Abstract
Concomitant HIV and hepatitis C virus (HCV) is a common yet complex coinfection. The present document is a practical guide for treating HCV infection in people coinfected with HIV. Effective antiretroviral therapies have prolonged survival rates for HIV-infected people over the past decade, which have made latent complications of HCV major causes of morbidity and mortality in these patients. Advances in the treatment of HCV (eg, combined pegylated interferon and ribavirin) offer the possibility of eradicating HCV infection in coinfected persons. The treatment of HCV must be considered in all cases. Intensive management of the adverse effects of HCV treatment is one of the factors for the success of these therapies. HCV eradication is predicted to decrease the mortality associated with coinfection and reduce the toxicity of HIV treatment.
Keywords: Antiretrovirals, Coinfection, Hepatitis C virus, HIV, Practical guide
Abstract
La co-infection par le VIH et le virus de l’hépatite C (VHC) est courante, mais complexe. Le présent document se veut un guide pratique pour le traitement de l’infection par le VHC chez les personnes co-infectées par le VIH. Des thérapies antirétrovirales efficaces ont prolongé les taux de survie des personnes infectées par le VIH depuis dix ans, ce qui fait des complications latentes du VHC des causes importantes de morbidité et de mortalité chez ces patients. Les progrès dans le traitement du VHC (p. ex., PEG-interféron associé à la ribavirine) offrent la possibilité d’éradiquer l’infection par le VHC chez les personnes co-infectées. Il faut envisager le traitement du VHC dans tous les cas. La prise en charge intensive des effets indésirables du traitement du VHC fait partie des facteurs de réussite de ces thérapies. L’éradication du VHC devrait réduire la mortalité associée à la co-infection ainsi que la toxicité du traitement du VIH.
Concomitant HIV and hepatitis C virus (HCV) infection is a complex and frequent problem. The increased survival rates since 1996 for HIV-infected people following highly active antiretroviral therapy have led to latent complications of HCV becoming major causes of morbidity and mortality in people living with HIV. Interactions with respect to pathogenesis, complexity of clinical management and treatment of HCV and/or HIV are a challenge for clinicians and the health care system. The present document is a practical guide for treating HCV infection in people coinfected with HIV. It promotes optimal follow-up and comprehensive case management by multidisciplinary teams.
The current practical guide was developed by the Comité consultatif sur la prise en charge des personnes vivant avec le VIH (Consultant Committee). This Quebec-based panel includes members with extensive experience in the care of HIV patients. They are mandated by the Ministère de la Santé et des Services sociaux (Ministry of Health and Social Services) to establish clinical guidelines on the management and treatment of persons living with HIV. A subgroup of the panel, in conjunction with HCV experts, was formed to develop the present practical guide to provide guidance to health care providers on the treatment of HCV in the setting of HIV-HCV coinfection. These recommendations are based on the current standard of care in treatment, published clinical guidelines, literature review and extensive clinical expertise by the members of the panel. Recommendations were obtained by consensus, and a medical writing subcommittee was established. The document was then reviewed and endorsed by the Consultant Committee. These recommendations are not intended to be a formal review of the published literature or to supersede the judgment of clinicians who are knowledgeable in the care of HIV-HCV coinfected persons. This is an evolving science; the availability of new clinical data with existing agents and the availability of new therapeutic options periodically change treatment options and preferences.
EPIDEMIOLOGY
It is estimated that approximately 20% of HIV-infected people in Canada are coinfected by HCV (1). The two viruses have certain common modes of transmission. HCV is approximately 10 times more transmissible than HIV by percutaneous exposure to contaminated needles (2). Transmission of one or the other is frequent in cases of transfusion or use of coagulation factors, as evidenced by the high rate of coinfection in hemophiliacs before the universal implementation of blood donor screening (3,4). The risk of HIV transmission following a single, high-risk, unprotected incident of sexual contact has been estimated to be 0.5% to 3% (5). HCV sexual transmission is rare (6,7). Vertical transmission of HIV from mother to child in the absence of antiretroviral therapy has been found to occur in approximately 20% to 30% of cases (2,8). Vertical HCV transmission is rare (2% to 5%) in mothers with HCV alone (2), but has been found to occur in up to 36% of mothers with HIV-HCV coinfection (9,10). HIV-HCV coinfection also increases the risk of sexual transmission of HCV (2,7,11), particularly in homosexual men (10,12). Transmission rates are shown in Table 1 (13). The number of HCV carriers in Canada has been estimated to be 250,000 (1). In studies examining injection drug users (IDUs) in two Canadian cities before 1999, the annual incidence of HCV was reported to be 26% in Vancouver and 27% in Montreal. The prevalence of HCV among IDUs in those two cities was 85% and 70%, respectively (14). In 2003, it was estimated that approximately 14.7% of Quebec IDUs were infected by HIV (15) and other, American, data (7) showed that 85% of persons infected with HIV through injection drug use were also infected by HCV.
TABLE 1.
Risk of transmission in the absence of treatment or postexposure prophylaxis
| Event | HIV | HCV (reference) |
|---|---|---|
| High-risk sexual exposure | 0.5%–3% | Rare; 3% if coinfected (2) |
| Percutaneous exposure (small amounts) | 0.3% | 1.8% (3) |
| Vertical transmission | 20%–30% | 2% to 5%; up to 36% if coinfected (9,12,16) |
HCV Hepatitis C virus
NATURAL HISTORY OF HIV-HCV COINFECTION
The independent natural histories of HIV and HCV has been reported in detail elsewhere (2,16–27). These natural histories can be significantly changed in the presence of coinfection.
HIV infection has a significant effect on the evolution of HCV. HIV increases the frequency of persistence of HCV after infection (27,28). Spontaneous clearance of HCV only occurs in 5% to 10% of HIV-positive people. HIV infection can also be associated with an increase in the HCV viral load (2), which can result in a decreased response to eradication treatments (2).
HIV has been associated with a faster progression toward cirrhosis (29), hepatic decompensation and, in some cases, hepatocellular carcinoma (30). Median progression for the development of cirrhosis in people with HCV-HIV coinfection can occur up to 10 years earlier than in those with HCV infection alone. A 1997 cross-sectional study (30) comparing the results of liver biopsies showed a median interval to cirrhosis of 6.9 years in coinfected people, compared with 23.2 years in monoinfected people. A more severe immunodeficiency (CD4 count less than 200 cells/μL) (31), consumption of more than 50 g of alcohol per day (equivalent to 3.6 standard drinks [where one standard drink equals one beer, one glass of wine, one aperitif or one hard liquor]) (31,32) and being older than 20 years of age at the time of HCV infection are independent aggravating factors in coinfection. The effect of HIV antiretroviral therapy on the evolution of HCV is not known (33), but some cases of decreased inflammation and fibrosis have been reported (31).
The influence of HCV infection on the natural history of HIV infection is less significant. Different cohorts have produced discordant findings, which can be explained by the selection bias of populations with negative prognostic factors for HIV, including alcohol and substance abuse, poor nutritional status and low adherence with antiretroviral treatment. Antiretroviral hepatotoxicity may be increased in the presence of HCV (34) and has been reported to result in more frequent treatment interruptions in coinfected patients (35). A recent Canadian study (36) has shown that HCV seropositivity is an independent predictor of mortality, especially death related to HIV infection.
LABORATORY TESTS AND THEIR INTERPRETATION
Diagnosis of coinfection
HCV infection is diagnosed using a serological test for anti-HCV. However, a positive anti-HCV test does not distinguish between the majority of patients with chronic infection and a minority of those who have spontaneously cleared the virus. Hence, a positive anti-HCV test should be confirmed by a qualitative HCV RNA polymerase chain reaction test. All HIV-infected people should be screened for HCV infection. At HIV diagnosis, HCV testing should be repeated for those who were initially HCV seronegative based on risk factors and clinical manifestations.
In immunocompromised people, HCV infection can be present despite a negative HCV antibody test (3% to 7% of cases) (37,38). Qualitative HCV RNA testing is recommended in HIV-positive people, along with a negative HCV antibody test, to detect serologically negative HCV infections in people with a sustained increase in liver enzymes or with risk factors for HCV (eg, IDUs and pre-1990 transfusion recipients).
