Abstract
OBJECTIVE
Extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli are increasingly common in nosocomial and community settings. Furthermore, fluoroquinolone (FQ) and even multidrug resistance (MDR) appear to be associated with certain ESBL genotypes. The purpose of the present study was to determine which ESBL genotypes are associated with FQ and MDR in E coli urinary isolates in Manitoba.
METHODS
The authors determined the antimicrobial susceptibility, genetic similarity and ESBL genotype of 27 FQ-resistant and seven FQ-susceptible, ESBL-producing urinary isolates submitted to the clinical microbiology laboratories of two teaching hospitals between October 2000 and April 2005. Susceptibilities to beta-lactams, FQs, trimethoprim-sulfamethoxazole (SXT), doxycycline (DOX), gentamicin (GM) and tigecycline were determined by microbroth dilution; pulsed-field gel electrophoresis (PFGE) was used to determine genetic relatedness, and ESBL genotype was determined by polymerase chain reaction and sequencing.
RESULTS
Of 34 ESBL-producing organisms, 27 (79.4%) were found to be ciprofloxacin (CIP) resistant, 27 (79.4%) were SXT resistant, eight (23.5%) were GM resistant and 29 (85.3%) were DOX resistant. Twenty-three (67.6%) had MDR, with concomitant resistance to CIP and SXT; 16 had concomitant resistance to CIP, SXT and DOX; and seven (20.6%) had MDR, with concomitant resistance to CIP, SXT, DOX and GM. All isolates were susceptible to tigecycline. Of 27 FQ-resistant ESBL-producing organisms, seven (25.9%) were genotype CTX-M-14, 19 (70.4%) were genotype CTX-M-15 and one (3.7%) was genotype CTX-M-24. Among the seven FQ-susceptible strains, three (42.8%) expressed SHV-type enzymes, three (42.8%) expressed TEM-type enzymes and one (14.3%) expressed CTX-M-9. CTX-M-15 was the most common MDR-associated genotype. Of a total of 19 strains, 18 (94.7%) were resistant to FQs and SXT; 15 (78.9%) were resistant to FQs, SXT and DOX; and five (26.3%) were resistant to FQs, SXT, DOX and GM. PFGE analysis revealed genetic similarity within CTX-M-15-producing isolates only.
CONCLUSION
CTX-M-15 in E coli is strongly associated with an MDR phenotype compared with other genotypes. CTX-M-14 is associated with FQ resistance only. PFGE suggests clonality of CTX-M-15-producing isolates within and among hospitals.
Keywords: CTX-M-15, ESBL, Escherichia coli, Fluoroquinolone-resistant, Molecular epidemiology, Multidrug-resistant
Abstract
BUT
Les souches d’Escherichia coli productrices de bêta-lactamases à spectre étendu (BLSE) sont de plus en plus souvent mises en causes dans les infections nosocomiales et les infections extrahospitalières. De plus, la résistance aux fluoroquinolones (FQ) et même la multirésistance aux antibiotiques (MRA) semblent associées à certains génotypes de BLSE. La présente étude avait pour but de déterminer quels génotypes de BLSE étaient associés aux FQ et à la MRA dans des isolats urinaires d’E. coli, au Manitoba.
MÉTHODE
Les auteurs ont procédé à la détermination de la sensibilité antimicrobienne, de la ressemblance génétique et du génotype de BLSE de 27 isolats urinaires, producteurs de BLSE, résistants aux FQ et de 7 isolats sensibles aux FQ, soumis aux laboratoires de microbiologie clinique de deux hôpitaux universitaires, entre octobre 2000 et avril 2005. La sensibilité aux bêtalactamines, aux FQ, au triméthoprime, au sulfaméthoxazole (Sul), à la doxycycline (Dox), à la gentamycine (Gen) et à la tigécycline a été déterminée par la technique de microdilution en milieu liquide; les ressemblances génétiques ont été déterminées par l’électrophorèse en champ pulsé (ECP) et le génotype des BLSE a été déterminé par l’amplification en chaîne par polymérase et par le séquençage.
