Abstract
As a site of high metabolic activity, the brain is particularly susceptible to oxidative damage. We explored the association between plasma antioxidants and cognition. In 858 female participants of the Nurses’ Health Study, aged 70+ years, we measured plasma carotenoids and tocopherols in 1989–1990, and assessed cognitive function by telephone beginning in 1995–2001; assessments were repeated twice at two-year intervals. We used linear regression to estimate multivariable-adjusted mean cognitive performance at the initial assessment by quartile of antioxidants, and longitudinal models for analyzing cognitive decline over four years. Higher antioxidant levels were not associated with initial performance or decline. Mean difference in initial global composite score (averaging all 6 cognitive tests) for the top versus bottom quartile of total carotenoids was −0.05 standard units (95% confidence interval [CI] −0.19, 0.09), and 0.04 units for total tocopherols (95% CI −0.10, 0.18). Individual antioxidants were not associated with cognition. Overall, total plasma carotenoids or tocopherols were not related to cognition in women.
INTRODUCTION
Brain tissue readily undergoes oxidative damage, and long-term oxidative stress likely contributes to declining cognitive processes.[29] A wide variety of antioxidants including carotenoids, vitamins E and C, as well as fruits and vegetables high in antioxidants, has been found to improve cognitive performance in animals.[8,26,36]
Epidemiologic data on antioxidant vitamins are not as clear, however, and several randomized trials[17,21,35,48] of antioxidant supplements have found no relation with cognitive function or development of Alzheimer disease. However, these studies examined only a few select antioxidants from supplements, and recent data suggest that the larger variety of antioxidants available in foods might be important to consider.[7,20,31].
In addition, the majority of epidemiologic studies have utilized questionnaires on dietary or supplement intake. In contrast to antioxidants measured in plasma, such dietary data cannot incorporate individual variations in metabolism,[19] and thus may not be able to directly assess relations between bioavailable antioxidants and cognition. Although several studies have found possible inverse associations between high plasma levels of antioxidants and either cognitive decline[3,22,34,37] or dementia,[4,6,13,24,28,49] these studies have mostly been cross-sectional; because plasma antioxidants were measured at the same time as diagnosis, it is difficult to disentangle causes and effects in these cross-sectional studies. Prospective investigations are scarce[18].
Thus, we prospectively studied the association between plasma retinol and antioxidants and cognitive function, measured 10 years later among 858 healthy older women participating in the Nurses’ Health Study.
METHODS
Nurses’ Health Study
In 1976, the Nurses’ Health Study (NHS) began when 121,700 female registered nurses aged 30 to 55 years, residing in 11 US States, returned a mailed questionnaire about their lifestyle and health. Participants complete biennial questionnaires to update this information. Follow-up rates remain high (>90%).
From 1989 to 1990, blood samples were collected from one-third of participants; details of the blood collection have been published elsewhere.[16] Briefly, participants volunteered to send a blood sample by overnight mail, shipped on ice, to our laboratory. Approximately 70% were fasting samples. Ninety-seven percent of the samples were received within 26 hours of being drawn, and the stability of a variety of biomarkers in whole blood for 24–48 hours has been previously documented.[15] Specifically, measurements for β-carotene and α-tocopherol from samples collected in this manner showed between-person variation that was at least twice the within-person variation, [15] thus implying that the variability in plasma levels across participants could be captured. Samples were processed and separated into plasma, red blood cells, and white blood cells, and have been stored in liquid nitrogen freezers.
These blood samples have been used to study the relation of several plasma biomarkers, including plasma antioxidants, to chronic diseases in various nested case-control studies.[14,40,41] Health and lifestyle characteristics were similar between the whole NHS cohort and those who returned blood samples (e.g., 43% of the entire cohort versus 46% of those who provided blood never smoked; mean alcohol intake was 5 grams/day for both groups), thus there is little chance of meaningful bias in the subcohort who provided specimens.
Cognitive Function Subcohort
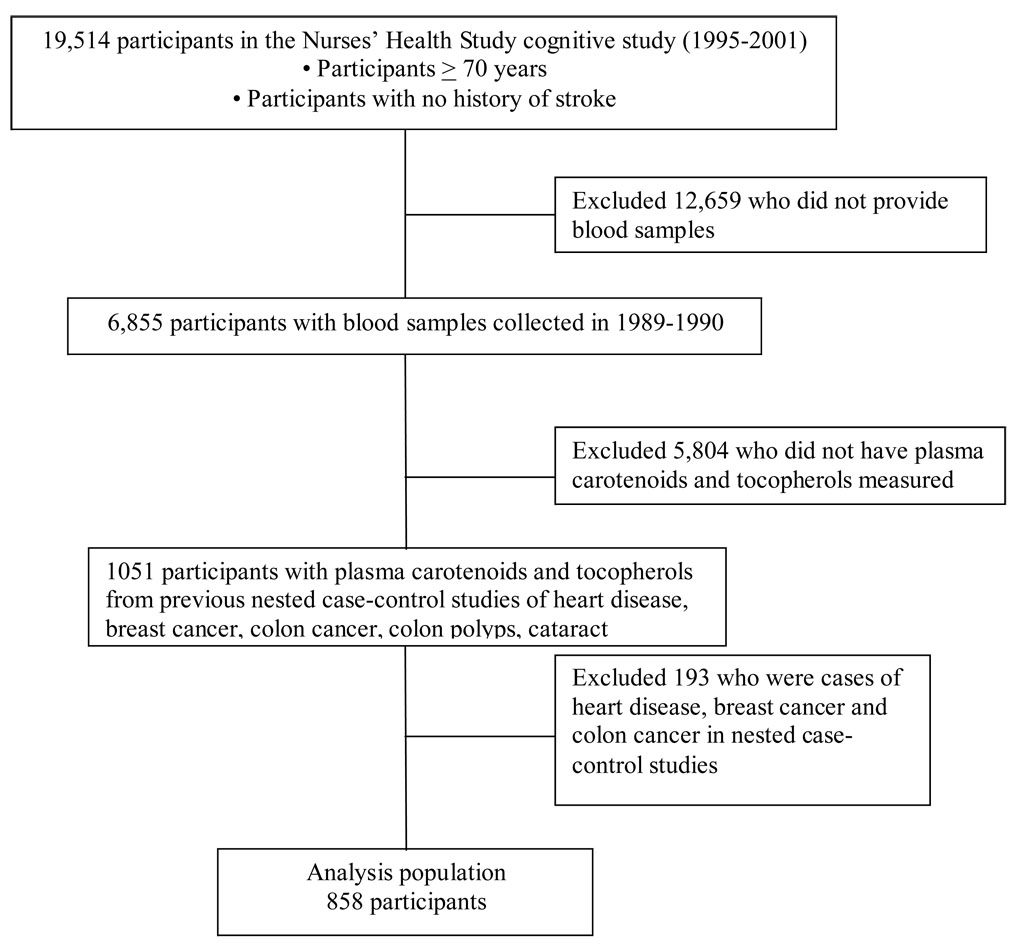
From 1995–2001, we selected 22,715 Nurses’ Health Study participants aged 70 years and older, free of diagnosed stroke, to participate in a telephone cognitive assessment. Of those whom we contacted, 92% completed the interview (n=19,514) (Figure 1). We have been administering follow-up cognitive assessments at approximately 2-year intervals, with very high follow-up rates; the second cognitive assessment administration is complete (with 91% participation, 5% refusal and 4% dead) and a third assessment is ongoing (86% complete to date; also with 91% participation to date).