HIV infection is diagnosed using a serological test for anti-HIV. Note that HIV viral load is not a screening test for HIV infection. HCV-infected people should be screened for HIV infection, particularly when a treatment for HCV infection is being considered.
ASSESSMENT OF COINFECTED PATIENTS
The baseline assessment and follow-up for HIV includes CD4 lymphocyte quantitation and HIV viral load testing. HIV plasma viral load testing and CD4 lymphocyte quantitation should be performed at the time of HIV diagnosis and every three to six months thereafter in untreated people. Recommendations for HIV monitoring, treatment and patient management are extensively covered in the literature (39–41).
In addition to HIV-specific biological parameters, monitoring of a coinfected individual can include many other laboratory and imaging tests as determined on a case-by-case basis.
Patients with clinical signs of liver failure or cirrhosis can be classified into stage A, B or C using the Child-Pugh scale, which considers biochemical markers, as well as the presence or absence of ascites or encephalopathy. A score from 1 to 3 is attributed to each of the parameters, and the total determines the Child-Pugh stage (42). The stage determines the five-year survival prognosis: 80% for stage A, 50% for stage B and 20% for stage C. Clinicians should be aware that the Child-Pugh score may be artificially high in HIV-infected patients receiving atazanavir- or indinavir-containing regimens. These patients may have increased total serum bilirubin levels on the basis of impaired glucuronidation, which is not indicative of liver disease.
If the coinfected patient can potentially be treated for HCV (see indications and contraindications below), the assessment should be more thorough and should include:
HCV genotyping, which provides an indication of the sensitivity to treatment. (There are at least six HCV genotypes [43]. Genotype 1 is the most frequent in Canada.)
Liver biopsy. The findings of a liver biopsy are interpreted based on semiquantitative criteria of the degree of inflammatory activity, hepatocellular necrosis and fibrosis.
There are at least four histological scores for inflammation and fibrosis – the METAVIR, the Knodell, the Ishak and the Scheuer scores are the most used (44,45). Table 2 shows an example of the METAVIR and the Knodell scoring systems. Interpretation of biopsy findings must be discussed with a HCV treatment specialist. Note that a liver biopsy is useful but not essential to the decision of whether to treat; its indication is controversial, particularly in the presence of a recent infection or an HCV genotype 2 or 3 infection. The higher rate of efficacy in treating HCV of these two genotypes makes the decision of whether to treat less dependent on biopsy results.
TABLE 2.
The METAVIR scoring system taking into account inflammatory activity and fibrosis
| Activity | Grade | Fibrosis | Stage | ||||
|---|---|---|---|---|---|---|---|
| No activity | A0 | No fibrosis | F0 | ||||
| Minimal activity | A1 | Portal fibrosis without septa | F1 | ||||
| Moderate activity | A2 | Portal fibrosis and some septa | F2 | ||||
| Severe activity | A3 | Septal fibrosis without cirrhosis | F3 | ||||
| Cirrhosis
|
F4
|
||||||
| Histology activity index – Knodell scoring system* | |||||||
|
Periportal ± bridging necrosis |
Score |
Intralobular degeneration and focal necrosis |
Score |
Portal inflammation |
Score |
Fibrosis |
Score |
| None | 0 | None | 0 | No portal inflammation | 0 | No fibrosis | 0 |
| Mild piecemeal necrosis | 1 | Mild (acidophilic bodies, ballooning degeneration and/or scattered foci of hepatocellular necrosis in 1/3 of lobules or nodules) | 1 | Mild (sprinkling of inflammatory cells in <1/3 of portal tracts) | Fibrous portal expansion | 1 | |
| Moderate piecemeal necrosis (involves <50% of the circumference of most portal tracts | 3 | Moderate (involvement of 1/3–2/3 of lobules or nodules) | 3 | Moderate (increased inflammatory cells in 1/3–2/3 of portal tracts) | 3 | Bridging fibrosis (portal-portal or portal-central linkage) | 3 |
| Marked piecemeal necrosis (involves >50% of the circumferenceof most portal tracts) | 4 | Marked (involvement of >2/3 of lobules or nodules) | 4 | Marked (dense packing of inflammatory cells in >2/3 of portal tracts) | 4 | Cirrhosis | 4 |
| Moderate piecemeal necrosis plus bridging necrosis | 5 | ||||||
| Marked piecemeal necrosis plus bridging necrosis | 6 | ||||||
| Multilobular necrosis | 10 | ||||||
Warning signs of liver disease
Assessment by an expert is recommended when the following warning signs are present:
Persistent increase in aspartate aminotransferase levels or alanine aminotransferase (ALT) levels to more than 10 times the upper limit of normal;
Signs of liver failure (spider angioma in the cava territory, palmar erythema, jaundice, hepatic encephalopathy, white nails, clubbing and ascites);
Signs of portal hypertension (gastrointestinal bleeding, splenomegaly, abdominal portal systemic collateral venous circulation and ascites); and
Signs of cirrhosis decompensation (jaundice, ascites and hepatic encephalopathy).
Patients with biopsy-proven cirrhosis should be monitored more closely.
Lifestyle recommendations
Screening for alcohol problems is extremely important, with the goal being complete abstinence. Alcohol consumption should be minimized and should not exceed 50 g/day. Another lifestyle issue to consider is the degree of adherence to anti-retroviral treatment; steps should be taken to optimize adherence. Steps should also be taken to prevent HCV reinfections because prior infection does not confer protective immunity. Finally, it is imperative to provide weight maintenance counselling, because weight gain can lead to faster development of fibrosis in the presence of fatty liver (46).
Assessment of psychological and social stability
The psychological and social assessment and case management of coinfected patients involves several important aspects. First, these patients should all undergo screening for mental health problems, because untreated psychosis and depression with suicidal ideation are contraindications to interferon (IFN) treatment, and a history of severe depression can sometimes be a relative contraindication for treatment.
Addictions are also a key consideration; one should aim for psychological and social stabilization, which, in cases of active addiction, can be assessed via the following elements:
Having stable lodging;
Being able to cooperate in a context of care;
Keeping appointments and taking medication as prescribed;
Having access to drug insurance and providing for essential needs – food, hygiene and transportation;
Evaluating, informing and involving the support network with respect to side effects of medications;
Promoting awareness of friends and family as to the impact of treatment; and
Assessing the patient's motivation to follow the treatment program.
Vaccination
People with HCV-HIV coinfection should be protected against other viral or bacterial infections. Of particular importance are hepatitis B virus, hepatitis A virus, influenza and pneumococcus (27,47–50). Clinicians should note that the immunogenicity of most vaccines is reduced in the HIV-infected population. Serological testing should be performed first to identify the presence of pre-existing antibody and/or those who are susceptible. However, vaccination should not be delayed when exposure can be predicted, particularly in IDUs.
MONITORING OF COINFECTED PERSONS
In Canada, more than 70% of HCV-infected people have a history of injection drug use (14). Although it would be appropriate to treat many of these patients for HIV and/or HCV, in actual fact, only a few are treated particularly for HCV.
Active consumption of illicit psychotropic substances for recreational use can lower the chances of responding to treatment, because adherence is often reduced. Drug or alcohol consumption should not, however, be an absolute contraindication to treatment. The decision to initiate treatment should be made on an individual basis.
Adverse or toxic effects should be minimized, and drug interactions should be avoided if possible. Physical, psychological and social needs also have to be met, not only at the beginning, but throughout treatment.
The monitoring of a coinfected person is complex. Several tests are indicated for the optimal monitoring of patients with HCV and those with HCV-HIV coinfection. Table 3 shows these tests and their optimal frequency.
TABLE 3.
