Abstract
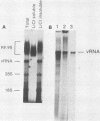
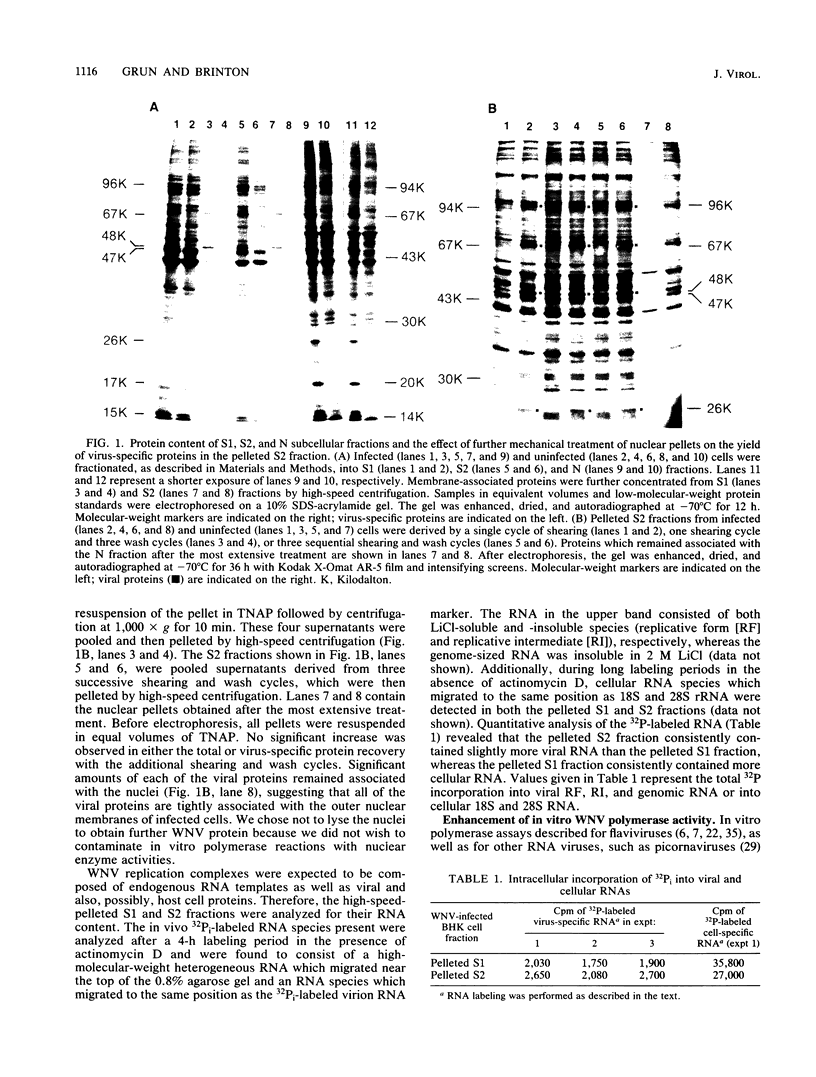
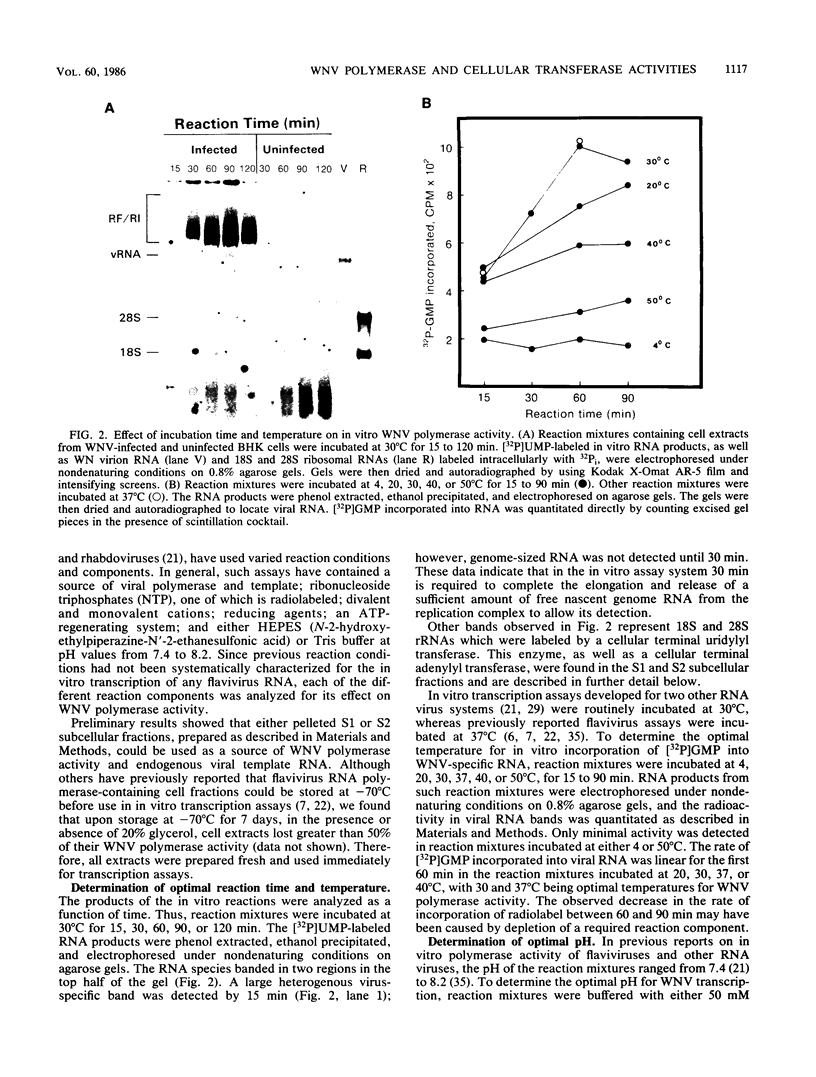
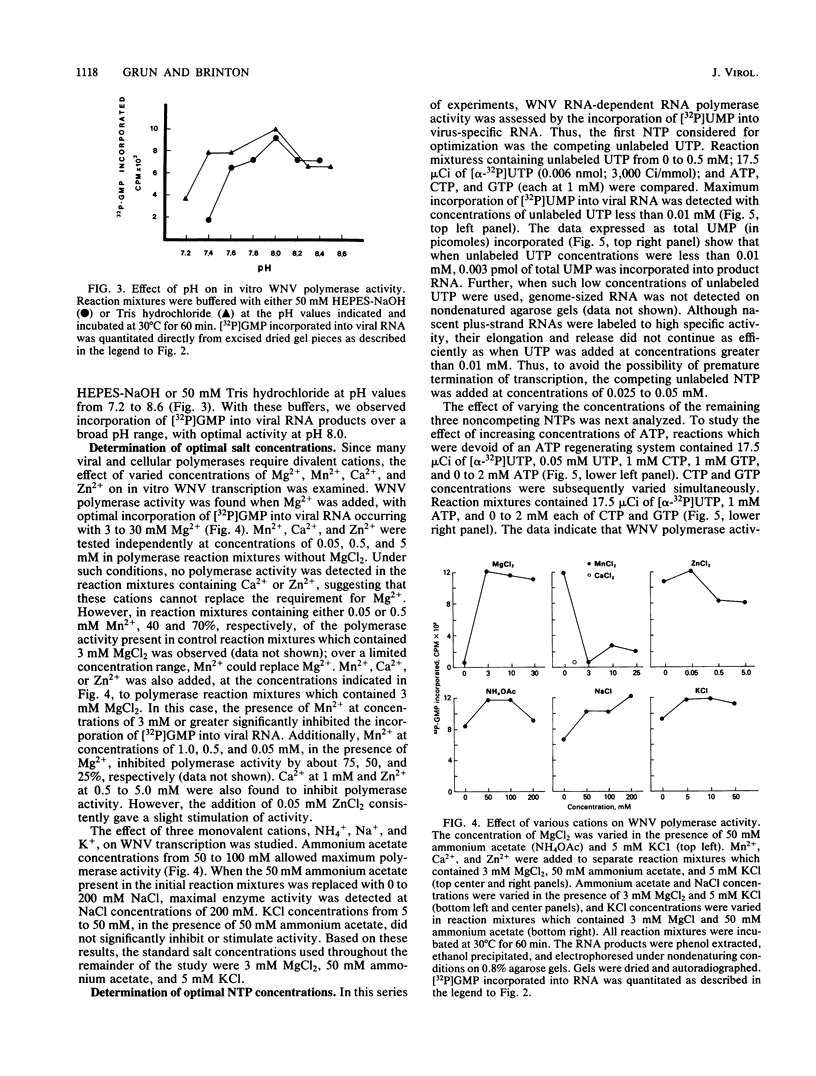
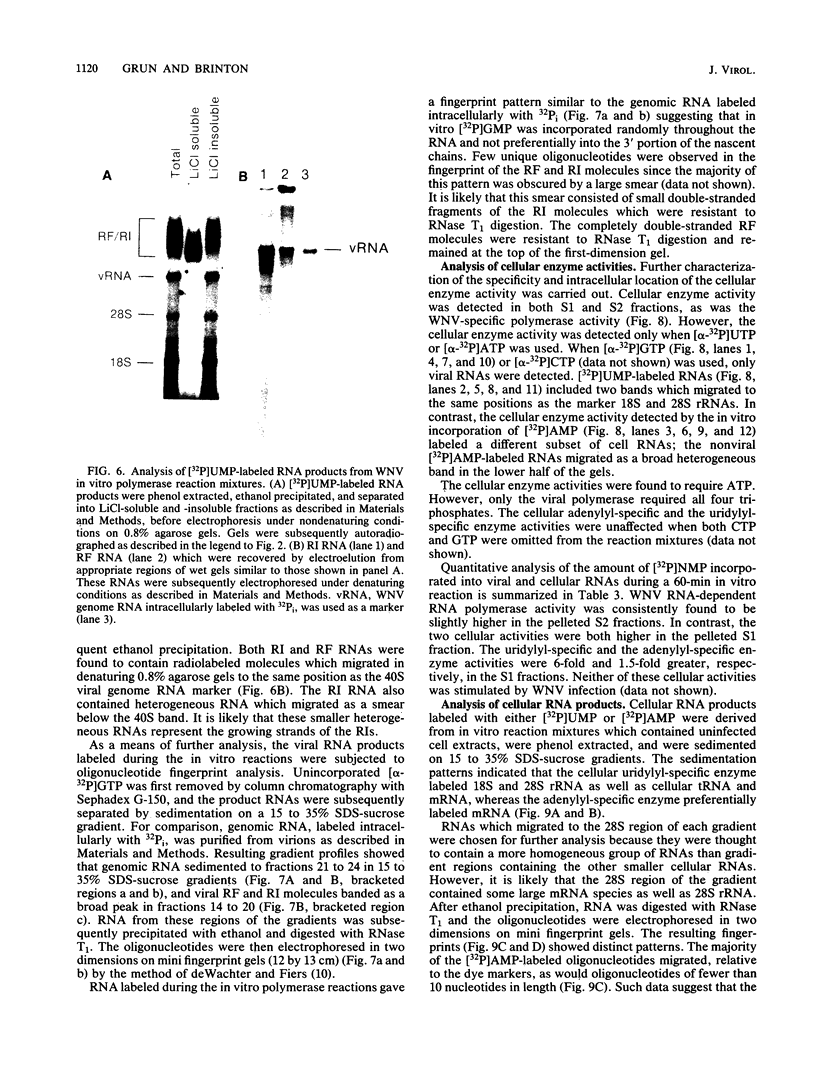
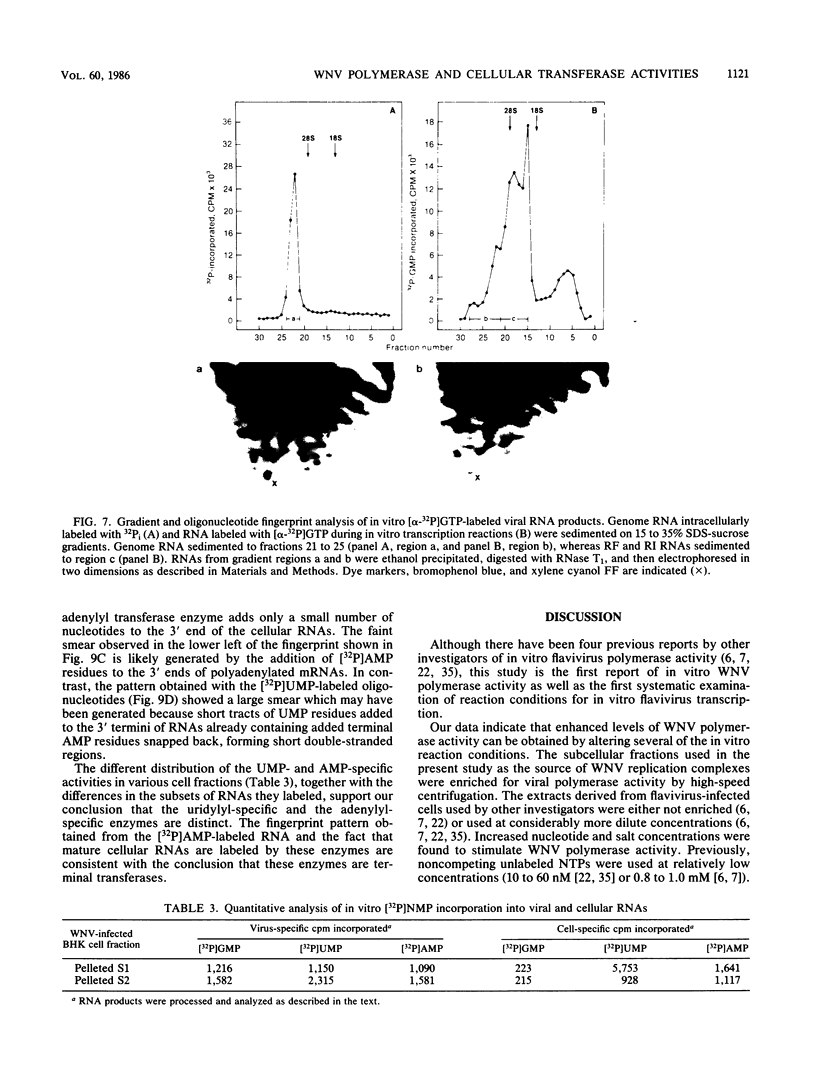
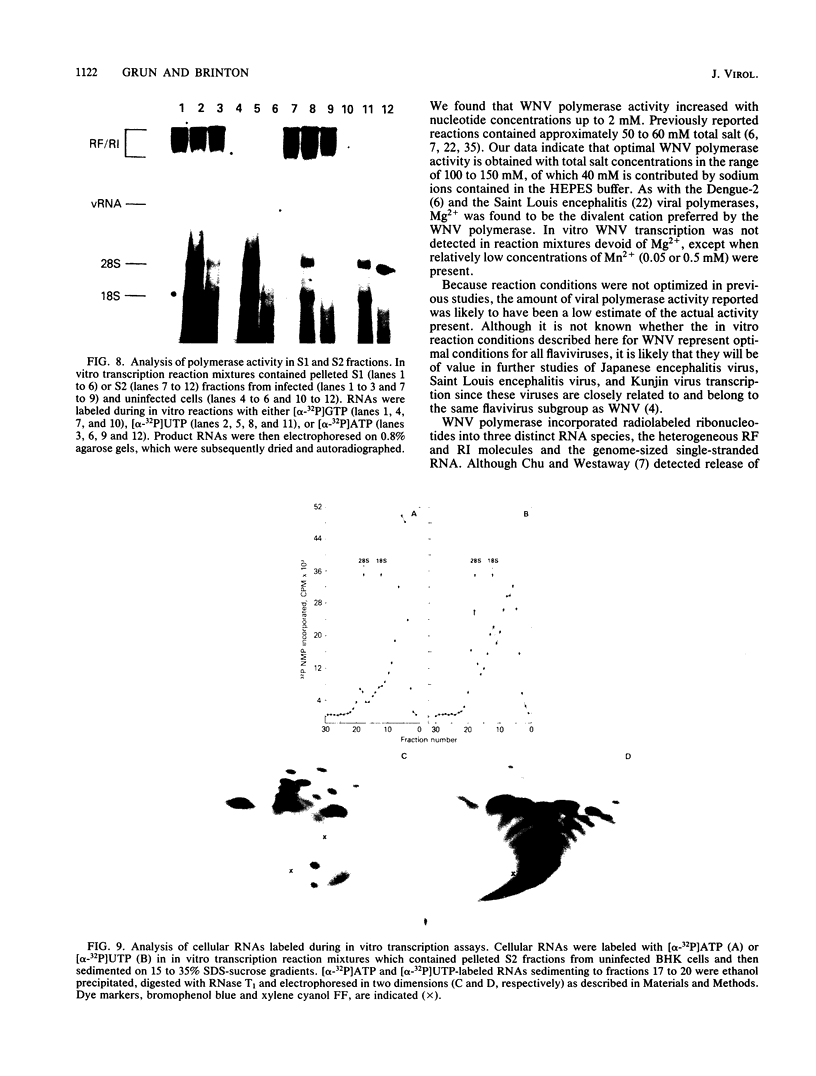
To facilitate further studies of flavivirus transcription, cell extraction methods and in vitro reaction conditions which increased West Nile virus (WNV) RNA-dependent RNA polymerase activity were determined. Subcellular fractions from WNV-infected BHK-21/W12 cells were characterized with regard to their protein and RNA content and in vitro polymerase activity. In both a cytoplasmic fraction, designated S1, and a fraction enriched for outer nuclear membranes, designated S2, seven virus-specific proteins, NS5 (96 kilodaltons [kDa]), NS3 (67 kDa), E (48 kDa), NS1 (47 kDa), ns4a (26 kDa), ns2a (17 kDa), and ns2b (14.5 kDa), were detected. The fractions also contained virus-specific RNA and cellular rRNA and mRNA. Polymerase activity in S1 and S2 fractions from WNV-infected cells was concentrated by pelleting and consisted of two types of enzyme activities: the WNV RNA-dependent RNA polymerase and terminal transferases of cellular origin. Enhanced levels of WNV polymerase activity were obtained from these cell fractions by altering several of the in vitro reaction conditions. Although Mg2+ was the divalent cation preferred by WNV polymerase, virus-specific in vitro transcription was detected at reduced levels when Mn2+ (0.05 or 0.5 mM) was present as the sole divalent cation. Product analysis revealed that the viral polymerase incorporated radiolabeled ribonucleotides into three distinct RNA species. Free single-stranded genome-sized RNA which was LiCl insoluble and RNase sensitive was found by fingerprint analysis to have an oligonucleotide pattern similar to that of WNV genomic RNA. RNA molecules which comigrated as a broad band near the top of the gel were separable into LiCl-insoluble, partially RNase-sensitive replicative-intermediate RNA and LiCl-soluble, RNase-resistant replicative-form RNA. The cellular transferases added UMP or AMP residues to the 3'-termini of cellular mRNA, tRNA, and 18S and 28S rRNA. Although a cellular terminal transferase has been reported to function in initiation of poliovirus transcription, no labeling of the WNV RNA by either of these cellular enzymes was detected. Therefore, they appear to play no specific role in flavivirus RNA synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Levin D., Baltimore D. Poliovirus replicase stimulation by terminal uridylyl transferase. J Biol Chem. 1985 Jun 25;260(12):7628–7635. [PubMed] [Google Scholar]
- Blumenthal T. Qbeta RNA replicase and protein synthesis elongation factors EF-Tu and EF-Ts. Methods Enzymol. 1979;60:628–638. doi: 10.1016/s0076-6879(79)60059-9. [DOI] [PubMed] [Google Scholar]
- Brinton M. A. Analysis of extracellular West Nile virus particles produced by cell cultures from genetically resistant and susceptible mice indicates enhanced amplification of defective interfering particles by resistant cultures. J Virol. 1983 Jun;46(3):860–870. doi: 10.1128/jvi.46.3.860-870.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton M. A., Fernandez A. V., Dispoto J. H. The 3'-nucleotides of flavivirus genomic RNA form a conserved secondary structure. Virology. 1986 Aug;153(1):113–121. doi: 10.1016/0042-6822(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Dalrymple J. M., Russell P. K. RNA polymerase in group B arbovirus (dengue-2) infected cells. Brief report. Arch Gesamte Virusforsch. 1973;40(3):392–396. doi: 10.1007/BF01242561. [DOI] [PubMed] [Google Scholar]
- Chu P. W., Westaway E. G. Replication strategy of Kunjin virus: evidence for recycling role of replicative form RNA as template in semiconservative and asymmetric replication. Virology. 1985 Jan 15;140(1):68–79. doi: 10.1016/0042-6822(85)90446-5. [DOI] [PubMed] [Google Scholar]
