Abstract
INTRODUCTION
While Staphylococcus aureus is an uncommon but serious cause of traditional community-acquired pneumonia (CAP), it is a predominant cause of nosocomial pneumonia in addition to the unique clinical entity of health care-associated pneumonia (HCAP). A cohort of bacteremic S aureus pneumonia cases was reviewed to determine the role of HCAP among the cohort, and to assess for differences between CAP and HCAP.
PATIENTS AND METHODS
Bacteremic S aureus pneumonia cases were identified from a prospective study of all patients diagnosed with CAP who presented to hospitals in Edmonton, Alberta, between November 2000 and November 2002. These cases were subsequently reviewed retrospectively. Demographic, clinical and microbiological data were obtained, and patients were classified as having CAP or HCAP. Relatedness of isolates was determined by pulsed-field gel electrophoresis analysis in conjunction with epidemiological information.
RESULTS
There were 28 cases of bacteremic S aureus pneumonia identified. Fifty-seven per cent were reclassified as having HCAP, and 43% remained classified as having CAP. The CAP cohort was significantly younger than the HCAP cohort (mean age 49.0±23.7 years versus 67.8±18.6 years; P=0.035) with higher rates of intravenous drug use (50% versus 0%; P=0.002). Long-term care facility residence (44%) was common in the HCAP cohort. The HCAP cohort presented with more severe illness, having a higher mean pneumonia severity index score (143.1±41.1 versus 98.2±54.6; P=0.028), and despite fewer embolic complications, there was a trend toward a significantly higher mortality rate (31% versus 0%; P=0.052). Two community-acquired isolates cultured in the setting of intravenous drug use were methicillin-resistant, and no isolates were positive for Panton-Valentine leukocidin. There was evidence of relatedness involving 44% of the HCAP isolates by pulsed-field gel electrophoresis analysis.
CONCLUSION
HCAP accounts for a significant number of cases that, when using traditional definitions, would be classified as CAP. Severity of illness and mortality was excessive within the HCAP group. There was evidence of relatedness and spread of common strains in the HCAP cohort. The present study supports recommendations for treatment guidelines directed toward the entity of HCAP and the empirical coverage of S aureus among certain high-risk groups.
Keywords: Community-acquired pneumonia, Health care-associated pneumonia, Staphylococcus aureus
Abstract
INTRODUCTION
Le staphylocoque doré est une cause peu courante mais grave de pneumonie non nosocomiale (PNN) classique, mais c’est une cause prédominante de pneumonie nosocomiale en plus de l’entité clinique de pneumonie associée aux soins (PAS). On a évalué une cohorte de cas de pneumonie à staphylocoque doré bactériémique pour établir le rôle de la PAS au sein de la cohorte et pour évaluer les différences entre la PNN et la PAS.
PATIENTS ET MÉTHODOLOGIE
On a repéré les cas de pneumonie à staphylocoque doré bactériémique au moyen d’une étude prospective de tous les patients atteints d’une PNN diagnostiquée qui ont consulté dans les hôpitaux d’Edmonton, en Alberta, entre novembre 2000 et novembre 2002. Ces cas ont ensuite fait l’objet d’une analyse rétrospective. On a obtenu les données démographiques, cliniques et microbiologiques, et on a reclassé les patients entre une PNN et une PAS. On a déterminé le rapprochement des isolats au moyen d’une analyse d’électrophorèse en champ pulsé conjointement avec l’information épidémiologique.
RÉSULTATS
On a repéré 28 cas de pneumonie à staphylocoque doré bactériémique. Cinquante-sept pour cent ont été reclassés parmi les PAS, et 43 % parmi les PNN. La cohorte de PNN était considérablement plus jeune que celle de PAS (âge moyen de 49,0±23,7 ans par rapport à 67,8±18,6 ans; P=0,035) et s’associait à des taux plus élevés de médicamentation intraveineuse (50 % par rapport à 0 %; P=0,002). La cohorte de PAS habitait couramment (44 %) dans un établissement de soins de longue durée. Cette cohorte présentait une maladie plus grave et un indice moyen de gravité de la pneumonie plus élevé (143,1±41,1 par rapport à 98,2±54,6; P=0,028), et malgré le moins grand nombre de complications emboliques, on remarquait une tendance vers un taux de mortalité considérablement plus élevé (31 % par rapport à 0 %; P=0,052). Deux isolats non nosocomiaux cultivés pendant une médicamentation intraveineuse étaient méthicillinorésistants, et aucun isolat n’était positif à la leucocidine de Panton-Valentine. On a constaté un rapprochement touchant 44 % des isolats de PAS au moyen de l’analyse d’électrophorèse en champ pulsé.
CONCLUSION
La PAS représente un nombre important de cas qui, selon les définitions classiques, seraient classés parmi les PNN. La gravité de la maladie et la mortalité étaient excessives au sein du groupe de PAS. On a constaté un rapprochement et une propagation des souches courantes au sein de la cohorte de PAS. La présente étude étaye les recommandations de lignes directrices de traitement orientées vers l’entité de la PAS et la couverture empirique du staphylocoque doré au sein de certains groupes très vulnérables.
Health care-associated pneumonia (HCAP) is being increasingly recognized as a clinical entity with significant morbidity and higher mortality than traditional community-acquired pneumonia (CAP) (1). With unique epidemiological, clinical and bacteriological characteristics, HCAP should be considered in the differential diagnosis of individuals with recent health care contact who present to hospitals with signs and symptoms of lower respiratory tract infection. Although presenting from the community, relevant health care contact is considered to include long-term care facility (LTCF) residence, ambulatory clinic hemodialysis, intravenous chemotherapy or home intravenous therapy, specialized home nursing care and recent hospitalization in an acute care hospital. When an individual presents with health care contact of this nature, empirical therapy may be tailored to the probable causative pathogens of HCAP, including Staphylococcus aureus. The American Thoracic Society and the Infectious Diseases Society of America have recently published guidelines that address the management of HCAP (2).