RÉSULTATS
Sur 34 micro-organismes producteurs de BLSE, 27 (79,4 %) étaient résistants à la ciprofloxacine (Cip); 27 (79,4 %), au Sul; 8 (23,5 %), à la Gen; et 29 (85,3 %), à la Dox. Vingt-trois (67,6 %) présentaient une résistance concomitante à la Cip et au Sul; 16, une résistance concomitante à la Cip, au Sul et à la Dox; et 7 (20,6 %) une résistance concomitante à la Cip, au Sul, à la Dox et à la Gen. Tous les isolats se sont montrés sensibles à la tigécycline. Sur les 27 micro-organismes producteurs de BLSE, résistants aux FQ, 7 (25,9 %) présentaient le génotype CTX-M-14; 19 (70,4 %), le génotype CTX-M-15; et 1 (3,7 %), le génotype CTX-M-24. Parmi les 7 souches sensibles aux FQ, 3 (42,8 %) exprimaient des enzymes de type SHV; 3 (42,8 %), des enzymes de type TEM; et 1 (14,3 %), le génotype CTX-M-9. Le génotype le plus souvent associé à la MRA était le CTX-M-15. Sur un total de 19 souches, 18 (94,7 %) étaient résistantes aux FQ et au Sul; 15 (78,9 %), aux FQ, au Sul et à la Dox; et 5 (26,3 %), aux FQ, au Sul, à la Dox et à la Gen. L’analyse par ECP a révélé des ressemblances génétiques parmi les isolats ayant le génotype CTX-M-15 seulement.
CONCLUSIONS
Le génotype CTX-M-15 présent dans les souches d’E. coli est fortement associé au phénotype de la MRA, comparativement aux autres génotypes. Le génotype CTX-M-14 est associé à la résistance aux FQ seulement. Les résultats de l’ECP semblent confirmer la clonalité des isolats producteurs du génotype CTX-M-15, que ce soit dans les hôpitaux ou entre les hôpitaux.
Extended-spectrum beta-lactamase (ESBL)-producing organ isms have spread worldwide since their first description in 1983 (1). Over 300 Ambler class A ESBL genotypes have now been described, and they have been reported in numerous species of the Enterobacteriaceae family, including Escherichia coli, Klebsiella pneumoniae and Salmonella species, as well as in non-Enterobacteriaceae (1). Furthermore, the spread of ESBL producers is increasingly becoming a local issue. Recent studies (2,3) from Calgary, Alberta, have clearly demonstrated the increasing prevalence of ESBL-producing E coli isolates in the community and hospital setting; local surveillance data from one hospital microbiology laboratory in Winnipeg, Manitoba, demonstrated that the number of ESBL-producing E coli isolates had increased dramatically from one (0.05% of E coli isolates) in 2000, when surveillance of ESBL isolates began, to 24 (0.8% of E coli isolates) in 2005.
ESBLs, named because of their activity against third- and fourth-generation cephalosporins, have a broad spectrum of activity and hydrolyze, to some degree, all cephalosporins and penicillins (1). In addition, there are increasing reports of ESBL-producing clinical isolates expressing multidrug resistance (MDR), defined as concomitant resistance to at least three different antibiotic classes (1,4–6). Organisms with varying resistance to fluoroquinolones (FQs), sulfonamides, aminoglycosides and tetracyclines, in addition to their ESBL phenotype, are not uncommon and may severely limit therapeutic options (1,5,7). In fact, carbapenems are the only antimicrobials with consistent activity against almost all ESBL-producing organisms and remain the drug of choice in severe infections (1,7).
Most ESBLs are members of the Ambler class A beta-lactamases and consist of three main genotypes based on sequence and structure: SHV, TEM and CTX-M. These and other rarer ESBLs exist and have recently been summarized in a review by Paterson and Bonomo (1). SHV and TEM ESBLs are derivatives of the narrow-spectrum SHV-1 and TEM-1/2 enzymes that are commonly found in many members of the Enterobacteriaceae family. CTX-M enzymes, named because of their strong hydrolytic activity for cefotaxime, are newer additions to the ESBL family (1,8). Studies have shown that most ESBLs are coded by plasmids, some of which also carry genes conferring resistance to other antimicrobials such as FQs, trimethoprim-sulfamethoxazole (SXT), tetracyclines and aminoglycosides (6,9,10). The purpose of the present study was to analyze and compare the ESBL genotypes and genetic relatedness of ESBL-producing E coli urinary isolates, with and without FQ resistance, in outpatient areas of Manitoba hospitals to determine whether any genotype is more strongly associated with FQ or MDR. In the present study, ESBLs with MDR were defined as those with concomitant resistance to two antimicrobial classes in addition to the resistance phenotype afforded by the ESBL.
