Abstract
Since 1999, Cryptococcus gattii has been identified as a primary pathogen on Vancouver Island in British Columbia, and it has resulted in infection of both people and animals living in that area. A previously healthy 45-year-old female resident of Alberta developed C gattii infection 11 months after travelling to an endemic region of Vancouver Island. A case of an immunocompetent patient, with an atypically long incubation time, who presented with subacute meningitis secondary to disseminated pulmonary cryptococcosis is presented. The present report highlights the need for clinical vigilance in treating patients presenting with atypical pulmonary infections or meningitis who have been holiday travellers to endemic areas of Vancouver Island.
Keywords: Cryptococcus gattii, Disseminated cryptococcosis, Immunocompetent, Meningitis, Pneumocryptococcosis
Abstract
Depuis 1999, on constate que le Cryptococcus gattii est un pathogène primaire sur l’île de Vancouver, en Colombie-Britannique, et il a provoqué des infections tant chez les humains que chez les animaux de cette région. Une habitante de l’Alberta de 45 ans auparavant en santé a développé un C gattii onze mois après avoir voyagé dans une région endémique de l’île de Vancouver. On présente le cas d’une patiente immunocompétente, à la période d’incubation d’une durée atypique, qui a consulté en raison d’une méningite subaiguë causée par une cryptococcose pulmonaire disséminée. Le présent rapport souligne l’importance de la vigilance clinique dans le traitement des patients qui consultent en raison d’infections pulmonaires ou de méningites atypiques et qui ont pris des vacances dans des régions endémiques de l’île de Vancouver.
Cryptococcus gattii is a basidiomycetous, encapsulated yeast that is typically found in tropical and subtropical climate zones (1–3) and predominantly infects immunocompetent hosts (4–6). Traditionally, the natural habitat of C gattii was mainly associated with the environmental source, the Eucalyptus camaldulensis tree (7). Due to an increased incidence of infections in both humans and animals since 1999, C gattii was recognized in 2002 as having colonized the eastern portion of Vancouver Island in British Columbia (8,9). It resulted in an estimated rate of infection of 8.5 to 37 million persons per year between 1999 and 2003 (10). By June 2005, there were 132 cases, with three of those individuals living outside of Vancouver Island in the Vancouver Coastal and Fraser Health regions of British Columbia (8). A case of disseminated cryptococcosis due to C gattii infection is presented in an immunocompetent traveller to Vancouver Island to highlight the need for heightened vigilance for C gattii infections in travellers to this endemic area.
CASE PRESENTATION
A 45-year-old, previously healthy, female resident of Alberta presented to the emergency department with a two-week history of increasingly intense and persistent headaches, five days of blurred vision and photosensitivity, two days of nausea and vomiting, and an occasional nonproductive cough of three to four months duration. The patient's history was remarkable for yearly travel for the past six years of summer holidays to the town of Parksville, which is located on the southeastern shore of Vancouver Island. The patient had regular excursions to Rathtrevor Beach and MacMillan (Cathedral Grove) provincial parks, and her last visit was 11 months before her presentation to hospital.
On physical examination, the patient was alert and oriented and had no signs of meningismus except a complaint of slight neck pain on passive range of motion. She was afebrile, her vital signs were normal and she required no supplementary oxygen. The remainder of her physical examination was otherwise unremarkable. Visual examination was normal and no papilledema was noted. No cutaneous lesions were identified. Her complete blood count and serum electrolyte levels were normal, blood cultures were negative and she was seronegative for HIV. Liver enzyme levels were elevated with an alanine transaminase (ALT) level of 103 U/L and a gamma glutamyl transpeptidase (GGT) level of 167 U/L. Her beta-human chorionic gonadotropin level was less than 2 U/L.
