Abstract
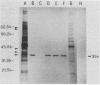
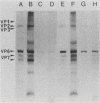
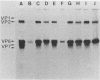
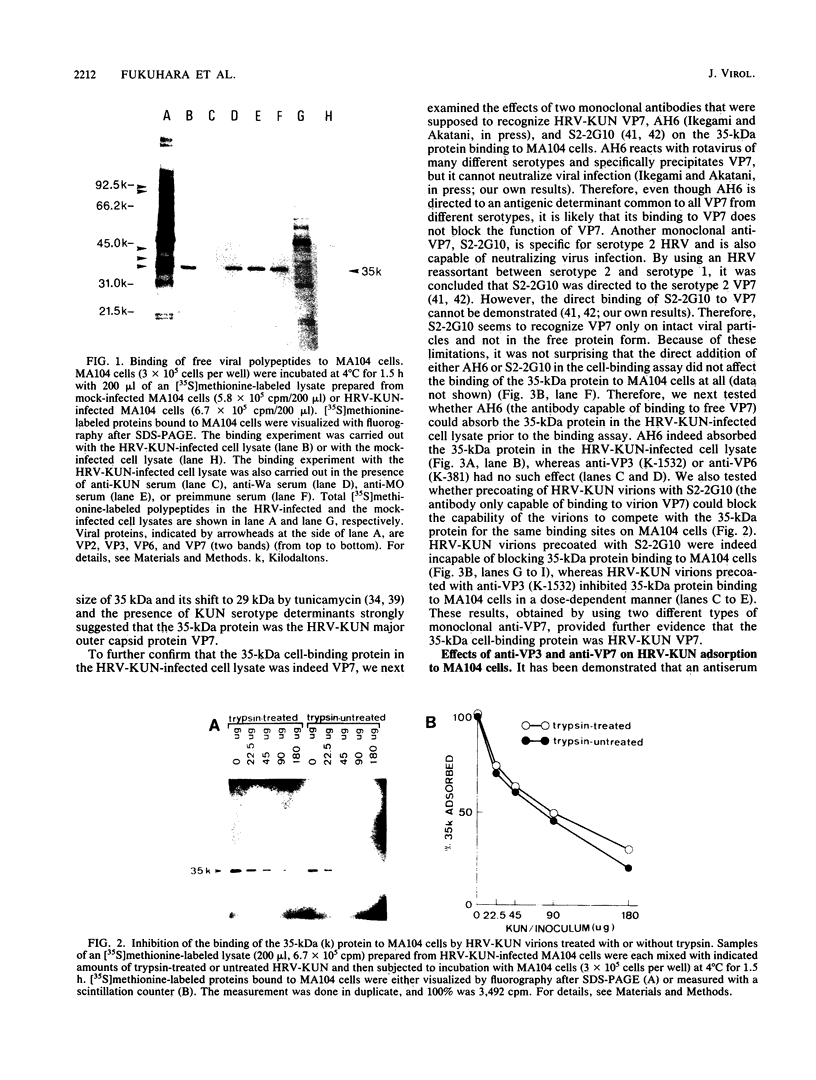
A cell lysate prepared from MA104 cells that had been infected with human rotavirus KUN strain (HRV-KUN) contained a 35-kilodalton protein capable of binding to MA104 cells. The binding of the 35-kilodalton protein was inhibited by a serotype 2-specific antiserum but not by antisera to other serotypes. Not only trypsin-treated, infectious HRV-KUN but also untreated, noninfectious virions effectively competed with the 35-kilodalton protein for the same cell surface binding sites. One monoclonal anti-VP7 (AH6) absorbed the 35-kilodalton protein from the HRV-KUN-infected cell lysate, whereas another monoclonal anti-VP7 (S2-2G10) inhibited the virions to compete with the 35-kilodalton protein for the cell surface binding sites. Both anti-VP7 (S2-2G10) and anti-VP3 (K-1532, K-376) monoclonal antibodies had the virus-neutralization activity, but only anti-VP7 inhibited virus adsorption. On the other hand, anti-VP3 monoclonal antibodies were capable of completely inhibiting the infection of preadsorbed HRV-KUN as long as virions were not yet internalized. Subsequent studies with [35S]methionine-labeled and purified HRV-KUN showed that not only trypsin-treated, infectious virions but also untreated, noninfectious virions were capable of efficient target cell binding and internalization. The internalization modes of these two HRV-KUN preparations were, however, quite different. Only the components of the inner capsid were internalized from trypsin-treated virions, whereas no such selective internalization was seen with untreated virions. Furthermore, anti-VP3 inhibited this selective internalization of the inner capsid from the infectious virions. From these results we conclude that VP7 is the HRV-KUN cell attachment protein and that adsorption of HRV-KUN via VP7 is independent of trypsin treatment, whereas the limited cleavage of VP3 by trypsin, which is essential for the development of HRV-KUN infectivity, is needed for the selective internalization of the inner capsid components, a process that is apparently essential for HRV-KUN infection.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banfield W. G., Kasnic G., Blackwell J. H. Further observations on the virus of epizootic diarrhea of infant mice. An electron microscopic study. Virology. 1968 Nov;36(3):411–421. doi: 10.1016/0042-6822(68)90166-9. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973 Dec 8;2(7841):1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Borsa J., Morash B. D., Sargent M. D., Copps T. P., Lievaart P. A., Szekely J. G. Two modes of entry of reovirus particles into L cells. J Gen Virol. 1979 Oct;45(1):161–170. doi: 10.1099/0022-1317-45-1-161. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Penaranda M. E., Crawford S. E., Estes M. K. Two glycoproteins are produced from the rotavirus neutralization gene. Virology. 1986 Jun;151(2):243–252. doi: 10.1016/0042-6822(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Barnett B. B., Spendlove R. S. Production of high-titer bovine rotavirus with trypsin. J Clin Microbiol. 1979 Mar;9(3):413–417. doi: 10.1128/jcm.9.3.413-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Roth J. R., Clark M. L., Barnett B. B., Spendlove R. S. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J Virol. 1981 Sep;39(3):816–822. doi: 10.1128/jvi.39.3.816-822.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Greenberg H. B., Myslinski J., Kalica A. R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Use of transcription probes for genotyping rotavirus reassortants. Virology. 1982 Sep;121(2):288–295. doi: 10.1016/0042-6822(82)90168-4. [DOI] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T., Ishida N. Evidence for endocytosis-independent infection by human rotavirus. Arch Virol. 1987;97(1-2):93–99. doi: 10.1007/BF01310737. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Estes M. K. Proteolytic enhancement of rotavirus infectivity: biology mechanism. Virology. 1980 Mar;101(2):432–439. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Fukuhara N., Tazawa F., Suzuki H., Sato T., Konno T., Ebina T., Ishida N. Characterization of monoclonal antibodies against human rotavirus hemagglutinin. J Med Virol. 1986 Aug;19(4):313–323. doi: 10.1002/jmv.1890190404. [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Suzuki H., Numazaki T., Sato T., Konno T., Ebina T., Ishida N., Nakagomi O., Nakagomi T. Hemagglutination by human rotavirus strains. J Med Virol. 1984;13(3):215–222. doi: 10.1002/jmv.1890130303. [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Suzuki H., Numazaki Y., Konno T., Ishida N. The effect of trypsin on the growth and infectivity of human rotavirus. Tohoku J Exp Med. 1986 Aug;149(4):437–447. doi: 10.1620/tjem.149.437. [DOI] [PubMed] [Google Scholar]
- Kouvelos K., Petric M., Middleton P. J. Comparison of bovine, simian and human rotavirus structural glycoproteins. J Gen Virol. 1984 Jul;65(Pt 7):1211–1214. doi: 10.1099/0022-1317-65-7-1211. [DOI] [PubMed] [Google Scholar]
- Kouvelos K., Petric M., Middleton P. J. Oligosaccharide composition of calf rotavirus. J Gen Virol. 1984 Jul;65(Pt 7):1159–1164. doi: 10.1099/0022-1317-65-7-1159. [DOI] [PubMed] [Google Scholar]
- Kutsuzawa T., Konno T., Suzuki H., Kapikian A. Z., Ebina T., Ishida N. Isolation of human rotavirus subgroups 1 and 2 in cell culture. J Clin Microbiol. 1982 Oct;16(4):727–730. doi: 10.1128/jcm.16.4.727-730.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Protein sigma 1 is the reovirus cell attachment protein. Virology. 1981 Jan 15;108(1):156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- Lenard J., Miller D. K. Uncoating of enveloped viruses. Cell. 1982 Jan;28(1):5–6. doi: 10.1016/0092-8674(82)90368-3. [DOI] [PubMed] [Google Scholar]
- Ludert J. E., Michelangeli F., Gil F., Liprandi F., Esparza J. Penetration and uncoating of rotaviruses in cultured cells. Intervirology. 1987;27(2):95–101. doi: 10.1159/000149726. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982 Apr 15;156(3):609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Inouye S. Purification of an outer capsid glycoprotein of neonatal calf diarrhea virus and preparation of its antisera. Infect Immun. 1983 Jan;39(1):155–158. doi: 10.1128/iai.39.1.155-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Graham D. Y., Estes M. K. Identification of rotavirus particle types. Intervirology. 1981;16(1):20–28. doi: 10.1159/000149243. [DOI] [PubMed] [Google Scholar]
- Quan C. M., Doane F. W. Ultrastructural evidence for the cellular uptake of rotavirus by endocytosis. Intervirology. 1983;20(4):223–231. doi: 10.1159/000149395. [DOI] [PubMed] [Google Scholar]
- Sabara M., Gilchrist J. E., Hudson G. R., Babiuk L. A. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J Virol. 1985 Jan;53(1):58–66. doi: 10.1128/jvi.53.1.58-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Suzuki H., Kitaoka S., Konno T., Ishida N. Patterns of polypeptide synthesis in human rotavirus infected cells. Arch Virol. 1986;90(1-2):29–40. doi: 10.1007/BF01314142. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kitaoka S., Konno T., Sato T., Ishida N. Two modes of human rotavirus entry into MA 104 cells. Arch Virol. 1985;85(1-2):25–34. doi: 10.1007/BF01317003. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kitaoka S., Sato T., Konno T., Iwasaki Y., Numazaki Y., Ishida N. Further investigation on the mode of entry of human rotavirus into cells. Arch Virol. 1986;91(1-2):135–144. doi: 10.1007/BF01316734. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Konno T., Numazaki Y., Kitaoka S., Sato T., Imai A., Tazawa F., Nakagomi T., Nakagomi O., Ishida N. Three different serotypes of human rotavirus determined using an interference test with coxsackievirus B 1. J Med Virol. 1984;13(1):41–44. doi: 10.1002/jmv.1890130105. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Sato T., Konno T., Kitaoka S., Ebina T., Ishida N. Effect of tunicamycin on human rotavirus morphogenesis and infectivity. Brief report. Arch Virol. 1984;81(3-4):363–369. doi: 10.1007/BF01310008. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Ohnishi S. Uncoating of influenza virus in endosomes. J Virol. 1984 Aug;51(2):497–504. doi: 10.1128/jvi.51.2.497-504.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]