Although generally uncommon as a cause of CAP, S aureus has been implicated as a predominant pathogen in HCAP (1). S aureus pneumonia has classically been described as a secondary bacterial infection in the setting of primary influenza virus upper respiratory tract infection (3–8). However, S aureus has a long history as a cause of nosocomial pneumonia (9,10), and has been known for years to play a significant role in nursing home-acquired pneumonia (11). Thus, as health care expands further into the community with shorter inpatient stays and more expansive outpatient management, the predominance of HCAP, and thus S aureus pneumonia, can be expected to increase.
In the present observational study, a cohort of S aureus pneumonia cases presenting from the community were reviewed to determine the role of HCAP. Cases were identified from a larger prospective study of CAP cases in Edmonton, Alberta, over a two-year period and reviewed retrospectively. Cases were determined to be community acquired or health care associated. Epidemiological, clinical and microbiological data were analyzed.
PATIENTS AND METHODS
Patients older than 17 years of age who presented to hospitals in Edmonton, Alberta, between November 2000 and November 2002 were eligible for the study. Four teaching hospitals, two community hospitals and one freestanding emergency centre in Edmonton participated. Patients presenting with at least two of the following were managed according to a critical pathway for treatment of CAP: fever (body temperature higher than than 38°C), productive cough, chest pain, shortness of breath and crackles on auscultation, in addition to a chest radiograph interpreted as pneumonia (12). Pregnant or nursing women, neutropenic patients (white blood cell count less than 1.0×109/L), critical care patients, cystic fibrosis patients and patients hospitalized within 48 h before presentation were ineligible for the treatment pathway.
As per the critical pathway protocol, patients who were admitted to hospital had blood cultures drawn, and those who were treated for pneumonia on an ambulatory basis had blood cultures performed at the discretion of the attending physician. Data collected on all patients included demographic variables, signs and symptoms at presentation, comorbidities and predisposing factors, laboratory values, microbiology results and diagnostic imaging, as well as course of treatment and outcome. A pneumonia severity index (PSI) score was calculated using a validated prognostic scoring model (13). Given the low sensitivity and specificity of sputum cultures, the causative organism was determined to be the definite source of infection only when isolated from blood or pleural fluid (14). To provide a strict analysis of patients with S aureus pneumonia, only patients with blood cultures positive for S aureus were included in the study cohort and underwent further chart and radiological review.
Clinical analysis
All cases were initially considered to be community acquired based on traditional definitions. However, on further review, a case was reclassified as health care associated if one of the following criteria was fulfilled: the patient received home intravenous therapy or specialized home nursing care within the 30 days before the infection; attended a hospital or hemodialysis clinic, or received intravenous chemotherapy within the 30 days before the infection; was hospitalized in an acute care hospital for a minimum of two days in the 30 days before infection; or resided in an LTCF. To determine the appropriate classification, information gathered by research nurses at the time of entry into the study was reviewed, and cases were retrospectively reviewed by one of the authors (DW) using inpatient hospital charts in every case and outpatient clinic charts as available.
On retrospective review of each case, the pathogenesis of the bacteremic pneumonia was also assessed. If S aureus infection was determined to have originated in the respiratory tract, it was defined as a primary S aureus pneumonia. This designation was based on clinical signs and symptoms of respiratory tract infection preceding or coinciding with the isolation of S aureus from blood, with conditions predisposing to primary pulmonary infection and without a further extrapulmonary source that may have predisposed to hematogenous seeding of the lungs. Infection determined to have originated outside of the respiratory tract with subsequent seeding of the lungs was defined as a secondary S aureus pneumonia. This classification was based on clinical evidence of an extra-pulmonary source of S aureus infection, such as endocarditis, that appeared to precede the development of pulmonary infection, or radiographic evidence, such as multiple nodular cavitary lesions, that was suggestive of hematogenous spread to the lungs.
Demographic and clinical data of the CAP and HCAP groups were compared. Statistical analysis of means was performed using the unpaired t test with Welch correction. Nominal data were analyzed using Fisher's exact test. A two-tailed P value was calculated, and P<0.05 was considered significant.
Microbiological analysis
S aureus isolates were identified by routine laboratory procedures. Susceptibility assays were performed using VITEK instrumentation (bioMerieux Inc, USA). VITEK cards (GPS-105) for susceptibility assays were inoculated and incubated according to the manufacturer's recommendations (bioMerieux Inc). Methicillin-resistant S aureus (MRSA) was determined as per the Clinical and Laboratory Standards Institute guidelines (15) using an oxacillin screen plate assay. Any isolates exhibiting growth on the screen plate were further characterized by detection of the penicillin-binding protein 2′ (PBP2′) using the PBP2′ latex agglutination test (Oxoid Ltd, United Kingdom) according to the manufacturer's instructions. All S aureus isolates exhibiting growth on the oxacillin screen plate and that were PBP2′-positive were considered to be MRSA. All isolates were tested for the presence of the Panton-Valentine leukocidin gene by polymerase chain reaction using primers established by Lina et al (16). A positive control was run concurrently with the test samples.
Molecular typing with pulsed-field gel electrophoresis (PFGE) was used to further characterize all the S aureus strains. The protocol was carried out as described by Mulvey et al (17). Analysis was performed using the Bio-Rad Gel Documentation System (Bio-Rad Laboratories, USA) and BioNumerics Software (Applied Maths, USA). A dendrogram was generated using the Dice coefficient with 1% tolerance. The molecular relatedness of strains was interpreted by PFGE analysis according to criteria described by Bannerman et al (18). Identical PFGE patterns were considered to be the same strain. Banding patterns with three or less band differences were considered to be subtypes. Banding patterns with more than three band differences were interpreted to be different strains. Epidemiological data were used to guide the interpretation of the molecular data.
RESULTS
Clinical analysis
Of 3043 patients who met the inclusion criteria for entry into the larger prospective CAP study between November 2000 and November 2002, 2008 patients (66%) had blood cultures performed, and 28 patients with bacteremic S aureus pneumonia were identified. Of these 28 patients, 23 were admitted on initial presentation, while five patients had blood cultures drawn in conjunction with initial ambulatory management. Twelve of the 28 patients (43%) remained classified as having CAP while 16 patients (57%) met criteria for HCAP.