METHODS
Isolates were obtained from urine specimens submitted to the clinical microbiology departments of the St Boniface General Hospital and the Health Sciences Centre in Winnipeg, Manitoba, between October 2000 and April 2005. Both laboratories are centralized and process samples from four community hospitals in addition to their own hospital specimens. Specimens were from outpatient hospital settings, including emergency departments and ambulatory care clinics. FQ-resistant organisms were collected from both hospitals as part of a larger study of all FQ-resistant outpatient urinary isolates submitted to these laboratories (North American Urinary Tract Infection Collaborative Alliance) (11). FQ-susceptible isolates were collected from the same period of time, but only from the St Boniface General Hospital microbiology laboratory because it stocks all ESBL-producing isolates as a matter of surveillance policy. The organisms were identified as E coli with a potential ESBL phenotype using the Vitek automated system (bioMérieux, France). The ESBL phenotype was confirmed using the confirmation method described by the Clinical and Laboratory Standards Institute (CLSI; USA) (12) at the laboratory of origin and at the National Microbiology Laboratory in Winnipeg, Manitoba, using cefpodoxime, cefotaxime and ceftazidime disks (Mast Diagnostics, United Kingdom) with and without clavulanate. Isolates with cefoxitin resistance were excluded from the study to exclude possible concomitant overproduction of a Class C (AmpC) beta-lactamase. Antimicrobial susceptibility to ampicillin, cephalexin, cefuroxime, cefotaxime, cefdinir, cefepime, ciprofloxacin (CIP), levofloxacin, meropenem, doxycycline (DOX), gentamicin (GM), SXT and tigecycline was determined using the microbroth dilution method in cation-adjusted Mueller-Hinton broth as per CLSI guidelines (13). Pulsed-field gel electrophoresis (PFGE) was performed as described by Barrett et al (14), with some modifications. XbaI restriction fragments were resolved on a Bio-Rad CHEF DRIII (Bio-Rad Laboratories, USA), with switch times of 2.2 s to 54.2 s at 14°C for 19 h. PGFE patterns were interpreted according to the criteria of Tenover et al (15) and digitized for analysis using BioNumerics software version 3.0 (Applied Maths, USA). Polymerase chain reaction (PCR) of the blaCTX-M, blaSHV and blaTEM genes was performed using methods described by Mulvey et al (16). Briefly, whole DNA was extracted from cells, and was subjected to PCR amplification using universal in-house primers for blaCTX-M, blaTEM and blaSHV. Subsequently, amplicons were purified using amicon filters (Millipore Corporation, Canada) and sequenced using an ABI 3100 Genetic Analyser (Applied Biosystems, USA) at the DNA core facility at the National Microbiology Laboratory to identify the ESBL genotype responsible for cephalosporin resistance. The universal blaCTX-M primers amplified only part of the CTX-M gene, insufficient for identification beyond the genetically distinct CTX-M groups: CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9 and CTX-M-25. Isolates with CTX-M genes were subjected to a second PCR using a separate set of in-house primers specific to the CTX-M group, identified to sequence the entire CTX-M gene in question for final identification. In isolates coexpressing CTX-M and SHV genes, only the CTX-M gene was assumed to be the gene responsible for the ESBL phenotype. The authors justified this approach because a study by Mulvey et al (16) had shown that enzyme isoelectrical focusing of local ESBL producers with both CTX-M and SHV genes expressed only SHV-1. Statistical associations between resistance phenotypes and ESBL genotypes were determined using JMP software (SAS Institute, USA) with a Tukey-Kramer all-pairs comparison test, and possible associations between resistance phenotypes were determined by multiple regression analysis using a significance level of P=0.05.