Figure 1.
Determination of the population for analysis
Telephone cognitive assessment
Trained nurses administered all interviews by telephone. When we began cognitive testing, we used only the Telephone Interview for Cognitive Status (TICS; mean in this population =34, SD=3, range 8–41),[5] a telephone adaptation of the Mini-Mental State Examination. We gradually added five other cognitive tests to the initial testing; immediate and delayed recalls of the East Boston Memory Test (EBMT; immediate recall: mean=9, SD=2, range=2–12; delayed recall: mean=9, SD=2, range=0–12);[1] a test of category fluency in which women name animals during one minute (mean=18, SD=5, range=0–37); a delayed recall of the TICS 10-word list (mean=2, SD=2, range=0–9); and digit span-backwards, in which women repeat backwards increasingly long series of digits (mean=7, SD=2, range=1–12). Thus, the sample size differs somewhat for each test.
Our telephone method showed high validity: we found a correlation of 0.81 comparing overall performance on our brief telephone interview to overall performance measured from an in-person interview. In tests of instrument reliability, we administered the TICS twice to 61 Nurses’ Health Study participants at an interval of one month and found a correlation of 0.70.
We focused on three main outcomes, the global performance on our test battery; verbal memory, a strong predictor of Alzheimer disease development[11,38]; and the TICS. We calculated a global composite score across our 6 cognitive tests by averaging the z-scores for each test. We also calculated a verbal memory score by similarly averaging the z-scores of 4 tests: immediate and delayed recalls of both the EBMT and TICS 10-word list. The global score and the verbal memory score were only calculated for participants who completed all the component tests. Such composite scores integrate information from a variety of sources and provide a more stable representation of overall cognitive function than a single test. [47]
Population for Analysis
Figure 1 is a flow-chart of the selection of 858 participants for analysis. We first included women who met the following criteria: 1) completed the initial cognitive interview (1995–2001); 2) provided a blood sample (1989–1990); and 3) had plasma levels of antioxidants measured previously, as part of nested case-control studies of cataracts, heart disease, breast cancer, colon cancer, or colon polyps. From these 1051 women, we excluded the 93 women who were selected as cases of heart disease, breast cancer or colon cancer as their disease status may be associated with both antioxidant levels and cognition; however, we did not exclude cases of colon polyps or cataract, since having a history of either condition is unlikely to have substantial influence on cognition.
Characteristics of the 858 women in the primary analyses were quite similar to those in the entire cognitive study. For example, mean age at cognitive assessment was identical (74 years); mean TICS score at first interview was 34 in both the subset and entire group; and prevalence of current multivitamin use at blood draw was 42% in the subset and 40% in the entire group. Thus the analytic sample was representative of the cognitive cohort. This study was approved by the Institutional Review Board of Brigham and Women’s Hospital, Boston, MA.
Ascertainment of Plasma Antioxidants
All antioxidants were assessed in the same lab using reversed phase high performance liquid chromatography (HPLC) methods[10] at the Department of Nutrition, Harvard School of Public Health. Briefly, samples were deproteinized with alcohol and extracted with hexane to remove lipid analytes. The extracted samples were dried and reconstituted in 250µl of a 3:1:1 mixture of acetonitrile:ethanol:dioxane. Total cholesterol, an important determinant of the bioavailability of carotenoids and tocopherols, was assayed from plasma using enzymatic methods.[2] From each of the 8 batches of different nested case-control studies, blinded replicate samples were included for quality control; the median coefficients of variation (CV) indicated high overall intra-assay reliability: for α-carotene the CV was 5.3%, β-carotene was 4.9%, β-cryptoxanthin was 5.3%, lycopene was 5.1%, lutein/zeaxanthin was 5.5%, α-tocopherol was 5.0%, γ-tocopherol was 5.4% and retinol was 6.3%.
Not all samples were fasting (approximately 70% were fasting samples). The plasma nutrient levels from non-fasting samples, while generally slightly higher, were quite comparable to fasting samples; thus we included all measurements. In alternate analyses where we excluded the non-fasting samples, we found similar results.