The demographic features are presented in Table 1. Two distinct populations presented from the community with S aureus pneumonia. The CAP cohort was significantly younger than the HCAP cohort (mean age 49.0±23.7 years versus 67.8±18.6 years; P=0.035), with higher rates of smoking (67% versus 12%; P=0.005) and intravenous drug use (IVDU) (50% versus 0%; P=0.002). In contrast, the HCAP cohort was generally older, with higher rates of noninfectious chronic disease, such as diabetes mellitus (38% versus 0%; P=0.024). As expected with systemic S aureus infection, both cohorts tended to present with severe illness. Overall, 75% of the entire cohort presented with a risk class IV or V PSI score; however, the HCAP group presented with more severe illness overall and a significantly higher mean PSI score (143.1±41.1 versus 98.2±54.6; P=0.028)
TABLE 1.
Demographics and severity of pneumonia among Staphylococcus aureus pneumonia study population
| Demographics | Overall (n=28) | CAP (n=12) | HCAP (n=16) | P |
|---|---|---|---|---|
| Mean age ± SD, years | 59.7±22.6 | 49.0±23.7 | 67.8±18.6 | 0.035 |
| Age ≥65 years, n (%) | 14 (50) | 4 (33) | 10 (62) | 0.252 |
| Male sex, n (%) | 14 (50) | 5 (42) | 9 (56) | 0.704 |
| Current smoker, n (%) | 10 (36) | 8 (67) | 2 (12) | 0.005 |
| COPD, n (%) | 6 (21) | 2 (17) | 4 (25) | 0.673 |
| Congestive heart failure, n (%) | 6 (21) | 2 (17) | 4 (25) | 0.673 |
| Hypertension, n (%) | 8 (29) | 3 (25) | 5 (31) | 1.000 |
| Diabetes mellitus, n (%) | 6 (21) | 0 (0) | 6 (38) | 0.024 |
| Active neoplasm, n (%) | 2 (7) | 0 (0) | 2 (12) | 0.492 |
| HIV, n (%) | 2 (7) | 2 (17) | 0 (0) | 0.175 |
| Hepatitis C, n (%) | 7 (25) | 5 (42) | 2 (12) | 0.103 |
| Intravenous drug use, n (%) | 6 (21) | 6 (50) | 0 (0) | 0.002 |
| Pneumonia severity risk class, n (%) | ||||
| I | 2 (7) | 2 (17) | 0 (0) | 0.175 |
| II | 2 (7) | 2 (17) | 0 (0) | 0.175 |
| III | 3 (11) | 1 (8) | 2 (12) | 1.000 |
| IV | 11 (39) | 5 (42) | 6 (38) | 1.000 |
| V | 10 (36) | 2 (17) | 8 (50) | 0.114 |
| Mean PSI ± SD | 123.8±51.6 | 98.2±54.6 | 143.1±41.1 | 0.028 |
| Seasonal distribution, n (%) | ||||
| Winter | 8 (29) | 4 (33) | 4 (25) | 0.691 |
| Spring | 6 (21) | 1 (8) | 5 (31) | 0.196 |
| Summer | 9 (32) | 5 (42) | 4 (25) | 0.432 |
| Autumn | 5 (18) | 2 (17) | 3 (19) | 1.000 |
CAP Community-acquired pneumonia;
COPD Chronic obstructive pulmonary disease
HCAP Health care-associated pneumonia
PSI Pneumonia severity index
There was no trend in seasonal distribution and none in the two cohorts were reported to have had a preceding influenza-like illness; none were tested for influenza. The most common presenting symptoms overall (data not shown) included dyspnea (79%), fever (64%) and cough (61%). Common laboratory findings included anemia (85% had a hemoglobin level lower than 135 g/L), neutrophilic leukocytosis (64% had a white blood cell count higher than 11.0×109/L, 78% had a neutrophil count higher than 7.5×109/L) and renal dysfunction (50% had a creatinine level higher than 130 μmol/L). Chest radiographs revealed effusion in 54% of individuals and cavitation in 18% of inidividuals.
Complications and outcomes are shown in Table 2, and distinct differences between the CAP and HCAP cohorts are again noted, although none reached statistical significance. Those with community-acquired S aureus pneumonia were noted to have higher rates of complications generally associated with S aureus bacteremia. Complications included endocarditis (50% versus 19%; P=0.114), cerebral emboli (8% versus 0%; P=0.429), epidural abscesses (17% versus 0%; P=0.175) and septic arthritis (33% versus 6%; P=0.133). However, despite fewer embolic complications, the HCAP cohort suffered a higher mortality rate (31% versus 0%; P=0.052), with a trend toward statistical significance in this latter category. A shorter mean length of stay among the HCAP cohort was due, in part, to this higher mortality rate, because death occurred within 48 h of admission in two HCAP patients.
TABLE 2.