RESULTS
The exact incidence and distribution of these urinary ESBL isolates cannot be determined by the present study because the sampling of FQ-resistant and FQ-susceptible isolates came from different pools of ESBL-producing organisms, and, therefore, the denominators were not known. Because the FQ-resistant isolates all came from both hospital microbiology laboratories, an estimate can be made of the incidence of FQ-resistant, ESBL-producing E coli submitted to these laboratories. Twenty-seven isolates were identified over a period of 54 months, or one isolate every two months. However, there has been a great increase in the rate of isolation, from one isolate in 2000 to 21 isolates in 2004.
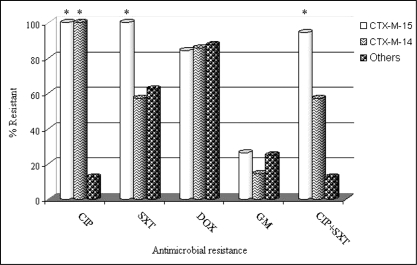
PCR and sequencing of ESBL genes identified 34 ESBL enzymes in 34 isolates: 19 CTX-M-15 (members of the CTX-M-1 group), seven CTX-M-14, one CTX-M-24, one CTX-M-9 (all members of the CTX-M-9 group), three TEM (TEM-11, -52 and -52) and three SHV (SHV-2a, -5 and -12) genes. Three of the CTX-M-15-producing organisms also expressed an SHV enzyme, which was not sequenced because previous studies had shown that all local CTX-M-producing stains coexpressed only SHV-1 (see Methods). As expected, all ESBL-producing isolates were resistant to ampicillin (minimal inhibitory concentration [MIC] of at least 32 mg/L) and had reduced susceptibility to at least one third-generation cephalosporin. Furthermore, many isolates had additional resistance phenotypes. Relationships between genotype and resistance phenotypes are shown in Table 1. One isolate was resistant only to beta-lactams, three had one additional resistance phenotype, 10 had two additional resistance phenotypes, 13 had three additional resistance phenotypes and seven had four additional resistance phenotypes. For all 34 ESBL-producing isolates, coresistance to SXT, DOX and GM were significantly correlated (P=0.0376). Within the CTX-M-15 ESBL genotype, resistance to SXT and CIP were correlated (P<0.001), as was resistance to DOX and CIP (P=0.0419). No correlations for antibiotic resistance were noted within other genotype subgroups. The CTX-M-15 ESBL genotype was significantly associated with CIP resistance (P<0.01), SXT resistance (P<0.01) resistance, and the combination of CIP and SXT resistance (P<0.01) compared with other genotypes. The CTX-M-14 genotype was significantly associated with CIP resistance only (P<0.01) compared with other non-CTX-M-15 ESBL producers. Figure 1 demonstrates the relative frequency of the resistance phenotypes by ESBL genotype.
TABLE 1.
Distribution of genotypes by resistance phenotype
| Genotype | CIP resistance, n (%) | SXT resistance, n (%) | DOX resistance, n (%) | GM resistance, n (%) |
|---|---|---|---|---|
| CTX-M-15 (n=19) | 19 (100) | 18 (94.7) | 16 (84.2) | 5 (26.3) |
| CTX-M-14 (n=7) | 7 (100) | 4 (57.1) | 6 (85.7) | 1 (14.3) |
| CTX-M-9 (n=1) | 0 (0) | 1 (100) | 1 (100) | 0 (0) |
| CTX-M-24 (n=1) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| SHV (n=3) | 0 (0) | 2 (66.7) | 3 (100) | 0 (0) |
| TEM (n=3) | 0 (0) | 1 (33.3) | 2 (66.7) | 1 (33.3) |
Resistance breakpoints were ciprofloxacin (CIP) (minimal inhibitory concentration [MIC] of at least 4 mg/L), trimethoprim-sulfamethoxazole (SXT) (MIC of at least 8:152 mg/L), doxycycline (DOX) (MIC of at least 16 mg/L) and gentamicin (GM) (MIC of at least 16 mg/L)
Figure 1.