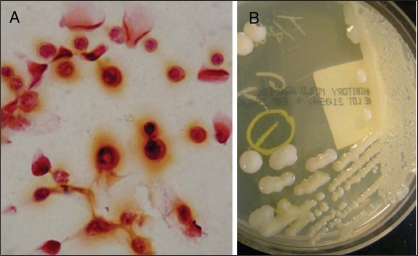
A lumbar puncture was performed, and the clear cerebral spinal fluid (CSF) collected showed a glucose level of 2.2 mmol/L and a protein concentration of 0.38 g/L. An elevated opening pressure was not noted. The CSF white blood count was elevated at 201×106/L and was predominantly lymphocytic (89%) in nature. Figure 1A shows CSF Gram stain demonstrating the presence of encapsulated yeast forms. CSF cryptococcus antigen test was positive at a titre of 1:4096. Figure 1B shows the CSF inoculated on inhibitory mold agar culture medium that grew mucous colonies indicative of capsule formation, and were subsequently identified as Cryptococcus neoformans using Vitek 2 (Vitek AMS; bioMérieux Inc, USA). The serotype of the isolate was determined by using a slide agglutination assay (Cryptocheck; Mitsubishi Kagaku Iatron, Japan) and revealed that the isolate belonged to serotype B (C gattii) (11). Antimicrobial susceptibilities using Sensititre YeastOne microdilution susceptibility plates (Trek Diagnostics, USA) showed the yeast to be sensitive to flucytosine, amphotericin B and fluconazole. Minimum inhibitory concentrations were interpreted with the Clinical and Laboratory Standards Institute criteria for yeast broth dilution (12).
Figure 1.
Cerebral spinal fluid Gram stain and culture. A Gram staining clearly showing the presence of encapsulated yeast forms. B Cerebral spinal fluid inoculated on inhibitory mold agar culture medium growing mucous colonies indicative of capsule formation
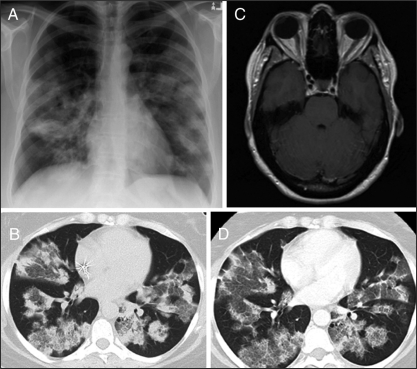
Chest radiography on admission showed significant airspace disease with poorly defined parenchymal opacities in both lungs (Figure 2A). Chest computed tomography (CT) demonstrated patchy, mixed ground-glass opacities and airspace consolidation involving both upper and lower lung regions (Figure 2B). Brain CT was normal, but subsequent magnetic resonance imaging revealed a slightly increased leptomeningeal enhancement over the inferior aspect of both cerebellar hemispheres and within the cerebellar folia bilaterally, consistent with disseminated cryptococcal meningitis (Figure 2C).
Figure 2.
Patient diagnostic imaging. A Chest radiography showing significant airspace disease with poorly defined bilateral parenchymal opacities. B Chest computed tomograpy (CT) (1.5 mm section thickness) demonstrating bilateral patchy, mixed ground-glass opacities and airspace consolidation with air bronchograms involving both upper and lower lung regions. C Brain magnetic resonance imaging (postgadolinium axial T1 weighted image) demonstrating increased leptomeningeal enhancement over the inferior aspect of both cerebellar hemispheres. D Repeat chest CT (1.5 mm section thickness, same level as chest CT shown in B) at week 7 showing some partial clearing of the airspace opacities by less dense ground-glass opacification with no new areas of involvement
The patient was admitted to hospital and started on amphotericin B at 1 mg/kg/day according to recognized practice guidelines (13). During the patient's remaining course in hospital, she did not demonstrate any deterioration in neurological or respiratory function. On postadmission day 2, the patient was started on flucytosine 25 mg/kg/6 h and amphotericin B was decreased to 0.7 mg/kg/day. On postadmission day 5, the patient began to develop nausea with abdominal cramping and diarrhea. Her serum creatinine level had climbed from a baseline of 90 μmol/L to over 150 μmol/L and liver enzyme levels had also increased (alkaline phosphatase levels increased from a baseline of 80 U/L to 197 U/L, GGT levels increased from a baseline of 167 U/L to 198 U/L, and ALT levels remained elevated at 101 U/L). She also developed hypoglycemia and hypokalemia requiring daily oral supplementation. Due to these significant toxicities attributed to her antifungal therapy, she was switched to 800 mg daily of intravenous fluconazole, and she experienced an immediate subjective symptomatic improvement. She was discharged from hospital on day 8 with an alkaline phosphatase level of 218 U/L and a serum creatinine level of 149 μmol/L. She continued treatment (ie, 800 mg daily of intravenous fluconazole) as an outpatient.