Monitoring tests
| Frequency | Workup for HCV | Additional workup in case of proven or suspected cirrhosis | Additional workup specific to HIV |
|---|---|---|---|
| Baseline | CBC, AST, ALT, alkaline phosphatase bilirubin, GGT, albumin, creatinine, INR, HCV RNA, HBsAG, anti-HBs, anti-HBc, Igg, liver biopsy, alpha-fetoprotein, liver ultrasound | Gastroscopy | CD4 lymphocyte quantitation, HIV viral load, HIV genotyping testing for antiretroviral resistance |
| Every 3 to 6 months | CBC, AST, ALT, alkaline phosphatase, bilirubin, GGT, albumin, creatinine, INR | Alpha-fetoprotein | CD4 lymphocyte quantitation, HIV viral load |
| Every 6 months | Alpha-fetoprotein, liver ultrasound (can be performed more often if alpha-fetoproteins increase) | ||
| Every 2 to 5 years | Liver biopsy | Gastroscopy |
Repeat every month for the first three months after starting anti-HIV therapy in coinfected patients. ALT Alanine aminotransferase;
AST Aspartate aminotransferase;
CBC Complete blood count;
GGT Gamma-glutamyl transferase;
HBc Hepatitis B core;
HBsAg Hepatitis B surface antigen;
Igg Immunoglobulin G;
INR International normalized ratio
Optimal monitoring is achieved when there is access to a multidisciplinary health team with experience in considering HIV, HCV, addiction, mental health, nutrition, and psychological and social aspects.
WHICH INFECTION TO TREAT FIRST
It is not recommended to begin HIV and HCV treatments simultaneously due to their complexity and overlapping drug toxicities. Clinical experience has shown that adherence is better when treatments are introduced separately. The efficacy of and tolerance to a single treatment should be determined before beginning another.
Ideally, treatment of HCV should be initiated before anti-HIV treatment to decrease the risk of hepatotoxicity of anti-HIV medication, to decrease the potential risks of hepatic decompensation during immune reconstitution syndrome, and to avoid overlapping drug toxicity between anti-HIV and HCV treatments.
HCV treatment should be initiated before HIV therapy when the HIV infection is stable and does not require immediate therapeutic intervention. This is usually the case when the CD4 cell count is higher than 350 cells/μL.
Treating HIV first is clearly indicated when the CD4 lymphocyte count is very low (less than 200 cells/μL). In some cases, however, when CD4 counts are between 200 cells/μL and 350 cells/μL, antiretroviral treatment can be delayed, particularly in the absence of opportunistic infections or markers of rapid progression (elevated viral load or clinical symptoms). The caregiving team needs to evaluate the risks and benefits of treating HIV or HCV first on a case-by-case basis.
TREATMENT OF HCV
The relevance of treating HCV should be considered for each coinfected patient. The decision of whether to treat must be made on an individual basis, considering the risk of progression of liver disease, the possibility of response to treatment, the risk of side effects, and the patient's condition and motivation (51).
When the decision to treat is taken, additional tests are called for to assess response to treatment and side effects, including quantitative HCV RNA and HCV genotype.
HCV treatment is contraindicated in certain cases. Absolute contraindications include (52,53):
Pregnancy, breastfeeding or the inability to use an effective means of contraception;
Hypersensitivity to IFNs, ribavirin (RBV) or excipients in the medication;
Active autoimmune disease, such as uncontrolled thyroid diseases or autoimmune hepatitis;
Kidney failure with a creatinine clearance of less than 50 mL/min (in these cases, pegylated [PEG] IFN-α-2a-based treatment without RBV can be considered with dose reduction if the creatinine clearance is less than 20 mL/min); and
Decompensated liver disease.
Relative contraindications include:
Problems with taking medication and keeping medical appointments;
Major uncontrolled depression and repeated suicide attempts – a psychological evaluation is necessary to assess suicide risk;
An uncontrolled psychotic state – if the psychosis is stable, HCV treatment may be undertaken if there is appropriate follow-up;
Severe coronary and valvular heart disease;
Uncontrolled epilepsy;
Retinopathy;
Platelet count less than 50,000×109/L;
Neutropenia less than 750×109/L;
Severe anemia – hemoglobin (Hb) level lower than 100 g/L; and
CD4 count less than 200 cells/μL.
To consider a treatment for HCV, liver biopsy is highly desirable (2). It should show significant damage – for example, a METAVIR score of F2 or higher, or equivalent (Table 2). Studies have shown that anti-HCV therapy treatment response is reduced in the presence of hepatic cirrhosis (54). Some authors believe that due to the prognosis of HCV in coinfection, treatment can be justified even in patients with little histological damage (55). The patient's refusal to undergo a biopsy is not in itself a contraindication to treatment. This is even truer for patients with a genotype 2 or 3 HCV infection because of the good response rate in these cases.
In cases of acute HCV infection (less than six months), studies (56) have reported very high response rates for monoinfection (98%). There are little similar published data for coinfection (57,58). However, in the presence of acute HCV infection and when not contraindicated, treatment should be initiated quickly, without a liver biopsy, as soon as possible following the 12-week postdiagnosis period (symptoms or viremia outbreak). Chances of spontaneous disappearance of HCV infection are low after 12 weeks.
Treatment of HCV infection
The most effective known treatment for HCV is PEG IFN with RBV. There are two formulations marketed in Canada.
Pegasys RBV (Hoffmann-La Roche Ltd, Canada): It consists of PEG IFN-α-2a at a fixed dose of 180 μg, once a week, associated with RBV. For genotype 1 or 4, RBV is given at a daily weight-adapted dose of 1000 mg for people weighing less than 75 kg and 1200 mg for people weighing 75 kg or more. For genotype 2 or 3, the dose of RBV is 800 mg (regardless of weight) (Table 4) (52).
Pegetron (Schering-Plough Canada Inc): It consists of PEG IFN α-2b administered in variable doses based on weight 1.5 μg/kg (as a subcutaneous injection once a week), with a variable oral dose of between 800 mg/day and 1200 mg/day of RBV based on weight (Table 4) (53).
TABLE 4.
| Genotype | Pegasys dose weekly | Ribavirin dose daily | ||
|---|---|---|---|---|
| 1 or 4 | 180 μg | Weight <75 kg = 1000 mg/day | ||
| Weight ≥75 kg = 1200 mg/day | ||||
| 2 or 3
|
180 μg
|
800 mg (regardless of weight)
|
||
| Pegetron* (pegylated interferon-alpha-2b) and ribavirin doses | ||||
|
Weight (kg) |
Pegetron RediPen* or vial strength to use |
Amount of Pegetron to administer weekly (μg) |
Volume of Pegetron to administer weekly (mL) |
Ribavirin dose to administer daily (mg) |
| <40 | 50 μg/0.5 mL | 50 | 0.5 | 800 |
| 40–<50 | 80 μg/0.5 mL | 64 | 0.4 | 800 |
| 50–<64 | 80 μg/0.5 mL | 80 | 0.5 | 800 |
| 64–<75 | 120 μg/0.5 mL | 96 | 0.4 | 1000 |
| 75–<85 | 120 μg/0.5 mL | 120 | 0.5 | 1000 |
| ≥85 | 150 μg/0.5 mL | 150 | 0.5 | 1200 |
Hoffmann-La Roche Ltd, Canada;
Hoffmann-La Roche Inc, USA
Schering-Plough Canada Inc
There are no comparative studies showing whether one of the PEG IFN/RBV formulations available is more effective than the other for coinfection. In December 2005, Health Canada approved Pegasys RBV formulation for use in HIV-HCV coinfected patients with a RBV dose of 800 mg (52). However, clinical studies following pivotal trials have demonstrated superior results with increased doses of RBV. RBV doses of between 1000 mg/day and 1200 mg/day (based on weight) as used in monotherapy are, therefore, recommended when tolerated (51,59,60).
Studies (54,60–62) have shown that response to HCV treatment is reduced in coinfected patients. HCV is treated for 48 weeks, regardless of genotype, in the setting of coinfection. For monoinfection, the duration of treatment depends on the genotype. Genotype 2 or 3 is treated for 24 weeks and genotype 1 or 4 is treated for 48 weeks.
HOW TO EVALUATE THE RESPONSE TO TREATMENT
Response to treatment is evaluated at different follow-up stages:
Early virological response (EVR) – negative qualitative HCV RNA test or two-log10 decrease at 12 weeks in quantitative HCV RNA, followed by a negative qualitative HCV RNA test at week 24 (54);
End-of-treatment virological response (ETR) – negative qualitative HCV RNA test at 48 weeks; and
Sustained virological response (SVR) – negative qualitative HCV RNA test 24 weeks after the end of treatment (week 72) and sustained normal liver enzyme values.