Statistical Analysis
For the analysis of performance in the initial cognitive interview, a mean of 10 years after blood draw, we used linear regression to estimate age and education-adjusted, and multivariable-adjusted mean differences in performance across plasma nutrient quartiles. To take into account any batch-to-batch variation in the measurement of antioxidants, we created quartiles with batch-specific cut-points. The significance level was α = 0.05.
The two main exposures in this analysis were quartiles of total carotenoids (defined as the sum of the concentrations (µg/L) of the 5 individual carotenoids: α-carotene, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin) and quartiles of total tocopherols (defined as the sum of the concentration (µg/L) of α-tocopherol and γ-tocopherol). We also examined each individual carotenoid and tocopherol, as well as retinol.
For longitudinal analysis using data from a subset of 788 participants who completed the first interview and at least one follow-up interview to date, we used repeated measures models incorporating random effects for intercepts and slopes.[23] This approach permits description of individual paths of decline and provides explicit tests regarding the relation of exposures to rates of cognitive change, while taking into account the within-person correlation.
In multivariable models, we included a wide array of potential confounding variables, selected a priori from the published literature as risk factors for poor cognition. Information on potential confounding variables was determined from the biennial questionnaire responses immediately before the blood collection. The covariates included: time between blood draw and cognitive interview (years), age at interview (years), highest attained education (Registered Nurse, Bachelor’s, Master’s or above), history of diabetes, history of high blood pressure, history of high cholesterol, postmenopausal hormone use (current, past, never), age at menopause (<50, 50–52, 53+), body mass index (<22, 22–24, 25–29, 30+ kg/m2), cigarette smoking (current, past, never), antidepressant use, aspirin use (<3/week, 3+/week), alcohol intake (0,<5g/day, 5–14g/day, 15+ g/day), physical activity (quintiles of metabolic-equivalent-hours/week), mental health index (0–51, 52–100) and energy-fatigue index (0–49, 50–100) from the SF-36 (Medical Outcomes Short Form),[45] and use of vitamin E supplements (except for analyses for tocopherols). Current multivitamin use (at blood draw) was not included as a covariate, since this would be equivalent to partially adjusting for our exposures of interest. Because many attributes of participants may change over time, we performed secondary analyses using covariates as of the baseline cognitive interview, rather than at blood draw; however, there were negligible differences from the main results.
In alternate analyses among a subset of participants with plasma folate or plasma total cholesterol (for tocopherol analysis) levels available, we performed analyses adjusting for these potential confounding variables; the results were not meaningfully different.
We performed several secondary analyses. First, we conducted analyses stratified by current supplement use (defined as use of multivitamins, vitamin A or vitamin E) as of the biennial questionnaire immediately preceding the blood draw. Second, because current cigarette smoking reduces the levels of plasma antioxidants, we also examined effect modification by smoking status at blood draw. Finally, when we examined individual tocopherols, we conducted stratified analyses where we examined the association between γ-tocopherol and cognition in those with low and high levels of α-tocopherol, as there is evidence that high α-tocopherol levels may lower γ-tocopherol levels in plasma. [25,44]
RESULTS
There was a wide range in the distribution of plasma nutrient levels (Table 1), with at least a two-fold difference in the median levels of plasma carotenoids and tocopherols between the top and bottom quartiles. Mean age at blood draw (1989–1990) was very similar across categories of carotenoids or tocopherols. As expected, women in the highest plasma carotenoid quartile took more multivitamins, were somewhat more educated and healthier (e.g. less obesity, less hypertension, less antidepressant use) and engaged in healthful behaviors (e.g. more physical activity, less cigarette smoking, more vitamin E supplement use) compared to women in the lowest quartile. Women with the highest plasma tocopherol levels, however, had more cardiovascular risk factors of hypercholesterolemia and hypertension, took more aspirin and vitamin E supplements compared with women in the lowest levels. Cognitive performance on the first assessment was generally similar across plasma nutrient levels.
Table 1.
Characteristics of participants in the top and bottom quartiles of plasma carotenoids and tocopherols (1989 – 1990)
| Characteristics at blood draw (1989 – 1990)* | Total Population | By Quartile of Total Plasma Carotenoids | By Quartile of Total Plasma Tocopherols | ||
|---|---|---|---|---|---|
| 1st | 4th | 1st | 4th | ||
| Number of participants | 858 | 213 | 216 | 212 | 217 |
| Mean age (years) | 65 | 64 | 65 | 64 | 65 |
| Mean level of retinol† | 621 | 592 | 649 | 546 | 698 |
| Mean level of carotenoids† | 1122 | 617 | 1754 | 926 | 1285 |
| Mean level of tocopherol† | 15779 | 14128 | 17591 | 10704 | 22581 |
| Masters/Doctorate degree (%) | 8 | 5 | 11 | 8 | 7 |
| Hypertension (%) | 34 | 42 | 32 | 26 | 43 |
| Hypercholesterolemia (%) | 33 | 26 | 39 | 14 | 54 |
| Current postmenopausal hormone use (%) | 31 | 32 | 33 | 27 | 37 |
| Vitamin E use (%) | 24 | 21 | 34 | 11 | 45 |
| Multivitamin use (%) | 42 | 40 | 50 | 23 | 64 |
| Aspirin 2+/week (%) | 23 | 22 | 24 | 17 | 28 |
| Antidepressant use (%) | 5 | 10 | 3 | 7 | 5 |
| Current smoking (%) | 14 | 23 | 6 | 20 | 11 |
| Mean alcohol use (mean g/day) | 7 | 7 | 6 | 7 | 7 |
| (mean METs/week) | 19 | 17 | 20 | 16 | 19 |
| Obesity (BMI ≥ 30kg/m2; %) | 12 | 23 | 4 | 13 | 11 |
| Cognitive Performance at first assessment (1995 – 2001) | |||||
| TICS (n=858) ‡ | 33.9 | 33.9 | 33.8 | 33.8 | 34.0 |
| Verbal score (n=693) ‡ | −0.02 | −0.03 | −0.10 | −0.01 | 0.05 |
| Global score (n=693) ‡ | −0.01 | −0.07 | −0.07 | 0.02 | 0.01 |
Based on n=858
Unit: µg/L, mean weighted by batch
TICS = Telephone Interview of Cognitive Status; Verbal memory score is an average of the z-scores of four tests among those with complete data on all tests (10 word list Immediate recall, East Boston Immediate recall, East Boston Delayed recall, 10 words list Delayed recall); Global score is an average of the z-scores of six tests among those with complete data on all tests (TICS, East Boston Immediate recall, East Boston Delayed recall, 10 words list Delayed recall, verbal / category function, digit backwards)
Initial Cognitive Function
After adjusting for potential confounding factors, we observed no differences in mean initial test scores for the three outcomes across quartiles of total plasma carotenoids or tocopherol (Table 2). For example, for the top versus bottom quartiles of plasma carotenoids, the multivariable-adjusted mean difference on the global score was −0.05 standard units (95% confidence interval [CI] −0.19, 0.09). Similarly, for plasma tocopherol, for the top versus bottom quartiles, the mean difference on the global score was 0.04 (95% CI −0.10, 0.18). When we included both nutrients together in models, the estimates for each nutrient changed very little (data not shown). Alternatively, when we modeled plasma levels of both carotenoids and tocopherols as continuous variables, we did not observe evidence of statistically significant dose-response trends for any of the cognitive outcomes.