Comparative frequency of ciprofloxacin (CIP), trimethoprim-sulfamethoxazole (SXT), doxycycline (DOX), gentamicin (GM) and multidrug resistance by extended-spectrum beta-lactamase genotype. Multidrug resistance phenotypes not included were not independently associated with a particular genotype. *P<0.01
MDR occurred more commonly with the CTX-M-15 genotype than with other observed genotypes. The majority of the FQ-resistant isolates (70.3%) were CTX-M-15 ESBL producers. Furthermore, 94.7% of CTX-M-15 ESBL producers were resistant to FQs and SXT, and 84.2% were resistant to FQs, SXT and DOX, although only the former resistance phenotype was significantly and independently associated with CTX-M-15. Although five antibiotic class resistance was more common in CTX-M-15 isolates (26.3%) than either CTX-M-14 (14.2%) or other (12.5%) isolates, this did not achieve significance. CTX-M-14 was also associated with FQ resistance compared with non-CTX-M-producing isolates, but it was not significantly associated with either SXT, GM, DOX or an MDR phenotype compared with other ESBL-producing organisms in the present study (Figure 1). All ESBL-producing isolates in the present study were susceptible to tigecycline, with an MIC of 1 mg/L or less.
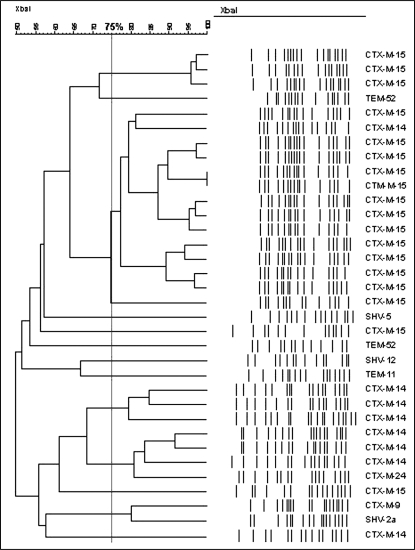
Figure 2 demonstrates the genetic relationship among the isolates harbouring an ESBL. PFGE revealed that the isolates of the CTX-M-15 genotype were more genetically homogeneous than other ESBL genotypes. Dendrogram analysis identified a predominant cluster with a coefficient of similarity of at least 75% containing 14 of 19 (73.7%) of the CTX-M-15 isolates. A small degree of clustering, although to a much lesser extent, was also observed among the CTX-M-14 isolates.
Figure 2.
Dendrogram of 34 extended-spectrum beta-lactamase-producing Escherichia coli urinary isolates identified in the present study, with corresponding genotypes. There is considerable heterogeneity among CTX-M-15 isolates
DISCUSSION
One possible explanation for the significant coresistance among CTX-M-15 ESBL-producing isolates is the presence of multiple plasmid-borne resistance loci (9). Many resistance genes, including most ESBLs, are encoded by plasmid-integrated transposable elements (1,6). When analyzing our resistance data from all the ESBL-producing isolates for any associations among resistance phenotypes, the only significant correlation was coresistance to DOX, SXT and GM. This correlation may be due to a single plasmid carrying resistance to these three agents, as well as the ESBL gene. Indeed, this phenomenon has been observed in a number of plasmid analysis studies. In one such study of an ESBL producer outbreak in Toronto, Ontario (6), the CTX-M-15 plasmid also carried transposable resistance determinants to multiple classes of antimicrobials, including aminoglycosides via drug modifying enzymes and tetracyclines via efflux pumps. Earlier studies of plasmids harbouring ESBLs also demonstrated multiple resistance genes, including aminoglycoside, sulfonamide and tetracycline resistance genes (9). The presence of a similar plasmid could explain MDR in our CTX-M-15 isolates. FQ resistance was not correlated with any other antibiotic resistance overall, but was associated with STX and DOX resistance in the CTX-M-15 and CTX-M-14 genotypes. Because most FQ resistance is chromosomally mediated through quinolone resistance determining region (QRDR) mutations, it is not surprising that no statistical association with common plasmid-mediated resistance genes was observed overall. However, the strong correlation of FQ resistance to CTX-M-14 and CTX-M-15/SXT/DOX resistance raises the possibility of plasmid-mediated quinolone resistance. Plasmid analysis in some ESBL-producing isolates has shown the presence of the qnr gene (10,17). The qnr gene confers partial resistance to FQs by protecting DNA gyrase from the effects of FQs (17). FQ resistance through plasmid-mediated efflux pumps, often conferring low-level resistance to multiple antimicrobials, has also been described (17,18). In addition, enhancers of chromosomal efflux pump expression have been localized to plasmids, leading to increased expression of chromosomal efflux pumps (19,20). Although FQ resistance is not typically encoded on plasmids, and high-level resistance is generally conferred by chromosomal mutations of gyrA and/or parC (together known as the QRDR), low-level, plasmid-mediated resistance may encourage the development of high-level resistance under antimicrobial pressure by increasing the mutation prevention concentration (17,21). Therefore, the coexpression of a qnr gene and efflux pumps on plasmids carrying ESBLs may encourage the development of gyrA- and parC-mediated FQ resistance.