HCV treatment efficacy is reduced in the presence of coinfection. Recent studies (54,60–62) have compared coinfected patient treatment efficacy with that of monoinfected patients (Table 5) (54,62,63). In coinfected patients, HCV treatment efficacy varies between 14% and 38% for genotype 1, compared with between 41% and 52% in monoinfections. For genotypes 2 and 3, efficacy is between 44% and 73%, compared with approximately 80% in monoinfected patients.
TABLE 5.
Summary of sustained virological response in arms treated with pegylated interferon-alpha (PEG IFN-α) + ribavirin (RBV) in HIV-hepatitis C virus (HCV) coinfected patients compared with HCV monoinfected patients
| Study (reference) | Treatment population | n | RBV dose (mg) | PEG IFN | Length of treatment (weeks) | Genotype 1 response (%) | Genotype 2 + 3 response (%) |
|---|---|---|---|---|---|---|---|
| CLINIVIC, Lagunoet al (60)† | Coinfected | 52 | 800/1200 | α-2b | 48 | 38* | 53 |
| RIBAVIC- ANRS(61) | Coinfected | 412 | 800 | α-2b | 48 | 17 | 44 |
| APRICOT (54) | Coinfected | 289 | 800 | α-2a | 48 | 29 | 62 |
| ACTGA5071 (62) | Coinfected | 67 | 600/1000 | α-2a | 48 | 14 | 73 |
| Hadziyannis et al (63) | Monoinfected | 361 | 800 | α-2a | 48 | 41 | 79 |
| Monoinfected | 436 | 1000/1200 | α-2a | 48 | 52 | 80 | |
| Friedet al (87) | Monoinfected | 1121 | 1000/1200 | α-2a | 48 | 46 | 76 |
| Manns et al (88) | Monoinfected | 1530 | 1000/1200 | α-2b | 48 | 42 | 82 |
Genotype 1 and genotype 4 patients combined. ACTG AIDS Clinical Trial Group;
APRICOT AIDS Pegasys Ribavirin International Co-infection Trial;
ANRS Agence nationale de recherches sur le sida
Treatment efficacy and indication to continue treatment for 48 weeks with PEG IFN plus RBV are evaluated as a function of kinetic viral response (HCV viral clearance) at 12 weeks. Before starting treatment, quantitative (viral load) testing of HCV RNA must be performed. After 12 weeks of treatment, HCV RNA qualitative and quantitative testing must be repeated. Quantitative RNA testing is only to be perfomed if qualitative RNA is positive.
A negative qualitative HCV RNA at week 12 or a minimal two-log decrease in HCV RNA from initiation of anti-HCV therapy is required to continue treatment (54). If qualitative polymerase chain reaction is still positive at week 12, a quantitative HCV RNA viral load test should be performed. If the quantitative viral load shows a two-log decrease (100 times) compared with baseline, treatment should be continued and a qualitative HCV RNA test should be performed at week 24 to ensure that results are negative (54). If the quantitative HCV RNA viral load has not decreased by more than two-log, treatment should be discontinued. The probability of virological response in these cases is less than 2% (54).
HCV relapse is defined by a repositivation of qualitative HCV RNA at week 72 in the absence of clinical factors indicative of possible reinfection.
PROPOSED MONITORING AT THE BEGINNING OF AND DURING TREATMENT
An electrocardiogram should be performed in patients older than 50 years of age or in those with risk factors for arteriosclerotic heart disease. A chest x-ray and a tuberculin skin test (baseline purified protein derivative) should be performed in all patients who have not previously had these tests completed as part of their baseline HIV assessment. In general, medical visits should be scheduled for every 15 days for the first two months and every month thereafter. Frequency and monitoring are suggestions and can be adapted on a case-by-case basis. At the first visit, the patient should undergo a CD4/CD8 profile and HIV viral load test. At each visit, a complete blood count (CBC) should be performed, and electrolyte, glucose, creatinine, bicarbonate, aspartate aminotransferase, ALT, alkaline phosphatase, gamma-glutamyl transferase, bilirubin, albumin, amylase and uric acid levels should be measured. Once a month, a CD4/CD8 profile and a urinalysis should be performed. Also, venous lactic acid should be measured in the presence of symptoms of lactic acidosis or lowered bicarbonates. Once every three months, thyroid-stimulating hormone, HIV viral load and amylase should be measured and a fasting lipid profile should be obtained. Table 6 shows a representation of an optimal monitoring regimen.
TABLE 6.
Monitoring of coinfected patients before and during treatment of hepatitis C virus (HCV) infection
| Test | Start | 2 | 4 | 6 | 8 | 10 | 12 | 16 | 20 | 24 | 28 | 32 | 34 | 36 | 40 | 44 | 48 | 60 | 72 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECG | • | |||||||||||||||||||
| Chest x-ray | • | |||||||||||||||||||
| Qualitative HCV RNA | • | • | •* | • | •† | |||||||||||||||
| Quantitative HCV RNA | • | •‡ | ||||||||||||||||||
| CD4/CD8 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||||
| HIV RNA | • | • | • | • | • | • | • | |||||||||||||
| CBC | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| Electrolytes | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||
| Glucose | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||
| Creatinine | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| Bicarbonates | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||
| AST/ALT | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| ALKP/GGT | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| Bilirubin | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||
| Albumin | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| Amylase | • | • | • | • | • | • | • | • | ||||||||||||
| Chol/TGs | • | • | • | • | • | • | • | • | ||||||||||||
| Lactic acid | Do this test if bicarbonates are low or in presence of symptoms | |||||||||||||||||||
| TSH | • | • | • | |||||||||||||||||
| Urinalysis | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||||
| Pregnancy test§ | • | |||||||||||||||||||
Must be performed if qualitative HCV RNA is positive at week 12 and if there was a two-log10 drop in quantitative HCV RNA at week 12 to determine whether treatment must be continued;
Must be performed if qualitative HCV RNA is negative at week 48 to confirm sustained response;
Must be performed if qualitative HCV RNA is positive at week 12;
Ensure proper contraception is used while under treatment.
ALKP Alkaline phosphatase;
ALT Alanine aminotransferase;
AST Aspartate aminotransferase;
CBC Complete blood count;
Chol/TG Cholesterol/triglycerides;
ECG Electrocardiogram;
GGT Gamma-glutamyl transferase;
TSH Thyroid-stimulating hormone
Adverse effects of PEG IFN and RBV treatment
There are many common side effects of PEG IFN and RBV treatment. General symptoms include flu-like symptoms characterized by discomfort, joint pain, headaches, fever, weight loss and alopecia. Psychiatric and neurological symptoms can include irritability, anxiety, insomnia, depression and psychosis; there can also be convulsions, ototoxicity, neuropathy and altered vision (for symptoms that suggest retinal alteration, refer for urgent ophthalmology consultation).
Cutaneous symptoms are also common, such as eruptions, pruritus, dryness and local inflammation at the injection site. Many patients experience gastrointestinal symptoms such as anorexia, nausea, diarrhea, abdominal cramps and pancreatitis. Hematological effects include neutropenia, thrombocytopenia and anemia. Cardiovascular or pulmonary complications may also arise such as hypotension, arrhythmia, myocardial infarction, cardiomyopathy or pulmonary edema. Finally, there are several autoimmune or renal adverse effects associated with PEG IFN and RBV treatment, such as thyroid dysfunction, vasculitis, arthritis, proteinuria and electrolyte imbalance.
Complications associated with treatment of HCV in coinfection and their management
Flu-like symptoms: Acetaminophen can be used up to a maximum dose of 1000 mg orally, four times a day, as needed. Other options include hydration and nonsteroidal anti-inflammatory drugs.
Insomnia: Oxazepam, temazepam or lorazepam can be used as needed. Other benzodiazepines are metabolized in the liver and can have interactions with HIV protease inhibitors.