On day 14, the patient was neurologically intact and reported an improvement in fatigue and activity tolerance. Her CSF analysis showed a white blood count of 13.2×106/L (85% lymphocytes) and a positive cryptococcal antigen titre of 1:2048. Gram staining showed scant neutrophils and yeasts. A trial dose of 1200 mg of oral fluconazole was unsuccessful due to nausea and she remained on 800 mg of oral fluconazole given daily. Her CSF cultures were negative at week 4. Repeat chest CT on postadmission week 7 is shown in Figure 2D. The patchy, mixed ground-glass opacities and airspace consolidation involving both upper and lower lung regions were unchanged in distribution and extent. There was some partial clearing of the airspace opacities by less dense ground-glass opacification with no new areas of involvement. Her CSF cultures remained negative at week 12 and her antigen titre dropped to 1:128. The patient continued to remain clinically stable throughout the four-month follow-up period.
DISCUSSION
The present report demonstrates a case of disseminated C gattii infection in a holiday visitor to Vancouver Island and is similar to reports of previous cases (14) involving travel to other endemic regions. Similar to the majority of persons infected by C gattii, our patient was previously healthy and, apparently, immunocompetent. However, this patient, unlike those previously related to the Vancouver Island outbreak, was not a resident of British Columbia. The patient had made multiple trips during her long, one-month holiday in 2005 to Rathtrevor Beach and MacMillan (Cathedral Grove) provincial parks, areas known to be reservoirs of potentially infectious cryptococcal isolates (15). It has been shown that immuno-competent patients infected with C gattii have a clinical course more indolent than that described for immunocompromised patients (16). Our patient experienced at least an 11-month delay from exposure to illness. This is an unusually long incubation time, and is at the high end of those reported in a study of British Columbia mainland natives who travelled to Vancouver Island (two to 11 months) (17).
Our patient presented with symptoms and signs of subacute meningitis marked by intensifying headaches. Previous studies (4,5) have reported that headache is the dominant presenting feature in 60% to 85% of patients suffering from C gattii infection of the central nervous system. In addition, brain magnetic resonance imaging showed meningeal enhancement consistent with reports of cerebral cryptococcosis in immunocompetent patients (16). Several factors likely contributed to a good clinical response to therapy. It has been demonstrated that the majority of immunocompetent patients infected with C gattii who present with meningitis have low rates of cryptococcemia and associated mortality (4,16). An absence of poor mentation and lack of mass lesions or dilated ventricles on cerebral CT, as observed in 25% to 33% of patients at presentation (5,10), has also been correlated with cure and no or only mild sequelae (5).
A majority of immunocompetent patients infected with C gattii present with pulmonary disease in addition to meningitis (4,6). Interestingly, our patient had diffuse, but relatively asymptomatic, pulmonary involvement. It has been reported that while 77% of patients have mass lesions on chest CT, only 18% of patients complain of respiratory symptoms (5). In a study by Pappas et al (18), it was shown that in HIV-negative patients with pulmonary cryptococcosis, one-quarter of patients do not present with respiratory symptoms and, in addition, cough is the only symptom present in the majority of patients (18,19). One caveat of the present case report is that bronchial or parenchymal specimens were not obtained to confirm pulmonary involvement. In contrast to earlier reports, our patient displayed extensive mixed ground-glass opacities and airspace consolidation involving both upper and lower lung regions. It has been demonstrated that the most common chest CT findings are pulmonary nodules (83% to 91%) or masses, while associated ground-glass opacities occur in a minority of patients (19,20). Ground-glass opacities in the absence of nodules are infrequently observed (19).
