-dependent. Inclusion of cytochrome P450 inhibitors and reactive oxygen scavengers had no effect on MetSeO formation. These results suggest that FMOs may be responsible for this metabolic reaction in the microsomes. Inclusion of SeMet into L-methionine incubations significantly inhibited the formation of methionine sulfoxide. This also provides evidence for involvement of FMOs in SeMet oxidation. The SeMet Km value (mean ± S.D., n=4) determined with the rat liver microsomes is 0.91 ± 0.29 mM which is between the Km values determined with purified rat liver FMO1 (7.8 mM) and FMO3 (0.11 mM). This suggests that that both FMO isoforms may be involved in MetSeO formation in rat liver microsomes.
SeMet can be metabolized by several enzymes other than FMOs. It can serve as a substrate for methionine adenosyltransferase with a Km value of 0.6–0.8 mM (33,34). SeMet can also be metabolized by L-methionine γ-lyase with a Km value of 0.51 mM (35). It is also a good substrate for glutamine transaminase, having a Km value of 0.13 mM (11). With purified rat liver FMO3, the SeMet Km value was 0.11 mM. Collectively, these kinetic results suggest that FMOs may play an important role in the metabolism of SeMet at low exposure levels.
FMOs have been previously implicated in the metabolism of other selenium-containing compounds. For example, ebelsen, 2-selenylbenzanilide, and 2- (methylseleno)benzanilide are metabolized to their corresponding selenoxides by FMO1 (36). Dimethylselenide has been shown to be oxidized by rat liver and lung microsomes and purified pig liver FMO1 (37). Rooseboom, et al. also demonstrated that rat liver microsomes and cDNA-expressed human FMO1 and FMO3 are responsible for the selenoxidation of Se-benzyl-L-selenocysteine although the selenoxide product in this case readily undergoes a β-elimination reaction to yield pyruvate (38).
Oxidation of SeMet may influence its biological activities. Selenoxides may cause redox cycling resulting in depletion of glutathione as MetSeO has been shown to be reduced to SeMet, using two equivalents of glutathione which are converted to glutathione disulfide (39). In this regard, our MetSeO recovery experiment suggests no significant reduction of MetSeO had occurred in the microsomal incubations. This is likely due to the use of “washed” microsomes that presumably removed GSH from these microsomal preparations. Ebselen has also been shown to induce apoptosis in HepG2 cells by depleting intracellular thiols (40). Thus, formation of MetSeO may result in depletion of intracellular thiols, causing inhibition of significant cellular function and/or may lead to changes in gene expression. Therefore, the ability of FMOs to oxidize SeMet to its corresponding selenoxide may play a role in the anti-cancer, chemopreventive, and toxic properties of SeMet.
Acknowledgments
This research was supported by Grant DK44295 from the National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health.
Footnotes
Abbreviations: seleno-L-methionine, SeMet; L-methionine selenoxide, MetSeO; Lmethionine selenoxide hydrate, MetSe(OH)2; flavin-containing monooxygenases, FMOs; dinitrophenyl, DNP; electrospray ionization-mass spectrometry, ESI-MS;
Inquiries should be sent to: Dr. Adnan Elfarra, School of Veterinary Medicine, 2015 Linden Drive, Madison, WI 53706
References
- 1.Redman C, Scott JA, Baines AT, Basye JL, Clark LC, Calley C, Roe D, Payne CM, Nelson MA. Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines. Cancer Lett. 1998;125:103–110. doi: 10.1016/s0304-3835(97)00497-7. [DOI] [PubMed] [Google Scholar]
- 2.Goulet A-C, Chigbrow M, Frisk P, Nelson MA. Selenomethionine induces sustained ERK phosphorylation leading to cell-cycle arrest in human colon cancer cells. Carcinogenesis. 2005;26:109–117. doi: 10.1093/carcin/bgh306. [DOI] [PubMed] [Google Scholar]
- 3.Miki K, Xu M, Gupta A, Ba Y, Tan Y, Al-Refaie W, Bouvet M, Makuuchi M, Moossa AR, Hoffman RM. Methionase cancer gene therapy with selenomethionine as suicide prodrug substrate. Cancer Res. 2001;61:6805–6810. [PubMed] [Google Scholar]
- 4.Clark LC, Combs GF, Turnbull WB, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Park HK, Sanders BB, Smith CL, Taylor JR The Nutrition Prevention of Cancer Study Group. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. J Am Med Assoc. 1996;276:957–1963. [PubMed] [Google Scholar]