Rash: 10 mg to 25 mg of hydroxyzine taken orally, three times daily, can be used as needed. This medication also has a sedating effect that can be helpful at bedtime.
Depression: If depression develops during PEG IFN plus RBV treatment, the usual therapeutic options can be used. A psychiatric assessment is always preferable, with a joint follow-up during treatment if possible. HCV treatment should be discontinued in cases of de novo psychosis, hallucinations, significant paranoia, suicide attempts or ideation, and an emergency psychiatric opinion should be obtained. Antidepressants are often helpful or necessary. It is best to avoid bupropion.
-
Hematological impact: Interventions should be based on individual product monographs with respect to hematological complications and dose adjustments.
In general, the following interventions are recommended. Furthermore, adjustments may vary on a case-by-case basis, based on the individual treating physician's experience:
5a. Thrombocytopenia (less than 50,000×109/L): This mainly appears at the beginning of treatment. PEG IFN dosage may be reduced by 25% to 50%, and a CBC should be performed every 15 days. Treatment should be discontinued if thrombocytopenia does not improve despite reduced doses, if there are hemorrhagic complications or if the platelet count is less than 20,000×109/L.
5b. Neutropenia: If the neutrophil count drops to less than 750×109/L, PEG IFN dosage may be reduced by 25% to 50% and the neutrophil count should be retested every seven to 15 days. If the neutrophil count is less than 500×109/L, options are to reduce the dose of PEG, discontinue treatment or administer granulocyte-colony stimulating factor – filgrastim (Neupogen, Amgen Canada Inc), 5 μg/kg, subcutaneously, two or three times per week.
5c. Anemia: The combination of azidothymidine (AZT) (64) and RBV increases the risk of developing anemia. RBV in association with tenofovir or abacavir is better tolerated. These latter options reduce hematological toxicity caused by RBV and allow for optimal dosing for successful therapy. If anemia is present from the start, AZT can be replaced with another nucleoside reverse transcriptase inhibitor (NRTI) such as abacavir or tenofovir when possible (absence of resistance or intolerance), before the start of HCV treatment. In patients who develop anemia during treatment, the following can be considered on a case-by-case basis, taking into consideration the baseline Hb level, the rate of fall, and the presence or absence of symptoms:
Switch AZT for another NRTI.
In patients with stable cardiac disease or chronic obstructive pulmonary disease, if the drop in Hb level is greater than 20 g/L or if the Hb level drops below 100 g/L in any patient, the RBV dose should be reduced in 200 mg increments (one capsule) and CBC should be monitored every seven to 14 days. RBV dose can be reduced in this way, to a minimum of 600 mg/day.
If the Hb level falls below 85 g/L despite AZT discontinuation, RBV dose reduction, and/or use of recombinant erythropoietin (Eprex, Janssen-Ortho Inc, Canada) or darbepoetin (Aranesp, Amgen Canada Inc), RBV should be discontinued.
In the absence of response or if the anemia gets worse, other causes of anemia must be investigated (bleeding or bone marrow involvement), and recombinant erythropoetin can be administered subcutaneously two to three times a week (50 U/kg to 100 U/kg) to a maximum of 40,000 U/kg per week. Some authors recommend usage of recombinant erythropoietin before reducing the RBV dose to increase the chance of successful HCV treatment (64,65).
If the CD4 count decreases to less than 200 cells/μL, prophylactic anti-Pneumocystis jiroveci (carinii) treatment must be initiated. The relative percentage of CD4 cells is not impacted much by HCV treatment, and the risk of developing an opportunistic infection is low. It is not clear whether IFN-driven decreases in CD4 count below 200 cells/mm3, unassociated with a fall in CD4 percentage, are associated with any increase in opportunistic infections in general or in P jiroveci pneumonia in particular. Hence, it is not clear whether instituting P jiroveci pneumonia prophylaxis for such patients is essential. HCV treatment can be discontinued if opportunistic infections arise.
-
If the HIV viral load increases (rare occurrence), the patient's adherence to treatment must be checked and the merits of the following discussed with an HIV expert:
Observe only;
Introduce an antiretroviral treatment; and
Modify an antiretroviral treatment, if applicable.
Hypo- or hyperthyroidism: This complication does not usually require HCV treatment to be discontinued. Thyroxin substitution treatment is indicated in the presence of hypothyroidism. If hyperthyroidism is present, subacute thyroiditis, or more rarely, Graves disease, may be the cause. Diagnosis is made using a radioactive iodine scan and a thyroid-stimulating hormone receptor antibody test. Follow-up with an endocrinologist is recommended if required.
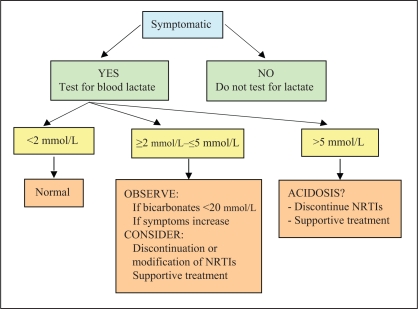
Lactic acidosis: Lactic acidosis is a rare complication of HIV treatments attributable to NRTI mitochondrial toxicity, resulting in depletion of mitochondrial DNA. Of 1000 people taking NRTIs, the frequency is approximately four to five people per year (66). Symptoms are nonspecific. Patients consult for significant general malaise, with lack of appetite, nausea, vomiting, weight loss, severe asthenia, dyspnea, cardiac arrhythmia and abdominal pain. These manifestations have been described mainly in women with fatty liver on stavudine, didanosine (ddI) or both (67,68). They result from the toxicity of medication on the mitochondria, which prevent these organelles from breaking down glucose via the usual metabolic pathways, resulting in excess production of lactic acid. The diagnosis is made in the presence of metabolic acidosis and an increase in blood lactic acid levels to more than 5 mmol/L. In the presence of symptoms, an increase in lactic acidemia to between 2 mmol/L and 5 mmol/L points to the possibility of this diagnosis in certain cases (Figure 1) (66). It should be noted that increases in blood lactic acid levels are common. Between 15% and 35% of patients treated with NRTIs may present asymptomatic hyperlactatemia (66), which is not predictive of lactic acidosis arising later (69). When interpreting an elevated lactic acid level, the patient's symptoms, blood bicarbonate levels and, if required, arterial gas analysis results must be taken into account. Blood for lactic acid testing is drawn without a tourniquet and sent immediately to the laboratory on ice. Unless the diagnosis is probable or certain, and discontinuation of treatment is an emergency, an HIV expert should be consulted before modifying or stopping antiretroviral therapy. It is believed that RBV may potentiate the mitochondrial toxicity of certain NRTIs (65,70), which is why the concomitant administration of ddI is avoided and stavudine-based (d4T) therapies are used with caution. Routine lactate testing is not recommended because of complexities associated with specimen collection, as well as the sensitivity and specificity limits of this marker.
Acute pancreatitis: There is an increased risk of pancreatitis in patients receiving ddI and the combination of PEG IFN with RBV. Some hepatic decompensation episodes have occurred in cirrhotic patients receiving RBV and ddI (71); concomitant use of ddI was identified as a strong independent risk factor for hepatic decompensation in patients with HIV-HCV coinfection receiving anti-HCV treatment (71).
Hepatic decompensation: In case of evidence of hepatic decompensation, HCV treatment should be discontinued (52,53).
Figure 1.
Investigation and treatment of hyperlactatemia. Adapted from reference 66. NRTIs Nucleoside reverse transcriptase inhibitors
Hepatotoxicity, HCV and HIV antiretroviral agents
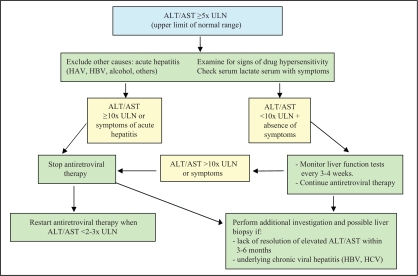
Most coinfected patients present elevated ALT levels to a certain degree after starting anti-HIV treatment. As many as 12% of patients develop severe hepatotoxicity (ALT levels greater than 10 times the upper limit of normal) (34,72–74). Table 7 shows recommendations for the use of antiretrovirals in cases of hepatotoxicity.