Complications and outcomes among 28 patients with Staphylococcus aureus pneumonia
| Complications and outcomes | Overall (n=28) | CAP (n=12) | HCAP (n=16) | P |
|---|---|---|---|---|
| Endocarditis, n (%) | 9 (32) | 6 (50) | 3 (19) | 0.114 |
| On clinical diagnosis | 4 (14) | 2 (17) | 2 (12) | 1.000 |
| On echocardiography | 5 (18) | 4 (33) | 1 (6) | 0.133 |
| Cerebral emboli, n (%) | 1 (4) | 1 (8) | 0 (0) | 0.429 |
| Epidural abscess, n (%) | 2 (7) | 2 (17) | 0 (0) | 0.175 |
| Osteomyelitis, n (%) | 5 (18) | 3 (25) | 2 (12) | 0.624 |
| Septic arthritis, n (%) | 5 (18) | 4 (33) | 1 (6) | 0.133 |
| Mortality, n (%) | 5 (18) | 0 (0) | 5 (31) | 0.052 |
| Mean LOS ± SD, days | 24±18.9 | 31±21.2 | 19±15.8 | 0.126 |
CAP Community-acquired pneumonia;
HCAP Health care-associated pneumonia;
LOS Length of stay
An assessment of the pathogenesis and summary of predisposing factors is provided in Table 3. In 11 of 12 cases (92%) of S aureus CAP, the pneumonia was determined to be secondary to hematogenous seeding of the lung parenchyma in the setting of a primary bacteremia. A break in skin integrity was the most common source of the primary bacteremia, with IVDU being the most frequent cause of the skin portal. A primary bloodstream infection with a secondary pneumonia was also common, although less so, among the HCAP cohort; this was observed in eight of 16 cases (50%). A break in skin integrity – frequently due to S aureus culture-positive decubitus ulcers, skin abrasions or infected intravascular catheters – was also the common source of the bacteremia among the HCAP cohort. LTCF residence was a prominent potential predisposing factor among the HCAP cohort, observed in seven of 16 (44%) cases. Readmission within 30 days of an acute care hospitalization was observed in four cases of HCAP (25%), although no single hospital predominated, because all four cases had been previously admitted to four different Edmonton hospitals.
TABLE 3.
Potential predisposing factors and pathogenesis among 28 patients with Staphylococcus aureus pneumonia
| Predisposing factors and pathogenesis | n (%) |
|---|---|
| Health care-associated pneumonia (n=28) | 16 (57) |
| Long-term care facility resident (n=16) | 7 (44) |
| Acute care hospitalization within 30 days (n=16) | 4 (25) |
| Skin portal (n=16) | 8 (50) |
| Device-related (n=16) | 3 (19) |
| Pacemaker (n=16) | 2 (12) |
| Intravenous catheter (n=16) | 1 (6) |
| Immunosuppression* (n=16) | 5 (31) |
| Active neoplasm and chemotherapy (n=16) | 2 (12) |
| Transplant (n=16) | 1 (6) |
| Primary pneumonia and secondary bloodstream infection (n=16) | 8 (50) |
| Primary bloodstream infection and secondary pneumonia (n=16) | 8 (50) |
| Community-acquired pneumonia (n=28) | 12 (43) |
| Intravenous drug use (n=12) | 6 (50) |
| Skin portal (n=12) | 9 (75) |
| Primary pneumonia and secondary bloodstream infection (n=12) | 1 (8) |
| Primary bloodstream infection and secondary pneumonia (n=12) | 11 (92) |
Immunosuppression is defined as hypogammaglobulinemia, CD4 lymphocyte count less than 200×106/L, treatment with conventional immunosuppressives and corticosteroid use of oral prednisone ≥10 mg daily
Microbiological analysis
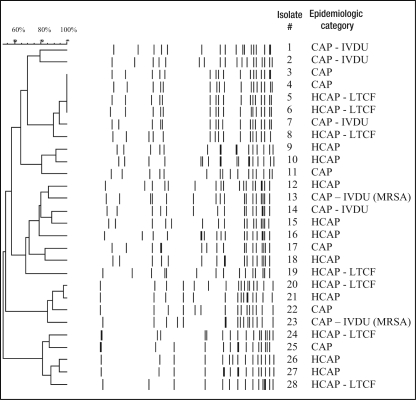
Susceptibilities were performed on 27 of 28 S aureus isolates because one of the isolates – isolate 12 shown in Figure 1 –failed to grow on the VITEK. Of the 27 isolates for which results were available, all were susceptible to vancomycin and rifampin. Eighty-five per cent of the isolates were beta-lactamase positive and, thus, penicillin resistant. One isolate was resistant to trimethoprim-sulfamethoxazole. Two isolates were found to be methicillin resistant, one of which was also resistant to clindamycin. Of these two MRSA isolates, both were cultured from individuals with a history of IVDU.
Figure 1.
Pulsed-field gel electrophoresis dendrogram analysis of Staphylococcus aureus isolates. CAP Community-acquired pneumonia; HCAP Health care-associated pneumonia; IVDU Intravenous drug use; LTCF Long-term care facility; MRSA Methicillin-resistant S aureus
Polymerase chain reaction revealed that all isolates were negative for the Panton-Valentine leukocidin gene. The PFGE patterns of the 28 study isolates are illustrated in Figure 1. Three isolates, numbers 4, 5 and 6, were interpreted as having indistinguishable band patterns. Dendrogram analysis with epidemiological information showed that identical isolates 5 and 6 along with subtype 8 were recovered from three elderly residents of three different LTCFs in Edmonton in 2002. The LTCFs were located in separate areas of the city, and no further epidemiological link was clearly identified in these cases. Isolate 4 was cultured from an individual living in the community and was classified as a CAP isolate. Isolates 3 and 7 were also subtypes within this group. Both were classified as CAP isolates, with the latter cultured in the setting of IVDU.
Isolates 20 and 21 were subtypes by PFGE pattern and were cultured in the setting of health care-associated infections less than one month apart in 2001. Both individuals were elderly with multiple comorbidities and were admitted to the same acute care hospital. Isolate 20 was cultured from the blood of an 89-year-old woman who was an LTCF resident with a left base infiltrate and endocarditis involving a porcine mitral valve. Isolate 21 was cultured from a 66-year-old man with Hodgkin's lymphoma and bilateral patchy infiltrates. He was a palliative patient and died within hours of his admission.
Isolates 26 and 27 were also subtypes cultured from individuals with health care-associated infections. These isolates were both cultured in 2002. The former isolate was cultured from the blood and sputum of a 71-year-old man with metastatic small cell lung cancer and recurrent admissions for pneumonia. The latter strain was cultured from the blood of a 59-year-old quadriplegic man with chronic pressure ulcers and a left upper lobe infiltrate.
The two MRSA isolates were numbers 13 and 23. Both were CAP isolates cultured in the setting of IVDU; however, PFGE analysis demonstrated no relatedness. Further assessment of the band patterns of these two isolates confirmed that the strains did not fall within a Canadian MRSA clonal prototype, or within the USA300 or USA400 prototypes.