Table 2.
Mean difference in cognitive test performance at first interview (1995–2001) by quartiles of total plasma carotenoids and total plasma tocopherols at blood draw (1989–1990)
| Mean Difference in Cognitive Test Performance (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|
| Total Plasma Carotenoids | 1st Q (REF | 2nd Q | 3rd Q | 4th Q | P for linear trend | Test score difference per 1 standard deviation increase | |
| TICS (n=858)* | |||||||
| Age-education adjusted | 0 | −0.05 (−0.53, 0.43) | 0.22 (−0.26, 0.70) | −0.13 (−0.61, 0.35) | |||
| Multivariable adjusted† | 0 | −0.13 (−0.62, 0.36) | 0.13 (−0.37, 0.63) | −0.25 (−0.76, 0.27) | 0.64 | −0.06 (−0.24, 0.12) | |
| Verbal score (n=673)* | |||||||
| Age-education adjusted | 0 | 0.09 (−0.05, 0.23) | 0.08 (−0.06, 0.22) | −0.05 (−0.19, 0.10) | |||
| Multivariable adjusted | 0 | 0.05 (−0.11, 0.20) | 0.03 (−0.12, 0.18) | −0.11 (−0.27, 0.05) | 0.19 | −0.02 (−0.08, 0.03) | |
| Global score (n=673)* | |||||||
| Age-education adjusted | 0 | 0.14 (0.01, 0.26) | 0.12 (−0.01, 0.24) | 0.01 (−0.12, 0.14) | |||
| Multivariable adjusted | 0 | 0.09 (−0.05, 0.22) | 0.07 (−0.07, 0.20) | −0.05 (−0.19, 0.09) | 0.44 | −0.02 (−0.07, 0.03) | |
| Mean Difference in Cognitive Test Performance (95% Confidence Interval) | |||||||
| Total Plasma Tocopherols | 1st Q (REF | 2nd Q | 3rd Q | 4th Q | P for linear trend | Test score difference per 1 standard deviation increase | |
| TICS (n=858)* | |||||||
| Age-education adjusted | 0 | 0.03 (−0.45, 0.51) | 0.03 (−0.45, 0.51) | 0.19 (−0.29, 0.67) | |||
| Multivariable adjusted | 0 | 0.05 (−0.43, 0.54) | 0.14 (−0.36, 0.63) | 0.24 (−0.27, 0.75) | 0.43 | 0.06 (−0.12, 0.24) | |
| Verbal score (n=673)* | |||||||
| Age-education adjusted | 0 | −0.02 (−0.16, 0.12) | −0.02 (−0.17, 0.12) | 0.09 (−0.06, 0.23) | |||
| Multivariable adjusted | 0 | −0.01 (−0.15, 0.14) | 0.00 (−0.16, 0.15) | 0.10 (−0.06, 0.26) | 0.13 | 0.01 (−0.04, 0.07) | |
| Global score (n=673)* | |||||||
| Age-education adjusted | 0 | −0.03 (−0.16, 0.09) | −0.06 (−0.19, 0.07) | 0.02 (−0.11, 0.15) | |||
| Multivariable adjusted | 0 | −0.02 (−0.15, 0.11) | −0.04 (−0.18, 0.09) | 0.04 (−0.10, 0.18) | 0.53 | 0.01 (−0.04, 0.05) | |
TICS = Telephone Interview of Cognitive Status; Verbal memory score is an average of the z-scores of four tests among those with complete data on all tests (10 word list Immediate recall, East Boston Immediate recall, East Boston Delayed recall, 10 words list Delayed recall); Global score is an average of the z-scores of six tests among those with complete data on all tests (TICS, East Boston Immediate recall, East Boston Delayed recall, 10 words list Delayed recall, verbal / category function, digit backwards)
Adjusted for age at blood draw, age at interview, highest attained education, history of diabetes, history of high blood pressure, history of high cholesterol, postmenopausal hormone use, age at menopause, body mass index, cigarette smoking, antidepressant use, aspirin use, alcohol intake, and physical activity
We found no suggestion of interactions between either plasma carotenoids (p for interaction=0.2) or tocopherols (p for interaction =0.8) and history of cigarette smoking at blood draw (Table 3). When we examined associations by strata of current use of vitamin supplements, we observed no significant interactions for plasma tocopherol (p for interaction = 0.3); there was no suggestion of inverse relations among either women who used supplements or those who did not. But we found a borderline significant interaction between carotenoids and supplement use (p for interaction=0.04). Specifically, among women who did not use supplements, there was a suggestion that those in the highest quartile performed better than those in the lowest quartile of carotenoids (mean difference = 0.10, 95% CI = −0.13, 0.33, p=0.08). However, for women who used supplements, there was a suggestion of worsening cognition with increased plasma carotenoids (mean difference = −0.08, 95% CI = −0.29, 0.14, p=0.10).