- Cleaves G. R., Ryan T. E., Schlesinger R. W. Identification and characterization of type 2 dengue virus replicative intermediate and replicative form RNAs. Virology. 1981 May;111(1):73–83. doi: 10.1016/0042-6822(81)90654-1. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Heinz F. X., Kunz C. Molecular epidemiology of tick-borne encephalitis virus: peptide mapping of large non-structural proteins of European isolates and comparison with other flaviviruses. J Gen Virol. 1982 Oct;62(Pt 2):271–285. doi: 10.1099/0022-1317-62-2-271. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Haruna I., Watanabe I., Mikoshiba K., Tsukada Y. Poly(U) polymerase in rat brain. Nature. 1975 Jul 24;256(5515):337–339. doi: 10.1038/256337a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landers T. A., Blumenthal T., Weber K. Function and structure in ribonucleic acid phage Q beta ribonucleic acid replicase. The roles of the different subunits in transcription of synthetic templates. J Biol Chem. 1974 Sep 25;249(18):5801–5808. [PubMed] [Google Scholar]
- Leung W. C., Leung M. F., Rawls W. E. Distinctive RNA transcriptase, polyadenylic acid polymerase, and polyuridylic acid polymerase activities associated with Pichinde virus. J Virol. 1979 Apr;30(1):98–107. doi: 10.1128/jvi.30.1.98-107.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubiniecki A. S., Henry C. J. Autoradiographic localization of RNA synthesis directed by arboviruses in the cytoplasm of infected BHK-21 cells. Proc Soc Exp Biol Med. 1974 Apr;145(4):1165–1169. doi: 10.3181/00379727-145-37973. [DOI] [PubMed] [Google Scholar]