DISCUSSION
In terms of epidemiology and pathogenesis, the clinical entity of S aureus pneumonia has evolved over time. A literature review, shown in Table 4, suggests some trends. Although a very heterogenous set of studies, the review suggested that the role of influenza has decreased in proportion to other risk factors over the past century, and has cycled in and out of the equation. Influenza remains an important predisposing factor; however, the contribution of other factors has grown. Table 4 shows the emergence of IVDU as an important risk factor for secondary S aureus pneumonia as highlighted by Julander et al (19), and MRSA as a major threat was prominent in a number of studies (20–22). S aureus pneumonia, as a significant nosocomial entity, is noted in Rebhan and Edwards’ (23) 1960 review of 329 cases of staphylococcal pneumonia at The Hospital for Sick Children (Toronto, Ontario). In this 1950s cohort, 32% of the cases involved recent hospital contact and 3% of all cases occured in hospitalized premature infants.
TABLE 4.
Literature review of Staphylococcus aureus pneumonia
| Reference, year | n | BC+ (%) | Resp+1° (%) | versus 2° (%) | BE (%) | Skin portal (%) | Influenza (%) | Mortality (%) | Location (years of study) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Current study | 28 | 100 | – | 32/68 | 32 | 61 | 0 | 18 | Edmonton, Alberta (2001–2002) | HCAP in 57% and CAP in 43% |
| Gonzalez et al (29), 2003 | 134 | 100 | – | 37/63 | – | 60 | 0 | 37 | Madrid, Spain (1990–1995) | 65 (48.2%) CAP patients with 47 (72.3%) 2° IVDU |
| Skull et al (30), 1999 | 13 | 100 | – | 23/77 | 0 | ‘Most’ | 15 | 46 | Darwin, Australia (1996–1997) | 12 of 13 cases (92%) were community acquired |
| Gonzalez et al (20), 1999 | 54 | 100 | – | – | – | 88 | – | 41 | Madrid, Spain (1990–1995) | All were MSSA, 63% had CVC and 70.4% were nosocomial |
| Gonzalez et al (20), 1999 | 32 | 100 | – | – | – | 63 | – | 56 | Madrid, Spain (1990–1995) | All were MRSA, 88% had CVC and 93.8% were nosocomial |
| Tumbarello et al (31), 1996 | 19 | 53 | 79* | 47/53 | 0 | 84 | – | 16 | Rome, Italy (1986–1994) | All were HIV-positive patients, 84% had IVDUs, 68% had CAP |
| Musher et al (32), 1994 | 19 | 37 | 100* | 74/26 | 26 | 26 | – | 32 | Texas, USA (1989–1992) | Among 162 S aureus infections; 37% had CAP |
| Tsao et al (33), 1992 | 34 | 100 | 21† | 21/79 | 12 | 32 | – | – | Taipei, Taiwan (1985–1986) | Among 138 S aureus BSI cases; 62% had CAP |
| Kaye et al (34), 1990 | 31 | 32 | 74‡ | 100/0 | 0 | 0 | – | 32 | New England, USA (1983–1988) | 81% nosocomial, 13% CAP and 6% LTCF cases |
| Levine et al (35), 1990 | 8 | 38 | 100* | – | – | 50 | 0 | 38 | New York, USA (1986–1987) | HIV-positive cohort; seven of eight cases defined as CAP |
| Cafferkey et al (21), 1988 | 11 | – | 100* | 64/36 | – | 36 | – | 18 | Dublin, Ireland (1985–1987) | Nosocomial MRSA infection cohort |
| Watanakunakorn (36), 1987 | 44 | 100 | 100* | 100/0 | – | 0 | – | 84 | Ohio, USA (1980–1984) | 66% nosocomial, 23% CAP and 11% LTCF cases |
| Woodhead et al (8), 1987 | 61 | 16 | 100§ | – | 1.6 | – | 25 | 30 | Trent, England (1974–1984) | All cases defined as community- acquired |
| Lentino et al (22), 1985 | 12 | 33 | 100* | – | – | 17 | 0 | 58 | llinois, USA (1982–1983) | MSSA cohort, five nosocomial cases |
| Lentino et al (22), 1985 | 17 | 53 | 100* | – | – | 65 | 6 | 82 | Illinois, USA (1982–1983) | MRSA cohort, 16 nosocomial cases |
| Julander et al (19), 1983 | 13 | 100 | – | 0/100 | 92 | 100 | – | – | Stockholm, Sweden (1977–1981) | BC+/PE/IVDU, prospective echo |
| Julander et al (19), 1983 | 16 | 100 | – | 0/100 | 75 | 100 | – | – | Stockholm, Sweden (1965–1981) | BC+/PE/IVDU, retrospective echo |
| Naraqi and McDonnell (37), 1981 | 12 | 100 | 25* | 16/83 | 8 | 83 | 0 | 25 | Papua, New Guinea (1977–1979) | 10 had SSTI; nine of 10 were community acquired |
| Musher and McKenzie (38), 1977 | 20 | 60 | 80* | 55/45 | 45 | – | – | 15 | Texas, USA (1971–1976) | Among 123 cases of S aureus infection |
| Rebhan and Edwards (23), 1960 | 329 | 2 | 100¶ | – | – | 17 | 0 | 14 | Toronto, Ontario (1950–1958) | 32% with recent hospital contact |
| Ede et al (4), 1959 | 36 | 6 | 100** | 94/6 | – | 3 | 50 | 3 | Illinois Naval Hospital, USA (1956–1958) | Includes the influenza epidemic of 1957–58 |
| Robertson et al (7), 1958 | 38 | – | 100* | – | – | – | ‘Most’ | 47 | Sheffield, England (1957) | During the influenza epidemic of 1957–58 |
| Fisher et al (39), 1958 | 21 | 48 | 81†† | 67/33 | – | 14 | 0 | 67 | Maryland, USA (1942–1956) | Six nosocomial cases with 100% mortality |
| Hausmann and Karlish (40), 1956 | 18 | – | 100* | – | – | – | 6 | 0 | Reading, England (1952–1954) | 17 community and one nosocomial case |
| Finland et al (5), 1942 | 66 | 17 | 100†† | 100/0 | – | – | 100 | 32 | Massachusetts, USA (1940–1941) | During the influenza epidemic of 1940–1941 |
| Reimann (6), 1933 | 6 | 0 | 100‡‡ | 100/0 | – | 0 | 33 | 33 | Minnesota, USA (1931–1933) | Four CAP and two nosocomial cases |
| Chickering and Park (3), 1919 | 155 | 1 | 100‡‡ | 100/0 | – | – | 100 | 99 | South Carolina, USA (1918) | During the influenza epidemic of 1918 |
Sputum cultures;
Bronchial brushing, secretion or sputum cultures;
Tracheal aspirate, direct lung aspirate, open lung biopsy, pleural fluid or bronchial brushing cultures;
Sputum, pleural fluid, tracheal aspirate or postmortem lung cultures;
Auger suction, bronchoscopic suction or pleural fluid cultures;
Throat, sputum or pleural fluid cultures;
Sputum, pleural fluid or postmortem lung cultures;
Sputum or postmortem lung cultures. BC+ Blood culture-positive;
BE Bacterial endocarditis;
BSI Bloodstream infection;
CAPCommunity-acquired pneumonia;
CVC Central venous catheter;
IVDU Intravenous drug use;
LTCF Long-term care facility resident;
MRSAMethicillin-resistant S aureus;
MSSA Methicillin-sensitive S aureus;
PE Pulmonary embolism;
Resp+ Respiratory specimen culture-positive;