TABLE 7.
Antiretroviral dose adjustment in patients with hepatic insufficiency
| Medication | Hepatic metabolism | Dose adjustment required in liver failure |
|---|---|---|
| Nucleoside/Nucleotide reverse transcriptase inhibitors | ||
| Abacavir | Yes | No recommended in patients with moderate to severe impairment |
| Child-Pugh score (dose): 5–6 (200 mg twice a day) | ||
| Zidovudine | Yes | No dosage adjustment |
| Lamivudine | No | No dosage adjustment |
| Didanosine | No | No dosage adjustment |
| Stavudine | No | No dosage adjustment |
| Emtricitabine | No | No dosage adjustment |
| Tenofovir | No | No dosage adjustment |
| Non-nucleoside reverse transcriptase inhibitors | ||
| Delavirdine | Yes | No dosage adjustment; use with caution in patients with hepatic impairment |
| Efavirenz | Yes | No dosage adjustment; use with caution in patients with hepatic impairment |
| Nevirapine* | Yes | Avoid initiation in women with a CD4 count >250 cells/μL or in men with a CD4 count >400 cells/μL; ifinitiated, close monitoring isrecommended (every 2 weeks for the first month, then monthly for for 3 months, then every 3 months) |
| Protease inhibitors | ||
| Atazanavir† | Yes | Child-Pugh score (dose): 7–9 (300 mg every day) >9 (not recommended) |
| Fosamprenavir | Yes | Child-Pugh score (dose): 5–8 (700 mg twice a day) 9–12 (not recommended) |
| Indinavir† | Yes | Mild to moderate hepatic insufficiency because of cirrhosis: 600 mg q8h |
| Nelfinavir | Yes | No dosage adjustment; use with caution in patients with hepatic impairment |
| Saquinavir | Yes | No dosage adjustment; use with caution in patients with hepatic impairment |
| Lopinavir/ritonavir | Yes | No dosage adjustment; use with caution in patients with hepatic impairment |
| Tipranavir | Yes | No dosage adjustment; use with caution in patients with hepatic impairment. TPV/RTV is contraindicated in patients with moderate to severe hepatic impairment (Child-Pugh classes B and C) |
| Darunavir Fusion inhibitors | Yes | No data with patients with hepatic impairment; use with caution in this population |
| Enfuvirtide (T-20) | No | No dosage adjustment |
Reported cases of severe fulminant hepatitis and death – use not recommended in cases of liver failure;
Associated with unconjugated hyperbilirubinemia – refer to product monograph. q8h Every 8 h; TPV/RTV Tipranavir/ritonavir. Data from reference 79
Intervention in cases of hepatotoxicity
It is essential, particularly in coinfected patients, to carefully monitor liver function after introducing a new HIV antiretroviral agent (see complications below). Transaminases should be tested every month for the first three months and every three months thereafter. If ALT levels increase, other potential causes should be investigated, such as alcohol or cocaine use, acute or chronic hepatitis B, hepatitis A, opportunistic diseases or the hepatotoxicity of a concomitant medication. Interruption of HIV antiretroviral treatment may be required if there are symptoms or in cases of severe hepatitis. Reintroduction should be performed with caution due to the risk of hepatotoxicity recurring. Eradicating HCV virus before initiating HIV treatment can improve tolerance to antiretrovirals and should be considered in coinfected people with a CD4 count higher than 350 cells/μL (2).
An algorithm for the management of hepatotoxicity is presented in Figure 2 (75).
Figure 2.
Clinical management of severe hepatotoxicity. Adapted from reference 75. ALT Alanine aminotransferase; AST Aspartate aminotransferase; HAV Hepatitis A virus; HBV Hepatitis B virus; HCV Hepatitis C virus; ULN Upper limit of normal
Antiretroviral metabolism
It has been shown that chronic liver disease can affect the pharmacokinetics of some anti-HIV agents. Increases in the plasma concentrations of most non-NRTIs and protease inhibitors have been reported with varying degrees of liver dysfunction (40,80–84). In some cases, these increases have been linked to toxicity (83). Therapeutic drug monitoring can possibly be helpful when available.
HIV medication to avoid while treating HCV
ddI is the only antiretroviral contraindicated during HCV treatment due to its increased toxicity in the presence of RBV. RBV has potentiated the antiretroviral effect of ddI in vitro and in animals by increasing the formation of the active triphosphate anabolite (ddATP). This observation raised the possibility that concomitant administration of RBV and ddI could increase the risk of adverse reactions to ddI (eg, peripheral neuropathy, pancreatitis and fatty liver accompanied by lactic acidosis). Increased frequency of pancreatitis and lactic acidosis has been observed with the coadministration of ddI and RBV (51,54,85,86).
The coadministration of zidovudine (AZT) and RBV has been associated with a greater risk of anemia (86). AZT is relatively contraindicated during the treatment of HCV; when used, it must be closely monitored. It is recommended that patients be switched to a nonzidovudine-containing regimen if at all possible.
The use of d4T with RBV increases the risk of lactic acidosis; particular attention should be paid to symptoms of hyperlactatemia or lactic acidosis (51,54).
CONCLUSIONS
Advances in the treatment of HCV using combined PEG IFN and RBV offer the possibility of eradicating HCV infection in coinfected people. Due to the risk for hepatic morbidity associated with HCV infection in HIV-positive people, HCV eradication treatment must be considered in all cases in which there is no contraindication. Intensive management of the side effects of HCV treatment is one of the factors for the success of these therapies. HCV eradication is predicted to decrease the mortality associated with coinfection and reduce the toxicity of HIV treatment.
Footnotes
NOTES: Pegasys RBV is a trademark of Hoffmann-La Roche Ltd Canada, Pegetron is a trademark of Schering-Plough Canada Inc and Eprex is a trademark of Jannsen-Ortho Inc, Canada.