Table 3.
Multivariable-adjusted* mean difference in global cognitive performance† (1995–2001) by quartiles of plasma carotenoids and tocopherol levels (1989–1990):effect modification by smoking status and multivitamin use
| Effect modification with smoking‡ | Mean Difference in Global Score (95% Confidence Interval) | ||||
|---|---|---|---|---|---|
| Cigarette Smoking | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | |
| Plasma Carotenoids | None (49%) | 0 (REF) | 0.14 (−0.05, 0.34) | 0.10 (−0.09, 0.29) | 0.04 (−0.15, 0.23) |
| Ever smoked (51%) | 0.06 (−0.12, 0.24) | 0.10 (−0.09, 0.29) | 0.09 (−0.10, 0.28) | −0.10 (−0.30, 0.10) | |
| p-interaction=0.23 | |||||
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
| Plasma Tocopherols | None (49%) | 0 (REF) | 0.00 (−0.19, 0.18) | 0.00 (−0.19, 0.20) | 0.06 (−0.15, 0.26) |
| Ever smoked (51%) | 0.01 (−0.17, 0.19) 0.19) | −0.03 (−0.22, 0.15) | −0.08 (−0.28, 0.12) | 0.03 (−0.16, 0.22) | |
| p-interaction=0.84 | |||||
| Effect modification with supplement use at blood draw‡ | Mean Difference in Global Score (95% Confidence Interval) | ||||
| Supplement Use | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | |
| Plasma Carotenoids | No (50%) | 0 (REF) | 0.09 (−0.13, 0.32) | 0.20 (−0.01, 0.42) | 0.10 (−0.13, 0.33) |
| Yes (50%) | 0.12 (−0.11, 0.34) | 0.17 (−0.06, 0.41) | 0.14 (−0.10, 0.38) | −0.08 (−0.29, 0.14) | |
| p-interaction=0.04 | |||||
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
| Plasma Tocopherols | No (50%) | 0 (REF) | −0.01 (−0.21, 0.18) | −0.08 (−0.30, 0.14) | −0.05 (−0.33, 0.23) |
| Yes (50%) | −0.09 (−0.35, 0.17) | −0.08 (−0.30, 0.15) | −0.16 (−0.38, 0.05) | 0.02 (−0.18, 0.21) | |
| p-interaction=0.27 | |||||
Adjusted for the same covariates in the footnotes to Table 2
Global score is an average of the z-scores of six tests: TICS, East Boston Immediate recall, East Boston Delayed recall, 10 words list Delayed recall, verbal / category function, digit backwards
Current use of multivitamins, vitamin E or vitamin A as of the biennial questionnaires immediately prior to blood draw
Because analyses of total carotenoids and tocopherols may obscure associations with individual nutrients, we examined whether levels of specific carotenoids or tocopherols may be associated with cognitive performance. We did not observe discernible associations with any of the carotenoids, tocopherols or retinol (Table 4). Similarly, when we evaluated each antioxidant as a continuous variable, we also did not observe any notable trends.
Table 4.
Multivariable-adjusted* mean difference in global cognitive performance†(1995–2001) by quartiles of individual carotenoids and tocopherols (1989–1990)
| Mean Difference in Global Score (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Plasma Antioxidant | 1st Q (REF | 2nd Q | 3rd Q | 4th Q | P for linear trend |
| α-carotene | 0 | 0.18 (−0.04, 0.31) | 0.07 (−0.07, 0.21) | −0.01 (−0.13, 0.15) | 0.57 |
| β-carotene | 0 | 0.07 (−0.06, 0.20) | 0.04 (−0.09, 0.18) | −0.05 (−0.19, 0.09) | 0.50 |
| Lutein / Zeaxanthin | 0 | 0.08 (−0.05, 0.21) | 0.12 (−0.01, 0.26) | 0.07 (−0.07, 0.20) | 0.37 |
| Lycopene | 0 | 0.13 (0.00, 0.26) | 0.06 (−0.08, 0.19) | −0.01 (−0.14, 0.12) | 0.24 |
| β-cryptoxanthin | 0 | −0.01 (−0.14, 0.12) | 0.01 (−0.13, 0.15) | 0.03 (−0.11, 0.17) | 0.83 |
| Retinol | 0 | 0.13 (−0.01, 0.26) | 0.05 (−0.09, 0.19) | 0.08 (−0.05, 0.22) | 0.44 |
| α-tocopherol | 0 | −0.05 (−0.18, 0.07) | −0.02 (−0.16, 0.11) | 0.06 (−0.08, 0.20) | 0.27 |
| γ-tocopherol | 0 | 0.01 (−0.12, 0.14) | −0.15 (−0.28, −0.01) | −0.03 (−0.17, 0.10) | 0.35 |
Adjusted for the same covariates in the footnotes to Table 2
Previous studies of vitamin E[12,30] suggest stronger inverse associations with vitamin E from diet than supplements. This may be due to the potential importance of γ tocopherol over α-tocopherol (the primary component of supplements); importantly, α-tocopherol may reduce the levels of γ tocopherol[32]. Thus we examined the association between γ tocopherol and cognitive performance within strata of plasma α-tocopherol levels. We observed a borderline significant interaction (p=0.08), where increasing levels of γ tocopherol appeared to be associated with better performance only among those with lower than median α-tocopherol levels (n=363): in this sub-group, mean difference in global score for the top quartile of γ tocopherol compared with bottom quartile = 0.08 (95% CI = −0.13, 0.29; p for linear trend=0.37). However, none of the trends or differences was statistically significant.