SSTI Skin and soft tissue infection
With the significant role of the health care system and its extension into the community, S aureus has become a more frequent pathogen among cases of pneumonia presenting from the community. The American Thoracic Society and the Infectious Diseases Society of America have acknowledged HCAP as a unique and important clinical entity, with recognition of S aureus as a common causal pathogen (2). This designation is supported by our present study, which also found a significant number of S aureus pneumonia cases within a traditional CAP cohort that were found to be more precisely defined as HCAP.
This new designation also finds support in a recently published retrospective cohort study (1), which analyzed a large multi-institutional database of American acute care hospitals. In the study, Kollef et al (1) hypothesized that HCAP would involve pathogens more commonly associated with nosocomial pneumonia. This large study included 2221 patients with CAP, 988 patients with HCAP, 835 patients with hospital-acquired pneumonia and 499 patients with ventilator-associated pneumonia. The distribution of pathogens was somewhat unusual, in that S aureus was the most common pathogen in all four groups. It was noted in the related editorial that this may have been an artifact of the inclusion criteria (24). Nonetheless, the frequency of occurrence of S aureus in the HCAP group (46.7%) was comparable with the hospital-acquired pneumonia (47.1%) and the ventilator-associated pneumonia (42.5%) groups, and was significantly higher than the CAP group (25.5%).
Other investigators have also noted the relevance of distinguishing health care-associated infection from community-acquired infection. In a study of 504 bloodstream infections in North Carolina, USA, Friedman et al (25) found that 37% of bacteremic episodes that would normally have been classified as community-acquired, were more accurately identified as health care-associated. Similar to nosocomial infections, S aureus was prominent and in fact the most common pathogen.
A significant predisposing factor among the small cohort in our present study was the acquisition of health care in the community setting. Among the cohort, it was found that HCAP was more common than CAP, with the former identified in 16 cases (57%) and the latter in 12 cases (43%). While one-half of the CAPs occurred in the setting of active IVDU, the most prominent predisposing factor in the older and sicker HCAP group was a break in skin integrity providing a portal of entry for bacteremia, which occured in 50% of cases; LTCF residence was a factor in 44% of cases. All five patients who died were from the HCAP group and three of the five were LTCF residents. Although the number of cases was small, a distinction in terms of predisposing factors, and perhaps outcomes, appeared to emerge, demonstrating the importance of distinguishing between these differing cohorts.
With epidemiological data to guide the interpretation of PFGE analysis, we were able to examine the relatedness of S aureus isolates in the present study. Within the community setting of the study, three identical isolates with related subtypes and two further subtype pairs were identified from the 28 isolates. Among these, seven were HCAP isolates. There is evidence of relatedness among 44% of the HCAP isolates and, therefore, modest evidence to suggest the spread of common strains.
Furthermore, additional evidence of relatedness may be observed through the application of the less stringent Tenover et al (26) rules. Although isolates 9 and 10 did not meet the predefined study criteria as subtypes, by the Tenover et al rules, they would be classified as ‘possibly related’ based on a four- to six-band difference, consistent with two genetic events. These isolates were cultured one month apart in 2001. Both involved individuals with chronic disease closely linked to the health care system. Isolate 9 was isolated from a 41-year-old woman with primary pulmonary hypertension, who developed bacteremic S aureus pneumonia in the setting of an infected intravascular Broviac catheter. Isolate 10 was cultured from a 45-year-old splenectomized man with hepatitis C and alcohol-related cirrhosis, who had been undergoing routine paracenteses when he developed a large left lower lobe pneumonia and S aureus bacteremia. Both had been receiving regular outpatient care at medical clinics within the same hospital.
The present study also provides some insight into the demographics of S aureus HCAP and is relevant in light of recently published guidelines for the management of HCAP (2). These guidelines recommend that HCAP be empirically treated as a multidrug-resistant infection with knowledge of local resistance patterns with further guidance by the principles of antibiotic stewardship and the targeting of modifiable risk factors. In our small study, MRSA accounted for 7% of cases. The need for empirical MRSA coverage with vancomycin, linezolid or an alternative may be best determined at a local level with periodic review.
However, given the exclusion criteria of the study, the findings may not be applicable to all populations, specifically pregnant women, critical care patients, individuals with cystic fibrosis or neutropenia, and those recently hospitalized. Prior studies and knowledge of these groups may provide some insight into the epidemiology of pneumonia in these specific settings. For example, observational studies of pneumonia in pregnancy with routine microbiological investigations have suggested that the range of causal pathogens is similar to those of the nonpregnant adult (27). It may be assumed that should a pregnant woman have exposures that would put her at risk for health care-associated infection, she would also be at increased risk for S aureus pneumonia. Although, admittedly, clear evidence from focused studies is lacking.