SUMMARY
For patients who travelled to Vancouver Island within a year, and presenting with meningitis or atypical pulmonary infections, suspicion of C gattii infection should be strongly entertained.
REFERENCES
- 1.Kwon-Chung KJ, Bennett JE. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984;120:123–30. doi: 10.1093/oxfordjournals.aje.a113861. [DOI] [PubMed] [Google Scholar]
- 2.Ellis DH. Cryptococcus neoformans var. gattii in Australia. J Clin Microbiol. 1987;25:430–1. doi: 10.1128/jcm.25.2.430-431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeiffer T, Ellis D. Environmental isolation of Cryptococcus neoformans gattii from California. J Infect Dis. 1991;163:929–30. doi: 10.1093/infdis/163.4.929. [DOI] [PubMed] [Google Scholar]
- 4.Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28–34. doi: 10.1093/clinids/21.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell DH, Sorrell TC, Allworth AM, et al. Cryptococcal disease of the CNS in immunocompetent hosts: Influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis. 1995;20:611–6. doi: 10.1093/clinids/20.3.611. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Sorrell T, Nimmo G, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis. 2000;31:499–508. doi: 10.1086/313992. [DOI] [PubMed] [Google Scholar]
- 7.Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1990;28:1642–4. doi: 10.1128/jcm.28.7.1642-1644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.British Columbia Centre for Disease Control. Fungal infection found in Vancouver and Fraser health regions. < http://http://www.bccdc.org/news.php?item=118> (Version current at June 7, 2007)
- 9.Stephen C, Lester S, Black W, Fyfe M, Raverty S. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can Vet J. 2002;43:792–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Hoang LM, Maguire JA, Doyle P, Fyfe M, Roscoe DL. Cryptococcus neoformans infections at Vancouver Hospital and Health Sciences Centre (1997–2002): Epidemiology, microbiology and histopathology. J Med Microbiol. 2004;53:935–40. doi: 10.1099/jmm.0.05427-0. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda R, Shinoda T, Fukazawa Y, Kaufman L. Antigenic characterization of Cryptococcus neoformans serotypes and its application to serotyping of clinical isolates. J Clin Microbiol. 1982;16:22–9. doi: 10.1128/jcm.16.1.22-29.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. < http://www.clsi.org/source/orders/free/m38-a.pdf> (Version current at June 7, 2007)
- 13.Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:710–8. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 14.Tsunemi T, Kamata T, Fumimura Y, et al. Immunohistochemical diagnosis of Cryptococcus neoformans var. gattii infection in chronic meningoencephalitis: The first case in Japan. Intern Med. 2001;40:1241–4. doi: 10.2169/internalmedicine.40.1241. [DOI] [PubMed] [Google Scholar]
- 15.Kidd SE, Hagen F, Tscharke RL, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc Natl Acad Sci USA. 2004;101:17258–63. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lui G, Lee N, Ip M, et al. Cryptococcus in apparently immunocompetent patients. QJM. 2006;99:143–51. doi: 10.1093/qjmed/hcl014. [DOI] [PubMed] [Google Scholar]
- 17.MacDougall L, Fyfe M. Emergence of Cryptococcus gattii in a novel environment provides clues to its incubation period. J Clin Microbiol. 2006;44:1851–2. doi: 10.1128/JCM.44.5.1851-1852.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappas PG, Perfect JR, Cloud GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–9. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 19.Zinck SE, Leung AN, Frost M, Berry GJ, Müller NL. Pulmonary cryptococcosis: CT and pathologic findings. J Comput Assist Tomogr. 2002;26:330–4. doi: 10.1097/00004728-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Fox DL, Müller NL. Pulmonary cryptococcosis in immunocompetent patients: CT findings in 12 patients. AJR Am J Roentgenol. 2005;185:622–6. doi: 10.2214/ajr.185.3.01850622. [DOI] [PubMed] [Google Scholar]