Although plasmid-mediated quinolone resistance has been reported, the presence of efflux-based resistance mechanisms has never been documented in ESBL-bearing plasmids; qnr-mediated quinolone resistance remains extremely rare in ESBL-bearing plasmids and has not yet been reported in Canadian E coli isolates. Indeed, an ongoing study of nosocomial FQ-resistant E coli isolates by our laboratory, including several CTX-M-15-producing isolates, has failed to demonstrate the presence of any of the three known qnr genes (data not shown). Only QRDR mutations have been implicated as mechanisms of FQ resistance in these local E coli isolates. Furthermore, all of the CIP-resistant isolates in the present study had MICs of at least 32 mg/L, suggesting high-level QRDR-mediated resistance. Therefore, the presence of qnr in these CTX-M-expressing plasmids is extremely remote, and, based on the findings of our ongoing nosocomial FQ resistance study, we elected not to pursue the identification of qnr genes from the isolates in the present study. However, it is worth mentioning that the absence of qnr in these ESBL-producing isolates substantially reduces the likelihood, but does not absolutely disprove, the plasmid-borne gene's role in the acquisition of high-level FQ resistance. The loss of qnr from the culprit plasmid could have occurred, even under antibiotic pressure, once qnr-assisted, QRDR-based resistance to FQs was established.
Resistance to SXT is most frequently encoded on plasmids and has been previously identified on plasmids coexpressing ESBLs (9,22). Most commonly, genes known as dhfr and dhfs express low-affinity enzymes that confer resistance to trimethoprim and sulfamethoxazole, respectively (22). The significant degree of SXT resistance in CTX-M-15-bearing isolates (94.7%) suggests that these resistance genes may indeed be present on this plasmid at a greater frequency than on other ESBL producers. Likewise, the high proportion of all the ESBL-producing isolates with DOX resistance (29 of 34 [85.3%]) suggests that these resistance genes may be coexpressed on plasmids with any of the several ESBL genes.
Another means of acquiring MDR in CTX-M-15 isolates may be sequential acquisition of resistance to multiple antimicrobial classes rather than acquisition through a single event or plasmid. Increased spread of ESBL-producing organisms in hospitals and personal care homes, where there is significant antimicrobial pressure, may preferentially select organisms that have acquired resistance to multiple classes of antimicrobials, thereby sequentially creating a clone with MDR. This has been observed with multidrug-resistant, methicillin-resistant S aureus, in which community-acquired isolates less often have MDR than nosocomial isolates (23). Coresistance to FQs, in particular, which is more commonly due to chromosomal mutations in genes coding for DNA gyrase rather than plasmids carrying genes for low-level resistance, is more likely to have been acquired independently of a plasmid-borne ESBL gene. Similarly, a proportion of aminoglycoside resistance is determined by chromosomal target site mutations rather than plasmid-mediated, aminoglycoside-modifying enzymes (24).