In terms of the critically ill, studies have found that S aureus is a more prominent etiological agent in the setting of severe CAP requiring admission to an intensive care unit compared with less severe CAP requiring hospitalization or ambulatory management (14). S aureus may also be more prominent as a cause of pneumonia presenting from the community among the other groups excluded from the study. Certainly, the airways of cystic fibrosis patients are commonly colonized by S aureus within the first few years of life, and these individuals are prone to developing respiratory tract infection due to this pathogen at rates higher than the general population (28). The risk factors for the neutropenic group excluded from the study depend on the cause and duration of the neutropenia, although in general, this would certainly be a group predisposed to receiving frequent and specialized medical care. In this respect, selected cases would also be at increased risk for S aureus infection, although, akin to the cystic fibrosis patients, there would frequently be other opportunistic pathogens that need to be considered. Finally, patients hospitalized within 48 h of presentation were excluded from the current study, because the cohort was derived from a larger prospective CAP study. However, these cases would meet the definition of HCAP, and would be considered to be at risk for developing S aureus infections, including pneumonia.
With regard to the role of influenza, which has been classically described as a risk factor for the development of S aureus pneumonia, it is difficult to comment on the role that this predisposing viral infection may have played in the present study. It was noted that there was no trend in seasonal distribution, and none in the cohorts were reported to have had a preceding influenza-like illness. Furthermore, preceding influenza infection was unlikely to have contributed to the development of S aureus pneumonia in the present study, because influenza A was not prominent in the region over the two-year study period, with only 314 confirmed cases in the Capital Health region (Edmonton, Alberta), which consists of over one million people. Yet, none of the cohort were tested for influenza, and thus, the role of this viral pathogen as a predisposing factor cannot be absolutely delineated in the present study. Note also that influenza would predispose a patient to a primary S aureus pneumonia rather than a secondary pneumonia via hematogenous spread. Primary pneumonia accounted for only a fraction of the total cases in the study.
CONCLUSION
In the present series of 28 cases of bacteremic S aureus pneumonia presenting to hospital from the community, health care-associated infection accounted for the majority of cases that, using traditional definitions, would have been classified as CAP. The HCAP cohort was significantly older, presented with a higher mean PSI score and showed a trend toward a higher mortality rate. LTCF residence and skin portals including decubitus ulcers and intravascular lines were important predisposing factors for the development of S aureus HCAP. The cohort with community-acquired infection was younger in age, and had higher rates of smoking and IVDU.
MRSA accounted for a small but significant percentage of these S aureus HCAP cases. There is evidence of relatedness among 44% of the HCAP isolates in the present study and modest evidence to suggest spread of common strains. S aureus is a predominant HCAP pathogen, and the present study supports distinguishing between HCAP and CAP, as well as the empirical coverage of S aureus among certain high-risk groups.
Acknowledgements
The authors thank Dr Kevin Forward for providing a critical review of the manuscript, Dr David Haldane for assistance with statistical analysis, Dr Ross Davidson for discussion regarding the molecular typing, Duane Leedell for assistance with dendrogram preparation, Theodore Chiu for assistance with Panton-Valentine leukocidin –polymerase chain reaction, Jane Huang for assistance with data collection, Infection Control at the Royal Alexandra Hospital (Edmonton, Alberta) and the Microbiology Laboratory staff at the University of Alberta Hospital.
REFERENCES
- 1.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: Results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–62. doi: 10.1378/chest.128.6.3854. (Erratum in 2006;129:831) [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Chickering HT, Park JH. Staphylococcus aureus pneumonia. JAMA. 1919;72:617–26. [Google Scholar]
- 4.Ede S, Davis GM, Holmes FH. Staphylococcic pneumonia. J Am Med Assoc. 1959;170:638–43. doi: 10.1001/jama.1959.03010060006002. [DOI] [PubMed] [Google Scholar]
- 5.Finland M, Peterson OL, Strauss E. Staphylococcic pneumonia occurring during an epidemic of influenza. Arch Intern Med. 1942;70:183–205. [Google Scholar]
- 6.Reimann HA. Primary staphylococcic pneumonia. JAMA. 1933;101:514–20. [Google Scholar]
- 7.Robertson L, Caley JP, Moore J. Importance of Staphylococcus aureus in pneumonia in the 1957 epidemic of influenza A. Lancet. 1958;2:233–6. doi: 10.1016/s0140-6736(58)90060-6. [DOI] [PubMed] [Google Scholar]
- 8.Woodhead MA, Radvan J, MacFarlane JT. Adult community-acquired staphylococcal pneumonia in the antibiotic era: A review of 61 cases. Q J Med. 1987;64:783–90. [PubMed] [Google Scholar]
- 9.Sanford JR, Pierce AK. Proceedings of the International Conference on Nosocomial Infections. Chicago: American Hospital Association; 1971. Current infection problems; pp. 77–81. [Google Scholar]
- 10.Thoburn R, Fekety FR, Jr, Cluff LE, Melvin VB. Infections acquired by hospitalized patients. An analysis of the overall problem. Arch Intern Med. 1968;121:1–10. [PubMed] [Google Scholar]
- 11.Garb JL, Brown RB, Garb JR, Tuthill RW. Differences in etiology of pneumonias in nursing home and community patients. JAMA. 1978;240:2169–72. [PubMed] [Google Scholar]