Cognitive Decline
Higher plasma levels of carotenoids or tocopherols were not associated with a slower decline in cognition (Table 5). For example, the difference in the mean annual rate of decline in the global score among women who were in the top quartile compared with women in the bottom quartile of total carotenoids was 0.00, (95% CI =−0.04, 0.04) and for total tocopherols was −0.01 (95% CI = −0.05, 0.03), with no discernible linear trends. Similar results were generally observed for cognitive decline as found in all the analyses described above for initial cognitive function.
Table 5.
Mean difference in rate of cognitive decline over four years by quartile of plasma carotenoids and tocopherols (n=788)
| Plasma Carotenoids | 1st Q | 2nd Q | 3rd Q | 4th Q | P for linear trend |
|---|---|---|---|---|---|
| TICS * Multivariate adjusted† |
0 (REF) | 0.00 (−0.17, 0.17) | 0.03 (−0.14, 0.20) | 0.01 (−0.16, 0.19) | 0.92 |
| Verbal Score * Multivariate adjusted |
0 (REF) | 0.00 (−0.05, 0.05) | 0.00 (−0.05, 0.05) | 0.01 (−0.04, 0.06) | 0.86 |
| Global Score * Multivariate adjusted |
0 (REF) | −0.02 (−0.06, 0.02) | 0.00 (−0.04, 0.04) | 0.00 (−0.04, 0.04) | 0.97 |
| Plasma Tocopherols | 1st Q | 2nd Q | 3rd Q | 4th Q | P for linear trend |
| TICS * Multivariate adjusted |
0 (REF) | −0.06 (−0.22, 0.11) | −0.12 (−0.29, 0.05) | −0.12 (−0.29, 0.05) | 0.13 |
| Verbal Score * Multivariate adjusted |
0 (REF) | 0.00 (−0.05, 0.04) | −0.02 (−0.07, 0.02) | −0.03 (−0.07, 0.02) | 0.08 |
| Global Score * Multivariate adjusted |
0 (REF) | −0.01 (−0.05, 0.03) | −0.02 (−0.06, 0.01) | −0.01 (−0.05, 0.03) | 0.24 |
TICS = Telephone Interview of Cognitive Status; Verbal memory score is average of the z-scores of four tests among those with complete data on all tests (10 word list Immediate recall, East Boston Immediate recall, East Boston Delayed recall, 10 words list Delayed recall); Global score is an average of the z-scores of six tests among those with complete data on all tests (TICS, East Boston Immediate recall, East Boston Delayed recall, 10 words list Delayed recall, verbal / category function, digit backwards)
Adjusted for the same covariates in the footnotes to Table 2
Denotes the estimate of the difference in rate of decline from longitudinal analyses
DISCUSSION
In this prospective study, we found no overall relation between plasma carotenoids or tocopherols or retinol levels measured when women were, on average, in their mid 60’s and cognitive function assessed 10 years later. Individual antioxidant vitamins were also not generally associated with cognitive function or decline.
We observed suggestive associations in secondary analyses that merit further investigation in future studies. First, we observed suggestions that those with higher plasma carotenoids performed better at cognitive tests in the stratum of women not taking any supplements at blood draw. It is possible that women who achieve high levels of carotenoids just from dietary sources are getting a larger variety of carotenoids that may act synergistically against brain oxidation. There is evidence that a mixture of carotenoids, as would be found in dietary sources, are more effective than individual carotenoids; such a synergistic antioxidant effect appears to be particularly strong for carotenoids mainly in diet, such as lutein and lycopene. [39]. However, because of the borderline significant results and reduced power within strata, it is possible that our findings within women not taking supplements may be due to chance.
Second, we observed a suggestive interaction between α-tocopherol and γ-tocopherol; γ-tocopherol is mainly from the diet, and plasma and tissue γ-tocopherol levels are lowered with α-tocopherol supplementation.[25,44] Consistent with this, we did observe a possible trend of higher γ-tocopherol levels associated with better cognition among those with lower than median α- tocopherol levels, 92% of whom were not taking vitamin E supplements. This is supportive of the hypothesis that different tocopherols interact in their metabolism and absorption and may thus exert independent effects on cognitive decline. [32] Again, however, our results were borderline significant and should be interpreted with caution.
An important strength of this study is the prospective design and the long-term follow-up period between blood-draw and cognitive testing. Impairments in cognition develop over many years, thus earlier exposures may have particular significance. In addition, plasma measurements have some key advantages over diet surveys, as they are not subject to errors due to recall, and better capture bioavailable nutrient levels.[19] Finally, we were able to adjust for a wide variety of potential confounding factors.
Limitations of our study should be considered. In this observational study, confounding may be an issue. However, we conducted numerous analyses to explore confounding; we adjusted for potential confounding factors at blood draw as well as at cognitive interview, and we stratified analyses by vitamin use or smoking status. Moreover, in this cohort of health professionals, with similar access to healthcare and health knowledge, many opportunities for confounding are inherently reduced. Nonetheless, it is not possible to rule out confounding in observational studies. On the other hand, the homogeneity of our study population who are all health professionals and mostly Caucasian, may limit the generalizeability of the results to other populations with different education levels and racial / ethnic backgrounds. In addition, the analyses were based on antioxidant levels from a single plasma measure, which may not represent long-term exposure, and may thus bias results toward the null. Data from similar cohorts[42,43] suggest that the correlations between repeated measurements of plasma antioxidants over time were high: e.g., intraclass correlations between single carotenoid measurements and average concentrations over a 3-year period ranged from 0.63 to 0.85, demonstrating that single samples reasonably reflect longer-term vitamin status. Finally, our analyses of cognitive decline were limited to a maximum of four years over which we measured decline. Thus, there may not yet be sufficient cognitive decline to identify modest relations with risk factors as longer follow-up is generally needed to observe clinically significant decline in cognition.[27,46] Although with approximately 10 years between blood draw and the initial cognitive assessment, our analyses of the initial cognitive interview likely reflect results from a long-term prospective study, nevertheless, we will re-examine this analysis with additional follow-up.