REFERENCES
- 1.Sherman M, Shafran S, Burak K, et al. Management of chronic hepatitis C: Consensus guidelines. Can J Gastroenterol. 2007;21(Suppl C):25C–34C. [PMC free article] [PubMed] [Google Scholar]
- 2.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected patient. Clin Liver Dis. 2003;7:179–94. doi: 10.1016/s1089-3261(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 3.Bove JR. Transfusion-associated hepatitis and AIDS. What is the risk? N Engl J Med. 1987;317:242–5. doi: 10.1056/NEJM198707233170411. [DOI] [PubMed] [Google Scholar]
- 4.Goedert JJ, Brown DL, Hoots K, Sherman KE. Human immunodeficiency and hepatitis virus infections and their associated conditions and treatments among people with hemophilia. Haemophilia. 2004;10:205–10. doi: 10.1111/j.1365-2516.2004.00997.x. [DOI] [PubMed] [Google Scholar]
- 5.Public Health Agency of Canada. HIV/AIDS Epi Updates. < http://www.phac-aspc.gc.ca/publicat/epiu-aepi/epi-05/pdf/epi_05_e.pdf> (Version current at August 27, 2007)
- 6.Eyster ME, Alter HJ, Aledort LM, Quan S, Hatzakis A, Goedert JJ. Heterosexual co-transmission of hepatitis C virus (HCV) and human immunodeficiency virus (HIV) Ann Intern Med. 1991;115:764–8. doi: 10.7326/0003-4819-115-10-764. [DOI] [PubMed] [Google Scholar]
- 7.US Public Health Service. Updated US public health service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1–52. [PubMed] [Google Scholar]
- 8.Landesman SH, Kalish LA, Burns DN, et al. Obstetrical factors and the transmission of human immunodeficiency virus type 1 from mother to child. The Women and Infants Transmission Study. N Engl J Med. 1996;334:1617–23. doi: 10.1056/NEJM199606203342501. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DL, Villano SA, Riester KA, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J Infect Dis. 1998;177:1480–8. doi: 10.1086/515315. [DOI] [PubMed] [Google Scholar]
- 10.Rauch A, Rickenbach M, Weber R, et al. Swiss HIV Cohort Study. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: The Swiss Cohort Study. Clin Infect Dis. 2005;41:395–402. doi: 10.1086/431486. [DOI] [PubMed] [Google Scholar]
- 11.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 12.Browne R, Asboe D, Gilleece Y. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex Transm Infect. 2004;80:326–7. doi: 10.1136/sti.2003.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benson CA, Kaplan JE, Masur H, Pau A, Holmes KK CDC; National Institutes of Health; Infectious Diseases Society of America. Treating opportunistic infections among HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm Rep. 2004;53(RR-15):1–112. (Erratum in 2005;54:311) [PubMed] [Google Scholar]
- 14.Health Canada. Hepatitis C – prevention and control: A public health consensus. < http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/99vol25/25s2/hepceb.html> (Version current at August 27, 2007)
- 15.Parent R, Alary M, Morrissettte C, et al. Rapport de surveillance 1995–30 juin 2004. Centre de prévention et de contrôle des maladies infectieuses. Ottawa: Santé Canada; 2004. Épidémiologie du VIH et du VHC chez les utilisateurs de drogue par injection; p. 48. [Google Scholar]
- 16.Zanetti AR, Tanzi E, Paccagnini S, et al. Mother-to-infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission. Lancet. 1995;345:289–91. doi: 10.1016/s0140-6736(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 17.Altfeld M, Walker BD. Acute HIV-1 infection. HIV Med. 2005:S33–37. [Google Scholar]
- 18.Lee LM, Karon JM, Selik R, Neal JJ, Fleming PL. Survival after AIDS diagnosis in adolescents and adults during the treatment era. United-States, 1984–1997. JAMA. 2001;285:1308–15. doi: 10.1001/jama.285.10.1308. [DOI] [PubMed] [Google Scholar]
- 19.Seef LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 20.de Lédinghen V. [Natural history of HCV infection] Gastroenterol Clin Biol. 2002;26:B9–22. [PubMed] [Google Scholar]
- 21.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–33. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 22.Flamm SL. Chronic hepatitis C virus infection. JAMA. 2003;289:2413–7. doi: 10.1001/jama.289.18.2413. [DOI] [PubMed] [Google Scholar]
- 23.Trinchet JC. [Natural history of HCV infection] Gastroenterol Clin Biol. 2002;26:B144–53. [PubMed] [Google Scholar]
- 24.Poynard T, Mathurin P, Lai CL, et al. PANFIBROSIS Group. A comparison of fibrosis progression in chronic liver disease. J Hepatol. 2003;38:257–65. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A, Keeffe EB. Chronic hepatitis C with normal aminotransferase levels. Gastroenterology. 2004;126:1409–15. doi: 10.1053/j.gastro.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 26.Mathurin P, Moussalli J, Cadranel JF, et al. Slow progression rate of fibrosis in hepatitis C virus patients with persistently normal alanine transaminase activity. Hepatology. 1998;27:868–72. doi: 10.1002/hep.510270333. [DOI] [PubMed] [Google Scholar]
- 27.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: Host, viral, and environmental factors. JAMA. 2000;284:450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 28.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–83. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 29.Soto B, Sánchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 30.García-Samaniego J, Rodríguez M, Berenguer J, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:179–83. doi: 10.1111/j.1572-0241.2001.03374.x. [DOI] [PubMed] [Google Scholar]
- 31.Benhamou Y, DiMartino V, Bochet M, et al. Factors affecting liver fibrosis in human immunodeficiency virus- and hepatitis C virus-coinfected patients: Impact of protease inhibitor therapy. Hepatology. 2001;34:283–7. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 32.Prakash O, Mason A, Luftig RB, Bautista AP. Hepatitis C virus (HCV) and human immunodeficiency virus type 1 (HIV-1) infections in alcoholics. Front Biosci. 2002;7:e286–300. doi: 10.2741/A924. [DOI] [PubMed] [Google Scholar]
- 33.Torre D, Tambini R, Cadario F, Barbarini G, Moroni M, Basilico C. Evolution of coinfection with human immunodeficiency virus and hepatitis C virus in patients treated with highly active antiretroviral therapy. Clin Infect Dis. 2001;33:1579–85. doi: 10.1086/322611. [DOI] [PubMed] [Google Scholar]
- 34.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 35.Mocroft A, Phillips AN, Soriano V EuroSIDA Study Group. Reasons for stopping antiretrovirals used in an initial highly active antiretroviral regimen: Increased incidence of stoping due to toxicity or patient/physician choice in patients with hepatitis C coinfection. AIDS Res Hum Retroviruses. 2005;21:743–52. doi: 10.1089/aid.2005.21.743. [DOI] [PubMed] [Google Scholar]
- 36.Braitstein P, Yip B, Montessori V, Moore D, Montaner JS, Hogg RS. Effect of serostatus for hepatitis C virus on mortality among antiretrovirally naive HIV-positive patients. CMAJ. 2005;73:160–4. doi: 10.1503/cmaj.045202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beld M, Penning M, van Putten M, et al. Low levels of hepatitis C virus RNA in serum, plasma, and peripheral blood mononuclear cells of injecting drug users during long antibody-undetectable periods before seroconversion. Blood. 1999;94:1183–91. [PubMed] [Google Scholar]
- 38.Kao JH, Lai MY, Hwang YT, et al. Chronic hepatitis C without anti-hepatitis C antibodies by second-generation assay. A clinicopathologic study and demonstration of the usefulness of a third-generation assay. Dig Dis Sci. 1996;41:161–5. doi: 10.1007/BF02208599. [DOI] [PubMed] [Google Scholar]
- 39.Baril JG, et al. [Antiretroviral therapy for HIV-infected adults] Quebec Ministry of Health and Social Services; 2002. p. 93. [Google Scholar]
- 40.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC) Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. < http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf> (Version current at August 27, 2007)
- 41.Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292:251–65. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 42.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 43.Murphy D, Willems B, Deschênes M, Hilzenrat N, Mousseau R, Sabbah S. Use of sequence analysis of the NS5B region for routine genotyping of hepatitis C virus with reference to C/E1 and 5– untranslated region sequences. J Clin Microbiol. 2007;45:1102–12. doi: 10.1128/JCM.02366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piroth L. Classification des hépatites chroniques. Société française de lutte contre le sida. < http://www.sfls.aei.fr/diaporamas/piroth_2/diaporama_piroth_2.asp> (Version current at August 27, 2007)
- 45.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 46.Powell EE, Jonsson JR, Clouston AD. Steatosis: Cofactor in other liver diseases. Hepatology. 2005;42:5–13. doi: 10.1002/hep.20750. [DOI] [PubMed] [Google Scholar]