PFGE of all strains studied revealed a largely heterogeneous genetic population (Figure 2). However, 13 of 20 CTX-M-15 isolates (65%) were clustered in a genetically homogeneous group, with at least 75% genetic concordance and an MDR phenotype. This clustering of isolates originating from a diverse outpatient population, including the emergency departments of one teaching hospital and two community hospitals, also raises the possibility that there is clonal spread within and between hospital outpatient areas. Such spread of ESBLs between individuals, hospitals and even large regions has been described by others (1,25). Clonal spread of ESBL-producing organisms has also been implicated in outbreaks in intensive care units (1). Furthermore, the finding of similar CTX-M-15 PFGE profiles from geographically distinct centres in our study suggests that there is a significant asymptomatic carrier population causing widespread distribution of ESBL-producing organisms by patient or caregiver-to-patient transfer. Indeed, recent studies (25,26) have documented significant asymptomatic carriage of ESBL organisms, even in nonoutbreak situations. Carriage rates were reported to be high as 11.8% in the inpatient population and 3.7% in the general population. Recent reports from Calgary, Alberta, have also reported increasing rates of CTX-M-15-producing E coli in the community setting (2,3). The possibility of clonal spread and significant carriage of such organisms in Canadian hospital outpatient areas and in the community is worrisome and warrants further study. Of particular concern is the common practice of transferring patients between hospital facilities to optimize bed utilization. Such practices may unknowingly lead to the clonal spread of ESBL-producing organisms and other antimicrobial-resistant organisms from unrecognized carriers to noncarriers, both within and between hospital departments. Interestingly, it was the FQ-resistant, CTX-M-15-producing isolates that appeared to be spreading in a clonal fashion in this study. Although the exact cause of this clonal spread remains unclear, horizontal spread of CTX-M-15-bearing organisms with acquired chromosomal FQ resistance may explain the high levels of FQ coresistance in CTX-M-14- and CTX-M-15-producing isolates in Manitoba.
CONCLUSION
MDR is common in E coli-expressing ESBLs. In particular, the CTX-M-15 genotype is associated with three- and four-class MDR. Although the exact mechanism of coresistance remains to be determined, plasmid-borne transposable resistance genes, besides those encoding for ESBL genes, are likely playing a role. This is particularly true of resistance genes commonly found on plasmids, such as those conferring aminoglycoside, DOX and SXT resistance. The transferable nature of these resistance genes is particularly worrisome, and treatment options for infections caused by these organisms are very limited. In addition, the clonal spread of these organisms with MDR may be occurring, and may be contributing to the burden of organisms with MDR in both the community and nosocomial settings. This calls for enhanced infection control and a better understanding of the resistance mechanisms, molecular epidemiology and the means by which spread occurs.
REFERENCES
- 1.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: A clinical update. Clin Microbiol Rev. 2005;18:657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitout JD, Hanson ND, Church DL, Laupland KB. Population-based laboratory surveillance for Escherichia coli-producing extended-spectrum beta-lactamases: Importance of community isolates with blaCTX-M genes. Clin Infect Dis. 2004;38:1736–41. doi: 10.1086/421094. [DOI] [PubMed] [Google Scholar]
- 3.Pitout JD, Nordmann P, Laupland KB, Poirel L. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005;56:52–9. doi: 10.1093/jac/dki166. [DOI] [PubMed] [Google Scholar]
- 4.Morosini MI, Garcia-Castillo M, Coque TM, et al. Antibiotic coresistance in extended-spectrum-beta-lactamase-producing Enterobacteriaceae and in vitro activity of tigecycline. Antimicrob Agents Chemother. 2006;50:2695–9. doi: 10.1128/AAC.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyle EP, Lipworth AD, Zaoutis TE, et al. Risk factors for increasing multidrug resistance among extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species. Clin Infect Dis. 2005;40:1317–24. doi: 10.1086/429239. [DOI] [PubMed] [Google Scholar]