Few previous studies have examined antioxidants in plasma, and these have been mostly cross-sectional. In an Austrian study (n=1,769) in which 10 antioxidants were assayed, α-tocopherol was significantly associated with better performance on the Mattis Dementia Rating Scale (p=0.02), and α-carotene, β-carotene, β-cryptoxanthin and retinol all demonstrated non-significant inverse relations.[37] The NHANES study found a significant association between low serum vitamin E and poor memory among 4,809 subjects, with no relation for several other antioxidants.[33] In the Rotterdam Study[9] where plasma carotenoids in 203 healthy older persons were measured and related to white matter lesions on MRI (which are associated with cognitive impairment), those with higher overall carotenoid levels had less severe periventricular white matter lesions. Thus, cross-sectional data are supportive of higher levels of plasma antioxidants, particularly tocopherols, being associated with better cognition, but it is likely in cross-sectional studies that poor cognition resulted in poor diet, rather than the reverse, thus results from cross-sectional studies are very difficult to interpret.
In the one prospective study of plasma antioxidants and cognitive decline over 7 years that we could find, the MacArthur Study of Successful Aging (n=455) reported that higher beta-carotene was related to less cognitive decline, but only among 23% of the population with APOE e4 allele.[18] However, the subset of subjects with an e4 allele was very small and the interaction could have been due to chance. Nonetheless, we do not have available APOE genotypes for our subjects in this analysis, thus future research on a possible interaction is needed.
In conclusion, in this population of generally well-nourished and healthy aging women, plasma carotenoid and tocopherol levels measured when women in their sixties were not associated with their cognitive function or decline ten years later. Further research is needed of specific subgroups however, such as those with high antioxidant intakes solely from diet rather than supplements, and more investigation is needed of the inter-relations of γ and α-tocopherol.
Acknowledgements
The sources of financial support related to this manuscript are grants AG023860, AG15424, CA49449, and CA40356 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Both Dr. Jae Hee Kang and Dr. Francine Grodstein do not have any actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence their work.
REFERENCES
- 1.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 2.Allain CC, Poon LS, Chan CS, Richmond W, Fu P. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 3.Berr C, Balansard B, Arnaud J, Roussel AM, Alperovitch A. Cognitive decline is associated with systemic oxidative stress: the EVA study. J Am Geriatr Soc. 2000;48:1285–1291. doi: 10.1111/j.1532-5415.2000.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 4.Bourdel-Marchasson I, Delmas-Beauvieux M-C, Peuchant E, Richard-Harston S, Decamps A, Regnier B, Emeriau J-P, Rainfray M. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing. 2001;30:235–241. doi: 10.1093/ageing/30.3.235. [DOI] [PubMed] [Google Scholar]
- 5.Brandt J, Folstein MF. Telephone Interview for Cognitive Status: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 6.Cherubini A, Martin A, Andres-Lacueva C, Di Iorio A, Lamponi M, Mecocci P, Bartali B, Corsi A, Senin U, Ferrucci L. Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol Aging. 2005;26:987–994. doi: 10.1016/j.neurobiolaging.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16:357–363. doi: 10.1023/a:1007614613771. [DOI] [PubMed] [Google Scholar]
- 8.Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002;23:809–818. doi: 10.1016/s0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 9.den Heijer T, Launer LJ, de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Breteler MM. Serum carotenoids and cerebral white matter lesions: the Rotterdam scan study. J Am Geriatr Soc. 2001;49:642–646. doi: 10.1046/j.1532-5415.2001.49126.x. [DOI] [PubMed] [Google Scholar]
- 10.El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr. 2002;76:172–176. doi: 10.1093/ajcn/76.1.172. [DOI] [PubMed] [Google Scholar]
- 11.Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 12.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 13.Foy C, Passmore A, Vahidassr M, Young I, Lawson J. Plasma chain-breaking antioxidants in Alzheimer's disease, vascular dementia, and Parkinson's disease. Q J Med. 1999;92:39–45. doi: 10.1093/qjmed/92.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Han J, Hankinson SE, De Vivo I, Spiegelman D, Tamimi RM, Mohrenweiser HW, Colditz GA, Hunter DJ. A prospective study of XRCC1 haplotypes and their interaction with plasma carotenoids on breast cancer risk. Cancer Res. 2003;63:8536–8541. [PubMed] [Google Scholar]
- 15.Hankinson SE, London SJ, Chute CG, Barbieri RL, Jones L, Kaplan LA, Sacks FM, Stampfer MJ. Effect of transport conditions on the stability of biochemical markers in blood. Clinical Chemistry. 1989;35:2313–2316. [PubMed] [Google Scholar]
- 16.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, Longcope C, Speizer FE. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–1302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 17.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 18.Hu P, Bretsky P, Crimmins EM, Guralnik JM, Reuben DB, Seeman TE. Association between serum beta-carotene levels and decline of cognitive function in high-functioning older persons with or without apolipoprotein E 4 alleles: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2006;61:616–620. doi: 10.1093/gerona/61.6.616. [DOI] [PubMed] [Google Scholar]
- 19.Hunter DJ. Biochemical indicators of dietary intake. New York: Oxford University Press; 1998. pp. 174–243. [Google Scholar]