- Milchev G. I., Hadjiolov A. A. Association of poly(A) and poly(U) polymerases with cytoplasmic ribosomes. Eur J Biochem. 1978 Mar;84(1):113–121. doi: 10.1111/j.1432-1033.1978.tb12147.x. [DOI] [PubMed] [Google Scholar]
- Morrow C. D., Lubinski J., Hocko J., Gibbons G. F., Dasgupta A. Purification of a soluble template-dependent rhinovirus RNA polymerase and its dependence on a host cell protein for viral RNA synthesis. J Virol. 1985 Jan;53(1):266–272. doi: 10.1128/jvi.53.1.266-272.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M. L., Pedersen J. S., Toh B. H., Westaway E. G. Immunofluorescent sites in vero cells infected with the flavivirus Kunjin. Arch Virol. 1983;78(3-4):177–190. doi: 10.1007/BF01311313. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Initiation and replication of vesicular stomatitis virus genome RNA in a cell-free system. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3198–3202. doi: 10.1073/pnas.80.11.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A. A., Trent D. W. Saint Louis encephalitis viral ribonucleic acid replication complex. J Virol. 1972 Apr;9(4):565–573. doi: 10.1128/jvi.9.4.565-573.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Lenches E. M., Eddy S. R., Shin S. J., Sheets R. L., Strauss J. H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985 Aug 23;229(4715):726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Jelinek W., Darnell J. E. 3'-Terminal addition to HeLa cell nuclear and cytoplasmic poly (A). J Mol Biol. 1977 Jun 15;113(1):219–235. doi: 10.1016/0022-2836(77)90051-1. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Monath T. P. Monoclonal antibodies distinguish between wild and vaccine strains of yellow fever virus by neutralization, hemagglutination inhibition, and immune precipitation of the virus envelope protein. Virology. 1983 Feb;125(1):8–17. doi: 10.1016/0042-6822(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Smith G. W., Wright P. J. Synthesis of proteins and glycoproteins in dengue type 2 virus-infected vero and Aedes albopictus cells. J Gen Virol. 1985 Mar;66(Pt 3):559–571. doi: 10.1099/0022-1317-66-3-559. [DOI] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegami T., Semler B. L., Anderson C. W., Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983 Jul 15;128(1):33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Tuschall D. M., Hiebert E., Flanegan J. B. Poliovirus RNA-dependent RNA polymerase synthesizes full-length copies of poliovirion RNA, cellular mRNA, and several plant virus RNAs in vitro. J Virol. 1982 Oct;44(1):209–216. doi: 10.1128/jvi.44.1.209-216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A., Maes R. Cytopathic effect of rubella virus in RHK21 cells and growth to high titers in suspension culture. Virology. 1965 Oct;27(2):239–241. doi: 10.1016/0042-6822(65)90170-4. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. A., Flanegan J. B. Identification of poliovirus polypeptide P63 as a soluble RNA-dependent RNA polymerase. J Virol. 1980 Sep;35(3):732–740. doi: 10.1128/jvi.35.3.732-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway E. G., Brinton M. A., Gaidamovich SYa, Horzinek M. C., Igarashi A., Käriäinen L., Lvov D. K., Porterfield J. S., Russell P. K., Trent D. W. Flaviviridae. Intervirology. 1985;24(4):183–192. doi: 10.1159/000149642. [DOI] [PubMed] [Google Scholar]
- Young R. A., Blumenthal T. Phage Q-beta ribonucleic acid replicase. Subunit relationships determined by intramolecular cross-linking. J Biol Chem. 1975 Mar 10;250(5):1829–1832. [PubMed] [Google Scholar]
- Zabel P., Dorssers L., Wernars K., Van Kammen A. Terminal uridylyl transferase of Vigna unguiculata: purification and characterization of an enzyme catalyzing the addition of a single UMP residue to the 3'-end of an RNA primer. Nucleic Acids Res. 1981 Jun 11;9(11):2433–2453. doi: 10.1093/nar/9.11.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebovitz E., Leong J. K., Doughty S. C. Involvement of the host cell nuclear envelope membranes in the replication of Japanese encephalitis virus. Infect Immun. 1974 Jul;10(1):204–211. doi: 10.1128/iai.10.1.204-211.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]