- 47.Brook MG, Gilson R, Wilkins E BHIVA Hepatitis Coinfection Guideline Committee; British HIV Association. BHIVA guidelines on HIV and chronic hepatitis: Coinfection with HIV and hepatitis B virus infection (2005) HIV Med. 2005;6:84–95. doi: 10.1111/j.1468-1293.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 48.Casseb J, Fonseca LA, Veiga AP, et al. Efficacy to hepatitis B vaccination and its relationship with T CD45RA+ (naive) and CD45RO+ (memory) subsets in HIV-1 infected subjects. XV International AIDS Conference; Bangkok. July 11 to 16, 2004. [Google Scholar]
- 49.Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161–5. doi: 10.1016/s0264-410x(99)00389-8. [DOI] [PubMed] [Google Scholar]
- 50.Ministère de la Santé et des Services Sociaux du Québec. Protocole d'immunisation du Québec. < http://publications.msss.gouv.qc.ca/acrobat/f/documentation/piq/mise_jour/09sept_05.pdf> (Version current at August 27, 2007)
- 51.Alberti A, Clumeck N, Collins S, et al. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co-infected patients. J Hepatol. 2005;42:615–24. doi: 10.1016/j.jhep.2005.03.003. (Erratum in 2005;43:1098) [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann-La Roche Limited. Pegasys RBV product monograph, 2006. < www.rochecanada.com> (Version current at October 9, 2007)
- 53.Schering Canada Inc. Pegetron® product monograph, 2005. < www.schering-plough.ca> (Version current at October 9, 2007)
- 54.Torriani FJ, Rodriguez-Torres M, Rockstroh JK et al. APRICOT Study Group. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 55.Soriano V, Puoti M, Sulkowski M, et al. Care of patients with hepatitis C and HIV co-infection. AIDS. 2004;18:1–12. doi: 10.1097/00002030-200401020-00001. [DOI] [PubMed] [Google Scholar]
- 56.Tocci G, Visco-Comandini U, Antonucci G. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2002;346:1091–2. [PubMed] [Google Scholar]
- 57.Dominguez S. Safety and efficacy of a 24-week course of pegylated interferon-alpha2a and ribavirin for the treatment of acute HCV infection in HIV. 10th European AIDS Conference; Dublin. November 17 to 20, 2005. [Google Scholar]
- 58.Dominguez S, Ghosn J, Valantin MA, et al. Efficacy of early treatment of acute hepatitis C infection with pegylated interferon and ribavirin in HIV-infected patients. AIDS. 2006;20:1157–61. doi: 10.1097/01.aids.0000226956.02719.fd. [DOI] [PubMed] [Google Scholar]
- 59.Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–7. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 60.Laguno M, Murillas J, Blanco JL, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18:F27–36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 61.Carrat F, Bani-Sadr F, Pol S, et al. ANRS HCO2 RIBAVIC Study Team. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: A randomized controlled trial. JAMA. 2004;292:2839–48. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 62.Chung RT, Andersen J, Volberding P, et al. AIDS Clinical Trials Group A5071 Study Term. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 64.Alvarez D, Dieterich DT, Moorehead L, Ball L, Sulkowski MS The Hepatitis Resource Network Clinical Trials Group. Impact of zidovudine on anemia and epoetin-alfa use during pegylated interferon and ribavirin therapy for HCV in HIV-infected patients. 12th Conference on Retroviruses and Opportunistic Infections; Boston. February 22 to 25, 2005. [Google Scholar]
- 65.Landovitz RJ, Sax PE. NRTI-associated mitochondrial toxicity. AIDS Clin Care. 2001;13:43-5, 48–9, 52. [PubMed] [Google Scholar]
- 66.Brinkman K. Management of hyperlactatemia: No need for routine lactate measurements. AIDS. 2001;15:795–7. doi: 10.1097/00002030-200104130-00016. [DOI] [PubMed] [Google Scholar]
- 67.Bonnet F, Bonarek M, Morlat P, et al. Risk factors for lactic acidosis in HIV-infected patients treated with nucleoside reverse-transcriptase inhibitors: A case-control study. Clin Infect Dis. 2003;36:1324–8. doi: 10.1086/374601. [DOI] [PubMed] [Google Scholar]
- 68.Carr A. Lactic acidemia in infection with human immunodeficiency virus. Clin Infect Dis. 2003;36(Suppl 2):S96–100. doi: 10.1086/367565. [DOI] [PubMed] [Google Scholar]
- 69.Moyle GJ, Datta D, Mandalia S, Morlese J, Asboe D, Gazzard BG. Hyperlactataemia and lactic acidosis during antiretroviral therapy: Relevance, reproductibility and possible risk factors. AIDS. 2002;16:1341–9. doi: 10.1097/00002030-200207050-00005. (Erratum in 2002;16:1708) [DOI] [PubMed] [Google Scholar]
- 70.Laguno M, Milinkovic A, de Lazzari E, et al. Incidence and risk factors for mitochondrial toxicity in treated HIV/HCV-coinfected patients. Antivir Ther. 2005;10:423–9. [PubMed] [Google Scholar]
- 71.Mauss S, Valenti W, DePamphilis J, et al. Risk factors for hepatic decompensation in patients with HIV/HCV coinfection and liver cirrhosis during interferon-based therapy. AIDS. 2004;18:F21–5. doi: 10.1097/00002030-200409030-00002. [DOI] [PubMed] [Google Scholar]
- 72.Sulkowski MS. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: Role of hepatitis C and B infections. Hepatology. 2002;35:182–9. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 73.Puoti M, Torti C, Ripamonti D, et al. HIV-HCV Co-Infection Study Group. Severe hepatotoxicity during combination antiretroviral treatment: Incidence, liver histology, and outcome. J Acquir Immune Defic Syndr. 2003;32:259–67. doi: 10.1097/00126334-200303010-00004. [DOI] [PubMed] [Google Scholar]
- 74.Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT cohort, Thailand, 1996–2001. AIDS. 2003;17:2191–9. doi: 10.1097/00002030-200310170-00007. [DOI] [PubMed] [Google Scholar]
- 75.Dore G, Sasadeusz J. Coinfection – HIV & viral hepatitis: A guide for clinical management. < http://www.ashm.org.au/uploads/File/coinfection-mono.pdf> (Version current at August 27, 2007)
- 76.Bossi P, Peytavin G, Lamotte C, et al. High indinavir plasma concentrations in HIV-positive patients co-infected with hepatitis B or C virus treated with low doses of indinavir and ritonavir (400/100 mg twice a day) plus two nucleoside reverse transcriptase inhibitors. AIDS. 2003;17:1108–10. doi: 10.1097/00002030-200305020-00030. [DOI] [PubMed] [Google Scholar]
- 77.Regazzi M, Maserati R, Villani P, et al. Clinical pharmacokinetics of nelfinavir and its metabolite M8 in human immunodeficiency virus (HIV)-positive and HIV-hepatitis C virus-coinfected subjects. Antimicrob Agents Chemother. 2005;49:643–9. doi: 10.1128/AAC.49.2.643-649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arribas J, Pulido F, Peng JZ, et al. Evaluation of the multiple-dose pharmacokinetics of lopinavir/ritonavir (lopinavir/R) in HIV co-infected subjects with mild or moderate hepatic insufficiency. Eighth European AIDS Conference; Varsovie. October 25 to 29, 2003. [Google Scholar]
- 79.Veronese L, Rautaureau J, Sadler BM, et al. Single-dose pharmacokinetics of amprenavir, a human immunodeficiency virus type 1 protease inhibitor, in subjects with normal or impaired hepatic function. Antimicrob Agents Chemother. 2000;44:821–6. doi: 10.1128/aac.44.4.821-826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gibbons SE, Taylor C, Waldron S, Back DJ, Weber J, Khoo SH. Therapeutic drug monitoring of NNRTIs in patients with hepatic dysfunction. Sixth International Congress on drug therapy in HIV infection; Glagow. November 17 to 21, 2002. [Google Scholar]
- 81.de Maat MM, Huitema AD, Mulder JW, Meenhorst PL, van Gorp EC, Beijnen JH. Population pharmacokinetics of nevirapine in an unselected cohort of HIV-1-infected individuals. Br J Clin Pharmacol. 2002;54:378–85. doi: 10.1046/j.1365-2125.2002.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aranzabal L, Casado J, Moya J, et al. HAART-associated hepatotoxicity in HIV/HCV co-infected patients: The role of liver histologic damage and drug levels. The Seventh International Congress on Drug Therapy in HIV Infection; Glasgow. November 14 to 18, 2004. [Google Scholar]
- 83.Barreiro P, Novoa SR, Carbonero LM, et al. Liver toxicity with nevirapine: Incidence and risk factors. The Seventh International Congress on Drug Therapy in HIV Infection; Glasgow. November 14 to 18, 2004. [Google Scholar]
- 84.Khaliq Y, Gallicano K, Seguin I, et al. Single and multiple dose pharmacokinetics of nelfinavir and CYP2C19 activity in human immunodeficiency virus-infected patients with chronic liver disease. Br J Clin Pharmacol. 2000;50:108–15. doi: 10.1046/j.1365-2125.2000.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Butt A. Fatal lactic acidosis and pancreatitis associated with ribavirin and didanosine therapy. AIDS Read. 2003;13:344–8. [PubMed] [Google Scholar]
- 86.Hester J, Keiser J, Berggren R. Pancreatitis: An emerging complication of HCV treatment in HIV co-infected patients treated with didanosine/stavudine containing regimens. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago. December 16 to 19, 2001. [Google Scholar]
- 87.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 88.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]