- 5.Ip C, Birringer M, Block E, Kotrebai M, Tyson JF, Uden PC, Lisk DJ. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J Agric Food Chem. 2000;48:2062–2070. doi: 10.1021/jf000051f. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay-Sardar S, Pada Rana M, Chatterjee M. Antioxidant associated chemoprevention by selenomethionine in murine tumor model. Mol Cell Biochem. 2000;206:17–25. doi: 10.1023/a:1007040705928. [DOI] [PubMed] [Google Scholar]
- 7.Willhite CC, Hawkes WC, Omaye ST, Choy WN, Cox DN, Cukierski MJ. Absorption, distribution and elimination of selenium as L-selenomethionine in non-human primates. Fd Chem Toxic. 1992;30:903–913. doi: 10.1016/0278-6915(92)90174-j. [DOI] [PubMed] [Google Scholar]
- 8.Schrauzer GN. Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr. 2000;130:1653–1656. doi: 10.1093/jn/130.7.1653. [DOI] [PubMed] [Google Scholar]
- 9.Kajander EO, Harvima RJ, Eloranta TO, Martikainen H, Kantola M, Kärenlampi SO, Åkerman K. Metabolism, cellular actions and cytotoxicity of selenomethionine in cultured cells. Biol Trace Elem Res. 1991;28:57–68. doi: 10.1007/BF02990463. [DOI] [PubMed] [Google Scholar]
- 10.Okuno T, Kubota T, Kuroda T, Ueno H, Nakamuro K. Contribution of enzymic α,γ-elimination reaction in detoxification pathway of selenomethionine in mouse liver. Toxicol Appl Pharmacol. 2001;176:18–23. doi: 10.1006/taap.2001.9260. [DOI] [PubMed] [Google Scholar]
- 11.Blarzino C, Coccia R, Pensa B, Cini C, De Marco C. Selenomethionine as substrate for glutamine transaminase. Biochem Mol Biol Inter. 1994;32:79–86. [PubMed] [Google Scholar]
- 12.Beilstein MA, Whanger PD. Chemical forms of selenium in rat tissues after administration of selenite or selenomethionine. J Nutr. 1986;116:1711–1719. doi: 10.1093/jn/116.9.1711. [DOI] [PubMed] [Google Scholar]
- 13.Duescher RJ, Lawton MP, Philpot RM, Elfarra AA. Flavin-containing monooxygenase (FMO)-dependent metabolism of methionine and evidence for FMO3 being the major FMO involved in methionine sulfoxidation in rabbit liver and kidney microsomes. J Biol Chem. 1994;269:17525–17530. [PubMed] [Google Scholar]
- 14.Krause RJ, Ripp SL, Sausen PJ, Overby LH, Philpot RM, Elfarra AA. Characterization of the methionine S-oxidase activity of rat liver and kidney microsomes: immunochemical and kinetic evidence for FMO3 being the major catalyst. Arch Biochem Biophys. 1996;333:109–116. doi: 10.1006/abbi.1996.0370. [DOI] [PubMed] [Google Scholar]
- 15.Ripp SL, Itagaki K, Philpot RM, Elfarra AA. Species and sex differences in expression of flavin-containing monooxygenase form 3 in liver and kidney microsomes. Drug Metab Dispos. 1999;27:46–52. [PubMed] [Google Scholar]
- 16.Ripp SL, Itagaki K, Philpot RM, Elfarra AA. Methionine Soxidation in human and rabbit liver microsomes: evidence for a high-affinity methionine S-oxidase activity that is distinct from flavin-containing monooxygenase 3. Arch Biochem Biophys. 1999;367:322–332. doi: 10.1006/abbi.1999.1247. [DOI] [PubMed] [Google Scholar]
- 17.Sausen PJ, Elfarra AA. Cysteine conjugate S-oxidase: characterization of a novel enzymatic activity in rat hepatic and renal microsomes. J Biol Chem. 1990;265:6139–6145. [PubMed] [Google Scholar]
- 18.Lopez J, Jao TC, Rudzinski WE. The Raman spectra of selenomethionine and selenocystine. J Inorg Biochem. 1981;14:177–181. doi: 10.1016/s0162-0134(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 19.Gammelgaard B, Cornett C, Olsen J, Bendahl L, Hansen SH. Combination of LC-ICP-MS, LC-MS and NMR for investigation of the oxidative degradation of selenomethionine. Talanta. 2003;59:1165–1171. doi: 10.1016/S0039-9140(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 20.Isab A. A 1H NMR study of the reaction of gold (III) with DL-selenomethionine in aqueous solution. Inorg Chim Acta. 1983;80:L3–L4. [Google Scholar]
- 21.Zainal HA, Wolf WR, Waters RM. An NMR spectroscopic investigation of the oxidation reactions of DL-selenomethionine. J Chem Technol Biotechnol. 1998;72:38–44. [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Lash LH, Sausen PJ, Duescher RJ, Cooley AJ, Elfarra AA. Roles of cysteine conjugate β-lyase and S-oxidase in nephrotoxicity: studies with S-(1,2- dichlorovinyl)-L-cysteine and S-(1,2-dichlorovinyl)-L-cysteine sulfoxide. J Pharmacol Exp Therap. 1994;269:374–383. [PubMed] [Google Scholar]