- 20.Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol. 2005;57:713–720. doi: 10.1002/ana.20476. [DOI] [PubMed] [Google Scholar]
- 21.Kang JH, Cook NR, Manson JE, Buring JE, Grodstein F. A Randomized Trial of Vitamin E and Cognitive Function in Women. Arch Intern Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 22.La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr. 1997;65:20–29. doi: 10.1093/ajcn/65.1.20. [DOI] [PubMed] [Google Scholar]
- 23.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 24.Larrieu S, Letenneur L, Helmer C, Dartigues JF, Barberger-Gateau P. Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J Nutr Health Aging. 2004;8:150–154. [PubMed] [Google Scholar]
- 25.Lehmann J, Rao DD, Canary JJ, Judd JT. Vitamin E and relationships among tocopherols in human plasma, platelets, lymphocytes, and red blood cells. Am J Clin Nutr. 1988;47:470–474. doi: 10.1093/ajcn/47.3.470. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci. 2002;99:7184–7185. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyketsos CG, Chen LS, Anthony JC. Cognitive decline in adulthood: an 11.5-year follow-up of the Baltimore Epidemiologic Catchment Area study. Am J Psychiatry. 1999;156:58–65. doi: 10.1176/ajp.156.1.58. [DOI] [PubMed] [Google Scholar]
- 28.Mecocci P, Polidori MC, Cherubini A, Ingegni T, Mattioli P, Catani M, Rinaldi P, Cecchetti R, Stahl W, Senin U, Beal MF. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch Neurol. 2002;59:794–798. doi: 10.1001/archneur.59.5.794. [DOI] [PubMed] [Google Scholar]
- 29.Meydani M. Antioxidants and cognitive function. Nutrition Reviews. 2001;59:S75–S82. doi: 10.1111/j.1753-4887.2001.tb05505.x. [DOI] [PubMed] [Google Scholar]
- 30.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal NT, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer's disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 31.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS, Aggarwal NT, Scherr PA. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr. 2005;81:508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- 33.Perkins AJ, Hendrie HC, Callahan CM, Gao S, Unverzagt FW, Xu Y, Hall KS, Hui SL. Association of antioxidants with memory in a multiethnic elderly sample using the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;150:37–44. doi: 10.1093/oxfordjournals.aje.a009915. [DOI] [PubMed] [Google Scholar]
- 34.Perrig W, Perrig P, Stahelin H. The relation between antioxidants and memory performance in the old and the very old. J Am Geriatr Soc. 1997;45:718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 35.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 36.Sack CA, Socci DJ, Crandall BM, Arendash GW. Antioxidant treatment with phenyl-a-tert-butyl nitrone improves the cognitive performance and survival of aging rats. Neurosci Lett. 1996;205:181–184. doi: 10.1016/0304-3940(96)12417-4. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt R, Hayn M, Reinhart B. Plasma antioxidants and cognitive performance in middle-aged and older adults: results of the Austrian Stroke Prevention Study. J Am Geriatr Soc. 1998;46:1407–1410. doi: 10.1111/j.1532-5415.1998.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 38.Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 39.Stahl W, Junghans A, de Boer B, Driomina ES, Briviba K, Sies H. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: synergistic effects of lycopene and lutein. FEBS Lett. 1998;427:305–308. doi: 10.1016/s0014-5793(98)00434-7. [DOI] [PubMed] [Google Scholar]
- 40.Tamimi RM, Hankinson SE, Campos H, Spiegelman D, Zhang S, Colditz GA, Willett WC, Hunter DJ. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am J Epidemiol. 2005;161:153–160. doi: 10.1093/aje/kwi030. [DOI] [PubMed] [Google Scholar]
- 41.Tamimi RM, Hankinson SE, Spiegelman D, Colditz GA, Hunter DJ. Manganese superoxide dismutase polymorphism, plasma antioxidants, cigarette smoking, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:989–996. [PubMed] [Google Scholar]
- 42.Toniolo P, van Kappel AL, Akhmedkanov A, Ferrari P, Kato I, Shore RE, Riboli E. Serum carotenoids and breast cancer. Am J Epidemiol. 2001;153:1142–1147. doi: 10.1093/aje/153.12.1142. [DOI] [PubMed] [Google Scholar]
- 43.van Kappel AL, Steghens JP, Zeleniuch-Jacquotte A, Chajes V, Toniolo P, Riboli E. Serum carotenoids as biomarkers of fruit and vegetable consumption in the New York Women's Health Study. Public Health Nutrition. 2001;4:829–835. doi: 10.1079/phn2000115. [DOI] [PubMed] [Google Scholar]
- 44.Vatassery GT, Morley JE, Kuskowski MA. Vitamin E in plasma and platelets of human diabetic patients and control subjects. Am J Clin Nutr. 1983;37:641–644. doi: 10.1093/ajcn/37.4.641. [DOI] [PubMed] [Google Scholar]
- 45.Ware J, Snow K, Kosinski M, Bandek B. SF-36 health survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 46.Wilson RS, Beckett LA, Bennett DA, Albert M, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer Disease. Arch Neurol. 1999;56:1274–1279. doi: 10.1001/archneur.56.10.1274. [DOI] [PubMed] [Google Scholar]
- 47.Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. The apolipoprotein E e4 allele and decline in different cognitve systems during a 6-year period. Arch Neurol. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 48.Yaffe K, Clemons TE, McBee WL, Lindblad AS. Impact of antioxidants, zinc, and copper on cognition in the elderly: a randomized, controlled trial. Neurology. 2004;63:1705–1707. doi: 10.1212/01.wnl.0000142969.19465.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaman Z, Roche S, Fielden P, Frost PG, Niriella DC, Cayley AC. Plasma concentrations of vitamins A and E and carotenoids in Alzheimer's disease. Age Ageing. 1992;21:91–94. doi: 10.1093/ageing/21.2.91. [DOI] [PubMed] [Google Scholar]