- 6.Boyd DA, Tyler S, Christianson S, et al. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob Agents Chemother. 2004;48:3758–64. doi: 10.1128/AAC.48.10.3758-3764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paterson DL, Ko WC, Von Gottberg A, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: Implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39:31–7. doi: 10.1086/420816. [DOI] [PubMed] [Google Scholar]
- 8.Shah AA, Hasan F, Ahmed S, Hameed A. Characteristics, epidemiology and clinical importance of emerging strains of Gram-negative bacilli producing extended-spectrum beta-lactamases. Res Microbiol. 2004;155:409–21. doi: 10.1016/j.resmic.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Jacoby GA, Sutton L. Properties of plasmids responsible for production of extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991;35:164–9. doi: 10.1128/aac.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Sahm DF, Jacoby GA, Hooper DC. Emerging plasmid-mediated quinolone resistance associated with the qnr gene in Klebsiella pneumoniae clinical isolates in the United States. Antimicrob Agents Chemother. 2004;48:1295–9. doi: 10.1128/AAC.48.4.1295-1299.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhanel GG, Hisanaga TL, Laing NM, et al. NAUTICA Group. Antibiotic resistance in outpatient urinary isolates: Final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA) Int J Antimicrob Agents. 2005;26:380–8. doi: 10.1016/j.ijantimicag.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Pennylvania: Wayne; 2005. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Pennylvania: Wayne; 2003. [Google Scholar]
- 14.Barrett TJ, Lior H, Green JH, et al. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J Clin Microbiol. 1994;32:3013–7. doi: 10.1128/jcm.32.12.3013-3017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulvey MR, Bryce E, Boyd D, et al. Canadian Hospital Epidemiology Committee, Canadian Nosocomial Infection Surveillance Program, Health Canada. Ambler class A extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp in Canadian hospitals. Antimicrob Agents Chemother. 2004;48:1204–14. doi: 10.1128/AAC.48.4.1204-1214.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XZ. Quinolone resistance in bacteria: Emphasis on plasmid-mediated mechanisms. Int J Antimicrob Agents. 2005;25:453–63. doi: 10.1016/j.ijantimicag.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Szczepanowski R, Krahn I, Linke B, Goesmann A, Pühler A, Schlüter A. Antibiotic multiresistance plasmid pRSB101 isolated from a wastewater treatment plant is related to plasmids residing in phytopathogenic bacteria and carries eight different resistance determinants including a multidrug transport system. Microbiology. 2004;150:3613–30. doi: 10.1099/mic.0.27317-0. [DOI] [PubMed] [Google Scholar]
- 19.Alekshun MN, Levy SB. Regulation of chromosomally mediated multiple antibiotic resistance: The mar regulon. Antimicrob Agents Chemother. 1997;41:2067–75. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chollet R, Chevalier J, Bollet C, Pages JM, Davin-Regli A. RamA is an alternate activator of the multidrug resistance cascade in Enterobacter aerogenes. Antimicrob Agents Chemother. 2004;48:2518–23. doi: 10.1128/AAC.48.7.2518-2523.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Martinez L, Pascual A, Jacoby GA. Quinolone resistance from a transferable plasmid. Lancet. 1998;351:797–9. doi: 10.1016/S0140-6736(97)07322-4. [DOI] [PubMed] [Google Scholar]
- 22.Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Clin Infect Dis. 2001;32:1608–14. doi: 10.1086/320532. [DOI] [PubMed] [Google Scholar]
- 23.Rybak MJ, LaPlante KL. Community-associated methicillin-resistant Staphylococcus aureus: A review. Pharmacotherapy. 2005;25:74–85. doi: 10.1592/phco.25.1.74.55620. [DOI] [PubMed] [Google Scholar]
- 24.Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: Activity and resistance. Antimicrob Agents Chemother. 1999;43:727–37. doi: 10.1128/aac.43.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisson G, Fishman NO, Patel JB, Edelstein PH, Lautenbach E. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species: Risk factors for colonization and impact of antimicrobial formulary interventions on colonization prevalence. Infect Control Hosp Epidemiol. 2002;23:254–60. doi: 10.1086/502045. [DOI] [PubMed] [Google Scholar]
- 26.Valverde A, Coque TM, Sanchez-Moreno MP, Rollan A, Baquero F, Canton R. Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spain. J Clin Microbiol. 2004;42:4769–75. doi: 10.1128/JCM.42.10.4769-4775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]