- 12.Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000;283:749–55. doi: 10.1001/jama.283.6.749. [DOI] [PubMed] [Google Scholar]
- 13.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 14.Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH Canadian Infectious Disease Society; Canadian Thoracic Society. Summary of Canadian guidelines for the initial management of community-acquired pneumonia: An evidence-based update by the Canadian Infectious Disease Society and the Canadian Thoracic Society. Can Respir J. 2000;7:371–82. doi: 10.1155/2000/412616. [DOI] [PubMed] [Google Scholar]
- 15.Clinical Laboratory and Standards Institute. Pennsylvania: CLSI; 2006. Performance standards for antimicrobial susceptibility testing: Sixteenth informational supplement – CLSI document M100-s16. [Google Scholar]
- 16.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 17.Mulvey M, Chui L, Ismail J, et al. Canadian Committee for the Standardization of Molecular Methods. Development of a Canadian standardized protocol for subtyping methicillin-resistant Staphylococcus aureus using pulsed-field gel electrophoresis. J Clin Microbiol. 2001;39:3481–5. doi: 10.1128/JCM.39.10.3481-3485.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bannerman TL, Hancock JA, Tenover FC, Miller JM. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–5. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julander I, Arneborn P, Back E, Hoglund C, Svanbom M. Intravenous drug addiction – staphylococcal septicemia –pulmonary embolism: A triad pathognomonic for tricuspid valve endocarditis? Scand J Infect Dis. 1983;15:257–65. doi: 10.3109/inf.1983.15.issue-3.05. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez C, Rubio M, Romero-Vivas J, Gonzales M, Picazo JJ. Bacteremic pneumonia due to Staphylococcus aureus: A comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin Infect Dis. 1999;29:1171–7. doi: 10.1086/313440. [DOI] [PubMed] [Google Scholar]
- 21.Cafferkey MT, Abrahamson E, Bloom A, Keane CT. Pulmonary infection due to methicillin-resistant Staphylococcus aureus. Scand J Infect Dis. 1988;20:297–301. doi: 10.3109/00365548809032455. [DOI] [PubMed] [Google Scholar]
- 22.Lentino JR, Hennein H, Krause S, et al. A comparison of pneumonia caused by gentamicin, methicillin-resistant and gentamicin, methicillin-sensitive Staphylococcus aureus: Epidemiologic and clinical studies. Infect Control. 1985;6:267–72. doi: 10.1017/s0195941700061737. [DOI] [PubMed] [Google Scholar]
- 23.Rebhan AW, Edwards HE. Staphylococcal pneumonia: A review of 329 cases. Can Med Assoc J. 1960;82:513–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Hiramatsu K, Niederman MS. Health-care-associated pneumonia: A new therapeutic paradigm. Chest. 2005;128:3784–7. doi: 10.1378/chest.128.6.3784. [DOI] [PubMed] [Google Scholar]
- 25.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 26.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim WS, Macfarlane JT, Colthorpe CL. Treatment of community-acquired lower respiratory tract infections during pregnancy. Am J Respir Med. 2003;2:221–33. doi: 10.1007/BF03256651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajan S, Saiman L. Pulmonary infections in patients with cystic fibrosis. Semin Respir Infect. 2002;17:47–56. doi: 10.1053/srin.2002.31690. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez C, Rubio M, Romero-Vivas J, Gonzalez M, Picazo JJ. Staphylococcus aureus bacteremic pneumonia: Differences between community and nosocomial acquisition. Int J Infect Dis. 2003;7:102–8. doi: 10.1016/s1201-9712(03)90004-x. [DOI] [PubMed] [Google Scholar]
- 30.Skull SA, Krause V, Coombs G, Pearman JW, Roberts LA. Investigation of a cluster of Staphylococcus aureus invasive infection in the top end of the Northeastern Territory. Aust NZ J Med. 1999;29:66–72. doi: 10.1111/j.1445-5994.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 31.Tumbarello M, Tacconelli E, Lucia MB, Cauda R, Ortona L. Predictors of Staphylococcus aureus pneumonia associated with human immunodeficiency virus infection. Respir Med. 1996;90:531–7. doi: 10.1016/s0954-6111(96)90145-6. [DOI] [PubMed] [Google Scholar]
- 32.Musher DM, Lamm N, Darouche RO, Young EJ, Hamill RJ, Landon GC. The current spectrum of Staphylococcus aureus infection in a tertiary care hospital. Medicine (Baltimore) 1994;73:186–208. doi: 10.1097/00005792-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Tsao TC, Tsai YH, Lan RS, Shieh WB, Lee CH. Pulmonary manifestations of Staphylococcus aureus septicemia. Chest. 1992;101:574–6. doi: 10.1378/chest.101.2.574. [DOI] [PubMed] [Google Scholar]
- 34.Kaye MG, Fox MJ, Bartlett JG, Braman SS, Glassroth J. The clinical spectrum of Staphylococcus aureus pulmonary infection. Chest. 1990;97:788–92. doi: 10.1378/chest.97.4.788. [DOI] [PubMed] [Google Scholar]
- 35.Levine SJ, White DA, Fels AO. The incidence and significance of Staphylococcus aureus in respiratory cultures from patients infected human immunodeficiency virus. Am Rev Respir Dis. 1990;141:89–93. doi: 10.1164/ajrccm/141.1.89. [DOI] [PubMed] [Google Scholar]
- 36.Watanakunakorn C. Bacteremic Staphylococcus aureus pneumonia. Scand J Infect Dis. 1987;19:623–7. doi: 10.3109/00365548709117196. [DOI] [PubMed] [Google Scholar]
- 37.Naraqi S, McDonnell G. Hematogenous staphylococcal pneumonia secondary to soft tissue infection. Chest. 1981;79:173–5. doi: 10.1378/chest.79.2.173. [DOI] [PubMed] [Google Scholar]
- 38.Musher DM, McKenzie SO. Infections due to Staphylococcus aureus. Medicine. 1977;56:383–409. doi: 10.1097/00005792-197709000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Fisher AM, Trever RW, Curtin JA, Schultz G, Miller DF. Staphylococcal pneumonia. N Engl J Med. 1958;258:919–28. doi: 10.1056/NEJM195805082581901. [DOI] [PubMed] [Google Scholar]
- 40.Hausmann W, Karlish AJ. Staphylococcal pneumonia in adults. Br Med J. 1956;2:845–7. doi: 10.1136/bmj.2.4997.845. [DOI] [PMC free article] [PubMed] [Google Scholar]