- 24.Davis FA, Reddy RT. Asymmetric oxidation of simple selenides to selenoxides in high entantiopurity. Stereochemical aspects of the allyl selenoxide/allyl selenenate rearrangement. J Org Chem. 1992;57:2599–2606. [Google Scholar]
- 25.Shimizu T, Kobayashi M, Kamigata N. Configurational stability of optically active selenoxides; racemization via achiral hydrate. Bull Chem Soc Jpn. 1988;61:3761–3763. [Google Scholar]
- 26.Reich HJ. Organoselenium Oxidations. In: Trahanovsky WS, editor. Oxidation in Organic Chemistry Part C. Academic Press Inc; New York: 1978. pp. 1–33. [Google Scholar]
- 27.Kondo N, Fueno H, Fujimoto H, Makino M, Nakaoka H, Aoki I, Uemura S. Theoretical and experimental studies of regioselectivity in selenoxide elimination. J Org Chem. 1994;59:5254–5263. [Google Scholar]
- 28.Elfarra AA, Krause RJ. Potential roles of flavin-containing monooxygenases in sulfoxidation reactions of L-methionine, N-acetyl-L-methionine and peptides containing L-methionine. Biochim Biophys Acta. 2005;1703:183–189. doi: 10.1016/j.bbapap.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Ripp SL, Overby LH, Philpot RM, Elfarra AA. Oxidation of cysteine S-conjugates by rabbit liver microsomes and cDNA-expressed flavin-containing monooxygenases: studies with S-(1,2-dichlorovinyl)-L-cysteine, S-(1,2,2-trichlorovinyl)- L-cysteine, S-allyl-L-cysteine, and S-benzyl-L-cysteine. Mol Pharmacol. 1997;51:507–515. [PubMed] [Google Scholar]
- 30.Chen G-P, Ziegler DM. Liver microsome and flavin-containing monooxygenase catalyzed oxidation of organic selenium compounds. Arch Biochem Biophys. 1994;312:566–572. doi: 10.1006/abbi.1994.1346. [DOI] [PubMed] [Google Scholar]
- 31.Korsmeyer KK, Guan S, Yang Z-C, Falick A, Ziegler DM, Cashman JR. N-Glycosylation of pig flavin-containing monooxygenase form 1: determination of the site of protein modification by mass spectrometry. Chem Res Toxicol. 1998;9:1183–1193. doi: 10.1021/tx980117p. [DOI] [PubMed] [Google Scholar]
- 32.Padmaja S, Squadrito GL, Lemercier JN, Cueto R, Pryor WA. Rapid oxidation of DL-selenomethionine by peroxynitrite. Free Rad Biol Med. 1996;21:317–322. doi: 10.1016/0891-5849(96)00132-3. [DOI] [PubMed] [Google Scholar]
- 33.Markman GD, Hafner EW, Tabor CW, Tabor H. S-Adenosylmethionine synthetase from Escherichia coli. J Biol Chem. 1980;255:9082–9092. [PubMed] [Google Scholar]
- 34.Konze JR, Kende H. Interactions of methionine and selenomethionine with methionine adenosyltransferase and ethylene-generating systems. Plant Physiol. 1979;63:507–510. doi: 10.1104/pp.63.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esaki N, Tanaka H, Uemura S, Suzuki T, Soda K. Catalytic action of Lmethionine γ-lyase on selenomethionine and selenols. Biochemistry. 1979;18:407–410. doi: 10.1021/bi00570a003. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler DM, Graf P, Poulsen LL, Stahl W, Sies H. NADPH-Dependent oxidation of reduced ebselen, 2-selenylbenzanilide, and 2- (methylseleno)benzanilide catalyzed by pig liver flavin-containing monooxygenase. Chem Res Toxicol. 1992;5:163–166. doi: 10.1021/tx00026a004. [DOI] [PubMed] [Google Scholar]
- 37.Goeger DE, Ganther HE. Oxidation of dimethylselenide to dimethylselenoxide by microsomes from rat liver and lung and by flavin containing monooxygenase from pig liver. Arch Biochem Biophys. 1994;310:448–451. doi: 10.1006/abbi.1994.1191. [DOI] [PubMed] [Google Scholar]
- 38.Rooseboom M, Commandeur JNM, Floor GC, Rettie AE, Vermeulen NPE. Selenoxidation by flavin-containing monooxygenases as a novel pathway for β-elimination of selenocysteine Se-conjugates. Chem Res Toxicol. 2001;14:127–134. doi: 10.1021/tx0001326. [DOI] [PubMed] [Google Scholar]
- 39.Assmann A, Briviba K, Sies H. Reduction of methionine selenoxide to selenomethionine by glutathione. Arch Biochem Biophys. 1998;349:201–203. doi: 10.1006/abbi.1997.0462. [DOI] [PubMed] [Google Scholar]
- 40.Yang C-F, Shen H-M, Ong CN. Ebselen induces apoptosis in HepG2 cells through rapid depletion of intracellular thiols. Arch Biochem Biophys. 2000;374:142– 152. doi: 10.1006/abbi.1999.1574. [DOI] [PubMed] [Google Scholar]